Effect of Vacuum and Modified Atmosphere Packaging on the Shelf Life and Quality of Gutted Rainbow Trout (Oncorhynchus mykiss) during Refrigerated Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Material and Experimental Design
2.2. Gas Analyses
2.3. Microbiological Analysis
2.4. Chemical Analysis
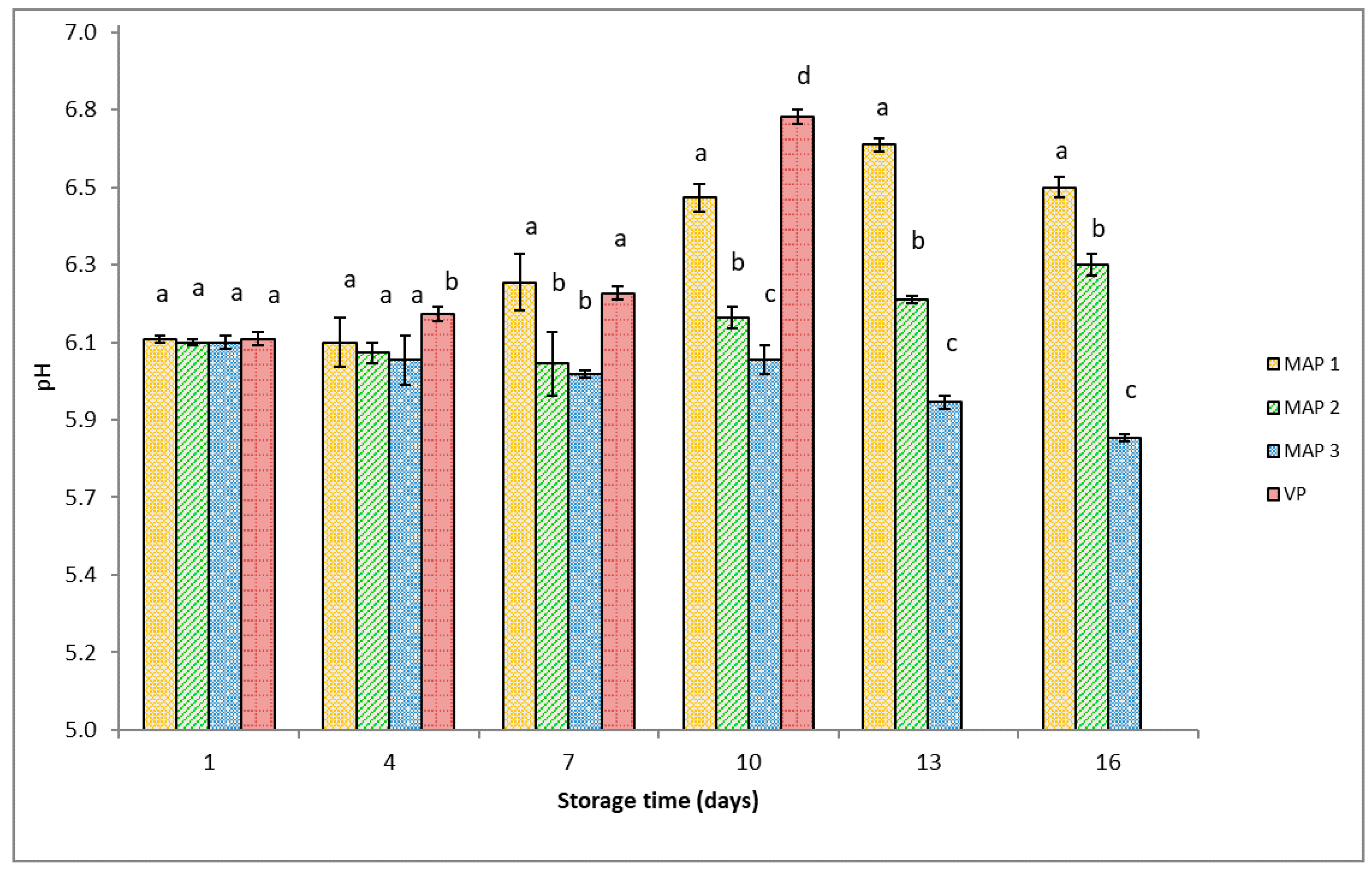
2.4.1. pH
2.4.2. Lipid Oxidation
2.4.3. Total Volatile Basic Nitrogen (TVB-N)
2.5. Sensory Assessment
2.6. Statistics
3. Results and Discussion
3.1. Gas Composition Analysis
3.2. Microbiological Analysis
3.3. Chemical Analysis
3.4. Sensory Assessment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Europen Market Observatory for Fisheries and Aquaculture Products—Case Study—Portion trout in the EU. October 2021. Available online: https://www.eumofa.eu/documents/20178/474612/PTAT+Portion+trout+in+PL+DE+IT_EN.pdf (accessed on 5 May 2023).
- Statistical Yearbook. Production and Catch of Fish, 2019–2021. 2022. Available online: https://publikacije.stat.gov.rs/G2022/PdfE/G20222055.pdf (accessed on 5 May 2023).
- DeWitt, C.A.M.; Oliveira, A.C.M. Modified atmosphere systems and shelf life extension of fish and fishery products. Foods 2016, 5, 48. [Google Scholar] [CrossRef]
- Provincial, L.; Gil, M.; Guillén, E.; Alonso, V.; Roncales, P.; Beltran, J.A. Effect of modified atmosphere packaging using different CO2 and N2 combinations on physical, chemical, microbiological and sensory changes of fresh sea bass (Dicentrarchus labrax) fillets. Int. J. Food Sci. Technol. 2010, 45, 1828–1836. [Google Scholar] [CrossRef]
- Rodrigues, B.L.; Alvares, T.S.; Sampaio, G.S.L.; Cabral, C.C.; Araujo, J.V.A.; Franco, R.M.; Mano, S.B.; Conte Junior, C.A. Influence of vacuum and modified atmosphere packaging in combination with UV-C radiation on the shelf life of rainbow trout (Oncorhynchus mykiss) fillets. Food Control 2016, 60, 596–605. [Google Scholar] [CrossRef]
- Esteves, E.; Guerra, L.; Anibal, J. Effects of vacuum and modified atmosphere packaging on the quality and shelf-life of gray triggerfish (Balistes capriscus) fillets. Foods 2021, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Milijašević, M.; Babić, J.; Baltić, M.; Spirić, A.; Velebit, B.; Borović, B.; Spirić, D. The influence of different gas mixtures on the changes of certain microbiological and chemical parameters in carp’s (Cyprinus carpio) cuts packed in modified atmosphere. Meat Technol. 2010, 51, 66–70. [Google Scholar]
- Olatunde, O.O.; Benjakul, S. Nonthermal processes for shelf-life extension of seafoods: A revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef]
- Siverstvik, M.; Jeksrud, W.K.; Rosnes, J.T. A review of modified atmosphere packaging of fish and fishery products-Significance of microbial growth, activities and safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Yang, X.; Liu, X.; Hong, H.; Luo, Y. Effects of oregano essential oil and nisin on the shelf life of modified atmosphere packed grass carp (Ctenopharyngodon idellus). Food Sci. Technol. 2021, 147, 111609. [Google Scholar] [CrossRef]
- Lerfall, J.; Thomassen, G.M.B.; Jakobsen, A.N. Quality of fresh saithe (Pollachius virens) in modified atmosphere packages as affected by the gas composition. Food Packag. Shelf Life 2018, 18, 147–156. [Google Scholar] [CrossRef]
- Randell, K.; Hattula, T.; Ahvenainen, R. Effect of packaging method on the quality of rainbow trout and Baltic herring fillets. Food Sci. Technol. 1997, 30, 56–61. [Google Scholar] [CrossRef]
- Rodriguez, C.J.; Besteiro, I.; Pascual, C. Biochemical changes in freshwater rainbow trout (Oncorhynchus mykiss) during chilled storage. J. Sci. Food Agric. 1999, 79, 1473–1480. [Google Scholar] [CrossRef]
- Gimenez, B.; Roncales, P.; Beltran, J.A. Modified atmosphere packaging of filleted rainbow trout. J. Sci. Food Agric. 2002, 82, 1154–1159. [Google Scholar] [CrossRef]
- Ježek, F.; Buchtova, H. The effect of vacuum packaging on physicochemical changes in rainbow trout (Oncorhynchus mykiss) during cold storage. Acta Vet. Brno 2014, 83, 51–58. [Google Scholar] [CrossRef]
- Tarladgis, B.S.; Pearson, A.M.; Dugan, L.R. Chemistry of the 2-thiobarbituric acid test for determination oxidative rancidity in foods. II Formation of the TBA malonaldehyde complex without acid-heat treatment. J. Sci. Food Agric. 1964, 15, 602–607. [Google Scholar] [CrossRef]
- Holland, C.D. Determination of Malonaldehyde as an index of Randicity of Nut Meats. J. AOAC 1971, 54, 1024–1026. [Google Scholar] [CrossRef]
- The European Union. Commission Regulation (EC) No. 2074/2005 of 5 December 2005. Off. J. Eur. Union 2005, L 338, 27–59. [Google Scholar]
- The Europen Communitiees. Council Regulation (EEC) No. 103/76 of 19 January 1976. Off. J. Eur. Commun. 1976, L 20, 29–34. [Google Scholar]
- Zhang, X.; Pan, C.; Chen, S.; Xue, Y.; Wang, Y.; Wu, Y. Effects of Modified Atmosphere Packaging with Different Gas Ratios on the Quality Changes of Golden Pompano (Trachinotus ovatus) Fillets during Superchilling Storage. Foods 2022, 11, 1943. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Rosnes, J.T.; Kleiberg, G.H. Effect of modified atmosphere packaging and superchilled storage on the microbial and sensory quality of atlantic salmon (Salmo salar) fillets. J. Food Sci. 2003, 68, 1467–1472. [Google Scholar] [CrossRef]
- ICMSF. International commission on microbiological specifications for foods, sampling plans for fish and shellfish. In ICMSF, Microorganisms in Foods. Sampling for Microbiological Analysis: Principles and Scientific Applications, 2nd ed.; University of Toronto Press: Toronto, ON, Canada, 1986; Volume 2, pp. 181–196. [Google Scholar]
- Fletcher, G.C.; Summers, G.; Corrigan, V.; Cumarasamy, S.; Dufour, J.P. Spoilage of king salmon (Oncorhynchus tshawytscha) fillets stored under different atmospheres. J. Food Sci. 2002, 67, 2362–2374. [Google Scholar] [CrossRef]
- Hansen, A.A.; Mørkøre, T.; Rudi, K.; Rødbotten, M.; Bjerke, F.; Eie, T. Quality changes of prerigor filleted Atlantic salmon (Salmo salar L.) packaged in modified atmosfhere using CO2 emitter, traditional MAP and vacuum. J. Food Sci. 2009, 74, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Özogul, F.; Polat, A.; Özogul, Y. The effects of modified atmosphere packaging and vacuum packaging on chemical, sensory and microbiological changes of sardines (Sardina pilchardus). Food Chem. 2004, 85, 49–57. [Google Scholar] [CrossRef]
- Stamatis, N.; Arkoudelos, J.S. Effect of modified atmosphere and vacuum packaging on microbial, chemical and sensory quality indicators of fresh, filleted Sardina pilchardus at 3 °C. J. Sci. Food Agric. 2007, 87, 1164–1171. [Google Scholar] [CrossRef]
- Milijasevic, M.; Babic, J.; Veskovic-Moracanin, S. Effect of vacuum and modified atmosphere on Enterobacteriaceae count determined in rainbow trout (Oncorhynchus mykiss) and carp (Cyprinus carpio) steaks. Procedia Food Sci. 2015, 5, 195–198. [Google Scholar] [CrossRef][Green Version]
- Pavlićević, N.; Dimitrijević, M.; Teodorović, V.; Karabasil, N.; Đorđević, V.; Baltić, M.Ž. Change number of enterobacteria during storage of cold smoked trout packed in vacuum and modified atmosphere. In Proceedings of the V International Conference “Aquaculture & Fishery”, Belgrade, Serbia, 1–3 June 2011; pp. 570–580. [Google Scholar]
- Ordonez, J.A.; Lopez-Galvez, D.E.; Fernandez, M.; Hierro, E.; Dela-Hoz, L. Microbial and physicochemical modifications of hake (Merluccius merluccius) steaks stored under carbon dioxide enriched atmospheres. J. Sci. Food Agric. 2000, 80, 1831–1840. [Google Scholar] [CrossRef]
- Gill, C.D.; Molin, G. Modified atmospheres and vacuum packaging. In Food Preservatives; Rusell, N.J., Gould, G.W., Eds.; Blackie: Glasgow, Scotland, 1991; pp. 172–199. [Google Scholar]
- Babić Milijašević, J. Pakovanje Ribe u Modifikovanu Atmosferu—Uticaj na Održivost Bezbednost; Posebna izdanja; Zadužbina Andrejević: Belgrade, Serbia, 2017; pp. 58–68. [Google Scholar]
- Stenstrom, I.M. Microbial flora of cod fillets (Gadus morhua) stored at 2 °C in different mixtures of carbon dioxide and nitrogen/oxygen. J. Food Prot. 1985, 48, 585–589. [Google Scholar] [CrossRef]
- Huss, H.H. The effect of anaerobic conditions and carbon dioxide. In FAO Fisheries Technical Paper 348; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995; Available online: https://www.fao.org/3/V7180E/V7180E00.htm (accessed on 5 May 2023).
- Lalitha, K.V.; Sonaji, E.R.; Manju, S.; Jose, L.; Gopal, T.K.S.; Ravisankar, C.N. Microbiological and biochemical changes in pearl spot (Etroplus suratensis Bloch) stored under modified atmospheres. J. Appl. Microbiol. 2005, 99, 1222–1228. [Google Scholar] [CrossRef]
- Masniyom, P.; Benjakul, S.; Visessanguan, W. Shelf-life extension of refrigerated seabass slices under modified atmosphere packaging. J. Sci. Food Agric. 2002, 82, 873–880. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Debevere, J.; Boskou, G. Effect of modified atmosphere packaging on the TVB/TMA producing microflora of cod fillets. Int. J. Food Microbiol. 1996, 31, 221–229. [Google Scholar] [CrossRef]
- Chan, S.S.; Skare, M.; Rotabakk, B.T.; Sivertsvik, M.; Lerfall, J.; Løvdal, T.; Roth, B. Evaluation of physical and instrumentally determined sensory attributes of Atlantic salmon portions packaged in modified atmosphere and vacuum skin. Food Sci. Technol. 2021, 146, 111404. [Google Scholar] [CrossRef]
- Yesudhason, P.; Lalitha, K.V.; Srinivasa Gopal, T.K.; Ravishankar, C.N. Retention of shelf life and microbial quality of seer fish stored in modified atmosphere packaging and sodium acetate pretreatment. Food Packag. Shelf Life 2014, 1, 123–130. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Moral, A. Residual effect of CO2 on hake (Merluccius merluccius L) stored in modified and controlled atmospheres. Eur. Food Res. Technol. 2001, 212, 413–420. [Google Scholar] [CrossRef]
- Goulas, A.E.; Kontominas, M.G. Effect of modified atmosphere packaging and vacuum packaging on the shelf-life of refrigerated chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Eur. Food Res. Technol. 2007, 224, 545–553. [Google Scholar] [CrossRef]
- Babić, J.; Dimitrijević, M.; Milijašević, M.; Đorđević, V.; Petronijević, R.; Grbić, S.; Spirić, A. Effect of modified atmospheric conditions and vacuum packaging on selected chemical parameters that define freshness of rainbow trout (Oncorhynchus mykiss) and carp (Cyprinus carpio). Hem. Ind. 2014, 68, 69–76. [Google Scholar] [CrossRef]
- Arashisar, Ş.; Hisar, O.; Kaya, M.; Yanik, T. Effect of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (Oncorhynchus mykiss) fillets. Int. J. Food Microbiol. 2004, 97, 209–214. [Google Scholar] [CrossRef]
- Fernández, J.; Pérez-Álvarez, J.A.; Fernández-López, J.A. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem. 1997, 59, 345–353. [Google Scholar] [CrossRef]
- Connell, J.J. Methods of assessing and selecting for quality. In Control of Fish Quality; Connell, J.J., Ed.; Fishing News Books: Oxford, UK, 1990; pp. 122–150. [Google Scholar]
- Masniyom, P.; Benjama, O.; Maneesri, J. Effect of modified atmosphere and vacuum packaging on quality changes of refrigerated tilapia (Oreochromis niloticus) fillets. Int. Food Res. J. 2013, 20, 1401–1408. [Google Scholar]
- Babić Milijašević, J.; Milijašević, M.; Vranić, D.; Đinović-Stojanović, J.; Lilić, S.; Korićanac, V. Effect of modified atmosphere packaging on the shelf life of chilled Common carp (Cyprinus carpio) steaks: Chemical and sensory attributes. Czech J. Food Sci. 2018, 36, 221–226. [Google Scholar] [CrossRef]


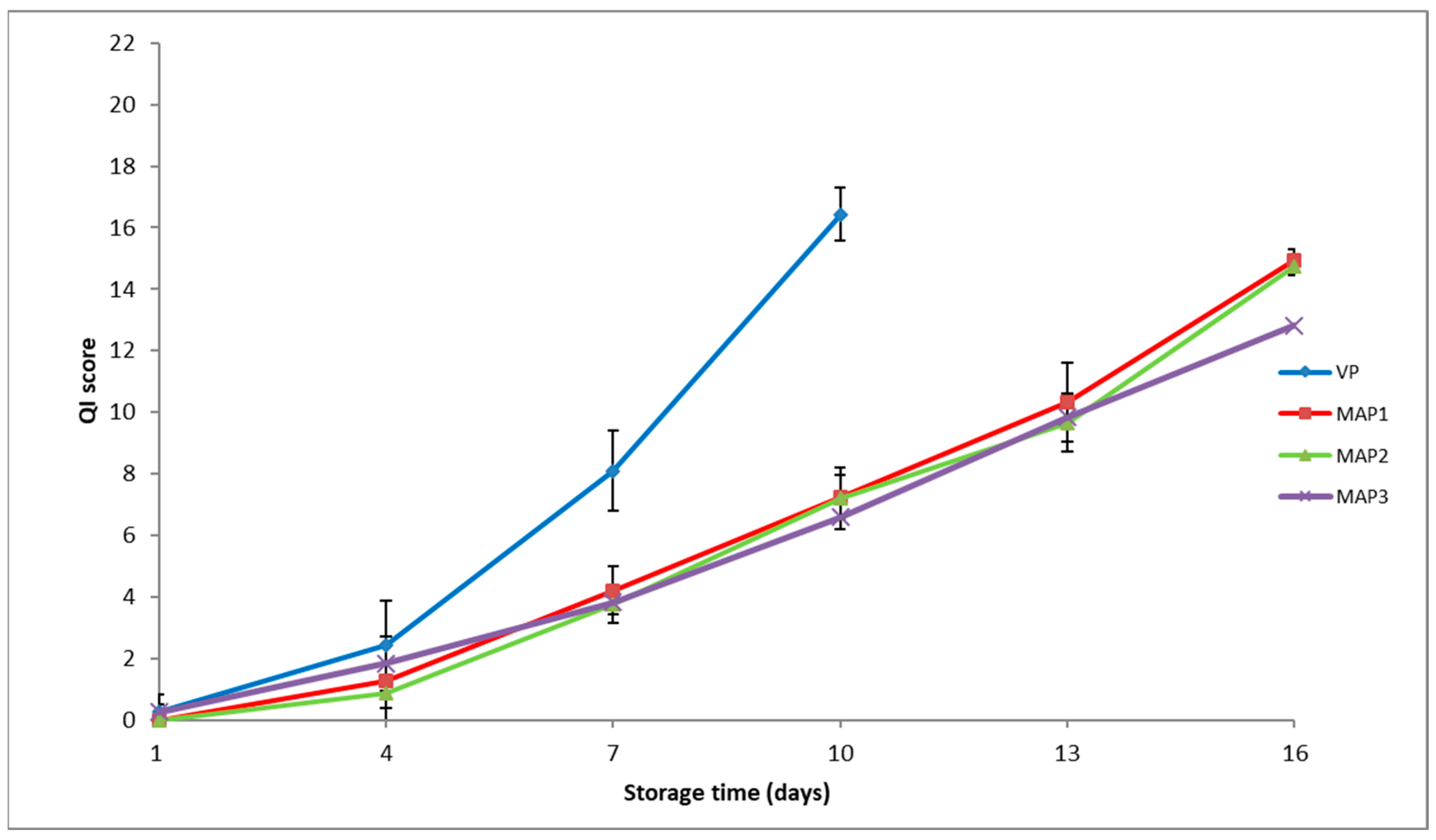
| Quality Attributes | Parameter | Score | |
|---|---|---|---|
| Eyes | Pupils | Black, bright with metallic radiancy | 0 |
| Black dull | 1 | ||
| Opaque grey | 2 | ||
| Shape | Convex | 0 | |
| Flat | 1 | ||
| Concave in the centre | 2 | ||
| Flesh | Color | Light rosy, smooth, shining | 0 |
| Velvety, waxy, dull, slightly changed | 1 | ||
| Slightly opaque | 2 | ||
| Opaque | 3 | ||
| Consistency | Firm and elastic, smooth surface | 0 | |
| Less elastic | 1 | ||
| Less elastic, slightly soft, dull surface | 2 | ||
| Soft, inclining to mealy | 3 | ||
| Skin | Color | Bright, pearly shine all over the body | 0 |
| Less pearly shine | 1 | ||
| Yellowish predominantly on the abdomen | 2 | ||
| Mucus | Aqueous, transparent | 0 | |
| Slightly cloudy, milky | 1 | ||
| Yellowish, opaque | 2 | ||
| Odor | Fresh, neutral, typical of fish | 0 | |
| Metallic, on cucumber, on hay | 1 | ||
| Rancid | 2 | ||
| Unpleasant (rancid and rotten) | 3 | ||
| Texture | Firm, elastic | 0 | |
| Finger mark disappears rapidly | 1 | ||
| Soft, finger mark delays disappear | 2 | ||
| Abdomen | Odor | Fresh, neutral | 0 |
| On cucumber, on melon | 1 | ||
| Rancid, on fermentation | 2 | ||
| Putrid | 3 | ||
| Total QI score | 0–22 |
| Parameter | Packaging Type | Days of Storage | |||||
|---|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 13 | 16 | ||
| APC | 40% CO2 + 60% N2 | 2.18 ± 0.80 a | 3.27 ± 0.31 a | 4.99 ± 0.44 a | 6.38 ± 0.14 a | 6.77 ± 0.43 a | 7.76 ± 0.56 a |
| 60% CO2 + 40% N2 | 2.20 ± 0.21 a | 3.14 ± 0.33 a | 4.77 ± 0.24 a | 5.84 ± 0.50 b | 6.59 ± 0.21 b | 7.28 ± 0.71 b | |
| 90% CO2 + 10% N2 | 2.18 ± 0.34 a | 2.76 ± 0.35 b | 3.52 ± 0.45 b | 4.25 ± 0.32 c | 4.98 ± 0.55 c | 5.79 ± 0.36 c | |
| Vacuum | 2.24 ± 0.34 a | 3.94 ± 0.56 c | 6.38 ± 0.32 c | 7.63 ± 0.26 d | ne | ne | |
| PBC | 40% CO2 + 60% N2 | 2.68 ± 0.52 a | 3.88 ± 0.09 a | 5.06 ± 0.20 a | 5.91 ± 0.33 a | 7.68 ± 0.32 a | 8.39 ± 0.18 a |
| 60% CO2 + 40% N2 | 2.70 ± 0.52 a | 3.92 ± 0.22 a | 4.97 ± 0.10 a | 6.06 ± 0.10 a | 7.28 ± 0.22 b | 8.79 ± 0.32 b | |
| 90% CO2 + 10% N2 | 2.68 ± 0.20 a | 3.13 ± 0.32 b | 4.09 ± 0.26 b | 4.94 ± 0.20 b | 5.58 ± 0.19 c | 6.50 ± 0.26 c | |
| Vacuum | 2.66 ± 0.30 a | 4.89 ± 0.20 c | 6.65 ± 0.13 c | 8.25 ± 0.13 c | ne | ne | |
| ENT | 40% CO2 + 60% N2 | 1.84 ± 0.13 a | 2.79 ± 0.54 a | 3.52 ± 0.20 a | 4.77 ± 0.11 a | 5.07 ± 0.32 a | 5.45 ± 0.53 a |
| 60% CO2 + 40% N2 | 1.86 ± 0.17 a | 2.74 ± 0.16 a | 3.25 ± 0.81 a | 3.94 ± 0.17 b | 4.16 ± 0.41 b | 4.99 ± 0.20 b | |
| 90% CO2 + 10% N2 | 1.86 ± 0.27 a | 2.40 ± 0.20 b | 2.88 ± 0.24 b | 3.10 ± 0.52 c | 3.71 ± 0.24 c | 4.25 ± 0.28 c | |
| Vacuum | 2.02 ± 0.25 a | 3.41 ± 0.26 c | 4.48 ± 0.53 c | 5.75 ± 0.23 d | ne | ne | |
| LAB | 40% CO2 + 60% N2 | 2.68 ± 0.10 a | 3.01 ± 0.28 a | 4.20 ± 0.35 a | 4.76 ± 0.48 a | 5.20 ± 0.31 a | 6.63 ± 0.13 a |
| 60% CO2 + 40% N2 | 2.60 ± 0.23 a | 3.57 ± 0.34 b | 4.86 ± 0.33 b | 5.84 ± 0.32 b | 6.72 ± 0.35 b | 7.48 ± 0.62 b | |
| 90% CO2 + 10% N2 | 2.49 ± 0.12 a | 3.74 ± 0.42 b | 4.83 ± 0.42 b | 6.23 ± 0.63 c | 6.69 ± 0.37 b | 7.73 ± 0.21 b | |
| Vacuum | 2.46 ± 0.37 a | 3.20 ± 0.34 a | 4.16 ± 0.38 a | 5.59 ± 0.37 b | ne | ne | |
| H2S | 40% CO2 + 60% N2 | 2.77 ± 0.35 a | 3.43 ± 0.54 a | 4.09 ± 0.20 a | 4.74 ± 0.11 a | 5.40 ± 0.32 a | 6.07 ± 0.53 a |
| 60% CO2 + 40% N2 | 2.80 ± 0.27 a | 3.50 ± 0.16 a | 4.12 ± 0.81 a | 4.89 ± 0.17 a | 5.60 ± 0.41 a | 6.20 ± 0.20 a | |
| 90% CO2 + 10% N2 | 2.53 ± 0.38 b | 3.10 ± 0.20 b | 3.94 ± 0.24 a | 4.48 ± 0.52 b | 4.94 ± 0.24 b | 5.77 ± 0.28 b | |
| Vacuum | 2.57 ± 0.30 b | 4.83 ± 0.26 c | 5.48 ± 0.53 b | 6.73 ± 0.23 c | ne | ne | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babic Milijasevic, J.; Milijasevic, M.; Lilic, S.; Djinovic-Stojanovic, J.; Nastasijevic, I.; Geric, T. Effect of Vacuum and Modified Atmosphere Packaging on the Shelf Life and Quality of Gutted Rainbow Trout (Oncorhynchus mykiss) during Refrigerated Storage. Foods 2023, 12, 3015. https://doi.org/10.3390/foods12163015
Babic Milijasevic J, Milijasevic M, Lilic S, Djinovic-Stojanovic J, Nastasijevic I, Geric T. Effect of Vacuum and Modified Atmosphere Packaging on the Shelf Life and Quality of Gutted Rainbow Trout (Oncorhynchus mykiss) during Refrigerated Storage. Foods. 2023; 12(16):3015. https://doi.org/10.3390/foods12163015
Chicago/Turabian StyleBabic Milijasevic, Jelena, Milan Milijasevic, Slobodan Lilic, Jasna Djinovic-Stojanovic, Ivan Nastasijevic, and Tamara Geric. 2023. "Effect of Vacuum and Modified Atmosphere Packaging on the Shelf Life and Quality of Gutted Rainbow Trout (Oncorhynchus mykiss) during Refrigerated Storage" Foods 12, no. 16: 3015. https://doi.org/10.3390/foods12163015
APA StyleBabic Milijasevic, J., Milijasevic, M., Lilic, S., Djinovic-Stojanovic, J., Nastasijevic, I., & Geric, T. (2023). Effect of Vacuum and Modified Atmosphere Packaging on the Shelf Life and Quality of Gutted Rainbow Trout (Oncorhynchus mykiss) during Refrigerated Storage. Foods, 12(16), 3015. https://doi.org/10.3390/foods12163015







