Effect of Vacuum Roasting on Total Selenium Content of Selenium-Enriched Rapeseed, Maillard Reaction Products, Oxidative Stability and Physicochemical Properties of Selenium-Enriched Rapeseed Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Roasting and Oil Extraction
2.3. Physicochemical Properties (AV, PV, CV, CD, CT, BaP)
2.4. Carotenoids
2.5. Total Phenolic Content (TPC)
2.6. Tocopherols
2.7. Radical Scavenging Activity (RSA)
2.8. Oxidative Stability Index (OSI)
2.9. Fatty Acid Composition (FAC)
2.10. Maillard Reaction Products (MRPs)
2.10.1. Browning Index (BI)
2.10.2. 5-Hydroxymethylfurfural (HMF)
2.10.3. Free Fluorescent Intermediate Compounds (FIC)
2.11. Total Selenium Content
2.12. Statistical Analysis
3. Results and Discussion
3.1. Oil Yield
3.2. Physicochemical Properties
3.2.1. Acid Value (AV)
3.2.2. Peroxide Value (PV)
3.2.3. Carbonyl Value (CV)
3.2.4. Conjugated Dienes and Trienes (CD and CT)
3.2.5. Benzo[a]pyrene (BaP)
3.3. Carotenoids
3.4. Total Phenolic Content (TPC)
3.5. Tocopherols
3.6. Radical Scavenging Activity (RSA)
3.7. Oxidative Stability Index (OSI)
3.8. Fatty Acid Composition (FAC)
3.9. Maillard Reaction Products (MRPs)
3.10. Total Selenium Content
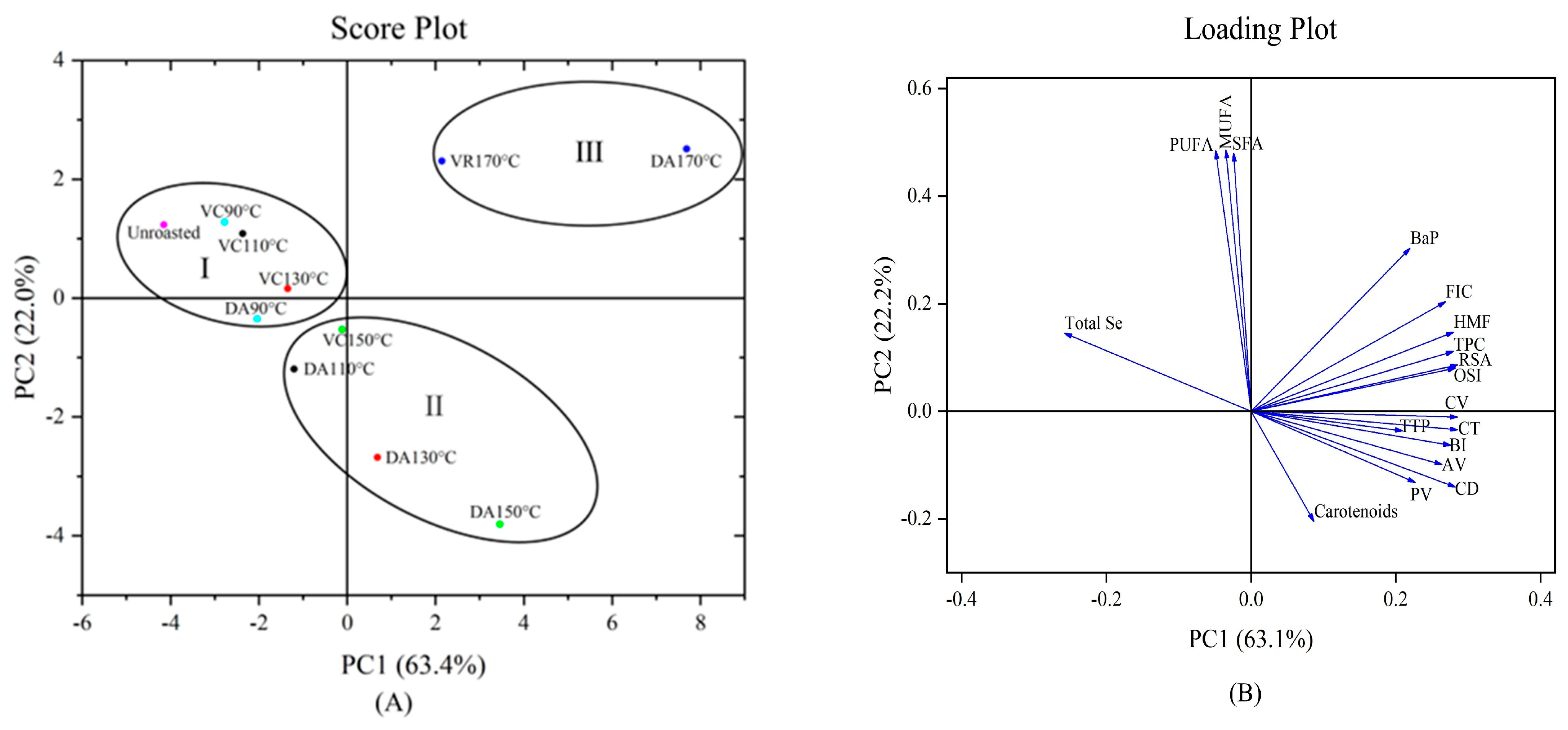
4. Principal Component Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Terms | Letter abbreviations |
| Selenium-enriched rapeseed | SER |
| Vacuum | VC |
| Dry air | DA |
| Lipid oxidation products | LOPs |
| Maillard reaction products | MRPs |
| Acid value | AV |
| Peroxide value | PV |
| Carbonyl value | CV |
| Conjugated dienes | CD |
| Conjugated trienes | CT |
| Total phenolic content | TPC |
| Radical scavenging activity | RSA |
| Oxidative stability index | OSI |
| Browning index | BI |
| 5-Hydroxymethylfurfural | HMF |
| Free fluorescent intermediate Compounds | FIC |
| Benzo(a)pyrene | BaP |
| Saturated fatty acids | SFA |
| Monounsaturated fatty acids | MUFA |
| Polyunsaturated fatty acids | PUFA |
| Total tocopherol | TTP |
References
- Szydlowska-Czerniak, A.; Tulodziecka, A.; Momot, M.; Stawicka, B. Physicochemical, Antioxidative, and Sensory Properties of Refined Rapeseed Oils. J. Am. Oil Chem. Soc. 2019, 96, 405–419. [Google Scholar] [CrossRef]
- Peeters, K.; Tenorio, A.T. Comparing Analytical Methods for Erucic Acid Determination in Rapeseed Protein Products. Foods 2022, 11, 815. [Google Scholar] [CrossRef]
- DB 61/T 556-2018; Standard for Selenium Content in Selenium-Enriched/Selenium-Containing Foods and Related Products. Administration of Quality and Technology Supervision of Shaanxi Province: Ankang, China, 2018.
- Kieliszek, M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Impact of infrared and dry air roasting on the oxidative stability, fatty acid composition, Maillard reaction products and other chemical properties of black cumin (Nigella sativa L.) seed oil. Food Chem. 2019, 295, 537–547. [Google Scholar] [CrossRef]
- Zou, Y.P.; Gao, Y.Y.; He, H.; Yang, T.K. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. Lwt-Food Sci. Technol. 2018, 90, 246–253. [Google Scholar] [CrossRef]
- Wei, G.-J.; Ho, C.-T.; Huang, A.S. Determination of volatile compounds formed in a glucose-selenomethionine model system by gas chromatography-atomic emission detector and gas chromatography-mass spectrometry. Food Chem. 2009, 116, 774–778. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Jing, B.; Guo, R.; Wang, M.; Zhang, L.; Yu, X. Influence of seed roasting on the quality of glucosinolate content and flavor in virgin rapeseed oil. Lwt-Food Sci. Technol. 2020, 126, 109301. [Google Scholar] [CrossRef]
- Gulcan, U.; Uslu, C.C.; Mutlu, C.; Arslan-Tontul, S.; Erba, M. Impact of inert and inhibitor baking atmosphere on HMF and acrylamide formation in bread. Food Chem. 2020, 332, 127434. [Google Scholar] [CrossRef]
- Anese, M.; Nicoli, M.C.; Verardo, G.; Munari, M.; Mirolo, G.; Bortolomeazzi, R. Effect of vacuum roasting on acrylamide formation and reduction in coffee beans. Food Chem. 2014, 145, 168–172. [Google Scholar] [CrossRef]
- Rattana, M.; Yongyut, C.; Supachet, P.; Sukhuntha, O. Effect of roasting and vacuum microwave treatments on physicochemical and antioxidant properties of oil extracted from black sesame seeds. Curr. Res. Nutr. Food Sci. 2020, 8, 798–814. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, D.; Zhang, X.; Zhao, H.; Gong, G.; Tang, X.; Li, L. Drying performance and energy consumption of Camelliaoleifera seeds under microwave-vacuum drying. Food Sci. Biotechnol. 2023, 32, 969–977. [Google Scholar] [CrossRef] [PubMed]
- AOCS; Firestone, D. Official methods and recommended practices of the American Oil Chemists’ Society. AOCS 1998, 5, 2–93. [Google Scholar]
- IUPAC; Paquot, C. Standard Methods for the Analysis of Oils, Fats and Derivatives; Blackwell Scientific Publications: Oxford, UK, 1987; pp. 99–102. [Google Scholar]
- Delfan-Hosseini, S.; Nayebzadeh, K.; Mirmoghtadaie, L.; Kavosi, M.; Hosseini, S.M. Effect of extraction process on composition, oxidative stability and rheological properties of purslane seed oil. Food Chem. 2017, 222, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Multescu, M.; Marinas, I.C.; Susman, I.E.; Belc, N. Byproducts (Flour, Meals, and Groats) from the Vegetable Oil Industry as a Potential Source of Antioxidants. Foods 2022, 11, 253. [Google Scholar] [CrossRef]
- Suri, K.C.; Singh, B.; Kaur, A.; Yadav, M.P.; Singh, N. Influence of microwave roasting on chemical composition, oxidative stability and fatty acid composition of flaxseed (Linum usitatissimum L.) oil. Food Chem. 2020, 326, 126974. [Google Scholar] [CrossRef]
- Rattanathanalerk, M.; Chiewchan, N.; Srichumpoung, W. Effect of thermal processing on the quality loss of pineapple juice. J. Food Eng. 2005, 66, 259–265. [Google Scholar] [CrossRef]
- Shrestha, K.; De Meulenaer, B. Antioxidant activity of Maillard type reaction products between phosphatidylethanolamine and glucose. Food Chem. 2014, 161, 8–15. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A. Impact of microwave roasting on physicochemical properties, maillard reaction products, antioxidant activity and oxidative stability of nigella seed (Nigella sativa L.) oil. Food Chem. 2022, 368, 130777. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, M.; Mujumdar, A.S. Effect of various pretreatments on the quality of vacuum-fried carrot chips. Dry. Technol. 2006, 24, 1481–1486. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission. Report of the twenty-first session of the Codex Committee on Fats and Oils. In FAO/WHO Food Standards Programme; ALINORM 09/32/17; Codex Alimentarius Commission: Rome, Italy, 2009; p. vi-46. [Google Scholar]
- Jose Crosa, M.; Skerl, V.; Cadenazzi, M.; Olazabal, L.; Silva, R.; Suburu, G.; Torres, M. Changes produced in oils during vacuum and traditional frying of potato chips. Food Chem. 2014, 146, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Wongso, I. Drying and quality characteristics of almonds and walnuts with different drying conditions. Masters Abstr. Int. 2021, 83. [Google Scholar]
- Albertos, I.; Martin-Diana, A.B.; Jaime, I.; Diez, A.M.; Rico, D. Protective role of vacuum vs. atmospheric frying on PUFA balance and lipid oxidation. Innov. Food Sci. Emerg. Technol. 2016, 36, 336–342. [Google Scholar] [CrossRef]
- Karademir, Y.; Goncuoglu, N.; Gokmen, V. Investigation of heat induced reactions between lipid oxidation products and amino acids in lipid rich model systems and hazelnuts. Food Funct. 2013, 4, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.M.B.; Khaneghah, A.M.; Koubaa, M.; Lopez-Cervantes, J.; Yousefabad, S.H.A.; Hosseini, S.F.; Karimi, M.; Motazedian, A.; Asadifard, S. Novel edible oil sources: Microwave heating and chemical properties. Food Res. Int. 2017, 92, 147–153. [Google Scholar] [CrossRef]
- Zyzelewicz, D.; Budryn, G.; Krysiak, W.; Oracz, J.; Nebesny, E.; Bojczuk, M. Influence of roasting conditions on fatty acid composition and oxidative changes of cocoa butter extracted from cocoa bean of Forastero variety cultivated in Togo. Food Res. Int. 2014, 63, 328–343. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Zhu, W.; Ma, Y.; Wang, H.; Li, C. Effects of roasting temperature on the flavor and comprehensive quality of fragrant Cyperus esculentus oil. China Oils Fats 2022, 47, 1–7. [Google Scholar]
- Cheng, W.; Liu, G.; Wang, X.; Liu, X.; Liu, B. Formation of Benzo(a)pyrene in Sesame Seeds During the Roasting Process for Production of Sesame Seed Oil. J. Am. Oil Chem. Soc. 2015, 92, 1725–1733. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A.; Singh, N. Influence of dry air and infrared pre-treatments on oxidative stability, Maillard reaction products and other chemical properties of linseed (Linum usitatissimum L.) oil. J. Food Sci. Technol.-Mysore 2022, 59, 366–376. [Google Scholar] [CrossRef]
- An, J.; Adelina, N.M.; Zhang, L.; Zhao, Y. Effect of roasting pre-treatment of two grafted pine nuts (Pinus koraiensis) on yield, color, chemical compositions, antioxidant activity, and oxidative stability of the oil. J. Food Process. Preserv. 2022, 46, e16145. [Google Scholar] [CrossRef]
- Feng, Z.; Aslam Khan, M.; Hao, C.; Li, L. Co-encapsulation of alpha-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Jiang, S.H.; Xie, Y.F.; Li, M.; Guo, Y.H.; Cheng, Y.L.; Qian, H.; Yao, W.R. Evaluation on the oxidative stability of edible oil by electron spin resonance spectroscopy. Food Chem. 2020, 309, 125714. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Han, Y.F.; Wang, N.N.; Liu, H.M.; Zheng, Y.Z.; Wang, X.D. Improvement of the oxidative stability of cold-pressed sesame oil using products from the Maillard reaction of sesame enzymatically hydrolyzed protein and reducing sugars. J. Sci. Food Agric. 2020, 100, 1524–1531. [Google Scholar] [CrossRef]
- Mohamed Ahmed, I.A.; Al Juhaimi, F.Y.; Magdi, A.O.; Al Maiman, S.A.; Amro, B.H.; Hesham, A.S.A.; Elfadil, E.B.; Kashif, G. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT-Food Sci. Technol. 2020, 131, 109825. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, V.; Kumar, S.; Majid, I.; Aggarwal, P.; Suri, S. 5-Hydroxymethylfurfural (HMF) formation, occurrence and potential health concerns: Recent developments. Toxin Rev. 2021, 40, 545–561. [Google Scholar] [CrossRef]
- Hang, Y.; Ruyue, Z.; Fangwei, Y.; Yunfei, X.; Yahui, G.; Weirong, Y.; Weibiao, Z. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- Rozanska, M.B.; Kowalczewski, P.L.; Tomaszewska-Gras, J.; Dwiecki, K.; Mildner-Szkudlarz, S. Seed-Roasting Process Affects Oxidative Stability of Cold-Pressed Oils. Antioxidants 2019, 8, 313. [Google Scholar] [CrossRef]
- Palazoglu, T.K.; Coskun, Y.; Tuta, S.; Mogol, B.A.; Gokmen, V. Effect of vacuum-combined baking of cookies on acrylamide content, texture and color. Eur. Food Res. Technol. 2015, 240, 243–249. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Deng, X.; Li, M.; Zhang, W.; Zhao, Z. Effects of sulfur and sulfate on selenium uptake and quality of seeds in rapeseed (Brassica napus L.) treated with selenite and selenate. Environ. Exp. Bot. 2017, 135, 13–20. [Google Scholar] [CrossRef]
- Wang, P.; Li, Y.; Yu, R.; Huang, D.; Chen, S.; Zhu, S. Effects of Different Drying Methods on the Selenium Bioaccessibility and Antioxidant Activity of Cardamine violifolia. Foods 2023, 12, 758. [Google Scholar] [CrossRef]
- Gao, L.; Luo, D.; Hu, X.; Wu, J. Se in Se-enriched peanut, and losses during peanut protein preparation. Int. J. Food Sci. Technol. 2017, 52, 843–850. [Google Scholar] [CrossRef]
| Parameters | Unroasted (Raw) | DA 90 °C | VC 90 °C | DA 110 °C | VC 110 °C | DA 130 °C | VC 130 °C | DA 150 °C | VC 150 °C | DA 170 °C | VC 170 °C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oil yield (%) | 32.38 ± 0.33 d | 31.71 ± 0.11 e | 31.89 ± 0.43 de | 32.45 ± 0.43 d | 31.55 ± 0.14 e | 33.51 ± 0.29 c | 35.58 ± 0.21 a | 35.13 ± 0.31 a | 35.13 ± 0.73 a | 30.01 ± 0.35 f | 34.18 ± 0.12 b |
| AV (mg KOH/g) | 0.14 ± 0.00 g | 0.17 ± 0.01 e | 0.15 ± 0.00 fg | 0.18 ± 0.00 d | 0.15 ± 0.01 f | 0.20 ± 0.00 b | 0.19 ± 0.00 c | 0.22 ± 0.01 a | 0.20 ± 0.01 bc | 0.22 ± 0.00 a | 0.22 ± 0.01 a |
| PV (mmol/kg) | 0.44 ± 0.01 f | 0.58 ± 0.02 e | 0.30 ± 0.02 h | 0.66 ± 0.02 d | 0.31 ± 0.01 gh | 0.77 ± 0.04 c | 0.35 ± 0.02 g | 0.84 ± 0.01 b | 0.35 ± 0.02 g | 1.13 ± 0.06 a | 0.35 ± 0.01 g |
| CV (meq/kg) | 1.16 ± 0.01 f | 1.39 ± 0.04 e | 1.31 ± 0.06 e | 1.41 ± 0.07 e | 1.23 ± 0.10 e | 2.11 ± 0.08 c | 1.56 ± 0.07 d | 2.27 ± 0.10 b | 2.16 ± 0.04 bc | 3.12 ± 0.16 a | 2.05 ± 0.19 bc |
| CD | 1.35 ± 0.06 c | 1.58 ± 0.02 bc | 1.47 ± 0.06 c | 1.63 ± 0.08 bc | 1.48 ± 0.06 c | 1.82 ± 0.02 b | 1.63 ± 0.06 bc | 2.16 ± 0.12 a | 1.78 ± 0.02 b | 2.16 ± 0.48 a | 1.82 ± 0.04 b |
| CT | 0.13 ± 0.01 h | 0.22 ± 0.01 ef | 0.19 ± 0.01 g | 0.23 ± 0.00 de | 0.19 ± 0.01 g | 0.25 ± 0.01 d | 0.21 ± 0.01 f | 0.50 ± 0.01 b | 0.24 ± 0.01 d | 0.58 ± 0.01 a | 0.32 ± 0.02 c |
| Carotenoids (mg/kg) | 42.87 ± 1.67 h | 46.89 ± 1.12 g | 56.86 ± 1.55 e | 50.85 ± 1.37 f | 62.54 ± 0.94 d | 71.87 ± 0.40 b | 69.08 ± 1.78 c | 68.91 ± 1.95 c | 76.48 ± 1.76 a | 55.26 ± 1.55 e | 61.93 ± 0.87 d |
| TPC (mg GAE/kg) | 151.29 ± 2.11 h | 188.48 ± 3.16 g | 184.74 ± 1.77 g | 200.01 ± 4.11 f | 199.46 ± 2.93 f | 258.81 ± 4.04 d | 219.18 ± 2.01 e | 491.47 ± 4.61 b | 253.50 ± 3.45 d | 967.49 ± 8.81 a | 391.96 ± 4.83 c |
| RSA (mmol/kg Trolox) | 2.42 ± 0.07 f | 2.51 ± 0.10 ef | 2.47 ± 0.07 ef | 2.77 ± 0.24 d | 2.51 ± 0.07 ef | 3.05 ± 0.06 c | 2.58 ± 0.01 ef | 3.34 ± 0.03 b | 2.64 ± 0.04 de | 4.64 ± 0.02 a | 3.33 ± 0.08 b |
| OSI(h) | 5.46 ± 0.07 e | 5.63 ± 0.06 e | 5.51 ± 0.08 e | 5.68 ± 0.12 e | 5.58 ± 0.07 e | 6.26 ± 0.04 d | 5.64 ± 0.04 e | 8.35 ± 0.01 b | 6.37 ± 0.21 d | 11.55 ± 0.52 a | 7.16 ± 0.20 c |
| BI (Abs 420 nm) | 0.17 ± 0.00 h | 0.20 ± 0.01 fg | 0.20 ± 0.02 g | 0.22 ± 0.01 e | 0.20 ± 0.02 ef | 0.25 ± 0.00 cd | 0.22 ± 0.01 e | 0.26 ± 0.01 bc | 0.24 ± 0.00 d | 0.28 ± 0.01 a | 0.27 ± 0.01 b |
| HMF (mg/kg) | 2.17 ± 0.01 g | 2.34 ± 0.05 g | 2.32 ± 0.04 g | 2.28 ± 0.01 g | 2.29 ± 0.01 g | 3.82 ± 0.02 e | 3.39 ± 0.03 f | 7.76 ± 0.23 c | 4.50 ± 0.08 d | 17.2 ± 0.17 a | 8.62 ± 0.02 b |
| FIC (%) | 6.46 ± 0.08 i | 7.32 ± 0.40 hi | 6.81 ± 0.01 hi | 9.15 ± 0.36 fg | 8.14 ± 0.00 gh | 11.19 ± 0.50 de | 10.30 ± 0.14 ef | 21.11 ± 1.10 c | 11.92 ± 0.20 d | 61.12 ± 1.14 a | 36.37 ± 2.39 b |
| Total Se in meal (μg/kg) | 218.92 ± 0.55 a | 187.86 ± 1.81 d | 203.22 ± 1.95 b | 182.99 ± 3.88 e | 197.53 ± 2.37 c | 172.20 ± 1.85 g | 196.90 ± 1.14 c | 170.97 ± 2.11 g | 189.80 ± 1.15 d | 165.17 ± 1.81 h | 175.66 ± 0.87 f |
| Total Se in oil (μg/kg) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| BaP (μg/kg) | ND | ND | ND | ND | ND | ND | ND | ND | ND | 0.68 ± 0.05 a | 0.59 ± 0.03 b |
| DF | Oil Yield | AV | PV | CV | CD | CT | Carotenoids | TPC | RSA | OSI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature | 4 | 116.91 ** | 10.91 ** | 166.78 ** | 178.09 ** | 81.67 ** | 607.54 ** | 248.50 ** | 13,186.57 ** | 226.99 ** | 269.69 ** |
| Vacuum | 1 | 70.99 ** | 46.60 ** | 1606.13 ** | 115.21 ** | 121.15 ** | 1014.27 ** | 166.00 ** | 11,420.08 ** | 254.21 ** | 268.24 ** |
| Interaction | 4 | 47.43 ** | 10.86 ** | 251.00 ** | 25.74 ** | 9.00 ** | 182.23 ** | 23.90 ** | 4707.09 ** | 38.43 ** | 85.29 ** |
| DF | α-tocopherol | γ-tocopherol | δ-tocopherol | HMF | FIC | Total Se | SFA | MUFA | PUFA | BI | |
| Temperature | 4 | 3.01 * | 5.45 ** | 2490.02 ** | 2050.61 ** | 131.10 ** | 6.90 ** | 3.92 * | 6.14 ** | 75.91 ** | |
| Vacuum | 1 | 10.82 ** | 18.86 ** | 948.08 ** | 449.51 ** | 499.12 ** | 18.98 ** | 15.98 ** | 22.88 ** | 25.83 ** | |
| Interaction | 4 | 426.26 ** | 184.80 ** | 10.06 ** |
| Tocopherols (mg/kg) | Unroasted (Raw) | DA 90 °C | VC 90 °C | DA 110 °C | VC 110 °C | DA 130 °C | VC 130 °C | DA 150 °C | VC 150 °C | DA 170 °C | VC 170 °C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| α-tocopherol | 339.67 ± 9.21 c | 360.22 ± 10.13 ab | 363.71 ± 6.84 ab | 359.27 ± 6.40 ab | 367.39 ± 5.81 ab | 359.24 ± 9.63 ab | 365.72 ± 6.36 ab | 351.35 ± 15.20 bc | 374.38 ± 5.09 a | 338.10 ± 23.11 c | 361.88 ± 4.34 ab |
| γ-tocopherol | 703.36 ± 10.84 c | 719.49 ± 29.82 abc | 747.62 ± 24.35 bc | 744.71 ± 8.90 abc | 752.52 ± 12.89 ab | 731.06 ± 26.62 bc | 746.13 ± 6.54 abc | 787.16 ± 38.81 a | 749.77 ± 12.16 ab | 787.29 ± 45.25 a | 758.73 ± 2.34 ab |
| δ-tocopherol | 27.79 ± 1.17 d | 33.00 ± 0.86 c | 37.25 ± 0.90 ab | 36.47 ± 0.61 ab | 38.60 ± 0.65 a | 36.79 ± 0.88 ab | 38.90 ± 2.39 a | 34.96 ± 1.44 bc | 37.48 ± 1.69 a | 38.38 ± 1.48 a | 37.89 ± 1.31 a |
| Total tocopherol | 1081.25 ± 19.23 b | 1123.75 ± 40.32 ab | 1156.38 ± 30.83 a | 1147.49 ± 15.57 a | 1165.00 ± 19.11 a | 1137.65 ± 36.52 ab | 1159.57 ± 14.78 a | 1190.21 ± 54.90 a | 1168.47 ± 18.29 a | 1188.46 ± 69.69 a | 1164.19 ± 5.11 a |
| Fatty Acids (g/100 g) | Unroasted (Raw) | DA 90 °C | VC 90 °C | DA 110 °C | VC 110 °C | DA 130 °C | VC 130 °C | DA 150 °C | VC 150 °C | DA 170 °C | VC 170 °C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C16:0 (Palmitic) | 3.71 ± 0.07 a | 3.58 ± 0.03 ab | 3.64 ± 0.08 ab | 3.49 ± 0.05 bc | 3.65 ± 0.03 a | 3.49 ± 0.13 bc | 3.64 ± 0.12 ab | 3.41 ± 0.02 cd | 3.60 ± 0.06 ab | 3.29 ± 0.09 d | 3.58 ± 0.11 ab |
| C18:0 (stearic) | 1.14 ± 0.02 a | 1.11 ± 0.07 a | 1.16 ± 0.03 a | 1.17 ± 0.09 a | 1.11 ± 0.09 a | 1.12 ± 0.07 a | 1.13 ± 0.08 a | 1.13 ± 0.03 a | 1.13 ± 0.05 a | 1.09 ± 0.02 a | 1.09 ± 0.04 a |
| C18:1n9c (Oleic) | 61.89 ± 0.73 a | 60.90 ± 0.38 ab | 61.54 ± 0.29 a | 59.68 ± 0.46 abc | 61.43 ± 0.32 a | 58.64 ± 2.65 bcd | 61.36 ± 2.10 ab | 57.88 ± 1.61 cd | 60.62 ± 1.02 ab | 56.88 ± 2.04 d | 60.21 ± 1.54 abc |
| C18:2n6c (Linoleic) | 16.01 ± 0.25 a | 15.76 ± 0.10 ab | 15.92 ± 0.12 a | 15.34 ± 0.21 abc | 15.93 ± 0.106 a | 15.22 ± 0.59 bc | 15.95 ± 0.61 a | 14.86 ± 0.33 cd | 15.64 ± 0.39 ab | 14.43 ± 0.37 d | 15.59 ± 0.42 ab |
| C18:3n3c (α-Linolenic) | 7.60 ± 0.15 ab | 7.53 ± 0.02 ab | 7.64 ± 0.19 a | 7.33 ± 0.11 abc | 7.61 ± 0.06 a | 7.24 ± 0.27 bc | 7.59 ± 0.30 ab | 7.03 ± 0.18 cd | 7.46 ± 0.18 ab | 6.81 ± 0.24 d | 7.37 ± 0.19 abc |
| C20:1n9c (Eicosanoic) | 1.30 ± 0.04 a | 1.28 ± 0.04 a | 1.29 ± 0.04 a | 1.28 ± 0.04 a | 1.31 ± 0.04 a | 1.27 ± 1.04 a | 1.30 ± 1.04 a | 1.20 ± 0.04 b | 1.29 ± 0.04 a | 1.24 ± 0.04 ab | 1.28 ± 1.04 a |
| C22:1n9c (Erucic) | 0.50 ± 0.01 ab | 0.49 ± 0.01 ab | 0.47 ± 0.01 b | 0.48 ± 0.01 ab | 0.49 ± 0.01 ab | 0.50 ± 0.02 ab | 0.49 ± 0.03 ab | 0.48 ± 0.01 ab | 0.49 ± 0.01 ab | 0.50 ± 0.01 a | 0.49 ± 0.02 ab |
| SFA | 4.85 ± 0.25 a | 4.69 ± 0.10 abc | 4.80 ± 0.12 ab | 4.66 ± 0.21 abc | 4.77 ± 0.16 ab | 4.61 ± 0.29 bc | 4.78 ± 0.31 ab | 4.54 ± 0.33 cd | 4.73 ± 0.39 ab | 4.38 ± 0.37 d | 4.67 ± 0.42 abc |
| MUFA | 63.69 ± 0.73 a | 62.68 ± 0.41 ab | 63.30 ± 0.23 a | 61.43 ± 0.47 abc | 63.23 ± 0.33 a | 60.40 ± 2.72 bcd | 63.14 ± 2.18 ab | 59.56 ± 1.59 cd | 62.40 ± 1.05 ab | 58.62 ± 2.04 d | 61.98 ± 1.53 abc |
| PUFA | 23.61 ± 0.40 a | 23.29 ± 0.12 ab | 23.55 ± 0.07 a | 22.67 ± 0.32 abc | 23.54 ± 0.20 a | 22.46 ± 0.86 bc | 23.53 ± 0.91 a | 21.89 ± 0.51 cd | 23.10 ± 0.57 ab | 21.24 ± 0.61 d | 22.96 ± 0.61 ab |
| Temperature | AV | PV | CV | CD | CT | TPC | RSA | OSI | Total Tocopherol | BI | HMF | FIC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AV | 0.920 ** | ||||||||||||
| CV | 0.849 ** | 0.873 ** | 0.725 * | ||||||||||
| CD | 0.801 ** | 0.882 ** | 0.815 ** | 0.948 ** | |||||||||
| CT | 0.709 * | 0.765 * | 0.809 ** | 0.872 ** | 0.961 ** | ||||||||
| TPC | 0.709 * | 0.699 * | 0.766 * | 0.895 ** | 0.894 ** | 0.939 ** | |||||||
| RSA | 0.744 * | 0.765 * | 0.801 ** | 0.907 ** | 0.902 ** | 0.920 ** | |||||||
| OSI | 0.727 * | 0.729 * | 0.784 * | 0.917 ** | 0.926 ** | 0.960 ** | |||||||
| Total tocopherol | 0.692 * | 0.671 * | 0.725 * | 0.684 * | 0.700 * | ||||||||
| BI | 0.945 ** | 0.948 ** | 0.921 ** | 0.912 ** | 0.828 ** | 0.798 * | 0.857 ** | 0.823 ** | 0.643 * | ||||
| HMF | 0.789 * | 0.758 * | 0.683 * | 0.910 ** | 0.883 ** | 0.913 ** | 0.985 ** | 0.973 ** | 0.979 ** | 0.679 * | 0.842 ** | ||
| FIC | 0.776 * | 0.724 * | 0.850 ** | 0.807 ** | 0.848 ** | 0.950 ** | 0.957 ** | 0.933 ** | 0.812 ** | 0.983 ** | |||
| Total Se | −0.694 * | −0.852 ** | −0.836 ** | −0.816 ** | −0.875 ** | −0.805 ** | −0.729 * | −0.830 ** | −0.760 * | −0.869 ** | −0.733 * | −0.708 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Wang, C.; Peng, L.; Dong, Y.; Gao, Y.; Xu, J.; Ping, H.; Liu, S. Effect of Vacuum Roasting on Total Selenium Content of Selenium-Enriched Rapeseed, Maillard Reaction Products, Oxidative Stability and Physicochemical Properties of Selenium-Enriched Rapeseed Oil. Foods 2023, 12, 3204. https://doi.org/10.3390/foods12173204
Xie Q, Wang C, Peng L, Dong Y, Gao Y, Xu J, Ping H, Liu S. Effect of Vacuum Roasting on Total Selenium Content of Selenium-Enriched Rapeseed, Maillard Reaction Products, Oxidative Stability and Physicochemical Properties of Selenium-Enriched Rapeseed Oil. Foods. 2023; 12(17):3204. https://doi.org/10.3390/foods12173204
Chicago/Turabian StyleXie, Qihui, Chengming Wang, Luqiu Peng, Yiyang Dong, Yu Gao, Jing Xu, Hongzheng Ping, and Shilin Liu. 2023. "Effect of Vacuum Roasting on Total Selenium Content of Selenium-Enriched Rapeseed, Maillard Reaction Products, Oxidative Stability and Physicochemical Properties of Selenium-Enriched Rapeseed Oil" Foods 12, no. 17: 3204. https://doi.org/10.3390/foods12173204
APA StyleXie, Q., Wang, C., Peng, L., Dong, Y., Gao, Y., Xu, J., Ping, H., & Liu, S. (2023). Effect of Vacuum Roasting on Total Selenium Content of Selenium-Enriched Rapeseed, Maillard Reaction Products, Oxidative Stability and Physicochemical Properties of Selenium-Enriched Rapeseed Oil. Foods, 12(17), 3204. https://doi.org/10.3390/foods12173204






