Recent Progress in Understanding the Impact of Food Processing and Storage on the Structure–Activity Relationship of Fucoxanthin
Abstract
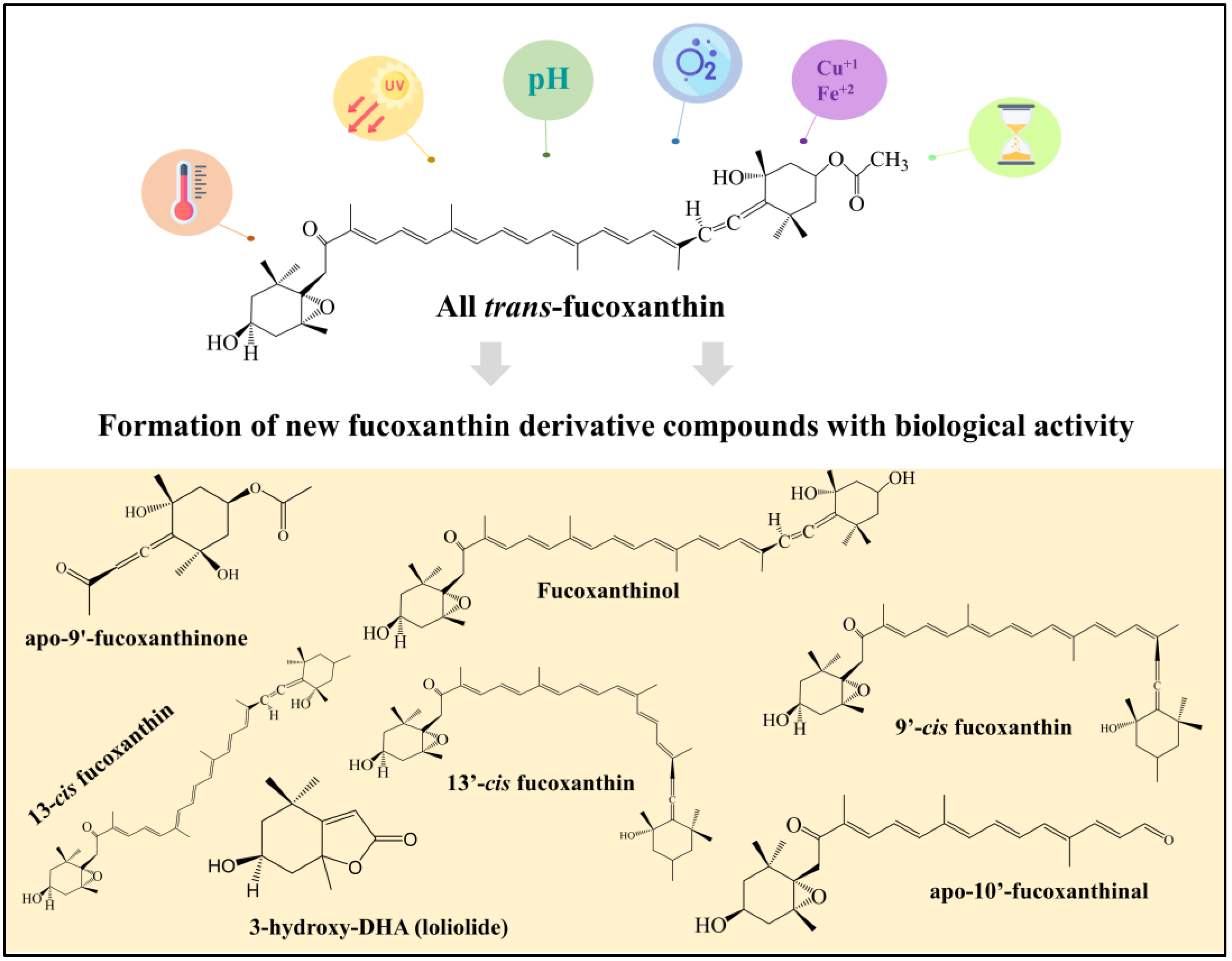
:1. Introduction
2. Food Processing and Storage Conditions Affecting Fucoxanthin Structure
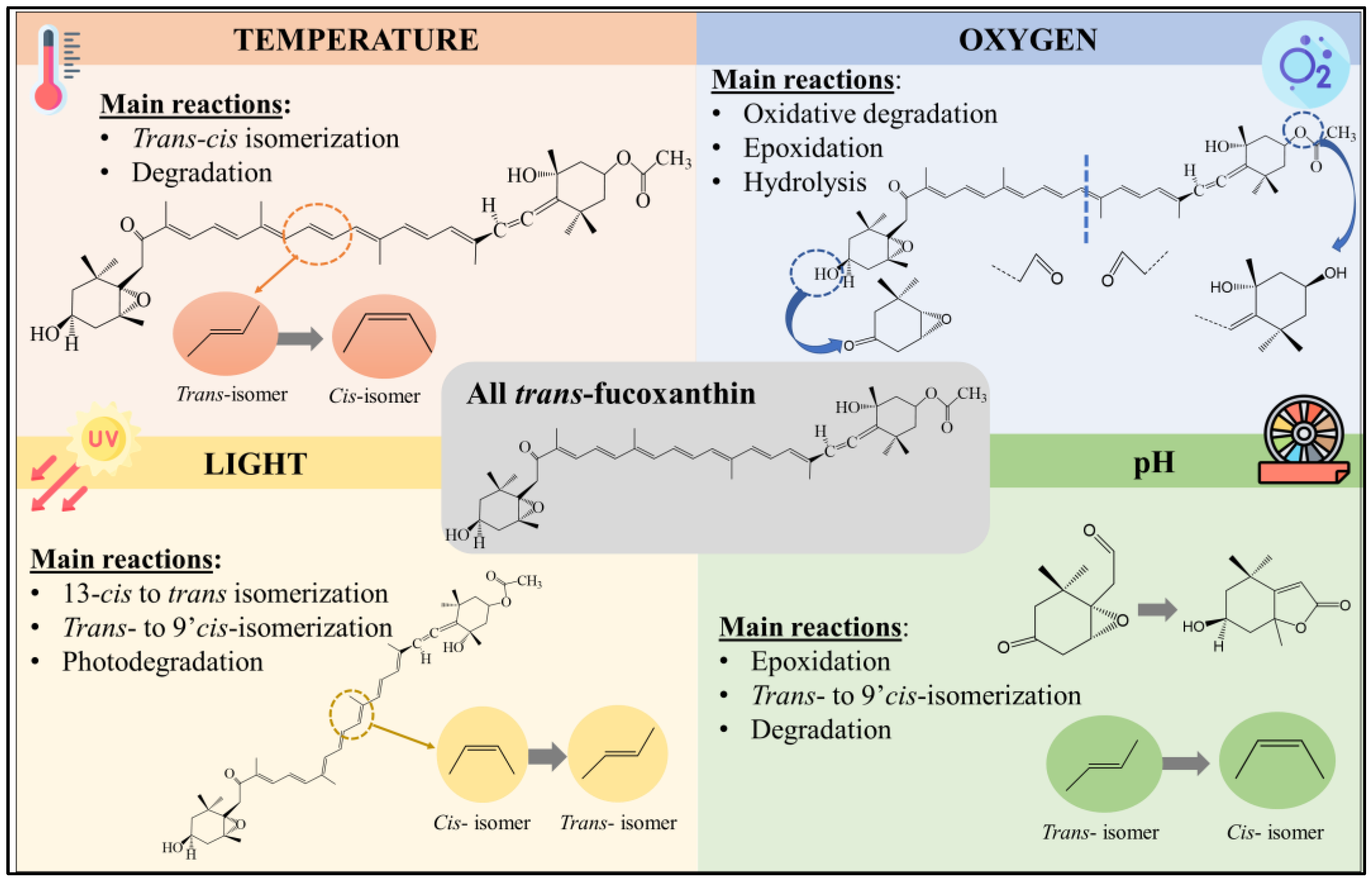
2.1. Heat Processing
2.2. Oxygen
2.3. Light Exposure
2.4. pH
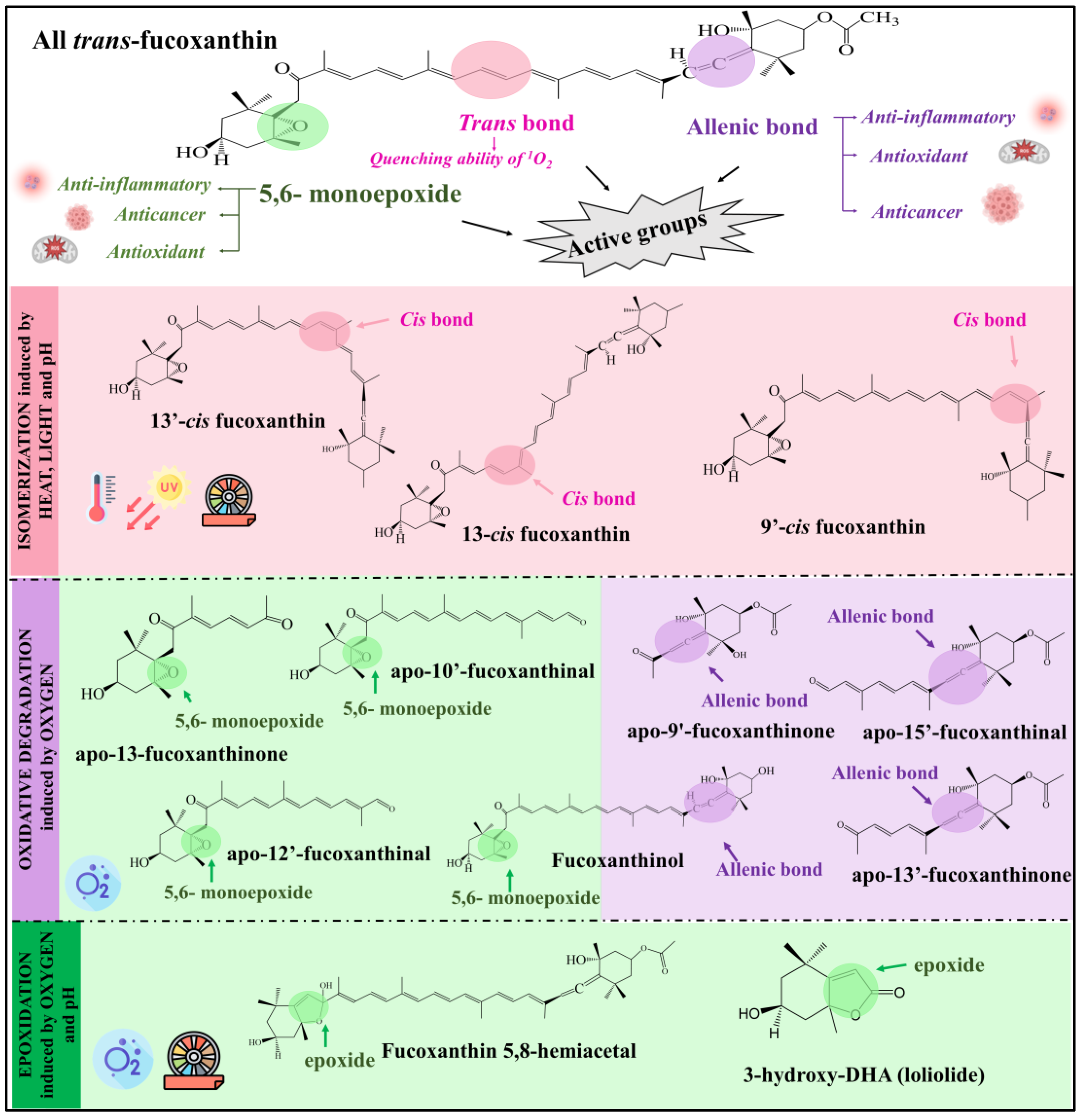
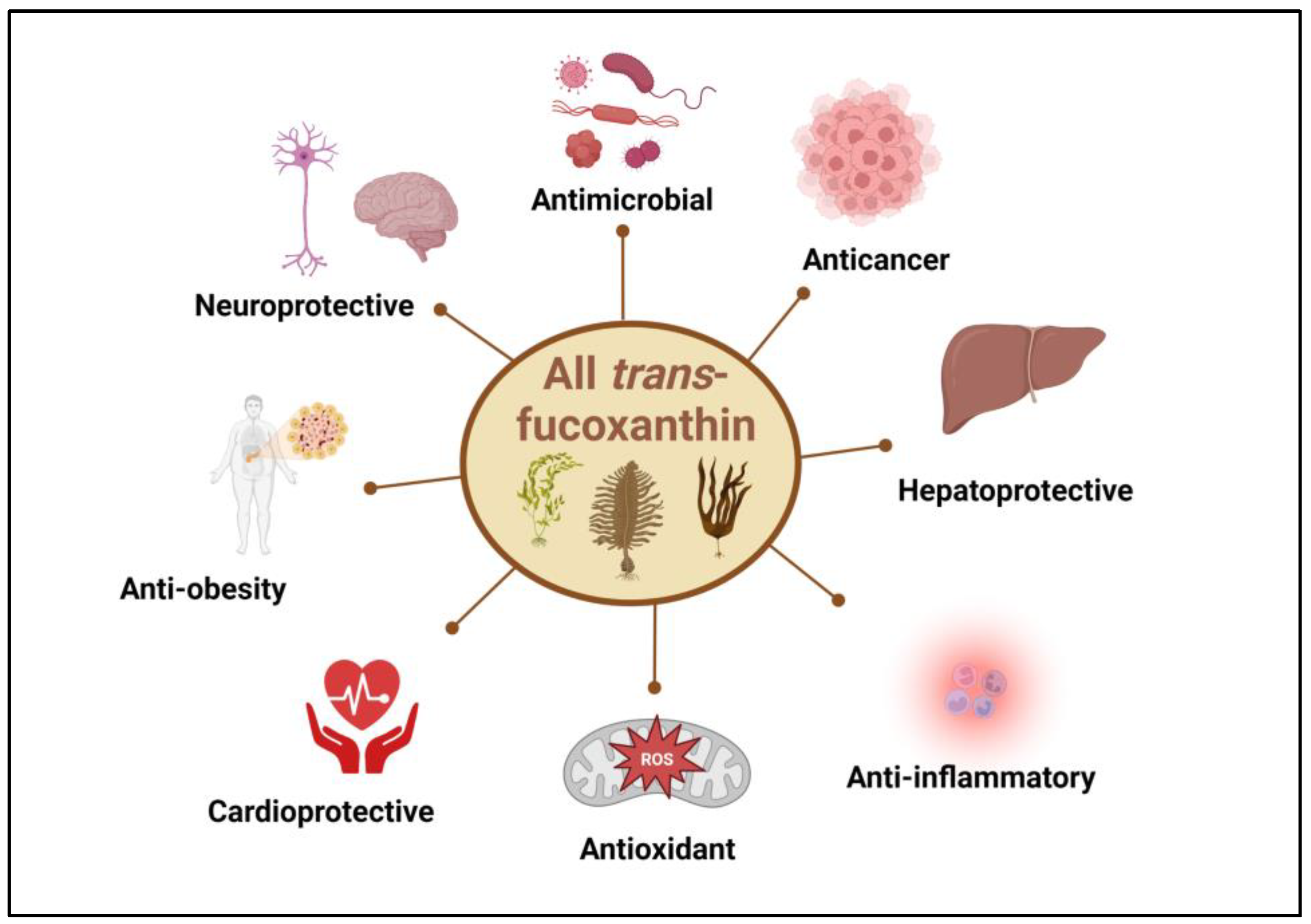
3. Biological Activity of Fucoxanthin Derivative Compounds
| Biological Activity | Fucoxanthin Derivative Compound | Source | Main Findings | Ref. |
|---|---|---|---|---|
| Anti-inflammatory | ||||
| 9′-cis fucoxanthin, and a complex including both 13-cis and 13′-cis fucoxanthin | Sargassum siliquastrum | 9′-cis fucoxanthin inhibited the production of NO, TNF-α, and IL-6 in RAW 264.7 cells. 13-cis and 13′-cis fucoxanthin showed cytotoxic effect. | [38] | |
| Apo-9′-fucoxanthinone | Sargassum muticum | Suppression of NO and PGE2 production, and iNOS and COX-2 expression in RAW 264.7 cells. Reduction of ROS and NO production, and level of iNOS, COX, TNF-α, and IL-1β in LPS-treated zebrafish. | [39] | |
| S. muticum | Suppression of NO and PGE2 production. | [40] | ||
| Sargassum horneri | Suppression of NO production in LPS-stimulated RAW 264.7 cells. | [41] | ||
| Loliolide | Sargassum horneri | Suppression of NO production in LPS-stimulated RAW 264.7 cells. | [41] | |
| S. horneri | Suppression of IL-1β, IL-6, and TNF-α, PGE2 COX-2, and iNOS expression in LPS-induced cells. | [42] | ||
| Apo-10′-fucoxanthinal | Prepared from fucoxanthinol | Suppression of MAPK and NF-κB signaling via downregulating the expression of inflammatory mediators in LPS-induced RAW264.7 cells. | [35] | |
| Fucoxanthinol | Nitzschia laevis | Suppression of NO, PGE2, and ROS production, and reduction of the expression of iNOS, COX-2, IL-1β, TNF-α, and IL-6, in LPS-induced BV-2 cells. | [25] | |
| Antioxidant | ||||
| Ratio of trans- to cis-fucoxanthin (100:3:7; 100:3:8, 100:3:10) | Phaeodactylum tricornutum | As the concentration of the cis isomers increased, the antioxidant activity (DPPH, superoxide anion, reducing power, and hydrogen peroxide) decreased. | [14] | |
| 9′-cis, 13-cis and 13′-cis fucoxanthin isomers | Laminaria japonica Aresch | DPPH and superoxide radical scavenging activity: 13-cis- and 13′-cis-isomers > all trans-fucoxanthin > 9′-cis-isomer. ABTS scavenging activity: 9′-cis isomer > all trans-fucoxanthin > 13-cis and 13′-cis isomers. | [43] | |
| Fucoxanthinol | Standard | Inhibition of cellular reactive oxygen species accumulation. | [44] | |
| Anticancer | ||||
| Apo-13-fucoxanthinone and apo-9′-fucoxanthinone | U. pinnatifida | Apo-9′-fucoxanthinone showed higher cytotoxic effect against Caco-2 cells than apo-13-fucoxanthinone. | [3] | |
| 9′-cis fucoxanthin, and a complex including both 13-cis and 13′-cis fucoxanthin | Sargassum siliquastrum | All isomers significantly inhibited human fibrosarcoma (HT1080) cell migration. Reduction of the MMP-2 and MMP-9 activities. | [45] | |
| Fucoxanthinol | Standard | Reduction of in colorectal cancer cells (HCT116, DLD-1, Caco-2, WiDr, SW620). | [46] | |
| Enzymatically prepared from fucoxanthin standard | Modulation of gene expression and core signaling pathways in human breast cancer cells (MCF-7 and MDA-MB-231). Induction of apoptosis in MCF-7 and MDA-MD-231 cells. | [47] | ||
| Immunomodulation | ||||
| Apo-9′-fucoxanthinone | S. muticum | Modulation of immune system by inhibition of IgE serum levels and cutaneous edema. Reduction of IL-4, IFN-γ, and TNF-α production. Decrease of lymph node size in atopic dermatitis mouse. | [48] | |
| Anti-hyaluronidase | ||||
| Trans-, 9′-cis, and 13′-cis fucoxanthin isomers. | Sargassum vulgare Keelakarai, Turbinaria ornata, Turbinaria conoides | Despite cis-isomers being able to react with hyaluronidase enzyme through hydrophobic interactions, these forms were less stable than trans-fucoxanthin. | [17] | |
| Hair growth stimulation | ||||
| Apo-9′-fucoxanthinone | S. muticum | Apo-9′-fucoxanthinone induced the dermal papilla cell growth and reduction of 5α-reductase activity, suggesting its potential use for hair growth. | [49] |
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Honda, M.; Murakami, K.; Takasu, S.; Goto, M. Extraction of Fucoxanthin Isomers from the Edible Brown Seaweed Undaria pinnatifida Using Supercritical CO2: Effects of Extraction Conditions on Isomerization and Recovery of Fucoxanthin. J. Oleo Sci. 2022, 71, ess22077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Kim, S.-M.; Pan, C.-H.; Chung, D. Effects of Heating, Aerial Exposure and Illumination on Stability of Fucoxanthin in Canola Oil. Food Chem. 2014, 145, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells. Mar. Drugs 2018, 16, 275. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, E.; Bai, Y.; Lu, Y.; Qi, H. Encapsulated Fucoxanthin Improves the Structure and Functional Properties of Fermented Yogurt during Cold Storage. Food Chem. 2023, 419, 136076. [Google Scholar] [CrossRef]
- Derwenskus, F.; Schäfer, B.; Müller, J.; Frick, K.; Gille, A.; Briviba, K.; Schmid-Staiger, U.; Hirth, T. Coproduction of EPA and Fucoxanthin with P. tricornutum—A Promising Approach for Up- and Downstream Processing. Chem. Ing. Tech. 2020, 92, 1780–1789. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Carreira-Casais, A.; Carperna, M.; Barral-Martinez, M.; Chamorro, F.; Jiménez-López, C.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Emerging Technologies to Extract Fucoxanthin from Undaria pinnatifida: Microwave vs. Ultrasound Assisted Extractions. Mar. Drugs 2023, 21, 282. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A Potential Commercial Source of Fucoxanthin Extracted from the Microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Li, D.; Liu, Y.; Ma, Y.; Liu, Y.; Wang, S.; Guo, Z.; Li, J.; Wang, Y.; Tan, B.; Wei, Y. Fabricating Hydrophilic Fatty Acid-Protein Particles to Encapsulate Fucoxanthin: Fatty Acid Screening, Structural Characterization, and Thermal Stability Analysis. Food Chem. 2022, 382, 132311. [Google Scholar] [CrossRef]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese Seaweed, Wakame (Undaria pinnatifida) as an Ingredient in Pasta: Chemical, Functional and Structural Evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, H.; Ding, Y.; Liu, S.; Ding, Y.; Lu, B.; Xiao, J.; Zhou, X. Dietary Protective Potential of Fucoxanthin as an Active Food Component on Neurological Disorders. J. Agric. Food Chem. 2023, 71, 3599–3619. [Google Scholar] [CrossRef]
- Nuñez de González, M.T.; Woldesenbet, S.; Mora-Gutierrez, A.; Jung, Y. Fucoxanthin as a Biofunctional Compound in Goat Milk Yogurt: Stability and Physicochemical Effects. Fermentation 2023, 9, 273. [Google Scholar] [CrossRef]
- Yusof, Z.; Khong, N.M.H.; Choo, W.S.; Foo, S.C. Opportunities for the Marine Carotenoid Value Chain from the Perspective of Fucoxanthin Degradation. Food Chem. 2022, 383, 132394. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, A.A.; Elim, P.E.; Heriyanto; Prihastyanti, M.N.U.; Yoewono, J.R.; Shioi, Y.; Limantara, L.; Brotosudarmo, T.H.P. Effect of Drying on the Production of Fucoxanthin Isomers from Brown Seaweeds. J. Food Process. Preserv. 2022, 46, e17057. [Google Scholar] [CrossRef]
- Kawee-aiab, A.; Kuntiyab, A.; Kima, S.M. Anticholinesterase and Antioxidant Activities of Fucoxanthin Purified from the Microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar]
- Nie, J.; Chen, D.; Lu, Y.; Dai, Z. Effects of Various Blanching Methods on Fucoxanthin Degradation Kinetics, Antioxidant Activity, Pigment Composition, and Sensory Quality of Sargassum fusiforme. LWT 2021, 143, 111179. [Google Scholar] [CrossRef]
- Gheysen, L.; Demets, R.; Devaere, J.; Bernaerts, T.; Goos, P.; Van Loey, A.; De Cooman, L.; Foubert, I. Impact of Microalgal Species on the Oxidative Stability of N-3 LC-PUFA Enriched Tomato Puree. Algal Res. 2019, 40, 101502. [Google Scholar] [CrossRef]
- Kurinjery, A.; Kulanthaiyesu, A. Anti-Hyaluronidase and Cytotoxic Activities of Fucoxanthin Cis/Trans Isomers Extracted and Characterized from 13 Brown Seaweeds. Process Biochem. 2022, 122, 53–68. [Google Scholar] [CrossRef]
- Semitsoglou-Tsiapou, S.; Meador, T.B.; Peng, B.; Aluwihare, L. Photochemical (UV–Vis/H2O2) Degradation of Carotenoids: Kinetics and Molecular End Products. Chemosphere 2022, 286, 131697. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, X.; Chen, X.; Wang, S.; Wang, D. Chemical Cleavage of Fucoxanthin from Undaria pinnatifida and Formation of Apo-Fucoxanthinones and Apo-Fucoxanthinals Identified Using LC-DAD-APCI-MS/MS. Food Chem. 2016, 211, 365–373. [Google Scholar] [CrossRef]
- Piovan, A.; Seraglia, R.; Bresin, B.; Caniato, R.; Filippini, R. Fucoxanthin from Undaria pinnatifida: Photostability and Coextractive Effects. Molecules 2013, 18, 6298–6310. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, D.; Kim, M.; Gu, M.-Y.; Kim, S.-M.; Pan, C.-H.; Kim, G.-H.; Chung, D. Effects of Temperature, Light, and PH on the Stability of Fucoxanthin in an Oil-in-Water Emulsion. Food Chem. 2019, 291, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Wang, F.; Xia, G.; Liu, H.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X.; et al. Isolation of Fucoxanthin from Sargassum thunbergii and Preparation of Microcapsules Based on Palm Stearin Solid Lipid Core. Front. Mater. Sci. 2017, 11, 66–74. [Google Scholar] [CrossRef]
- Sharoni, Y.; Linnewiel-Hermoni, K.; Khanin, M.; Salman, H.; Veprik, A.; Danilenko, M.; Levy, J. Carotenoids and Apocarotenoids in Cellular Signaling Related to Cancer: A Review. Mol. Nutr. Food Res. 2012, 56, 259–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, K.; He, S.; Tan, L.; Kong, X. Characterizations of High Purity Starches Isolated from Five Different Jackfruit Cultivars. Food Hydrocoll. 2016, 52, 785–794. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Sun, P.; Zhang, Y.; Wu, T.; Sun, H.; Cheng, K.-W.; Chen, F. Fucoxanthinol from the Diatom Nitzschia Laevis Ameliorates Neuroinflammatory Responses in Lipopolysaccharide-Stimulated BV-2 Microglia. Mar. Drugs 2020, 18, 116. [Google Scholar] [CrossRef]
- Martínez-Delgado, A.A.; Khandual, S.; Villanueva–Rodríguez, S.J. Chemical Stability of Astaxanthin Integrated into a Food Matrix: Effects of Food Processing and Methods for Preservation. Food Chem. 2017, 225, 23–30. [Google Scholar] [CrossRef]
- Hadjal, T.; Dhuique-Mayer, C.; Madani, K.; Dornier, M.; Achir, N. Thermal Degradation Kinetics of Xanthophylls from Blood Orange in Model and Real Food Systems. Food Chem. 2013, 138, 2442–2450. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Zhang, Y.; Diono, W.; Kanda, H.; Yamaguchi, R.; Takemura, R.; Fukaya, T.; Goto, M. Improved Carotenoid Processing with Sustainable Solvents Utilizing Z-Isomerization-Induced Alteration in Physicochemical Properties: A Review and Future Directions. Molecules 2019, 24, 2149. [Google Scholar] [CrossRef]
- Honda, M. Carotenoid Isomers: A Systematic Review of the Analysis, Biological Activity, Physicochemical Property, and Methods for Isomerization; Atta-ur Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9778-0-12-819485-0. [Google Scholar]
- Honda, M.; Kodama, T.; Kageyama, H.; Hibino, T.; Diono, W.; Kanda, H.; Goto, M. Enhanced Solubility and Reduced Crystallinity of Carotenoids, β-Carotene and Astaxanthin, by Z -Isomerization. Eur. J. Lipid Sci. Technol. 2018, 120, 1800191. [Google Scholar] [CrossRef]
- Murakami, K.; Honda, M.; Wahyudiono; Kanda, H.; Goto, M. Thermal Isomerization of (All-E-)-Lycopene and Separation of the Z -Isomers by Using a Low Boiling Solvent: Dimethyl Ether. Sep. Sci. Technol. 2017, 52, 2573–2582. [Google Scholar] [CrossRef]
- Cooperstone, J.L.; Ralston, R.A.; Riedl, K.M.; Haufe, T.C.; Schweiggert, R.M.; King, S.A.; Timmers, C.D.; Francis, D.M.; Lesinski, G.B.; Clinton, S.K.; et al. Enhanced Bioavailability of Lycopene When Consumed as Cis-Isomers from Tangerine Compared to Red Tomato Juice, a Randomized, Cross-over Clinical Trial. Mol. Nutr. Food Res. 2015, 59, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Murakami, K.; Osawa, Y.; Kawashima, Y.; Hirasawa, K.; Kuroda, I. Z-Isomers of Astaxanthin Exhibit Greater Bioavailability and Tissue Accumulation Efficiency than the All-E-Isomer. J. Agric. Food Chem. 2021, 69, 3489–3495. [Google Scholar] [CrossRef] [PubMed]
- Mohibbullah, M.; Haque, M.N.; Sohag, A.A.M.; Hossain, M.T.; Zahan, M.S.; Uddin, M.J.; Hannan, M.A.; Moon, I.S.; Choi, J.-S. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Mar. Drugs 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Takatani, N.; Taya, D.; Katsuki, A.; Beppu, F.; Yamano, Y.; Wada, A.; Miyashita, K.; Hosokawa, M. Identification of Paracentrone in Fucoxanthin-fed Mice and Anti-inflammatory Effect against Lipopolysaccharide-stimulated Macrophages and Adipocytes. Mol. Nutr. Food Res. 2021, 65, 2000405. [Google Scholar] [CrossRef]
- Mumu, M.; Das, A.; Emran, T.B.; Mitra, S.; Islam, F.; Roy, A.; Karim, M.M.; Das, R.; Park, M.N.; Chandran, D.; et al. Fucoxanthin: A Promising Phytochemical on Diverse Pharmacological Targets. Front. Pharmacol. 2022, 13, 929442. [Google Scholar] [CrossRef]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kyung Kim, O.; Koshimizu, K.; Ohigashi, H. Modifying Effects of Carotenoids on Superoxide and Nitric Oxide Generation from Stimulated Leukocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Oh, C.; Choi, Y.-U.; Yoon, K.-T.; Kang, D.-H.; Qian, Z.-J.; Choi, I.-W.; Jung, W.-K. Anti-Inflammatory Effect of Fucoxanthin Derivatives Isolated from Sargassum siliquastrum in Lipopolysaccharide-Stimulated RAW 264.7 Macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kim, S.-Y.; Ye, B.-R.; Kim, J.; Ko, S.-C.; Lee, W.W.; Kim, K.-N.; Choi, I.-W.; Jung, W.-K.; Heo, S.-J. Anti-Inflammatory Effect of Apo-9′-Fucoxanthinone via Inhibition of MAPKs and NF-KB Signaling Pathway in LPS-Stimulated RAW 264.7 Macrophages and Zebrafish Model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef]
- Jeon, H.; Yoon, W.-J.; Ham, Y.-M.; Yoon, S.-A.; Kang, S. Anti-Arthritis Effect through the Anti-Inflammatory Effect of Sargassum muticum Extract in Collagen-Induced Arthritic (CIA) Mice. Molecules 2019, 24, 276. [Google Scholar] [CrossRef]
- Kim, H.-S.; Fernando, I.P.S.; Lee, S.-H.; Ko, S.-C.; Kang, M.C.; Ahn, G.; Je, J.-G.; Sanjeewa, K.K.A.; Rho, J.-R.; Shin, H.J.; et al. Isolation and Characterization of Anti-Inflammatory Compounds from Sargassum horneri via High-Performance Centrifugal Partition Chromatography and High-Performance Liquid Chromatography. Algal Res. 2021, 54, 102209. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Kim, H.-S.; Asanka Sanjeewa, K.K.; Han, E.J.; Jee, Y.; Ahn, G.; Rho, J.-R.; Jeon, Y.-J. Loliolide, Isolated from Sargassum horneri; Abate LPS-Induced Inflammation via TLR Mediated NF-ΚB, MAPK Pathways in Macrophages. Algal Res. 2021, 56, 102297. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative Evaluation of the Radical-Scavenging Activities of Fucoxanthin and Its Stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Fucoxanthin Metabolites Exert Anti-Fibrogenic and Antioxidant Effects in Hepatic Stellate Cells. J. Agric. Food Res. 2021, 6, 100245. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Qian, Z.-J.; Lee, B.; Heo, S.-J.; Kim, K.-N.; Jeon, Y.-J.; Park, W.S.; Choi, I.-W.; Jang, C.H.; Ko, S.-C.; et al. Fucoxanthin Derivatives from Sargassum siliquastrum Inhibit Matrix Metalloproteinases by Suppressing NF-ΚB and MAPKs in Human Fibrosarcoma Cells. ALGAE 2014, 29, 355–366. [Google Scholar] [CrossRef]
- Takahashi, K.; Hosokawa, M.; Kasajima, H.; Hatanaka, K.; Kudo, K.; Shimoyama, N.; Miyashita, K. Anticancer Effects of Fucoxanthin and Fucoxanthinol on Colorectal Cancer Cell Lines and Colorectal Cancer Tissues. Oncol. Lett. 2015, 10, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, A.; Wagatsuma, M.; Murase, W.; Kubota, A.; Kojima, H.; Ohta, T.; Hamada, J.; Maeda, H.; Terasaki, M. Fucoxanthinol Promotes Apoptosis in MCF-7 and MDA-MB-231 Cells by Attenuating Laminins–IntegrinsaAxis. Onco 2022, 2, 145–163. [Google Scholar] [CrossRef]
- Han, S.-C.; Kang, N.-J.; Yoon, W.-J.; Kim, S.; Na, M.-C.; Koh, Y.-S.; Hyun, J.-W.; Lee, N.-H.; Ko, M.-H.; Kang, H.-K.; et al. External Application of Apo-9′-Fucoxanthinone, Isolated from Sargassum muticum, Suppresses Inflammatory Responses in a Mouse Model of Atopic Dermatitis. Toxicol. Res. 2016, 32, 109–114. [Google Scholar] [CrossRef]
- Kang, J.-I.; Yoo, E.-S.; Hyun, J.-W.; Koh, Y.-S.; Lee, N.H.; Ko, M.-H.; Ko, C.-S.; Kang, H.-K. Promotion Effect of Apo-9′-Fucoxanthinone from Sargassum muticum on Hair Growth via the Activation of Wnt/β-Catenin and VEGF-R2. Biol. Pharm. Bull. 2016, 39, 1273–1283. [Google Scholar] [CrossRef]
- Yokoyama, R.; Kushibiki, A.; Yamada, S.; Kubota, A.; Kojima, H.; Ohta, T.; Hamada, J.; Maeda, H.; Mutoh, M.; Terasaki, M. Requirement of CLIC4 Expression in Human Colorectal Cancer Cells for Sensitivity to Growth Inhibition by Fucoxanthinol. Cancer Genom. Proteom. 2022, 19, 428–444. [Google Scholar] [CrossRef]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, M. The Allenic Carotenoid Fucoxanthin, a Novel Marine Nutraceutical from Brown Seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef]
- Miyashita, K.; Maeda, H.; Tsukui, T.; Okada, T.; Hosokawa, M. Anti-Obesity Effect of Allene Carotenoids, Fucoxanthin and Neoxanthin from Seaweeds and Vegetables. Acta Hortic. 2009, 841, 167–172. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from Edible Seaweed, Undaria pinnatifida, Shows Antiobesity Effect through UCP1 Expression in White Adipose Tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. Marine Antioxidants: Polyphenols and Carotenoids from Algae. In Antioxidants and Functional Components in Aquatic Foods; Kristinsson, H.G., Ed.; Wiley: Hoboken, NJ, USA, 2014; pp. 219–235. ISBN 9780813813677. [Google Scholar]
- Shimoda, H.; Tanaka, J.; Shan, S.-J.; Maoka, T. Anti-Pigmentary Activity of Fucoxanthin and Its Influence on Skin MRNA Expression of Melanogenic Molecules. J. Pharm. Pharmacol. 2010, 62, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Berk, Z. Food Process Engineering and Technology; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128120187. [Google Scholar]
| Factor | Treatment Conditions | Algae | Main Findings | Ref. |
|---|---|---|---|---|
| FOOD PROCESSING | ||||
| Temperature | ||||
| Exposure at 25, 37, 50, 80, and 100 °C. | Phaeodactylum tricornutum | Fucoxanthin content slightly decreased from 25 to 80 °C. Beyond this value, a significant reduction was observed. | [14] | |
| Supercritical CO2 extraction (40–160 °C, and 20–40 MPa) using ethanol as co-solvent. | Undaria pinnatifida | Increasing extraction temperature promoted isomerization from all trans fucoxanthin to cis forms and recovery of 13-cis and 13′-cis isomers. | [1] | |
| Oven drying (40–100 °C). | Padina australis | Isomerization from all trans fucoxanthin to 13′-cis, 13-cis, and 9′-cis isomers (from 40 to 80 °C). Beyond 80 °C, degradations of all trans and cis isomers were found. | [13] | |
| Hot water blanching (HWB) and saltwater blanching (SWB, 0.5–16 min at 60–98.3 °C). Steam blanching (SB, 0.5–16 min at 100.1 °C). Microwave blanching (MWB, 0.5–16 min at 560 W). | Sargassum fusiforme | HWB allowed higher fucoxanthin recovery in comparison to other treatments. However, fucoxanthin degraded over time in all treatments applied. | [15] | |
| Tomato purees supplemented with microalgae and subjected to sterilization. | Isochrysis, and Phaeodactylum | Fucoxanthin content decreased by 40% due to sterilization | [16] | |
| −20 °C and room temperature. | Sargassum tenerrimum | Trans-fucoxanthin isomers remained stable when stored at both temperatures in the dark. | [17] | |
| Oxygen | ||||
| Exposure to irradiation (10, 20, 40, 60, 80 min) and hydrogen peroxide (20 mg/L). | Standard | Fucoxanthin showed the highest degradation rate. Degradation products were identified as: fucoxanthinals (12′-apo-fucoxanthinal and 15′-apo fucoxanthinal), fucoxanthinones (9′- apo-fucoxanthinone, 13′-apo-fucoxanthinone and 13-apo-fucoxanthinone), fucoxanthinol, and 3-hydroxy-DHA (loliolide). | [18] | |
| Exposure to potassium permanganate (KMnO4) and hypochlorous acid/hypochlorite (HClO/ClO−). | U. pinnatifida | Fucoxanthin degradation led to the formation of many cleavage compounds such as 3 apo-fucoxanthinones and 11 apo-fucoxanthinals. | [19] | |
| Ozone oxidation. | U. pinnatifida | Fucoxanthin oxidation led to the formation of apo-13-fucoxanthinone and apo-9′fucoxanthinone. | [3] | |
| Open air in amber color bottle at 30 °C. | S. tenerrimum | Approximately 55% of all-trans fucoxanthin was retained, while 30% of the fucoxanthin was observed to be oxidized. | [17] | |
| Light | ||||
| Direct daylight (2500 lux; 90 min) at room temperature | U. pinnatifida | Fucoxanthin content was reduced by 90%. | [20] | |
| Light at 175.77 mol/m2/s, room temperature | S. tenerrimum | Fucoxanthin underwent oxidation after exposing to light. | [17] | |
| pH | ||||
| Exposure at pH 2, 4, 6, 7, 8, and 10. | Phaeodactylum tricornutum | Fucoxanthin was sensitive to acidic conditions (pH 2–4), but stable in the neutral and alkaline systems (pH 6–10). | [14] | |
| STORAGE CONDITIONS | ||||
| Temperature | ||||
| 4 and 25 °C for six months. | Phaeodactylum tricornutum | Fucoxanthin stored at a low temperature (4 °C) was more stable during long-term storage than at 25 °C. | [14] | |
| Incubation at 25, 37, and 60 °C in a water bath in the dark. | C. costata | Degradation of all trans-fucoxanthin and 13-cis and 13′-cis isomers were observed at every temperature assayed during storage. However, the concentration of 9′-cis fucoxanthin was stable regardless of temperature studied during storage. | [21] | |
| Oxygen | ||||
| Sterilized tomato purees supplemented with microalgae stored for 12 weeks at 37 °C. | Isochrysis, and Phaeodactylum | Fucoxanthin was significantly degraded in purees during storage. | [16] | |
| Incubated in an oven in open air at 25 °C for 30 weeks. | C. costata | Fucoxanthin was degraded, resulting in the predominant formation of 9′-cis as the major product. | [2] | |
| Light | ||||
| Exposure at 300 or 2000 lux and incubation for 16 weeks. | C. costata | Isomerization from 13-cis and 13′-cis to all trans fucoxanthin was found at both light intensities. Isomerization from all trans to 9′-cis fucoxanthin was also detected and was more pronounced at 2000 lux. From half of the storage to the end, photodegradation of all trans and cis isomers was detected. | [2] | |
| Storage at room temperature with or without light for 30 days. | Sargassum thunbergii | A slight reduction of fucoxanthin content was observed during storage in the dark. On the contrary, a drastic decrease in fucoxanthin content was found when stored with light. | [22] | |
| pH | ||||
| Exposure at pH 1.2, 4.6, and 7.5 and incubation for 120 days. | C. costata | All trans and cis-fucoxanthin isomers were degraded at pH 1.2. However, in neutral conditions, degradation rate of all trans, 13-cis and 13′-cis fucoxanthin was reduced. The formation of 9′-cis isomer was observed. | [21] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomez-Zavaglia, A.; Barros, L.; Prieto, M.A.; Cassani, L. Recent Progress in Understanding the Impact of Food Processing and Storage on the Structure–Activity Relationship of Fucoxanthin. Foods 2023, 12, 3167. https://doi.org/10.3390/foods12173167
Gomez-Zavaglia A, Barros L, Prieto MA, Cassani L. Recent Progress in Understanding the Impact of Food Processing and Storage on the Structure–Activity Relationship of Fucoxanthin. Foods. 2023; 12(17):3167. https://doi.org/10.3390/foods12173167
Chicago/Turabian StyleGomez-Zavaglia, Andrea, Lillian Barros, Miguel A. Prieto, and Lucía Cassani. 2023. "Recent Progress in Understanding the Impact of Food Processing and Storage on the Structure–Activity Relationship of Fucoxanthin" Foods 12, no. 17: 3167. https://doi.org/10.3390/foods12173167
APA StyleGomez-Zavaglia, A., Barros, L., Prieto, M. A., & Cassani, L. (2023). Recent Progress in Understanding the Impact of Food Processing and Storage on the Structure–Activity Relationship of Fucoxanthin. Foods, 12(17), 3167. https://doi.org/10.3390/foods12173167










