Investigation of Antioxidant and Cytotoxicity Activities of Chocolate Fortified with Muscadine Grape Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of MGP Powder and MGP Chocolates
2.2. Chemicals
2.3. Preparation of Cocoa Beans and Chocolate Extracts
2.4. Analysis of Total Phenolic and Flavonoid Contents
2.5. DPPH Radical-Scavenging Activity
2.6. Ferric Reducing Antioxidant Potential (FRAP) Assay
2.7. Cell Culture
2.8. Anticancer Activity
2.9. Data Analysis
3. Results and Discussion
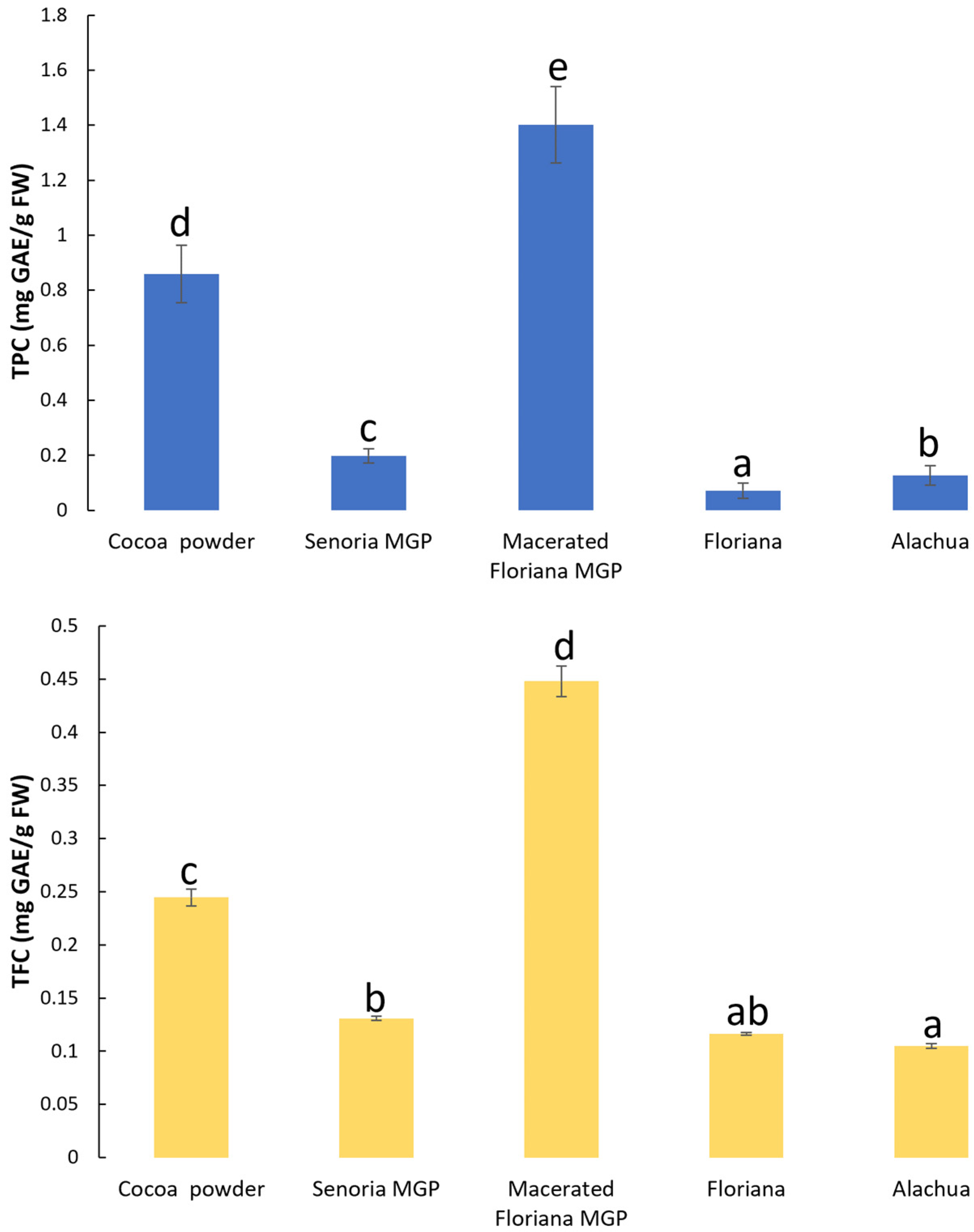
3.1. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) in MGP Powder and MGP Chocolates
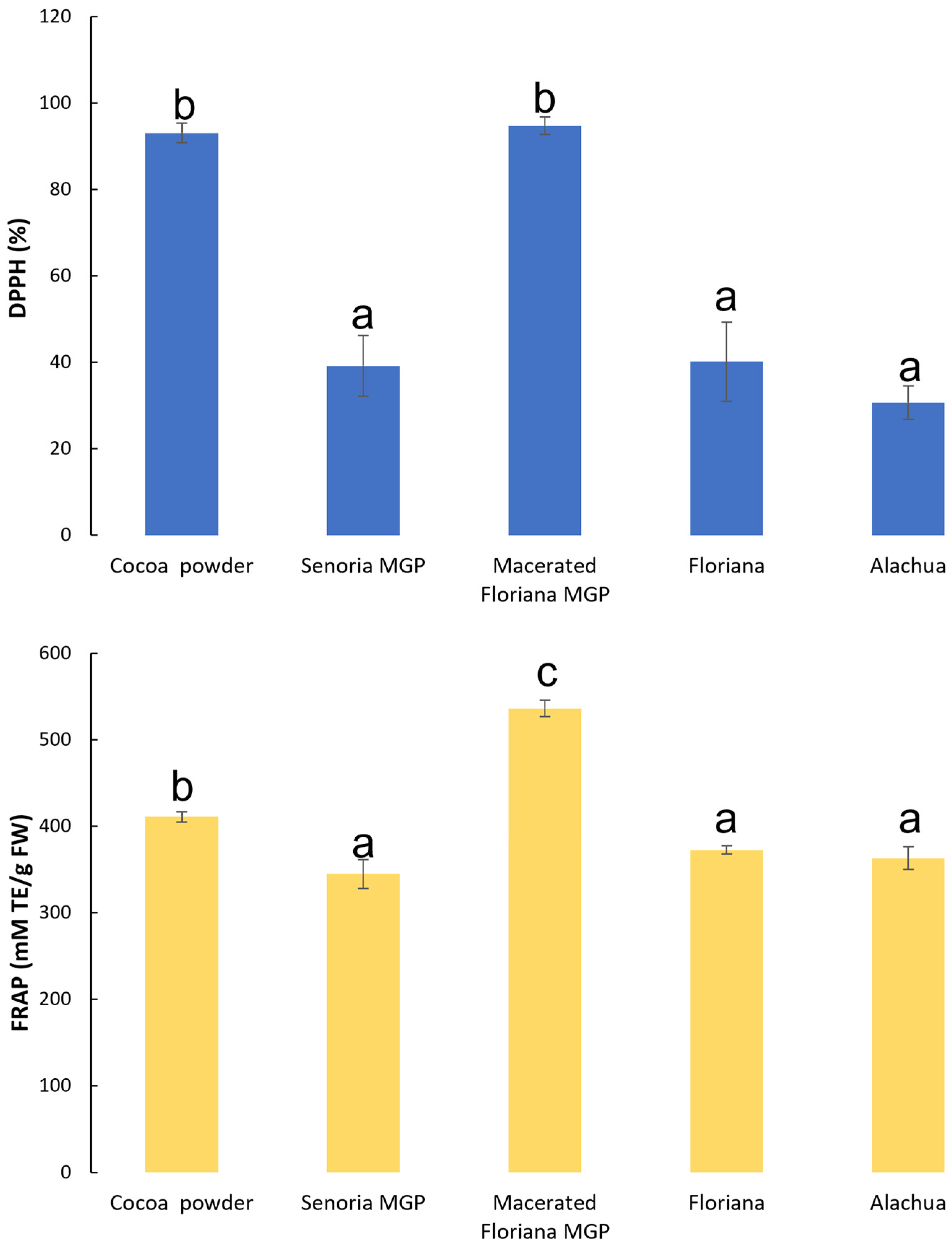
3.2. The Antioxidant Activities of MGP Chocolates
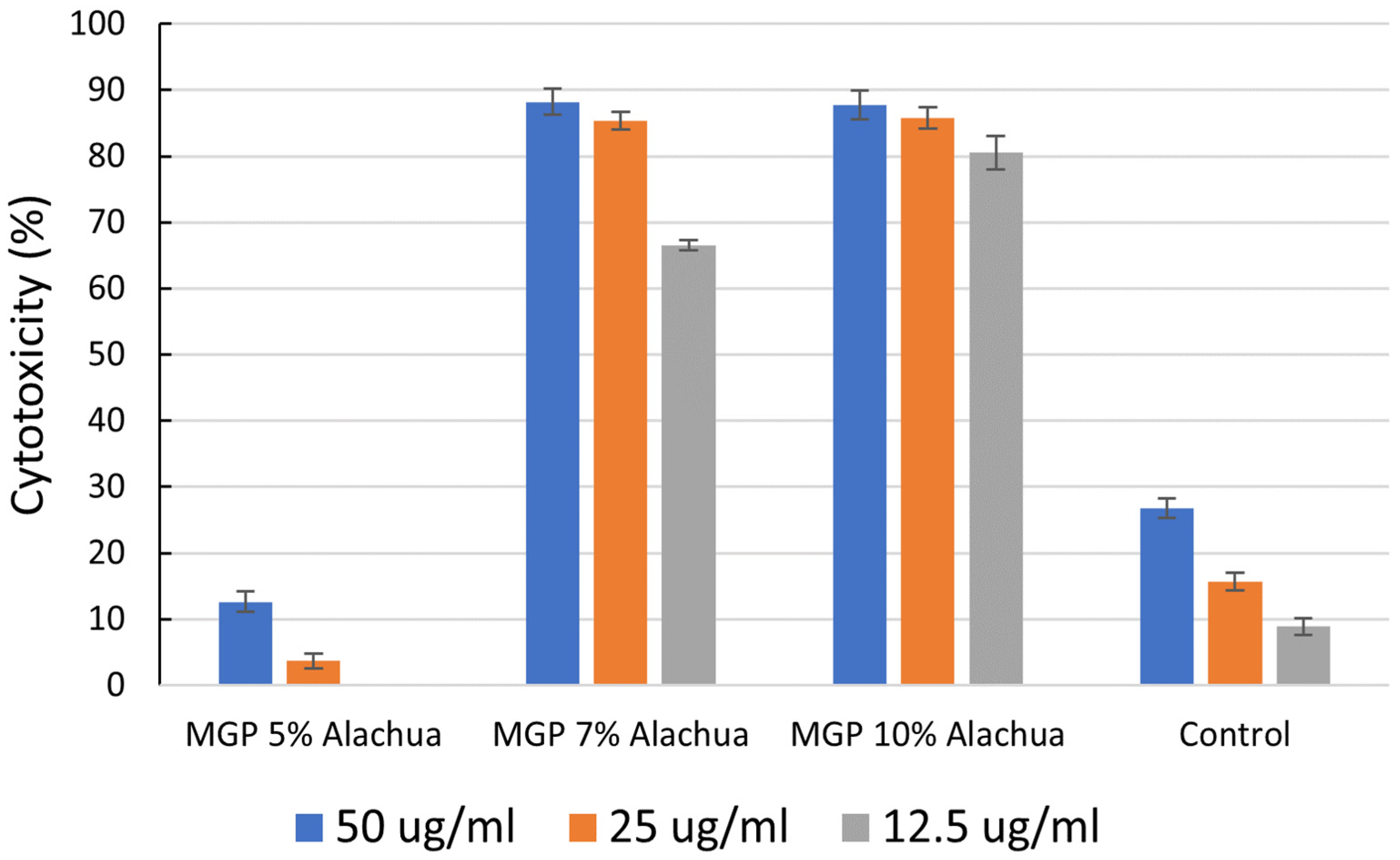
3.3. The Anticancer Activities of MGP Chocolates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Tong, H.; Chen, F.; Gangemi, J.D. Chemical characterization and antioxidant evaluation of muscadine grape pomace extract. Food Chem. 2010, 123, 1156–1162. [Google Scholar] [CrossRef]
- Li, R.; Wang, G.P.; Whitlock, J.A.; Zhao, S.; Yagiz, Y.; Gu, L. Muscadine grapes (Vitis rotundifolia) and dealcoholized muscadine wine alleviated symptoms of colitis and protected against dysbiosis in mice exposed to dextran sulfate sodium. J. Funct. Foods 2020, 65, 103746. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Chau, T.P.; Alwahibi, M.S.; Ali, M.A.; Shanmuganathan, R. Phytochemical profiling and GC–MS analysis of Vitis rotundifolia pulp extract (Jumbo muscadine). Appl. Nanosci. 2021, 13, 685–692. [Google Scholar] [CrossRef]
- Bitting, R.L.; Tooze, J.A.; Isom, S.; Petty, W.J.; Grant, S.C.; Thomas, A.; Thomas, C.Y.; Alistar, A.T.; Golden, S.L.; Pleasant, K. Phase I Study of Muscadine Grape Extract for Patients with Advanced Cancer. Am. J. Clin. Oncol. 2021, 44, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.G.; Das, P.R.; Ismail, A.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Sherif, S.M.; El-Sharkawy, I. Untargeted Metabolomics and Antioxidant Capacities of Muscadine Grape Genotypes during Berry Development. Antioxidants 2021, 10, 914. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Guven, M.; Yasar, K.; Karaca, O.B.; Hayaloglu, A.A. The effect of inulin as a fat replacer on the quality of set-type low-fat yogurt manufacture. Int. J. Dairy Technol. 2005, 58, 180–184. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef]
- Martínez-Cervera, S.; Salvador, A.; Muguerza, B.; Moulay, L.; Fiszman, S.M. Cocoa fibre and its application as a fat replacer in chocolate muffins. LWT-Food Sci. Technol. 2011, 44, 729–736. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Wang, Z.; Feng, Y.; Kim, Y.-T.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; He, Z.; Huang, H. Grape pomace and its secondary waste management: Biochar production for a broad range of lead (Pb) removal from water. Environ. Res. 2020, 186, 109442. [Google Scholar] [CrossRef] [PubMed]
- Kumanda, C.; Mlambo, V.; Mnisi, C.M. From landfills to the dinner table: Red grape pomace waste as a nutraceutical for broiler chickens. Sustainability 2019, 11, 1931. [Google Scholar] [CrossRef]
- Abt, E.; Fong Sam, J.; Gray, P.; Robin, L.P. Cadmium and lead in cocoa powder and chocolate products in the US Market. Food Addit. Contam. Part B 2018, 11, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Luo, J.; Huang, Y.; Guo, W.; Zhang, Y.; Guan, H.; Xu, C.; Lu, J. Profile of polyphenol compounds of five muscadine grapes cultivated in the United States and in newly adapted locations in China. Int. J. Mol. Sci. 2017, 18, 631. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Park, T.S.; Cha, Y.S. Grape seed extract (Vitis vinifera) partially reverses high fat diet-induced obesity in C57BL/6J mice. Nutr. Res. Pract. 2008, 2, 227–233. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Hsu, W.-Y.; Simonne, A.; Lu, J.; Marshall, M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014, 62, 6640–6649. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Kosir, V.; Yin, X.; Ross, K.; Diarra, M.S. Grape pomace as a promising antimicrobial alternative in feed: A critical review. J. Agric. Food Chem. 2019, 67, 9705–9718. [Google Scholar] [CrossRef]
- Vashisth, T.; Singh, R.K.; Pegg, R.B. Effects of drying on the phenolics content and antioxidant activity of muscadine pomace. LWT-Food Sci. Technol. 2011, 44, 1649–1657. [Google Scholar] [CrossRef]
- Iora, S.R.F.; Maciel, G.M.; Zielinski, A.A.F.; da Silva, M.V.; Pontes, P.V.d.A.; Haminiuk, C.W.I.; Granato, D. Evaluation of the bioactive compounds and the antioxidant capacity of grape pomace. Int. J. Food Sci. Technol. 2015, 50, 62–69. [Google Scholar] [CrossRef]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F.A. The anticancer and antioxidant effects of muscadine grape extracts on racially different triple-negative breast cancer cells. Anticancer Res. 2019, 39, 4043–4053. [Google Scholar] [CrossRef]
- Salehi, B.; Vlaisavljevic, S.; Adetunji, C.O.; Adetunji, J.B.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Uprety, Y.; Mileski, K.S.; Devkota, H.P. Plants of the genus Vitis: Phenolic compounds, anticancer properties and clinical relevance. Trends Food Sci. Technol. 2019, 91, 362–379. [Google Scholar] [CrossRef]
- Luo, J.; Wei, Z.; Zhang, S.; Peng, X.; Huang, Y.; Zhang, Y.; Lu, J. Phenolic fractions from muscadine grape “noble” pomace can inhibit breast cancer cell MDA-MB-231 better than those from european grape “cabernet sauvignon” and induce s-phase arrest and apoptosis. J. Food Sci. 2017, 82, 1254–1263. [Google Scholar] [CrossRef]
- Di Mattia, C.D.; Sacchetti, G.; Mastrocola, D.; Serafini, M. From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front. Immunol. 2017, 8, 1207. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Proch, J.; Zubik, L. Phenol antioxidant quantity and quality in foods: Cocoa, dark chocolate, and milk chocolate. J. Agric. Food Chem. 1999, 47, 4821–4824. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.B.; Stuart, D.A.; Smith, N.L.; Lee, C.Y.; McHale, N.L.; Flanagan, J.A.; Ou, B.; Hurst, W.J. Antioxidant activity and polyphenol and procyanidin contents of selected commercially available cocoa-containing and chocolate products in the United States. J. Agric. Food Chem. 2006, 54, 4062–4068. [Google Scholar] [CrossRef] [PubMed]
- Baharum, Z.; Akim, A.M.; Hin, T.Y.Y.; Hamid, R.A.; Kasran, R. Theobroma cacao: Review of the extraction, isolation, and bioassay of its potential anti-cancer compounds. Trop. Life Sci. Res. 2016, 27, 21. [Google Scholar] [PubMed]
- Ebuehi, O.A.T.; Anams, C.; Gbenle, O.D.; Ajagun-Ogunleye, M.O. Hydro-ethanol seed extract of Theobroma cacao exhibits antioxidant activities and potential anticancer property. J. Food Biochem. 2019, 43, e12767. [Google Scholar] [CrossRef]
- Acan, B.G.; Kilicli, M.; Bursa, K.; Toker, O.S.; Palabiyik, I.; Gulcu, M.; Yaman, M.; Gunes, R.; Konar, N. Effect of grape pomace usage in chocolate spread formulation on textural, rheological and digestibility properties. LWT 2021, 138, 110451. [Google Scholar] [CrossRef]
- Bolenz, S.; Glöde, L. Technological and nutritional aspects of milk chocolate enriched with grape pomace products. Eur. Food Res. Technol. 2021, 247, 623–636. [Google Scholar] [CrossRef]
- Xu, C.; Yagiz, Y.; Zhao, L.; Simonne, A.; Lu, J.; Marshall, M.R. Fruit quality, nutraceutical and antimicrobial properties of 58 muscadine grape varieties (Vitis rotundifolia Michx.) grown in United States. Food Chem. 2017, 215, 149–156. [Google Scholar] [CrossRef]
- God, J.M.; Tate, P.; Larcom, L.L. Anticancer effects of four varieties of muscadine grape. J. Med. Food 2007, 10, 54–59. [Google Scholar] [CrossRef]
- Guaita, M.; Bosso, A. Polyphenolic characterization of grape skins and seeds of four Italian red cultivars at harvest and after fermentative maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; De Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Abedini, A.; Dakhili, S.; Bazzaz, S.; Kamaladdin Moghaddam, S.; Mahmoudzadeh, M.; Andishmand, H. Fortification of chocolates with high-value-added plant-based substances: Recent trends, current challenges, and future prospects. Food Sci. Nutr. 2023, 11, 3686–3705. [Google Scholar] [CrossRef] [PubMed]
- Dand, R. Cocoa bean processing and the manufacture of chocolate. In International Cocoa Trade, 3rd ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 268–289. [Google Scholar]
- Gültekin-Özgüven, M.; Berktaş, İ.; Özçelik, B. Influence of processing conditions on procyanidin profiles and antioxidant capacity of chocolates: Optimization of dark chocolate manufacturing by response surface methodology. LWT-Food Sci. Technol. 2016, 66, 252–259. [Google Scholar] [CrossRef]
- Urbańska, B.; Derewiaka, D.; Lenart, A.; Kowalska, J. Changes in the composition and content of polyphenols in chocolate resulting from pre-treatment method of cocoa beans and technological process. Eur. Food Res. Technol. 2019, 245, 2101–2112. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar]
- Darwish, A.G.G.; Samy, M.N.; Sugimoto, S.; Otsuka, H.; Abdel-Salam, H.; Matsunami, K. Effects of hepatoprotective compounds from the leaves of Lumnitzera racemosa on acetaminophen-induced liver damage in vitro. Chem. Pharm. Bull. 2016, 64, 360–365. [Google Scholar] [CrossRef]
- Lee, L.-S.; Kim, S.-H.; Kim, Y.-B.; Kim, Y.-C. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules 2014, 19, 9173–9186. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, Y.J.; Lee, H.J.; Lee, C.Y. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J. Agric. Food Chem. 2003, 51, 7292–7295. [Google Scholar] [CrossRef] [PubMed]
- Subhashini, R.; Rao, U.S.M.; Sumathi, P.; Gunalan, G. A comparative phytochemical analysis of cocoa and green tea. Indian J. Sci. Technol. 2010, 3, 188–192. [Google Scholar] [CrossRef]
- Valls, J.; Agnolet, S.; Haas, F.; Struffi, I.; Ciesa, F.; Robatscher, P.; Oberhuber, M. Valorization of Lagrein grape pomace as a source of phenolic compounds: Analysis of the contents of anthocyanins, flavanols and antioxidant activity. Eur. Food Res. Technol. 2017, 243, 2211–2224. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Miñano, A.; López-Roca, J.M. Comparison of chromatic properties, stability and antioxidant capacity of anthocyanin-based aqueous extracts from grape pomace obtained from different vinification methods. Food Chem. 2006, 97, 87–94. [Google Scholar] [CrossRef]
- Salinas, M.R.; Garijo, J.; Pardo, F.; Zalacain, A.; Alonso, G.L. Influence of prefermentative maceration temperature on the colour and the phenolic and volatile composition of rosé wines. J. Sci. Food Agric. 2005, 85, 1527–1536. [Google Scholar] [CrossRef]
- Mazor Jolić, S.; Radojčić Redovniković, I.; Marković, K.; Ivanec Šipušić, Đ.; Delonga, K. Changes of phenolic compounds and antioxidant capacity in cocoa beans processing. Int. J. Food Sci. Technol. 2011, 46, 1793–1800. [Google Scholar] [CrossRef]
- Bordiga, M.; Locatelli, M.; Travaglia, F.; Coïsson, J.D.; Mazza, G.; Arlorio, M. Evaluation of the effect of processing on cocoa polyphenols: Antiradical activity, anthocyanins and procyanidins profiling from raw beans to chocolate. Int. J. Food Sci. Technol. 2015, 50, 840–848. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capacity of 68 Chinese herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant properties and phenolic compounds of vitamin C-rich juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Derradji-Benmeziane, F.; Djamai, R.; Cadot, Y. Antioxidant capacity, total phenolic, carotenoid, and vitamin C contents of five table grape varieties from Algeria and their correlations. OENO One 2014, 48, 153–162. [Google Scholar] [CrossRef]
- Silva, G.G.; Dutra, M.d.C.P.; de Oliveira, J.B.; Rybka, A.C.P.; Pereira, G.E.; dos Santos Lima, M. Processing methods with heat increases bioactive phenolic compounds and antioxidant activity in grape juices. J. Food Biochem. 2019, 43, e12732. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Kerr, W.L. Characterizing cocoa refining by electronic nose using a Kernel distribution model. LWT 2019, 104, 1–7. [Google Scholar] [CrossRef]
- Tan, J.; Balasubramanian, B.M. Particle size measurements and scanning electron microscopy (SEM) of cocoa particles refined/conched by conical and cylindrical roller stone melangers. J. Food Eng. 2017, 212, 146–153. [Google Scholar] [CrossRef]


| MGP Variety | MGP Concentration (w/w) | Macerated (Y/N) | TPC (mg GAE/g FW) | TFC (mg GAE/g FW) |
|---|---|---|---|---|
| Senoria | 5% | N | 0.33 ± 0.06 d | 0.13 ± 0.00 b |
| Senoria | 7% | N | 0.21 ± 0.02 b | 0.13 ± 0.00 b |
| Senoria | 10% | N | 0.21 ± 0.08 bc | 0.13 ± 0.00 b |
| Floriana | 5% | Y | 0.28 ± 0.04 c | 0.16 ± 0.01 c |
| Floriana | 7% | Y | 0.48 ± 0.04 e | 0.18 ± 0.00 d |
| Floriana | 10% | Y | 0.52 ± 0.01 f | 0.20 ± 0.00 e |
| Floriana | 5% | N | 0.13 ± 0.02 b | 0.13 ± 0.01 b |
| Floriana | 7% | N | 0.32 ± 0.11 d | 0.16 ± 0.00 c |
| Floriana | 10% | N | 0.04 ± 0.02 a | 0.12 ± 0.00 b |
| Alachua | 5% | N | 0.15 ± 0.04 bc | 0.12 ± 0.00 b |
| Alachua | 7% | N | 0.23 ± 0.14 bc | 0.14 ± 0.03 b |
| Alachua | 10% | N | 0.02 ± 0.01 a | 0.10 ± 0.00 a |
| Control | 0% | N | 0.36 ± 0.04 d | 0.13 ± 0.00 b |
| MGP Variety | MGP Concentration (w/w) | Macerated (Y/N) | DPPH % | FRAP (mM TE/g FW) |
|---|---|---|---|---|
| Senoria | 5% | N | 36.18 ± 3.66 abc | 316.13 ± 2.80 b |
| Senoria | 7% | N | 31.98 ± 3.55 ab | 308.80 ± 8.07 ab |
| Senoria | 10% | N | 43.49 ± 3.05 cd | 341.87 ± 7.77 cd |
| Floriana | 5% | Y | 36.45 ± 6.31 abc | 360.00 ± 3.49 e |
| Floriana | 7% | Y | 41.95 ± 4.20 c | 338.53 ± 6.55 cd |
| Floriana | 10% | Y | 50.72 ± 6.32 d | 404.87 ± 14.12 g |
| Floriana | 5% | N | 26.55 ± 4.03 a | 338.40 ± 6.12 cd |
| Floriana | 7% | N | 45.35 ± 8.33 c | 380.40 ± 5.44 f |
| Floriana | 10% | N | 29.43 ± 6.58 ab | 304.33 ± 2.83 ab |
| Alachua | 5% | N | 33.93 ± 8.09 abc | 335.20 ± 2.99 c |
| Alachua | 7% | N | 21.67 ± 9.15 a | 346.53 ± 4.87 d |
| Alachua | 10% | N | 21.27 ± 6.71 a | 287.40 ± 17.51 a |
| Control | 0% | N | 43.41 ± 3.20 c | 328.73 ± 6.71 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, A.G.; El-Sharkawy, I.; Tang, C.; Rao, Q.; Tan, J. Investigation of Antioxidant and Cytotoxicity Activities of Chocolate Fortified with Muscadine Grape Pomace. Foods 2023, 12, 3153. https://doi.org/10.3390/foods12173153
Darwish AG, El-Sharkawy I, Tang C, Rao Q, Tan J. Investigation of Antioxidant and Cytotoxicity Activities of Chocolate Fortified with Muscadine Grape Pomace. Foods. 2023; 12(17):3153. https://doi.org/10.3390/foods12173153
Chicago/Turabian StyleDarwish, Ahmed G., Islam El-Sharkawy, Chunya Tang, Qinchun Rao, and Juzhong Tan. 2023. "Investigation of Antioxidant and Cytotoxicity Activities of Chocolate Fortified with Muscadine Grape Pomace" Foods 12, no. 17: 3153. https://doi.org/10.3390/foods12173153
APA StyleDarwish, A. G., El-Sharkawy, I., Tang, C., Rao, Q., & Tan, J. (2023). Investigation of Antioxidant and Cytotoxicity Activities of Chocolate Fortified with Muscadine Grape Pomace. Foods, 12(17), 3153. https://doi.org/10.3390/foods12173153






