Cold Plasma-Assisted Extraction of Phytochemicals: A Review
Abstract
1. Introduction
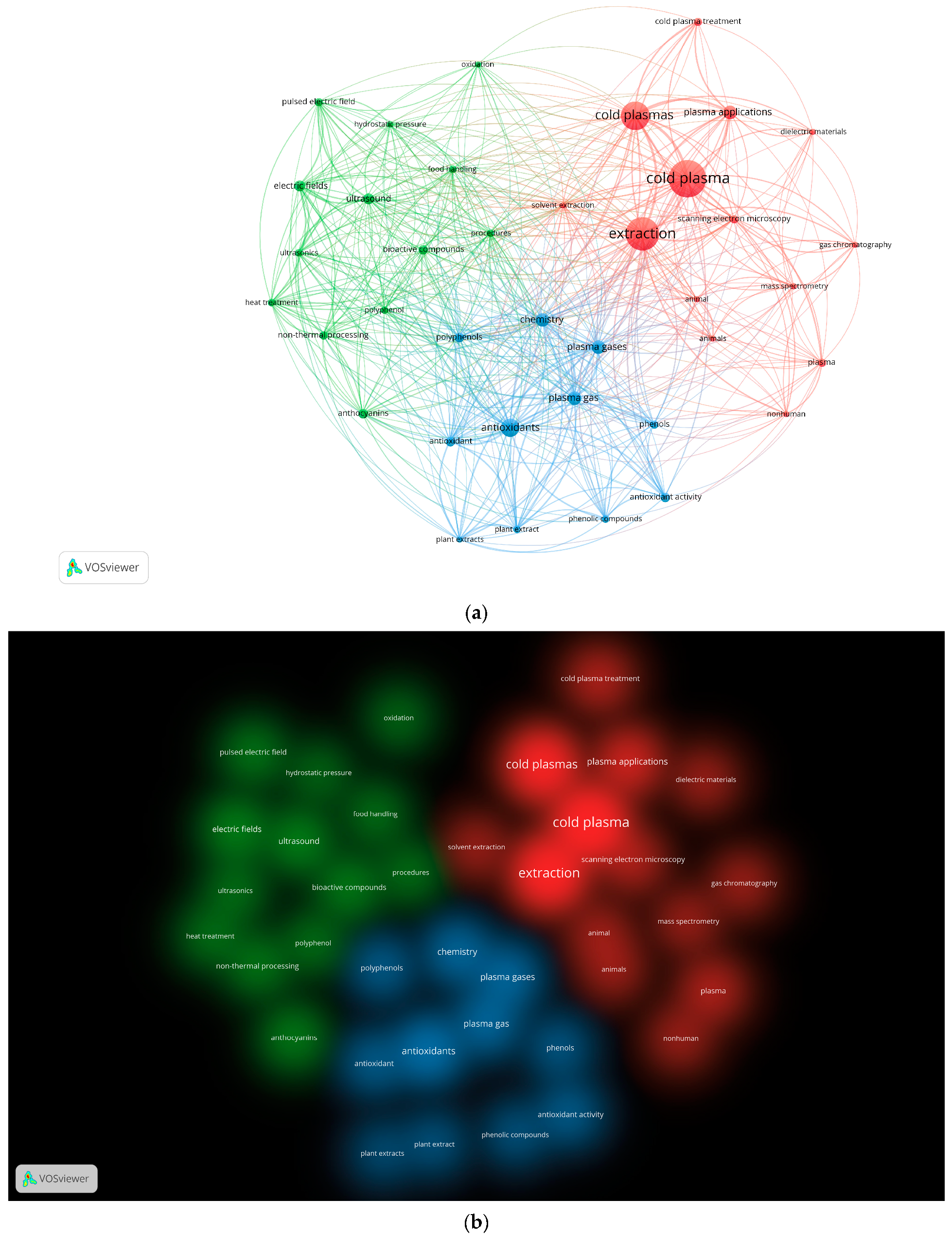
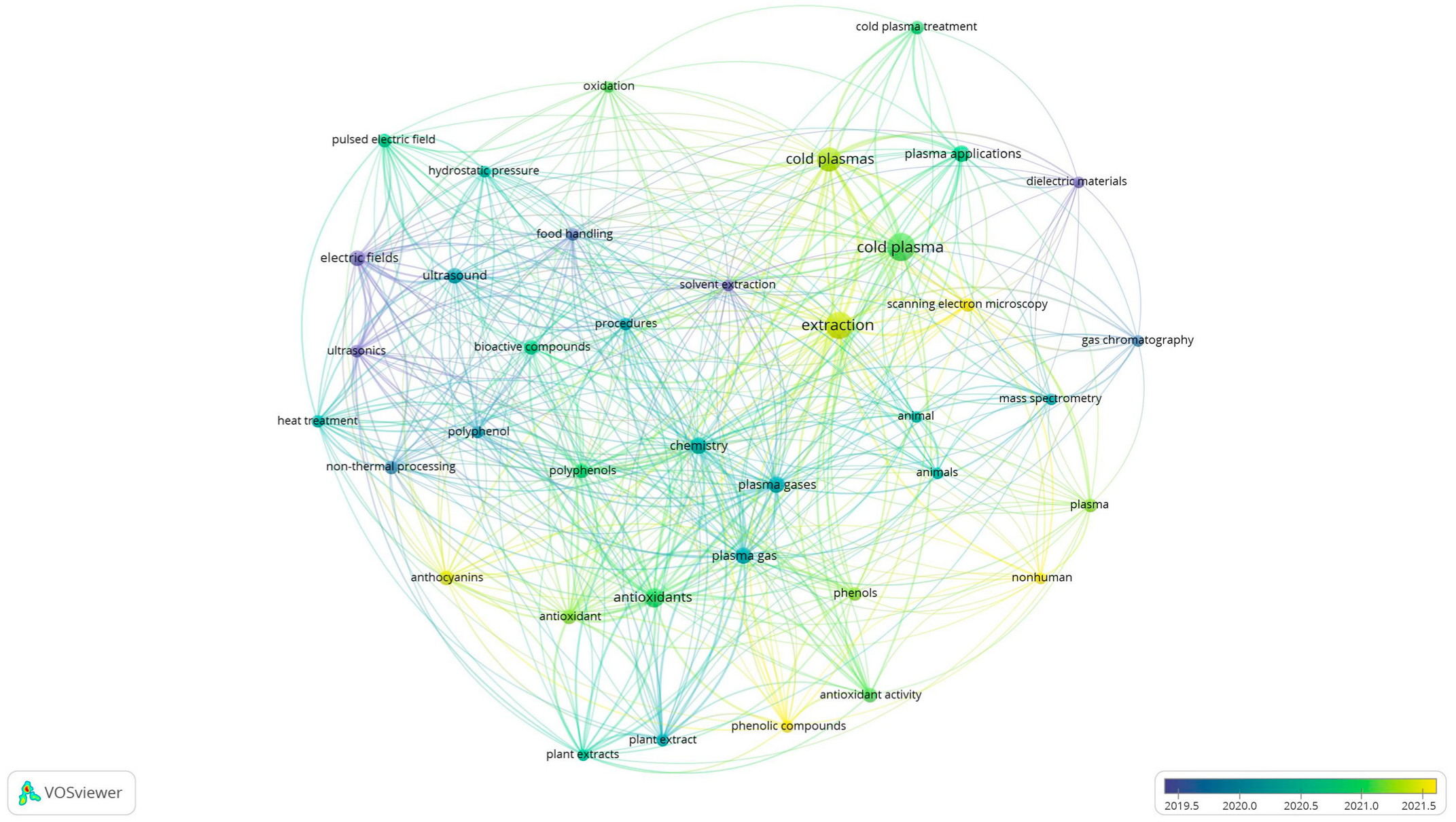
2. Scientometric Analysis
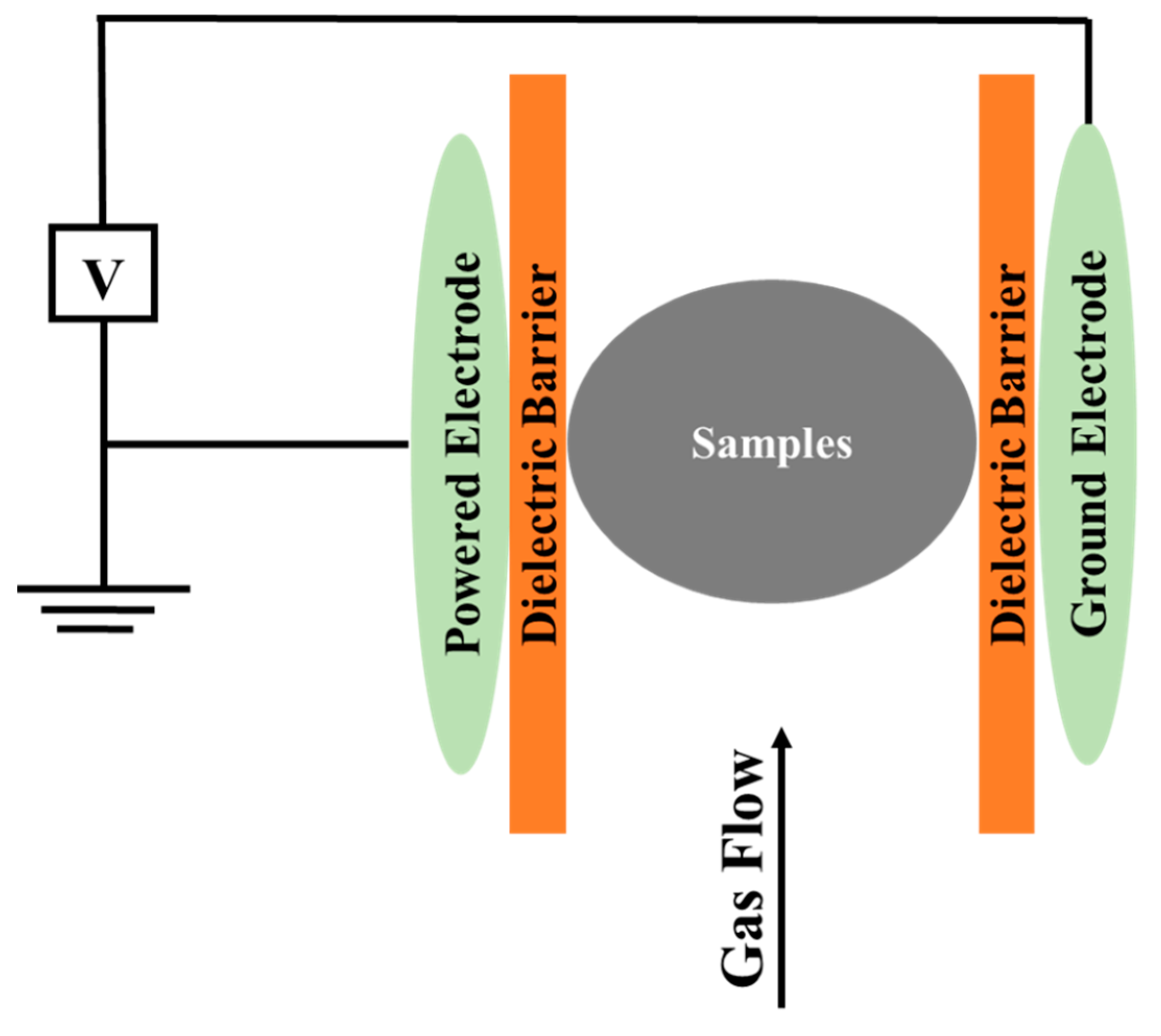
3. Principles, Types, and Sources of Cold Plasma Production
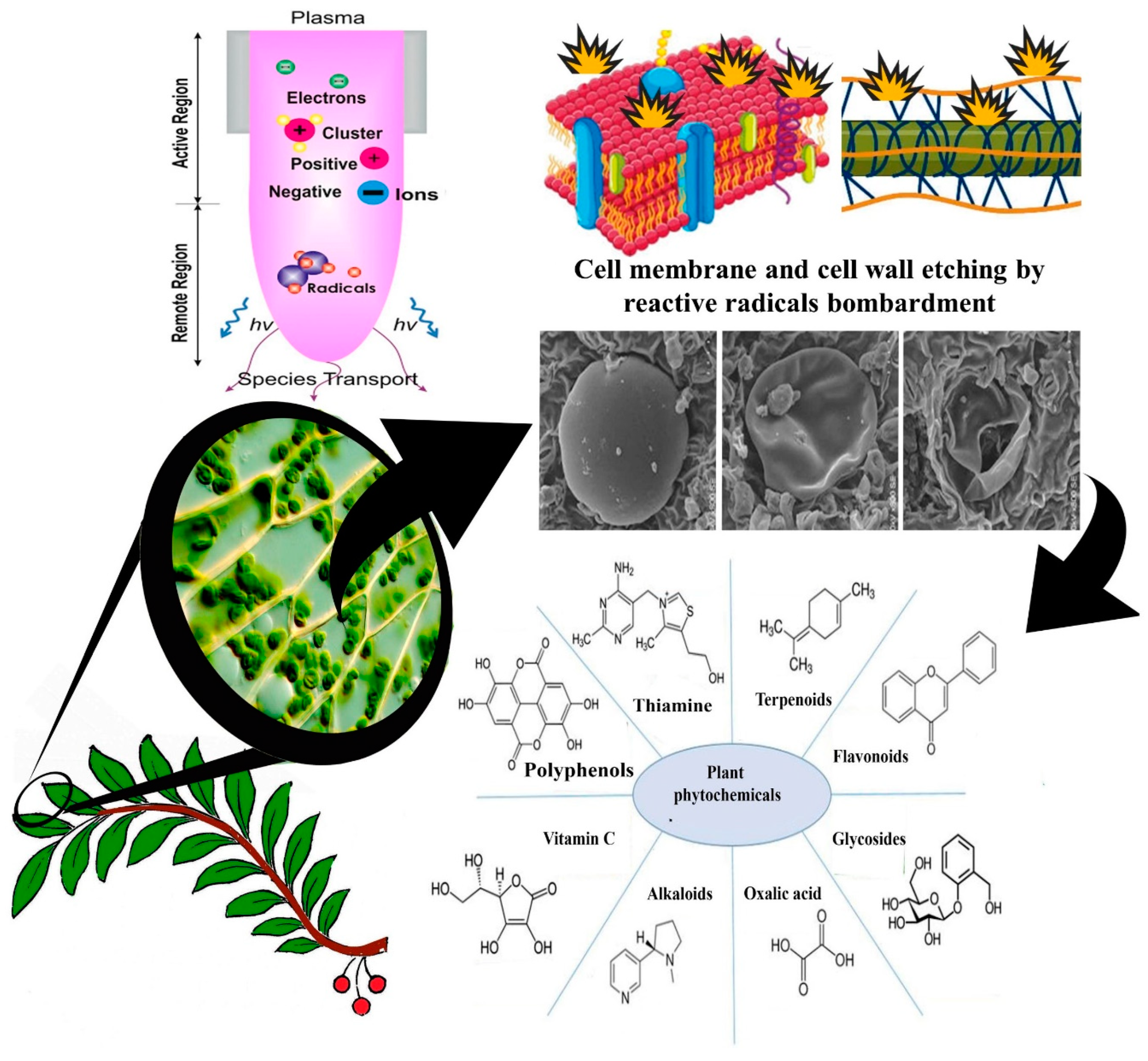
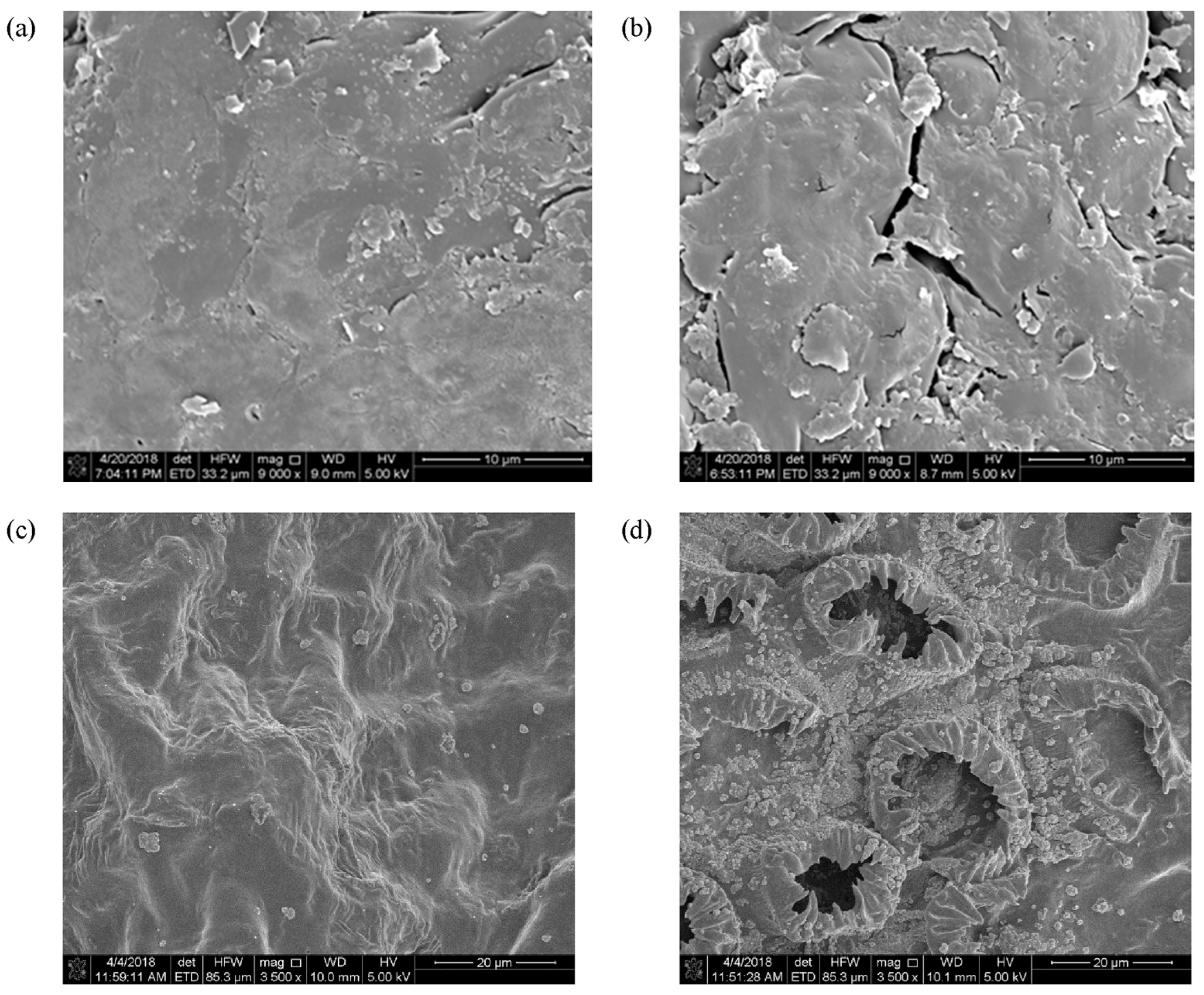
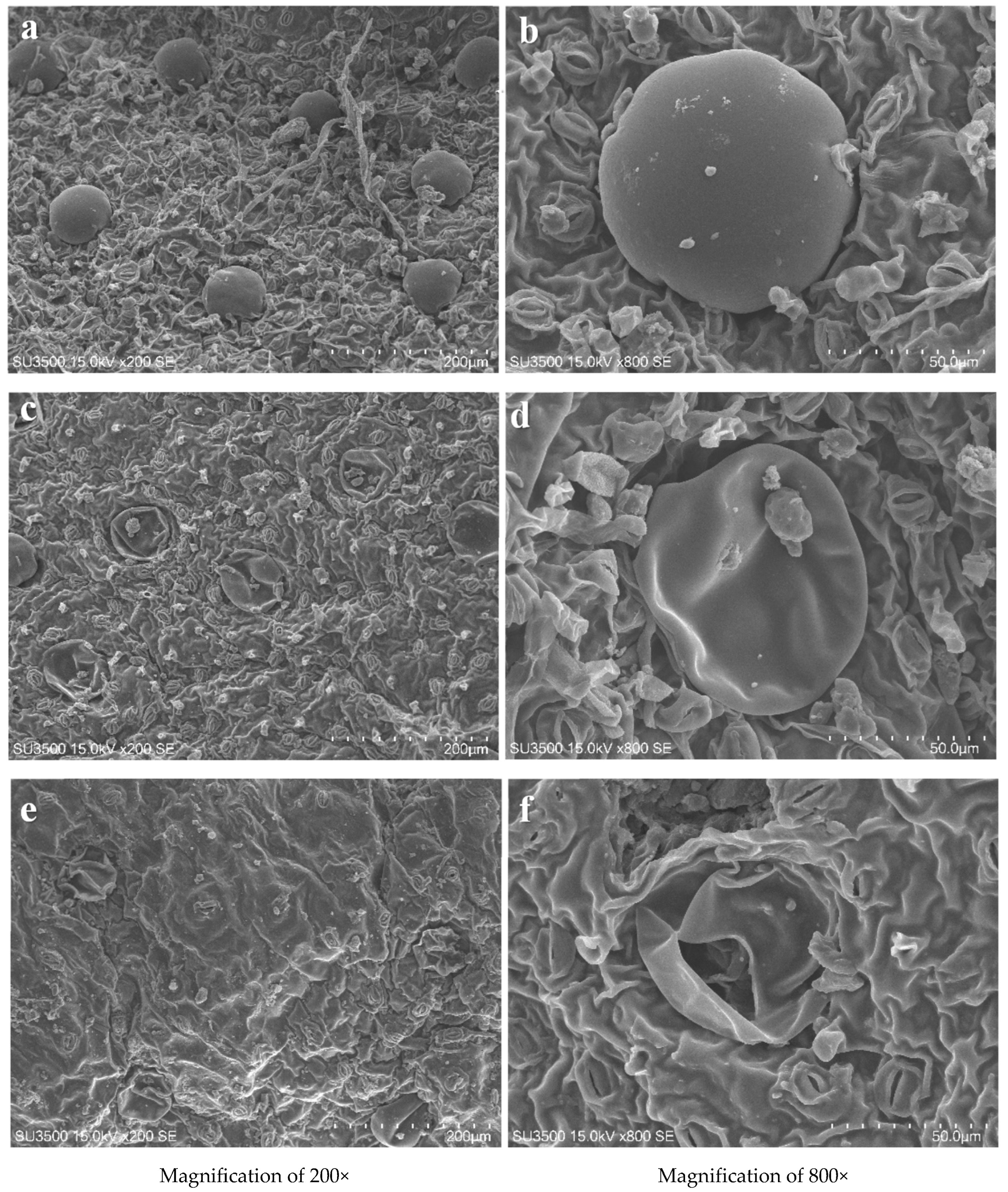
4. Mode of Action of Cold Plasma on the Plant Matrix and Its Critical Issues
5. Influence of Cold Plasma on the Phytochemical Profile and Biological Activity of Plant Extracts
5.1. Phytochemical Profile
5.2. Antioxidant Capacity
5.3. Antimicrobial Properties
5.4. Other Relevant Biological Properties
6. Conclusions and Future Perspective
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| Abbreviation | Definition |
| CP | Cold plasma |
| CARG | Compound Annual Growth Rate |
| SFE | Supercritical fluid extraction |
| MAE | Microwave-assisted extraction |
| UAE | Ultrasound-assisted extraction |
| SPE | Solid-phase extraction |
| LLE | Liquid-liquid extraction |
| ROS | Reactive oxygen species |
| DBD | Dielectric barrier discharge |
| HVACP | High voltage atmospheric cold plasma |
| LPCP | Low-pressure cold plasma |
| RNS | Reactive nitrogen species |
| TPC | Total polyphenol content |
| LC-MS | Liquid chromatography-mass spectroscopy |
| UPLC | Ultra-performance liquid chromatography |
| EGCG | Epigallocatechin gallate |
| SDBD | Surface dielectric barrier discharge |
| GDP | Glow discharge plasma |
| PGBR | Phenolic content of DBD-treated brown rice |
| CPJ | Cold plasma jet |
| CNG | Cyanidin-3-O-glucoside |
| TFC | Total flavan content |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid |
| FRAP | Fluorescence Recovery After Photobleaching |
| ORAC | Oxygen radical absorbance capacity |
| RSM | Response surface methodology |
| ANN | Artificial neural network |
| E. coli | Escherichia coli |
| S. neei | Salicornia neei |
| S. aureus | Staphylococcus aureus |
| E. faecalis | Enterococcus faecalis |
| S. Typhimurium | Salmonella typhimurium |
| L. monocytogenes | Listeria monocytogenes |
References
- Phytochemicals Market. Available online: https://www.persistencemarketresearch.com/market-research/phytochemicals-market.asp (accessed on 13 February 2023).
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals: Extraction process, safety assessment, toxicological evaluations, and regulatory issues. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–361. [Google Scholar]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Shirani, M.; Parandi, E.; Nodeh, H.R.; Akbari-Adergani, B.; Shahdadi, F. Development of a rapid efficient solid-phase microextraction: An overhead rotating flat surface sorbent based 3-D graphene oxide/lanthanum nanoparticles@ Ni foam for separation and determination of sulfonamides in animal-based food products. Food Chem. 2022, 373, 131421. [Google Scholar] [CrossRef]
- Shirani, M.; Aslani, A.; Sepahi, S.; Parandi, E.; Motamedi, A.; Jahanmard, E.; Nodeh, H.R.; Akbari-Adergani, B. An efficient 3D adsorbent foam based on graphene oxide/AgO nanoparticles for rapid vortex-assisted floating solid phase extraction of bisphenol A in canned food products. Anal. Methods 2022, 14, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Bidhendi, M.E.; Parandi, E.; Meymand, M.M.; Sereshti, H.; Nodeh, H.R.; Joo, S.-W.; Vasseghian, Y.; Khatir, N.M.; Rezania, S. Removal of lead ions from wastewater using magnesium sulfide nanoparticles caged alginate microbeads. Environ. Res. 2023, 216, 114416. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, N.; Ahranjani, P.J.; Parandi, E.; Nodeh, H.R.; Nawrot, N.; Rezania, S.; Sathishkumar, P. Titanium lanthanum three oxides decorated magnetic graphene oxide for adsorption of lead ions from aqueous media. Environ. Res. 2022, 214, 113831. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Aslani, A.; Ansari, F.; Parandi, E.; Nodeh, H.R.; Jahanmard, E. Zirconium oxide/titanium oxide nanorod decorated nickel foam as an efficient sorbent in syringe filter based solid-phase extraction of pesticides in some vegetables. Microchem. J. 2023, 189, 108507. [Google Scholar] [CrossRef]
- De Santis, D.; Carbone, K.; Garzoli, S.; Laghezza Masci, V.; Turchetti, G. Bioactivity and chemical profile of Rubus idaeus L. leaves steam-distillation extract. Foods 2022, 11, 1455. [Google Scholar] [CrossRef]
- Santarelli, V.; Neri, L.; Carbone, K.; Macchioni, V.; Pittia, P. Use of Conventional and Innovative Technologies for the Production of Food Grade Hop Extracts: Focus on Bioactive Compounds and Antioxidant Activity. Plants 2022, 11, 41. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Yousefi, S.; Heydari, M.; Seyedfatehi, F.; Jafarzadeh, S.; Mohammadi, R.; Rouhi, M.; Garavand, F. Application of red cabbage anthocyanins as pH-sensitive pigments in smart food packaging and sensors. Polymers 2022, 14, 1629. [Google Scholar] [CrossRef]
- Macchioni, V.; Carbone, K.; Cataldo, A.; Fraschini, R.; Bellucci, S. Lactic acid-based deep natural eutectic solvents for the extraction of bioactive metabolites of Humulus lupulus L.: Supramolecular organization, phytochemical profiling and biological activity. Sep. Purif. Technol. 2021, 264, 118039. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Lorenzo, J.M.; Barba, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Sarlak, Z.; Rouhi, M.; Mirza Alizadeh, A.; Sadeghi, E.; Hosseini, H.; Mousavi Khaneghah, A. Pb exposure from plant foods in Iran: A review. Int. J. Environ. Anal. Chem. 2021, 1–22. [Google Scholar] [CrossRef]
- Raesi, S.; Mohammadi, R.; Khammar, Z.; Paimard, G.; Abdalbeygi, S.; Sarlak, Z.; Rouhi, M. Photocatalytic detoxification of aflatoxin B1 in an aqueous solution and soymilk using nano metal oxides under UV light: Kinetic and isotherm models. LWT 2022, 154, 112638. [Google Scholar] [CrossRef]
- Paimard, G.; Mohammadi, R.; Bahrami, R.; Khosravi-Darani, K.; Sarlak, Z.; Rouhi, M. Detoxification of patulin from juice simulator and apple juice via cross-linked Se-chitosan/L-cysteine nanoparticles. LWT 2021, 143, 111146. [Google Scholar] [CrossRef]
- Sarlak, Z.; Khosravi-Darani, K.; Rouhi, M.; Garavand, F.; Mohammadi, R.; Sobhiyeh, M.R. Bioremediation of organophosphorus pesticides in contaminated foodstuffs using probiotics. Food Control 2021, 126, 108006. [Google Scholar] [CrossRef]
- Pogorzelska-Nowicka, E.; Hanula, M.M.; Brodowska-Trębacz, M.; Górska-Horczyczak, E.; Jankiewicz, U.; Mazur, T.; Marcinkowska-Lesiak, M.; Półtorak, A.; Wierzbicka, A. The Effect of Cold Plasma Pretreatment on Water-Suspended Herbs Measured in the Content of Bioactive Compounds, Antioxidant Activity, Volatile Compounds and Microbial Count of Final Extracts. Antioxidants 2021, 10, 1740. [Google Scholar] [CrossRef] [PubMed]
- Faria, G.; Souza, M.; Oliveira, J.; Costa, C.; Collares, M.; Prentice, C. Effect of ultrasound-assisted cold plasma pretreatment to obtain sea asparagus extract and its application in Italian salami. Food Res. Int. 2020, 137, 109435. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Najafi, G.; Ahmadi Gavlighi, H.; Seyfi, P.; Ghomi, H. Enhancement of polyphenolic content extraction rate with maximal antioxidant activity from green tea leaves by cold plasma. J. Food Sci. 2020, 85, 3415–3422. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U. A critical review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Aghel, B.; Gouran, A.; Parandi, E.; Jumeh, B.H.; Nodeh, H.R. Production of biodiesel from high acidity waste cooking oil using nano GO@ MgO catalyst in a microreactor. Renew. Energy 2022, 200, 294–302. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed]
- Heydari, M.; Rostami, O.; Mohammadi, R.; Banavi, P.; Farhoodi, M.; Sarlak, Z.; Rouhi, M. Hydrodistillation ultrasound-assisted green extraction of essential oil from bitter orange peel wastes: Optimization for quantitative, phenolic, and antioxidant properties. J. Food Process. Preserv. 2021, 45, e15585. [Google Scholar] [CrossRef]
- Carbone, K.; Macchioni, V.; Petrella, G.; Cicero, D.O. Exploring the potential of microwaves and ultrasounds in the green extraction of bioactive compounds from Humulus lupulus for the food and pharmaceutical industry. Ind. Crops Prod. 2020, 156, 112888. [Google Scholar] [CrossRef]
- Ramazzina, I.; Macchioni, V.; Carbone, K. Antioxidant and pro-oxidant phytochemicals in ultrasound and microwave assisted extracts from hop cones: A statistical modelling approach. Food Funct. 2022, 13, 9589–9601. [Google Scholar] [CrossRef]
- Hanula, M.; Wyrwisz, J.; Moczkowska, M.; Horbańczuk, O.K.; Pogorzelska-Nowicka, E.; Wierzbicka, A. Optimization of microwave and ultrasound extraction methods of açai berries in terms of highest content of phenolic compounds and antioxidant activity. Appl. Sci. 2020, 10, 8325. [Google Scholar] [CrossRef]
- Kumar, S.; Pipliya, S.; Srivastav, P.P. Effect of cold plasma on different polyphenol compounds: A review. J. Food Process Eng. 2023, 46, e14203. [Google Scholar] [CrossRef]
- Carbone, K.; Amoriello, T.; Iadecola, R. Exploitation of kiwi juice pomace for the recovery of natural antioxidants through microwave-assisted extraction. Agriculture 2020, 10, 435. [Google Scholar] [CrossRef]
- Patil, P.S.; Shettigar, R. An advancement of analytical techniques in herbal research. J. Adv. Sci. Res. 2010, 1, 8–14. [Google Scholar]
- Naude, Y.; De Beer, W.; Jooste, S.; Van Der Merwe, L.; Van Rensburg, S. Comparison of supercritical fluid extraction and Soxhlet extraction for the determination of DDT, DDD and DDE in sediment. Water 1998, 24, 205–214. [Google Scholar]
- Handa, S. An overview of extraction techniques for medicinal and aromatic plants. Extr. Technol. Med. Aromat. Plants 2008, 1, 21–40. [Google Scholar]
- Gupta, A.; Naraniwal, M.; Kothari, V. Modern extraction methods for preparation of bioactive plant extracts. Int. J. Appl. Nat. Sci. 2012, 1, 8–26. [Google Scholar]
- Mohammad Azmin, S.N.H.; Abdul Manan, Z.; Wan Alwi, S.R.; Chua, L.S.; Mustaffa, A.A.; Yunus, N.A. Herbal processing and extraction technologies. Sep. Purif. Rev. 2016, 45, 305–320. [Google Scholar] [CrossRef]
- Safaripour, M.; Parandi, E.; Aghel, B.; Gouran, A.; Saidi, M.; Nodeh, H.R. Optimization of the microreactor-intensified transesterification process using silver titanium oxide nanoparticles decorated magnetic graphene oxide nanocatalyst. Process Saf. Environ. Prot. 2023, 173, 495–506. [Google Scholar] [CrossRef]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef]
- Laghari, A.Q.; Memon, S.; Nelofar, A.; Laghari, A.H. Extraction, identification and antioxidative properties of the flavonoid-rich fractions from leaves and flowers of Cassia angustifolia. Am. J. Anal. Chem. 2011, 2, 871. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Zhao, L.-D.; Wang, Y. Ultrasonic-assisted extraction of epimedin C from fresh leaves of Epimedium and extraction mechanism. Innov. Food Sci. Emerg. Technol. 2009, 10, 54–60. [Google Scholar] [CrossRef]
- Zhang, H.-F.; Yang, X.-H.; Wang, Y. Microwave assisted extraction of secondary metabolites from plants: Current status and future directions. Trends Food Sci. Technol. 2011, 22, 672–688. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Abdellattif, M.H.; Saidi, M.; Bozorgian, A.; Nodeh, H.R.; Rezania, S. Biodiesel production from waste cooking oil using a novel biocatalyst of lipase enzyme immobilized magnetic nanocomposite. Fuel 2022, 313, 123057. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Oliver, C.M.; Kentish, S.; Ashokkumar, M. Use of power ultrasound to improve extraction and modify phase transitions in food processing. Food Rev. Int. 2013, 29, 67–91. [Google Scholar] [CrossRef]
- Wu, J.; Lin, L.; Chau, F.-t. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef]
- De Castro, M.L.; Priego-Capote, F. Soxhlet extraction: Past and present panacea. J. Chromatogr. A 2010, 1217, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Parandi, E.; Pero, M.; Kiani, H. Phase change and crystallization behavior of water in biological systems and innovative freezing processes and methods for evaluating crystallization. Discov. Food 2022, 2, 6. [Google Scholar] [CrossRef]
- Abedi-Firoozjah, R.; Parandi, E.; Heydari, M.; Kolahdouz-Nasiri, A.; Bahraminejad, M.; Mohammadi, R.; Rouhi, M.; Garavand, F. Betalains as promising natural colorants in smart/active food packaging. Food Chem. 2023, 424, 136408. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.L.; Garcıa-Ayuso, L. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Gao, X.; Yani, S.; Wu, H. Pyrolysis of spent biomass from mallee leaf steam distillation: Biochar properties and recycling of inherent inorganic nutrients. Energy Fuels 2014, 28, 4642–4649. [Google Scholar] [CrossRef]
- Alupului, A.; Calinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. UPB Sci. Bull. Ser. B 2012, 74, 129–142. [Google Scholar]
- Ridgway, K.; Lalljie, S.P.; Smith, R.M. Sample preparation techniques for the determination of trace residues and contaminants in foods. J. Chromatogr. A 2007, 1153, 36–53. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Szczepańska, N.; de La Guardia, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review. part ii. TrAC Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Jume, B.H.; Valizadeh Dana, N.; Rastin, M.; Parandi, E.; Darajeh, N.; Rezania, S. Sulfur-Doped Binary Layered Metal Oxides Incorporated on Pomegranate Peel-Derived Activated Carbon for Removal of Heavy Metal Ions. Molecules 2022, 27, 8841. [Google Scholar] [CrossRef]
- Sereshti, H.; Soltani, S.; Sridewi, N.; Salehi, E.; Parandi, E.; Rashid Nodeh, H.; Shahabuddin, S. Solid Phase Extraction Penicillin and Tetracycline in Human Serum Using Magnetic Graphene Oxide-Based Sulfide Nanocomposite. Magnetochemistry 2023, 9, 132. [Google Scholar] [CrossRef]
- Hadi Jume, B.; Parandi, E.; Nouri, M.; Aghel, B.; Gouran, A.; Rashidi Nodeh, H.; Kamyab, H.; Cho, J.; Rezania, S. Optimization of microreactor-assisted transesterification for biodiesel production using bimetal zirconium-titanium oxide doped magnetic graphene oxide heterogeneous nanocatalyst. Chem. Eng. Process. Process Intensif. 2023, 191, 109479. [Google Scholar] [CrossRef]
- Parandi, E.; Safaripour, M.; Mosleh, N.; Saidi, M.; Rashidi Nodeh, H.; Oryani, B.; Rezania, S. Lipase enzyme immobilized over magnetic titanium graphene oxide as catalyst for biodiesel synthesis from waste cooking oil. Biomass Bioenergy 2023, 173, 106794. [Google Scholar] [CrossRef]
- Hidayah, N.N.; Abidin, S.Z. The evolution of mineral processing in extraction of rare earth elements using liquid-liquid extraction: A review. Miner. Eng. 2018, 121, 146–157. [Google Scholar] [CrossRef]
- Silvestre, C.I.; Santos, J.L.; Lima, J.L.; Zagatto, E.A. Liquid–liquid extraction in flow analysis: A critical review. Anal. Chim. Acta 2009, 652, 54–65. [Google Scholar] [CrossRef]
- Kruk, Z.A.; Yun, H.; Rutley, D.L.; Lee, E.J.; Kim, Y.J.; Jo, C. The effect of high pressure on microbial population, meat quality and sensory characteristics of chicken breast fillet. Food Control 2011, 22, 6–12. [Google Scholar] [CrossRef]
- Kulawik, P.; Kumar Tiwari, B. Recent advancements in the application of non-thermal plasma technology for the seafood industry. Crit. Rev. Food Sci. Nutr. 2019, 59, 3199–3210. [Google Scholar] [CrossRef] [PubMed]
- Preis, S.; Klauson, D.; Gregor, A. Potential of electric discharge plasma methods in abatement of volatile organic compounds originating from the food industry. J. Environ. Manag. 2013, 114, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Niemira, B.A.; Sites, J. Cold plasma inactivates Salmonella Stanley and Escherichia coli O157: H7 inoculated on golden delicious apples. J. Food Prot. 2008, 71, 1357–1365. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Gao, J. Low temperature argon plasma sterilization effect on Pseudomonas aeruginosa and its mechanisms. J. Electrost. 2009, 67, 646–651. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold plasma: A novel non-thermal technology for food processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.; Milosavljević, V.; O’donnell, C.; Bourke, P.; Keener, K.; Cullen, P. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Misra, N.; Patil, S.; Moiseev, T.; Bourke, P.; Mosnier, J.; Keener, K.; Cullen, P. In-package atmospheric pressure cold plasma treatment of strawberries. J. Food Eng. 2014, 125, 131–138. [Google Scholar] [CrossRef]
- Yun, H.; Kim, B.; Jung, S.; Kruk, Z.A.; Kim, D.B.; Choe, W.; Jo, C. Inactivation of Listeria monocytogenes inoculated on disposable plastic tray, aluminum foil, and paper cup by atmospheric pressure plasma. Food Control 2010, 21, 1182–1186. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U. Consequences of non-thermal cold plasma treatment on meat and dairy lipids—A review. Future Foods 2021, 4, 100095. [Google Scholar] [CrossRef]
- Paixão, L.; Fonteles, T.V.; Oliveira, V.S.; Fernandes, F.A.; Rodrigues, S. Cold plasma effects on functional compounds of siriguela juice. Food Bioprocess Technol. 2019, 12, 110–121. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Keener, K.M. Effects of cold plasma on food quality: A review. Foods 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.; Gul, K.; Sehrawat, R.; Allai, F.M. Impact of in-package cold plasma treatment on the physicochemical properties and shelf life of button mushrooms (Agaricus bisporus). Food Biosci. 2023, 52, 102425. [Google Scholar] [CrossRef]
- Abouelenein, D.; Mustafa, A.M.; Nzekoue, F.K.; Caprioli, G.; Angeloni, S.; Tappi, S.; Castagnini, J.M.; Dalla Rosa, M.; Vittori, S. The Impact of Plasma Activated Water Treatment on the Phenolic Profile, Vitamins Content, Antioxidant and Enzymatic Activities of Rocket-Salad Leaves. Antioxidants 2023, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Li, Y.; Dhaliwal, H.; Feng, M.; Xiang, Q.; Roopesh, M.; Pan, D.; Du, L. The Application of Cold Plasma Technology in Low-Moisture Foods. Food Eng. Rev. 2023, 15, 86–112. [Google Scholar] [CrossRef]
- Qu, Z.; Chen, G.; Wang, J.; Xie, X.; Chen, Y. Preparation, structure evaluation, and improvement in foaming characteristics of fibrotic pea protein isolate by cold plasma synergistic organic acid treatment. Food Hydrocoll. 2023, 134, 108057. [Google Scholar] [CrossRef]
- Zhou, J.; Qaing, S.; Yang, B.; Wang, Y.; Wang, J.; Yang, T.; Zhang, Y.; Chen, Y.; Li, S. Cold plasma treatment with alginate oligosaccharide improves the digestive stability and bioavailability of nutrient-delivered particles: An in vitro INFOGEST gastrointestinal study. Int. J. Biol. Macromol. 2023, 232, 123309. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, Y.; Xu, G.; Zhou, L.; Liu, C.; Luo, S. Peroxidase inactivation by cold plasma and its effects on the storage, physicochemical and bioactive properties of brown rice. Food Biosci. 2023, 52, 102383. [Google Scholar] [CrossRef]
- Guo, J.; He, Z.; Ma, C.; Li, W.; Wang, J.; Lin, F.; Liu, X.; Li, L. Evaluation of cold plasma for decontamination of molds and mycotoxins in rice grain. Food Chem. 2023, 402, 134159. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Z.; Zhao, M.; Gao, C.; Wang, J.; Li, C.; Dong, X.; Liu, Z.; Zhou, D. Chitosan-wampee seed essential oil composite film combined with cold plasma for refrigerated storage with modified atmosphere packaging: A promising technology for quality preservation of golden pompano fillets. Int. J. Biol. Macromol. 2023, 224, 1266–1275. [Google Scholar] [CrossRef]
- Giannoulia, S.; Triantaphyllidou, I.-E.; Tekerlekopoulou, A.G.; Aggelopoulos, C.A. Mechanisms of Individual and Simultaneous Adsorption of Antibiotics and Dyes onto Halloysite Nanoclay and Regeneration of Saturated Adsorbent via Cold Plasma Bubbling. Nanomaterials 2023, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, N.; Najmi, M.; Parandi, E.; Nodeh, H.R.; Vasseghian, Y.; Rezania, S. Magnetic sporopollenin supported polyaniline developed for removal of lead ions from wastewater: Kinetic, isotherm and thermodynamic studies. Chemosphere 2022, 300, 134461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lavoine, N.; Salvi, D. Cold atmospheric pressure plasma for the sanitation of conveyor belt materials: Decontamination efficacy against adherent bacteria and biofilms of Escherichia coli and effect on surface properties. Innov. Food Sci. Emerg. Technol. 2023, 84, 103260. [Google Scholar] [CrossRef]
- Asghari, M.; Hosseinzadeh Samani, B.; Ebrahimi, R. Review on non-thermal plasma technology for biodiesel production: Mechanisms, reactors configuration, hybrid reactors. Energy Convers. Manag. 2022, 258, 115514. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Environmentally friendly techniques for the recovery of polyphenols from food by-products and their impact on polyphenol oxidase: A critical review. Appl. Sci. 2022, 12, 1923. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Yepez, X.; Illera, A.E.; Baykara, H.; Keener, K. Recent advances and potential applications of atmospheric pressure cold plasma technology for sustainable food processing. Foods 2022, 11, 1833. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Rodrigues, S. Cold plasma processing on fruits and fruit juices: A review on the effects of plasma on nutritional quality. Processes 2021, 9, 2098. [Google Scholar] [CrossRef]
- Stevens, C.; Wilson, C.; Lu, J.; Khan, V.; Chalutz, E.; Droby, S.; Kabwe, M.; Haung, Z.; Adeyeye, O.; Pusey, L. Plant hormesis induced by ultraviolet light-C for controlling postharvest diseases of tree fruits. Crop Prot. 1996, 15, 129–134. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Pimentel, T.C.; Esmerino, E.A.; de Freitas, M.Q.; Verruck, S.; Silva, M.C.; da Cruz, A.G. Cold Plasma. Nov. Technol. Food Sci. 2023, 109–169. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of non-thermal atmospheric pressure plasma with solid and liquid food systems: A review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.-Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Farias, T.R.; Rodrigues, S.; Fernandes, F.A. Effect of dielectric barrier discharge plasma excitation frequency on the enzymatic activity, antioxidant capacity and phenolic content of apple cubes and apple juice. Food Res. Int. 2020, 136, 109617. [Google Scholar] [CrossRef]
- Ahmadian, S.; Kenari, R.E.; Amiri, Z.R.; Sohbatzadeh, F.; Khodaparast, M.H.H. Effect of ultrasound-assisted cold plasma pretreatment on cell wall polysaccharides distribution and extraction of phenolic compounds from hyssop (Hyssopus officinalis L.). Int. J. Biol. Macromol. 2023, 233, 123557. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, Ó.; Gomes, W.F.; Rodrigues, S.; Fernandes, F.A. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.). LWT 2017, 84, 457–463. [Google Scholar] [CrossRef]
- Herceg, Z.; Kovačević, D.B.; Kljusurić, J.G.; Jambrak, A.R.; Zorić, Z.; Dragović-Uzelac, V. Gas phase plasma impact on phenolic compounds in pomegranate juice. Food Chem. 2016, 190, 665–672. [Google Scholar] [CrossRef]
- Hemmati, V.; Garavand, F.; Khorshidian, N.; Cacciotti, I.; Goudarzi, M.; Chaichi, M.; Tiwari, B.K. Impact of cold atmospheric plasma on microbial safety, total phenolic and flavonoid contents, antioxidant activity, volatile compounds, surface morphology, and sensory quality of green tea powder. Food Biosci. 2021, 44, 101348. [Google Scholar] [CrossRef]
- Zielinska, S.; Staniszewska, I.; Cybulska, J.; Zdunek, A.; Szymanska-Chargot, M.; Zielinska, D.; Liu, Z.-L.; Pan, Z.; Xiao, H.-W.; Zielinska, M. Modification of the cell wall polysaccharides and phytochemicals of okra pods by cold plasma treatment. Food Hydrocoll. 2022, 131, 107763. [Google Scholar] [CrossRef]
- Almeida, F.D.L.; Cavalcante, R.S.; Cullen, P.J.; Frias, J.M.; Bourke, P.; Fernandes, F.A.; Rodrigues, S. Effects of atmospheric cold plasma and ozone on prebiotic orange juice. Innov. Food Sci. Emerg. Technol. 2015, 32, 127–135. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, W.; Zhang, Q.; Gao, G.; Meng, Y. Effect of cold plasma treatment on sterilizing rate and quality of kiwi turbid juice. J. Food Process Eng. 2021, 44, e13711. [Google Scholar] [CrossRef]
- Leite, A.K.; Fonteles, T.V.; Miguel, T.B.; da Silva, G.S.; de Brito, E.S.; Alves Filho, E.G.; Fernandes, F.A.; Rodrigues, S. Atmospheric cold plasma frequency imparts changes on cashew apple juice composition and improves vitamin C bioaccessibility. Food Res. Int. 2021, 147, 110479. [Google Scholar] [CrossRef] [PubMed]
- Abedelmaksoud, T.G.; Hesarinejad, M.A.; Shokrollahi Yancheshmeh, B. The effect of cold plasma on the enzymatic activity and quality characteristics of mango pulp. Res. Innov. Food Sci. Technol. 2022, 10, 341–350. [Google Scholar]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-H.; Vidyarthi, S.K.; Zhong, C.-S.; Zheng, Z.-A.; An, Y.; Wang, J.; Wei, Q.; Xiao, H.-W. Cold plasma enhances drying and color, rehydration ratio and polyphenols of wolfberry via microstructure and ultrastructure alteration. LWT 2020, 134, 110173. [Google Scholar] [CrossRef]
- Dakshayani, R.; Paul, A.; Mahendran, R. Cold Plasma-Induced Effects on Bioactive Constituents and Antioxidant Potential of Lotus Petal Powder. IEEE Trans. Plasma Sci. 2020, 49, 507–512. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Park, S.; Kim, K.; Kim, T.H.; Choe, W.; Jo, C. Effect of atmospheric pressure dielectric barrier discharge plasma on the biological activity of naringin. Food Chem. 2014, 160, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, A.S.; Ramezan, Y.; Khani, M.R. Simultaneous study of the antioxidant activity, microbial decontamination and color of dried peppermint (Mentha piperita L.) using low pressure cold plasma. LWT 2020, 123, 109121. [Google Scholar] [CrossRef]
- Seelarat, W.; Sangwanna, S.; Panklai, T.; Chaosuan, N.; Bootchanont, A.; Wattanawikkam, C.; Subcharoen, A.; Subcharoen, N.; Chanchula, N.; Boonyawan, D. Enhanced fruiting body production and bioactive phytochemicals from white Cordyceps militaris by blending Cordyceps militaris and using cold plasma jet. Plasma Chem. Plasma Process. 2023, 43, 139–162. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Lin, Y.; Nasiru, M.M.; Zhang, J.; Abid, M.; Murtaza, M.A.; Zhao, L. Comparative study: Thermal and non-thermal treatment on enzyme deactivation and selected quality attributes of fresh carrot juice. Int. J. Food Sci. Technol. 2022, 57, 827–841. [Google Scholar] [CrossRef]
- Rashid, F.; Bao, Y.; Ahmed, Z.; Huang, J.-Y. Effect of high voltage atmospheric cold plasma on extraction of fenugreek galactomannan and its physicochemical properties. Food Res. Int. 2020, 138, 109776. [Google Scholar] [CrossRef]
- Rezaei, S.; Ghobadian, B.; Ebadi, M.T.; Ghomi, H. Qualitative and quantitative assessment of extracted oil from Camelina sativa seed treated by dielectric-barrier discharge cold plasma. Contrib. Plasma Phys. 2020, 60, e202000032. [Google Scholar] [CrossRef]
- Afshar, S.; Ramezan, Y.; Hosseini, S. Physical and chemical properties of oil extracted from sesame (Sesamum indicum L.) and sunflower (Helianthus annuus L.) seeds treated with cold plasma. J. Food Meas. Charact. 2022, 16, 740–752. [Google Scholar] [CrossRef]
- Rezaei, S.; Ebadi, M.-T.; Ghobadian, B.; Ghomi, H. Optimization of DBD-Plasma assisted hydro-distillation for essential oil extraction of fennel (Foeniculum vulgare Mill.) seed and spearmint (Mentha spicata L.) leaf. J. Appl. Res. Med. Aromat. Plants 2021, 24, 100300. [Google Scholar] [CrossRef]
- Pragna, C.; Gracy, T.R.; Mahendran, R.; Anandharamakrishnan, C. Effects of microwave and cold plasma assisted hydrodistillation on lemon peel oil extraction. Int. J. Food Eng. 2019, 15, 20190093. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, H.-J.; Lee, Y.; Yang, J.Y.; Song, J.S.; Woo, S.Y.; Kim, H.Y.; Song, S.-Y.; Seo, W.D.; Son, Y.-J. Cold Plasma Treatment Increases Bioactive Metabolites in Oat (Avena sativa L.) Sprouts and Enhances In Vitro Osteogenic Activity of their Extracts. Plant Foods Hum. Nutr. 2023, 78, 146–153. [Google Scholar] [CrossRef]
- Ebadi, M.T.; Abbasi, S.; Harouni, A.; Sefidkon, F. Effect of cold plasma on essential oil content and composition of lemon verbena. Food Sci. Nutr. 2019, 7, 1166–1171. [Google Scholar] [CrossRef]
- Kodama, S.; Thawatchaipracha, B.; Sekiguchi, H. Enhancement of essential oil extraction for steam distillation by DBD surface treatment. Plasma Process. Polym. 2014, 11, 126–132. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, S.Y.; Min, S.C. Improvement of the antioxidant activity, water solubility, and dispersion stability of prickly pear cactus fruit extracts using argon cold plasma treatment. J. Food Sci. 2019, 84, 2876–2882. [Google Scholar] [CrossRef]
- Buonopane, G.J.; Antonacci, C.; Lopez, J.L. Effect of cold plasma processing on botanicals and their essential oils. Plasma Med. 2016, 6, 315–324. [Google Scholar] [CrossRef]
- Poomanee, W.; Wattananapakasem, I.; Panjan, W.; Kiattisin, K. Optimizing anthocyanins extraction and the effect of cold plasma treatment on the anti-aging potential of purple glutinous rice (Oryza sativa L.) extract. Cereal Chem. 2021, 98, 571–582. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Ehlbeck, J.; Schlüter, O.; Kroh, L.W.; Rohn, S. Treating lamb’s lettuce with a cold plasma–Influence of atmospheric pressure Ar plasma immanent species on the phenolic profile of Valerianella locusta. LWT—Food Sci. Technol. 2011, 44, 2285–2289. [Google Scholar] [CrossRef]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 6, 21464. [Google Scholar] [CrossRef]
- Park, J.H.; Kumar, N.; Uhm, H.S.; Lee, W.; Choi, E.H.; Attri, P. Effect of nanosecond-pulsed plasma on the structural modification of biomolecules. RSC Adv. 2015, 5, 47300–47308. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Wende, K.; Schmidt, A.; Bekeschus, S. Safety aspects of non-thermal plasmas. In Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application; Springer: Berlin/Heidelberg, Germany, 2018; pp. 83–109. [Google Scholar]
- Mehta, D.; Yadav, K.; Chaturvedi, K.; Shivhare, U.; Yadav, S.K. Impact of cold plasma on extraction of polyphenol from de-oiled rice and corn bran: Improvement in extraction efficiency, in vitro digestibility, antioxidant activity, cytotoxicity and anti-inflammatory responses. Food Bioprocess Technol. 2022, 15, 1142–1156. [Google Scholar] [CrossRef]
- Heslin, C.; Boehm, D.; Gilmore, B.F.; Megaw, J.; Bourke, P. Safety evaluation of plasma-treated lettuce broth using in vitro and in vivo toxicity models. J. Phys. D Appl. Phys. 2020, 53, 274003. [Google Scholar] [CrossRef]
- Los, A.; Ziuzina, D.; Van Cleynenbreugel, R.; Boehm, D.; Bourke, P. Assessing the biological safety of atmospheric cold plasma treated wheat using cell and insect models. Foods 2020, 9, 898. [Google Scholar] [CrossRef]
- Carbone, K.; Garrigos, M.; Jimenez, A. Polyphenols: From wastes to high added value bio-products. In Frontiers in Natural Product Chemistry; Atta-ur-Rhaman, Ed.; Publishing Bentham Science: Cambridge, UK, 2016; Volume 2, pp. 115–178. [Google Scholar]
- Khezerlou, A.; Jafari, S.M. Nanoencapsulated bioactive components for active food packaging. In Handbook of Food Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 493–532. [Google Scholar]
- Tian, W.; Chen, G.; Zhang, G.; Wang, D.; Tilley, M.; Li, Y. Rapid determination of total phenolic content of whole wheat flour using near-infrared spectroscopy and chemometrics. Food Chem. 2021, 344, 128633. [Google Scholar] [CrossRef]
- Del Mondo, A.; Smerilli, A.; Ambrosino, L.; Albini, A.; Noonan, D.M.; Sansone, C.; Brunet, C. Insights into phenolic compounds from microalgae: Structural variety and complex beneficial activities from health to nutraceutics. Crit. Rev. Biotechnol. 2021, 41, 155–171. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Gao, Y.; Yeh, H.-Y.; Bowker, B.; Zhuang, H. Effects of different antioxidants on quality of meat patties treated with in-package cold plasma. Innov. Food Sci. Emerg. Technol. 2021, 70, 102690. [Google Scholar] [CrossRef]
- Gao, Y.; Zhuang, H.; Yeh, H.-Y.; Bowker, B.; Zhang, J. Effect of rosemary extract on microbial growth, pH, color, and lipid oxidation in cold plasma-processed ground chicken patties. Innov. Food Sci. Emerg. Technol. 2019, 57, 102168. [Google Scholar] [CrossRef]
- Yeh, H.-Y.; Line, J.E.; Hinton, A.; Gao, Y.; Zhuang, H. The effect of rosemary Extract and cold plasma treatments on bacterial community diversity in poultry ground meats. Heliyon 2019, 5, e02719. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Li, J.; Muhammad, A.I.; Suo, Y.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Application of a dielectric barrier discharge atmospheric cold plasma (Dbd-Acp) for Eshcerichia coli inactivation in apple juice. J. Food Sci. 2018, 83, 401–408. [Google Scholar] [CrossRef]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, N.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Khaneghah, A.M. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef]
- Farias, T.R.; Rodrigues, S.; Fernandes, F.A. Comparative study of two cold plasma technologies on apple juice antioxidant capacity, phenolic contents, and enzymatic activity. J. Food Process. Preserv. 2022, 46, e16871. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Ma, Q.; Zhou, Y. Enhancement of anthocyanins extraction from haskap by cold plasma pretreatment. Innov. Food Sci. Emerg. Technol. 2023, 84, 103294. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Maria do Socorro, M.R.; Alves, R.E.; de Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of cold plasma treatment on the functional properties of fresh-cut apples. J. Agric. Food Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef]
- Han, L.; Boehm, D.; Amias, E.; Milosavljević, V.; Cullen, P.; Bourke, P. Atmospheric cold plasma interactions with modified atmosphere packaging inducer gases for safe food preservation. Innov. Food Sci. Emerg. Technol. 2016, 38, 384–392. [Google Scholar] [CrossRef]
- Smet, C.; Baka, M.; Dickenson, A.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J.F. Antimicrobial efficacy of cold atmospheric plasma for different intrinsic and extrinsic parameters. Plasma Process. Polym. 2018, 15, 1700048. [Google Scholar] [CrossRef]
- Wiegand, C.; Beier, O.; Horn, K.; Pfuch, A.; Tölke, T.; Hipler, U.-C.; Schimanski, A. Antimicrobial impact of cold atmospheric pressure plasma on medical critical yeasts and bacteria cultures. Ski. Pharmacol. Physiol. 2014, 27, 25–35. [Google Scholar] [CrossRef]
- Hemmati, V.; Garavand, F.; Goudarzi, M.; Sarlak, Z.; Cacciotti, I.; Tiwari, B.K. Cold atmospheric-pressure plasma treatment of turmeric powder: Microbial load, essential oil profile, bioactivity and microstructure analyses. Int. J. Food Sci. Technol. 2021, 56, 2224–2232. [Google Scholar] [CrossRef]
- Mehta, D.; Purohit, A.; Bajarh, P.; Yadav, K.; Shivhare, U.; Yadav, S.K. Cold plasma processing improved the extraction of xylooligosaccharides from dietary fibers of rice and corn bran with enhanced in-vitro digestibility and anti-inflammatory responses. Innov. Food Sci. Emerg. Technol. 2022, 78, 103027. [Google Scholar] [CrossRef]
- Jeong, G.H.; Park, E.K.; Kim, T.H. Anti-diabetic effects of trans-resveratrol byproducts induced by plasma treatment. Food Res. Int. 2019, 119, 119–125. [Google Scholar] [CrossRef]



| Phytochemical Class | Representative Compounds | Biological Properties |
|---|---|---|
| 1. Terpenoids | ||
| Monoterpenes | Pinene, Citronellol, Limonene | Aroma, Digestion, Antiseptic |
| Diterpenes | Gibberellic acid, Steviol | Aroma, Antioxidant |
| Triterpenes | Saponins, Glycosidic tri-terpenoids | UV protector, anti-inflammatory, healing, anti-edema activity |
| Tetraterpenes, Carotenoids | β-Carotene, Lycopene, Xanthophylls, Lutein | Anticancer, Antioxidant, Countering macular degeneration |
| 2. Polyphenols | ||
| 2.1. Flavonoids compounds | ||
| Flavonols Flavones Flavanones Flavan-3-ols Isoflavones | Quercetin, Myricetin, Kaempferol Luteolin, Apigenin, Hesperetin, Naringenin (+)-Catechin, (-)-Epicatechin, (-)-Epigallocatechin Genistein, Daidzein, Formononetin | Antioxidant, Anticancer, Astringent, Phytoestrogenic |
| 2.2. Non-flavonoid compounds | ||
| Lignans Stilbenes Tannins | Secoisolariciresinol Resveratrol Tannin acids | Photosensitization, Antioxidant, Astringent |
| 2.3. Phenolic Acids Hydroxybenzoic acids Hydroxycinnamic acids | Gallic acid, Salicylic acid Ferulic acid, Coumaric acid, Caffeic acid | Antioxidant |
| 3. Sulfur-containing compounds | ||
| Thiosulfinates | Allicin | Aroma, Antiseptic, Anti-obesity, Anti-aging |
| Glucosinolates | Allylglucosinolate | Aroma, Thyroid hypertrophy, Inhibition of Helicobacter pylori |
| 4. Nitrogen-containing compounds | ||
| Capsaicinoids | Capsaicin | Burning taste, Analgesic |
| Betalains | Betacyanins, Betaxanthins | Color |
| Type of Extraction | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Supercritical fluid extraction (SFE) |
|
| [32,33,34,35,36,37] |
| Microwave-assisted extraction (MAE) |
|
| [38,39,40,41,42,43] |
| Ultrasound-assisted extraction (UAE) |
|
| [34,44,45,46,47,48,49] |
| Soxhlet extraction |
|
| [47,50] |
| Hydro-distillation extraction |
|
| [51,52] |
| Steam- distillation extraction |
|
| [53,54] |
| Solid phase extraction (SPE) |
|
| [55,56,57,58,59,60] |
| Liquid-liquid extraction (LLE) |
|
| [61,62,63,64,65] |
| Cold plasma |
|
| [1,65,66,67,68,69,70,71] |
| Food Sample | Plasma Process | Extraction Efficiency | Main Results | Ref | ||||
|---|---|---|---|---|---|---|---|---|
| Type of Plasma | Extraction Condition | Application | Antioxidant Capacity | Phenolic Compounds | Antimicrobial Activity | |||
| Tomato pomace | HVACP * | Gas: air, argon, helium, and nitrogen Voltage: 60 kV Time: 15 min | PC extraction | TPC extraction increased using He and N2 plasma by nearly 10% | Antioxidant potential increased by nitrogen CP (30%) in comparison with argon, helium, and air active gases. | No effect on trans-Ferulic acid, Gallic acid, Rutin and Isoquercetin Increase in Caffeic acid, Chlorogenic acid, Quercetin, and Naringenin | NA | [88] |
| Hyssop | CAP | Gas: air, nitrogen Voltage: 30 kV Frequency: 345 kHz Time: 5, 10, and 15 min | PC extraction | Increase in TPC extraction | FRAP: increased in both air and N2 plasma. DPPH: Decreased by air and increased by N2. The highest DPPH values were about 60.83 and 60.782% in nitrogen CP-pretreated samples for 5 and 15 min at a concentration of 1000 ppm, compared with un-pretreated sample prior to ultrasound-assisted extraction (DPPH: 77.939%) and conventional solvent extraction method (DPPH: 66.020%) at the same concentration. | NA | NA | [96] |
| Cashew apple juice | Cold Plasma (Nitrogen) | Gas: nitrogen Frequency: 80 kHz Time: 5, 10 and 15 min | Extract | TPC extraction increased using indirect nitrogen-cold plasma | FRAP: Increased by elevating N2 flow rate. The highest relative changes were about 164 and 163% after 15 min of treatment and flow rates of 30 and 50 mL/min. DPPH: Decreased by time extending. The highest antioxidant activity was observed in samples with minimum treatment duration (5 min) and flow rate of 50 mL/min (increased about 130%). | NA | NA | [97] |
| Pomegranate juice | Jet plasma | Voltage: 2.5 kV, 4 W Frequency: 25 kHz Time: 3–7 min | PC extraction | TPC extraction increased to the 33.03% by plasma operating condition (treatment time 3, 5, 7 min; gas flow 0.75, 1, 1.25 dm3 min−1) | NA | Increase in Catechin, Ellagic acid, Protocatechuic acid, Ferulic acid and p-Cumaric acid Decrease in Gallic acid, Chlorogenic acid, Caffeic acid and Punicalagin | NA | [98] |
| Green tea powder | CAP | Voltage: 20, 22 and 25 kV, Time: 2, 4, 6 and 8 min | Extract | Decrease in TPC extraction | FRAP and DPPH: decreased as the voltage increased and the treatment period lengthened compared with the untreated powder. | Decrease and Increase in Caffeine after 22 and 25 kV for 8 min, respectively Decrease in Linalool content (except at 20 kV for 2 or 4 min) Decrease in Geraniol after 20 kV for 8 min treatment | 0.97, 1.35, and 1.43 log reduction after 8 min and 25 kV exposure treatment in total microbial, mold, yeast, and coliform counts, respectively | [99] |
| Green tea leaves | DBD plasma | Time: 5 and 15 min Power: 5 to 15 W Frequency: 6 kHz Gas: Air and N2 | PC extraction | TPC extraction decreased 16.83% and 33.29% by the air and increased 41.14% by nitrogen CP, respectively | Antioxidant capacity decreased by air active gas and increased by nitrogen CP pretreatment. Nitrogen DBD treatment increased the antioxidant activity by 41.06% at 15 min and 15 W compared with un-pretreated green tea leaves. | Slightly increase in Gallic acid Increase in Catechin Decrease in Epicatechin and EGCG | NA | [21] |
| Okra pod | Cold Plasma and Air Atmospheric Pressure Plasma Jet | Time: 5, 15 and 30 s Power: 750 W Frequency: 20 kHz Gas: Air and N2 | PC extraction | 5, 15, and 30 s treatment decreased TPC by 5, 13, and 20% compared with non-treated sample | FRAP: increased by 20% until 5th s then decreased by 3 and 7% after 15th and 30th s of CP treatment. ABTS: increased by 4% until 5th s then decreased by 6 and 8% after 15th and 30th s of CP treatment. DPPH: no significant increase until 5th s and then decreased slightly by 5 and 9% after 15th and 30th s of CP treatment. | NA | NA | [100] |
| Orange juice | DBD-ACP | Time: 15, 30, 45 and 60 s Power: 70 kV Frequency: 50 Hz Gas: Air and N2 | PC pretreatment | Decrease in TPC from 2.52 g/L in control sample (without ozone or plasma treatment) to 2.37 and 1.93 g/L for direct and indirect exposure, respectively | ABTS: decreased about 50% after 60th s of direct exposure to plasma field. DPPH: no significant difference. | NA | NA | [101] |
| Water-Suspended Herbs | Plasma jet system | Activated by nitrogen plasma for 8 min at 20 kHz | Extract extraction | TPC extraction increased using N2 CP treatment in 11 herb extracts up to 77% compared with untreated extracts gained from non-treated water suspended herbs at 70° C for 10 min | Antioxidant potential increased and decreased in 9 and 1 herbs extracts, respectively. Maximum increase was observed in Vaccinium myrtillus (DPPH; 0.567, FRAP: 0.404 and ABTS: 0.596 M ascorbic acid/g dw). Lowest antioxidant activity was observed in Urtica dioica (DPPH: 0.005 M ascorbic acid/g dw) and Leonurus cardiaca (FRAP: 0.021 and ABTS: 0.054 M ascorbic acid/g dw). | Increase in Anthocyanins and Flavonoids content only in four herb extracts | 2.58 log CFU/g reduction in aerobic bacteria | [19] |
| Kiwi turbid juice | DBD | Voltage: 13, 22, 31 W; 15, 25, 35 kV Frequency: 60 Hz Time: 1–5 min | PC extraction | Decrease in TPC extraction | NA | NA | NA | [102] |
| Cashew apple juice | DBD | Voltage: 20 kV Frequency: 200, 700 Hz Time: 15 min | Extract | Decrease in TPC | NA | NA | NA | [103] |
| Mango Pulp | DBDP | Voltage: 25 kV Time: 0, 2, 4, 6, 8 and 10 min | Extract | Increase in TPC up to 6 min treatment time (19.39 mg GAE/100 mL) then reduced (16.43 mg GAE/100 mL) in comparison with untreated fresh extracted mango pulp (18.28 GAE/100 mL) | DPPH capacity percentage increased until 4th min of operation (about 7.9%) but then decreased by 3.4% until 10th min of operation. | NA | NA | [104] |
| Germinated brown rice (GBR) | DBD | Voltage: 100, 135, 170 and 200 W Time: 25–300 s | PC extraction | Increase in TPC in cold plasma-treated GBR from approximately 30% to 86% compared with un-pretreated germinated brown rice | No significant differences observed in all rice cultivars and control samples through DPPH assay. | Increase in Anthocyanins and Phenolic content | NA | [105] |
| Wolfberry | Cold Plasma and Air Atmospheric Pressure | Voltage: 750 V Frequency: 20 kHz Time: 15–60 s | Extract extraction | Decrease in TPC from 17.1 to 13.5 mg GAE/g DM compared with untreated sample (30 and 45 s had the highest value) | NA | NA | NA | [106] |
| Sea asparagus | GDP | Time: 5 and 60 min Power: 14 W | Extract extraction | NA | Antioxidant capacity increased after 5 min CP treatment. | NA | Reduction in E. coli No effect on S. aureus | [20] |
| Grape pomace | HVACP | Voltage: 60 kV Time: 5, 10, and 15 min | PC extraction | TPC extraction increased after CP treatment (5 and 15 min) by 10.9–22.8% compared with un- HVACP treated grape pomace | Increase by about 29.0 and 34.7% after 5 and 15 min treatment, along with a slight decrease in antioxidant capacity from 5 min to 10 min. | Increase in Quercetin Increase in Anthocyanins and Anthocyanidins content (after 5 and 15 min treatment) | NA | [94] |
| Lotus Petal Powder | DBD | Voltage: 0–11 kV Frequency: 50 Hz Time: 5 min | Extract | Increase in TPC by 5.37% compared with microwave dried LPP without CP treatment | FRAP: increased from 42.92% (Control sample:) to 76.63% (CP-treated LPP). | Increase in Nuciferine (7.07–8.85%), Pronuciferine (0.86–1.58%), α-Amyrin (1.03–7.36%) and β-Amyrin (0.62–4.64%) Decrease in Tetracosanoland (53.44–29.05%) and Phthalic acid (0.73–0.14%) | NA | [107] |
| Naringin | DBD | Power: 250 W Frequency: 15 kHz Time: 0–20 min | Naringin treatment | Increase in TPC from 172.50 to 225.83 ppm in comparison with untreated sample | DPPH: increased about 36.75% by 20 min plasma treatment. FRAP: a time-dependent increase. | NA | Decreased in S. Typhimurium, L. monocytogenes, S. aureus, and E. coli O157:H7 after 20 min treatment | [108] |
| Peppermint | LPCP | Power: 20, 50 and 60 W for 20 min | Extract | Increase in TPC from 262.81 to 292.53 mg GAE/g edw at 20 and 50 W then reduced at 60 W (255.28 mg GAE/g edw) | DPPH: increased by elevating the power to 50 W (Up to 83%) and then with using 60 W of power, decreased to the initial value of untreated peppermint powder as the control sample (74%). | NA | Reduction in E. coli after 50 and 60 W treatments | [109] |
| Blended scarlet caterpillarclub | CPJ | Voltage:12 kV Frequency: 25 kHz Time: 30–120 s | Extract extraction | Increase in TPC extraction compared with the Untreated blended C. militaris | ABTS: highest increase at 60 and 90 s (6.82% and 6.54%) then decreased in 120 s (9.08%). DPPH: After 60 to 120 s of treatment the antioxidant activity enhanced by 12 to 17% and the highest observed in 90 s. FRAP: After 60 s exhibited the highest increase (50.35%) and then decreased after 120 s (26.46%). | NA | NA | [110] |
| Carrot juice | HVACP | Voltage:70 kV Time: 3 min | Extract extraction | Increase in TPC from 9.15 to 11.89 μg/g by HVACP-UHP treatment | NA | NA | NA | [111] |
| Fenugreek (dried and soaked seeds) | HVACP | Frequency: 80 kHz Time: 30 min | Galactomannan extraction | Increase in extraction yield (sample DS: 67%, sample SS: 122%) | NA | NA | NA | [112] |
| Camelina seeds | DBD-Plasma | Voltage: 15, 18, and 21 kV Time: 2,4,8 and 16 min | Oil extraction | Increase in extraction yield (from 24.3 to 31.5%) | NA | NA | NA | [113] |
| Sesame Sunflower. seeds | DBD-Plasma | Voltage: 25 kV Frequency: 50 Hz Time: 0–30 min Gas: O2 and N2 | Oil extraction | Increase in extraction yield in comparison with the untreated control samples without storage time | Extending the treatment time and storage duration (from 0 to 30 min and 0–30 days) led to a significant decrease (25%) in the antioxidant potential of both oil types. | NA | NA | [114] |
| Fennel seed Spearmint leaf | DBD-Plasma | Voltage: 17–23 kV Time: 5–15 min | Essential oil extraction | Yield extraction increased after CP treatments at 15 kV for 5 min (1.83% v/w) and decreased after 23 kV for 17 min (1.81% v/w) compared with untreated sample | NA | NA | NA | [115] |
| Lemon peel | DBD-Plasma | Voltage: 1–11 kV Frequency: 50 kHz Gas: O2 and N2 | Oil extraction | Increase in extraction yield (149.34%) compared with microwave extraction method | NA | NA | NA | [116] |
| Oat sprout | SDBD | Frequency: 14.4 kHz Voltage:8 kVp Power: 51.7 W Time: 6 min/day for 3 days | Extract | Increase in TPC (8.5% higher than untreated control) | NA | Increase in hexacosanol (28% higher than untreated sample) | NA | [117] |
| Lemon verbena leaves | LPCP | Frequency: 50 kHz Gas: nitrogen (N2), argon (Ar), and oxygen (O2) | Essential oil extraction | Increase in anthocyanin extraction yield | NA | NA | NA | [118] |
| Lemon peel | DBD-Plasma | Frequency: 50 kHz Voltage: 30 kV Gas: nitrogen (N2), argon (Ar), and oxygen (O2) Time: 1–15 min | Essential oil extraction | Increase in extraction yield | NA | NA | NA | [119] |
| Prickly Pear Cactus Fruit | LPCP | Frequency: 2.45 GHz of microwave Time: 10 to 40 min Power: 600–900 W | CP treatment on extract | NA | The highest increase occurred at 40 min with a power setting of 750 W, resulting in a 1.77% increase. Conversely, the greatest decrease was observed at 25 min also with a power setting of 750 W, resulting in a decrease of about 4.01%. It is important to note that the untreated powdered Prickly Pear Cactus fruit was used as the control sample for comparison. | NA | NA | [120] |
| Sweet basil | Cold Plasma and Air/Helium Atmospheric Pressure Plasma Jet | Frequency: 1 kHz Time: 30 s Gas: air and helium | Seed pre treatment (Essential oil) | NA | EOs extracted from homegrown CP-treated Sweet basil seeds, demonstrated higher antioxidant capacity (81.55%) compared with the untreated conventional forms (46.36%) and commercial BHT (about 77.24%) at a concentration of 50 µg/mL. The antioxidant potential of CP-treated samples varied from 48% to 94.82% by increasing the concentration. | NA | NA | [121] |
| Purple glutinous rice | LPCP | Frequency: 13.56 MHz Time: 20 min Power: 100 W Pressure: 6 Torr | PC pretreatment | Increase in TPC (Luem Pua rice extract had the highest TPC) | The Luem Pua treated with CP (IC50 = 0.080 mg/mL) showed an increased antioxidant capacity compared with the untreated Luem Pua (IC50 = 0.187 mg/mL). | Increase in Anthocyanins and Flavonoids content especially in Luem Pua rice extracts | NA | [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydari, M.; Carbone, K.; Gervasi, F.; Parandi, E.; Rouhi, M.; Rostami, O.; Abedi-Firoozjah, R.; Kolahdouz-Nasiri, A.; Garavand, F.; Mohammadi, R. Cold Plasma-Assisted Extraction of Phytochemicals: A Review. Foods 2023, 12, 3181. https://doi.org/10.3390/foods12173181
Heydari M, Carbone K, Gervasi F, Parandi E, Rouhi M, Rostami O, Abedi-Firoozjah R, Kolahdouz-Nasiri A, Garavand F, Mohammadi R. Cold Plasma-Assisted Extraction of Phytochemicals: A Review. Foods. 2023; 12(17):3181. https://doi.org/10.3390/foods12173181
Chicago/Turabian StyleHeydari, Mahshid, Katya Carbone, Fabio Gervasi, Ehsan Parandi, Milad Rouhi, Omid Rostami, Reza Abedi-Firoozjah, Azin Kolahdouz-Nasiri, Farhad Garavand, and Reza Mohammadi. 2023. "Cold Plasma-Assisted Extraction of Phytochemicals: A Review" Foods 12, no. 17: 3181. https://doi.org/10.3390/foods12173181
APA StyleHeydari, M., Carbone, K., Gervasi, F., Parandi, E., Rouhi, M., Rostami, O., Abedi-Firoozjah, R., Kolahdouz-Nasiri, A., Garavand, F., & Mohammadi, R. (2023). Cold Plasma-Assisted Extraction of Phytochemicals: A Review. Foods, 12(17), 3181. https://doi.org/10.3390/foods12173181






