Short-Chain Fructooligosaccharide Synthesis from Sugarcane Syrup with Commercial Enzyme Preparations and Some Physical and Antioxidation Properties of the Syrup and Syrup Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymes and FOS Standards
2.2. Sugarcane Syrup Preparation
2.3. Determination of Sugar Compositions
2.4. Enzyme Assays
2.5. Production of sc-FOS from Sugarcane Syrup Using Two Commercial Enzymes
2.6. Preparation of FOS SS Powder with Foam-Mat Drying
2.7. Characterization of SS, sc-FOS Syrup and the Syrup Powder
2.7.1. Color
2.7.2. Viscosity
2.7.3. Evaluation of Antioxidant Compounds
Total Phenolic Content (TPC) Determination
Radical-Scavenging-Activity-DPPH-Assay and ABTS Assay (EC50)
2.7.4. Caloric Value
3. Results and Discussion
3.1. Effect of Sucrose Concentration on sc-FOS Synthesis from Sugarcane Syrup
3.2. Effect of SS Cultivars and Enzyme Sources on sc-FOS Synthesis from Sugarcane Syrup
3.3. Characterization of Sugarcane and sc-FOS Syrup
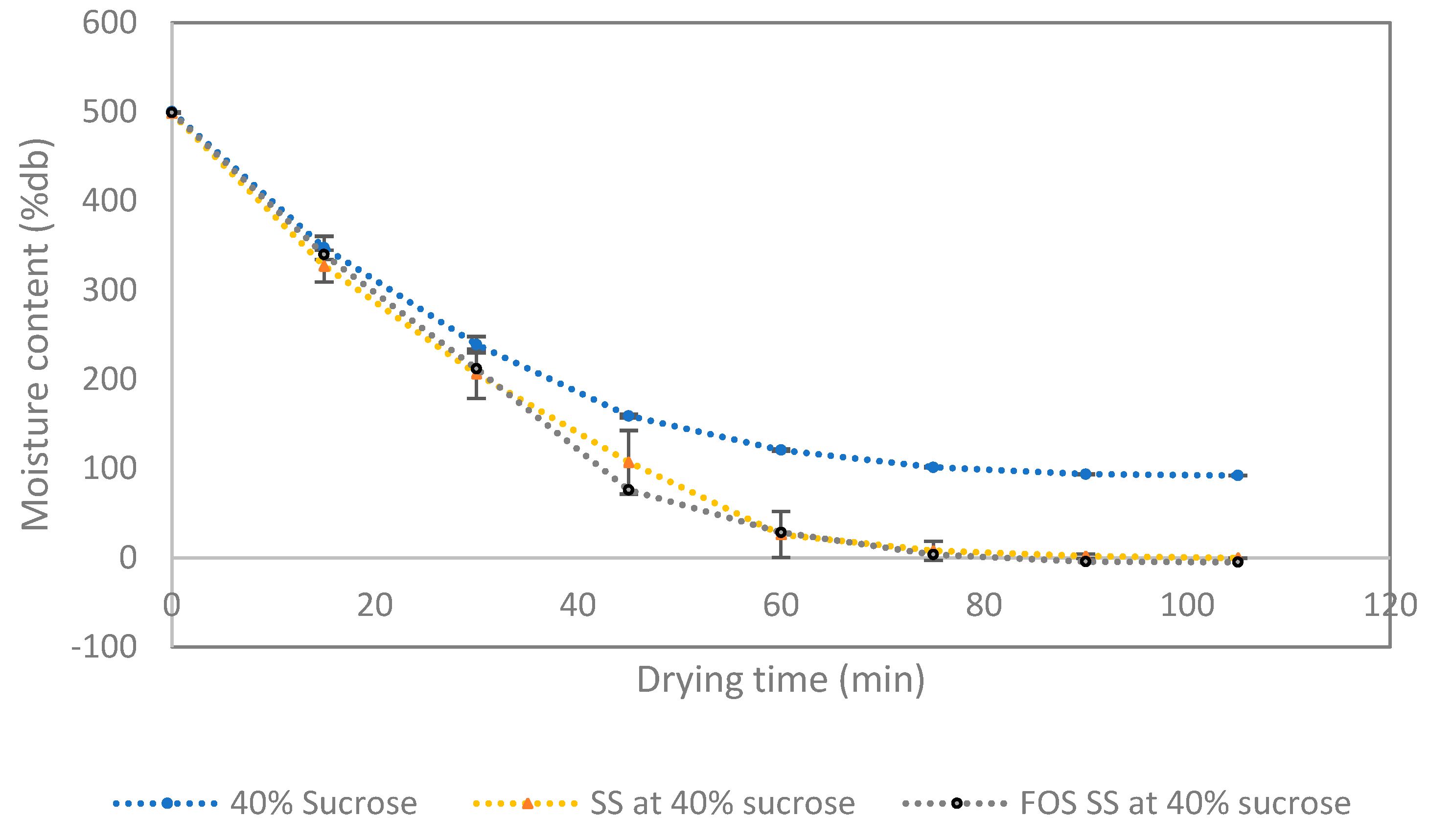
3.4. Drying the Syrup Foam and Characteristic of FOS SS Powder
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Crittenden, R.; Playne, M.J. Prebiotics. In Handbook of Probiotics and Prebiotics; Lee, Y.K., Salminen, S., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; pp. 535–561. [Google Scholar] [CrossRef]
- Yoshikawa, J.; Amachi, S.; Shinoyama, H.; Fujii, T. Production of fructooligosaccharides by crude enzyme preparations of b-fructofuranosidase from Aureobasidium pullulans. FEMS Microbiol. Lett. 2008, 30, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Smaali, I.; Jazzar, S.; Soussi, A.; Muzard, M.; Aubry, N.; Marzouki, M.N. Enzymatic synthesis of fructooligosaccharides from date by-products using an immobilized crude enzyme preparation of β-D-fructofuranosidase from Aspergillus awamori NBRC 4033. Biotechnol. Bioprocess Eng. 2012, 17, 385–392. [Google Scholar] [CrossRef]
- Vega-Paulino, R.J.; Zuniga-Hansen, M.E. Potential application of commercial enzyme preparations for industrial production of short-chain fructooligosaccharides. J. Mol. Catal. B Enzym. 2012, 76, 44–51. [Google Scholar] [CrossRef]
- Hajar-Azhari, S.; Rahim, M.H.A.; Wan-Mohtar, W.A.A.Q.I.; Sarbini, S.R.; Saari, N. Novel fructooligosaccharide conversion from sugarcane syrup using a specialised enzymatic pH-stat bioreactor. Process Biochem. 2020, 95, 55–63. [Google Scholar] [CrossRef]
- Lorenzoni, A.S.; Aydos, L.F.; Klein, M.P.; Rodrigues, R.C.; Hertz, P.F. Fructooligosaccharides synthesis by highly stable immobilized-fructofuranosidase from Aspergillus aculeatus. Carbohydr. Polym. 2014, 103, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Veljković, M.B.; Modi, A.; Petrov, A.I.; Ćorović, M.M.; Milivojević, A.D.; Banjanac, K.M.; Simović, M.B.; Bezbradica, D.I. Enzymatic synthesis of fructo-oligosaccharides using Pectinex® Ultra SP-L: A study of experimental conditions. Food Feed. Res. 2021, 48, 201–211. [Google Scholar] [CrossRef]
- Csanádi, Z.; Sisak, C. Immobilization of Pectinex Ultra SP-L pectinase and its application to production of fructooligosaccharides. Acta Aliment. 2006, 35, 205–212. [Google Scholar] [CrossRef]
- Lorenzoni, A.S.; Aydos, L.F.; Klein, M.P.; Ayub, M.A.; Rodrigues, R.C.; Hertz, P.F. Continuous production of fructooligosaccharides and invert sugar by chitosan immobilized enzymes: Comparison between in fluidized and packed bed reactors. J. Mol. Catal. B Enzym. 2015, 111, 51–55. [Google Scholar] [CrossRef]
- Romano, N.; Sciammaro, L.; Mobili, P.; Puppo, M.C.; Gomez-Zavaglia, A. Flour from mature Prosopis nigra pods as suitable substrate for the synthesis of prebiotic fructo-oligosaccharides and stabilization of dehydrated Lactobacillus delbrueckii subsp. bulgaricus. Food Res. Int. 2019, 121, 561–567. [Google Scholar] [CrossRef]
- Yoshikawa, J.; Amachi, S.; Shinoyama, H.; Fujii, T. Multiple β-fructofuranosidases by Aureobasidium pullulans DSM2404 and their roles in fructooligosaccharide production. FEMS Microbiol. Lett. 2006, 265, 159–163. [Google Scholar] [CrossRef]
- Magri, N.T.C.; Sartori, J.A.d.S.; Jara, J.L.P.; Eberlin, M.N.; Aguiar, C.L. Precipitation of nonsugars as a model of color reduction in sugarcane juice (Saccharum spp.) submitted to the hydrogen peroxide clarification of the crystal sugar process. J. Food Process. Preserv. 2019, 43, e14137. [Google Scholar] [CrossRef]
- Fard, A.E.; Dinani, S.T.; Moallemi-Oreh, A. An investigation on the effects of concentration and temperature on the time-independent rheological behavior of peach syrup. J. Food Meas. Charact. 2018, 12, 1303–1315. [Google Scholar] [CrossRef]
- Iqbal, E.; Abu Salim, K.; Lim, L.B. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ.-Sci. 2015, 27, 224–232. [Google Scholar] [CrossRef]
- Eggleston, G.; Boue, S.; Bett-Garber, K.; Verret, C.; Triplett, A.; Bechtel, P. Phenolic contents, antioxidant potential and associated colour in sweet sorghum syrups compared to other commercial syrup sweeteners. J. Sci. Food Agric. 2021, 101, 613–623. [Google Scholar] [CrossRef]
- Boue, S.M.; Shih, B.Y.; Burow, M.E.; Eggleston, G.; Lingle, S.; Pan, Y.-B.; Daigle, K.; Bhatnagar, D. Postharvest accumulation of resveratrol and piceatannol in sugarcane with enhanced antioxidant activity. J. Agric. Food Chem. 2013, 61, 8412–8419. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Eggleston, G.; Triplett, A.; Bett-Garber, K.; Boue, S.; Bechtel, P. Macronutrient and mineral contents in sweet sorghum syrups compared to other commercial syrup sweeteners. J. Agric. Food Res. 2022, 7, 100276. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Caloric value of inulin and oligofructose. J. Nutr. 1999, 129, 1436S–1437S. [Google Scholar] [CrossRef]
- Singh, J.; Rasane, P.; Kaur, S.; Kumar, V.; Dhawan, K.; Mahato, D.K.; Malhotra, S.; Sarma, C.; Kaur, D.; Bhattacharya, J. Nutritional Interventions and Considerations for the development of low calorie or sugar free foods. Curr. Diabetes Rev. 2020, 16, 301–312. [Google Scholar] [CrossRef]
- Hidaka, H.; Hirayama, M.; Sumi, N. A Fructooligosaccharide-producing enzyme from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 1988, 52, 1181–1187. [Google Scholar] [CrossRef][Green Version]
- Hirayama, M.; Sumi, N.; Hidaka, H. Purification and properties of a fructooligosaccharides-producing b-fructofuranosidase from Aspergillus niger ATCC 20611. Agric. Biol. Chem. 1989, 53, 667–673. [Google Scholar] [CrossRef][Green Version]
- Contesini, F.J.; de Lima, E.A.; Mandelli, F.; Borin, G.P.; Alves, R.F.; Terrasan, C.R.F. Carbohydrate active enzymes applied in the production of functional oligosaccharides. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 30–34. [Google Scholar] [CrossRef]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodríguez-Herrera, R.; Teixeira, J.A.; Aguilar, C.N. Biotechnological production and application of fructo-oligosaccharides. Crit. Rev. Biotechnol. 2016, 36, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, R.; Palai, T.; Bhattacharya, P.K. Kinetics and model development for enzymatic synthesis of fructo-oligosaccharides using fructosyltransferase. Bioprocess Biosyst. Eng. 2015, 38, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.; Zuniga-Hansen, M. A new mechanism and kinetic model for the enzymatic synthesis of short-chain fruc-tooligosaccharides from sucrose. Biochem. Eng. J. 2014, 82, 158–165. [Google Scholar] [CrossRef]
- Ghazi, I.; Fernandez-Arrojo, L.; De Segura, A.G.; Alcalde, M.; Plou, F.J.; Ballesteros, A. Beet sugar syrup and molasses as low-cost feedstock for the enzymatic production of fructo-oligosaccharides. J. Agric. Food Chem. 2006, 54, 2964–2968. [Google Scholar] [CrossRef]
- Hang, Y.D.; Woodams, E.E. Fructosyltransferase activity of commercial enzyme preparations used in fruit juice processing. Biotechnol. Lett. 1995, 17, 741–744. [Google Scholar] [CrossRef]
- Duan, K.J.; Chen, J.S.; Sheu, D.C. Kinetic studies and mathematical model for enzymatic production of fructooligosaccharides from sucrose. Enzym. Microb. Technol. 1994, 16, 334–339. [Google Scholar] [CrossRef]
- Alves, M.J.; Cavalcanti, V.; de Resende, M.M.; Cardoso, V.L.; Reis, M.H. Biodiesel dry purification with sugarcane bagasse. Ind. Crops Prod. 2016, 89, 119–127. [Google Scholar] [CrossRef]
- Ali, S.E.; El Gedaily, R.A.; Mocan, A.; Farag, M.A.; El-Seedi, H.R. Profiling metabolites and biological activities of sugarcane (Saccharum officinarum Linn.) juice and its product molasses via a multiplex metabolomics approach. Molecules 2019, 24, 934. [Google Scholar] [CrossRef]
- Kerdchan, K.; Srihanam, P. Fractionation of sugarcane extracts and determination of phenolic compounds and their antioxidant activity. Asian J. Chem. 2022, 34, 284–288. [Google Scholar] [CrossRef]
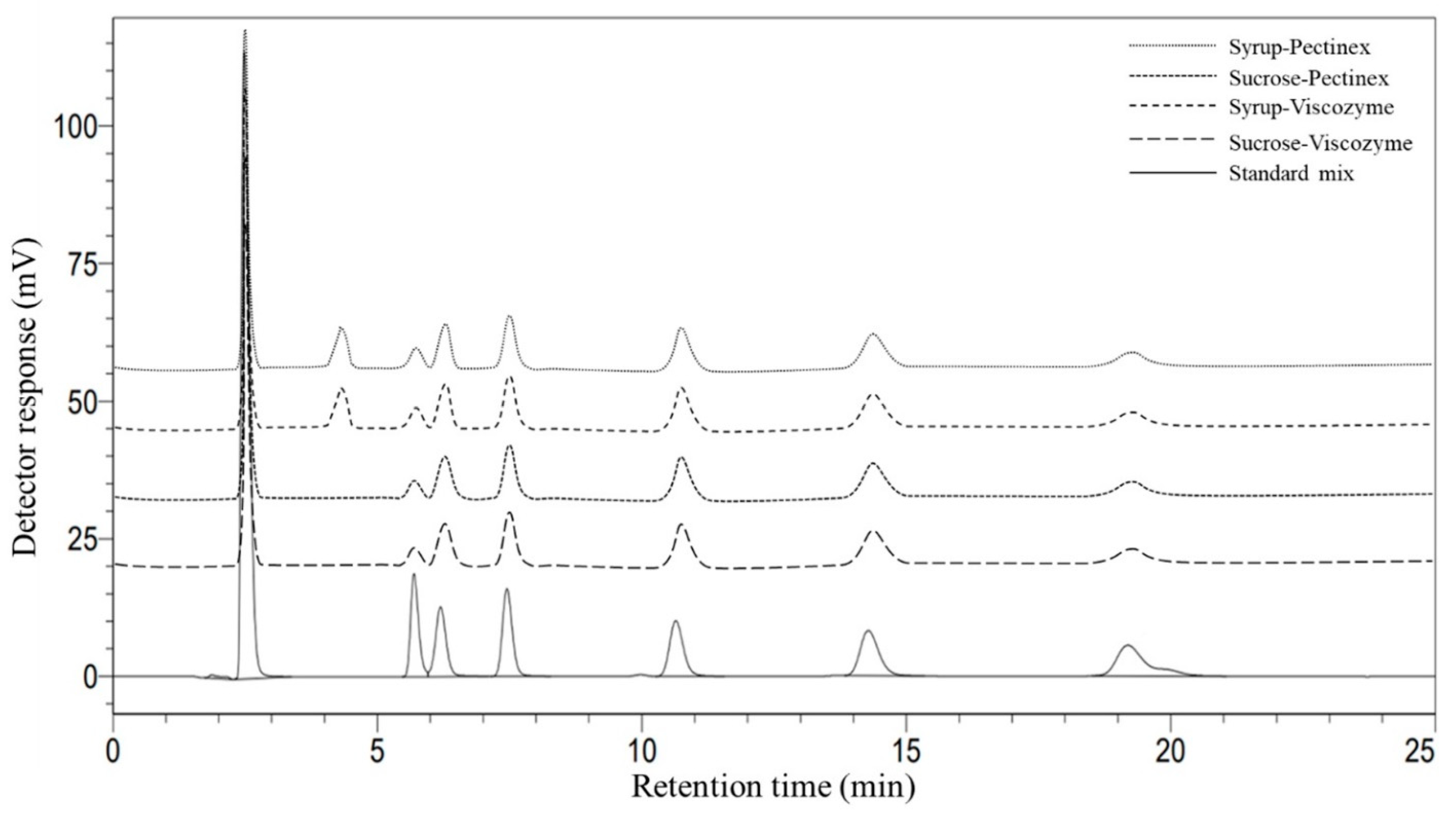
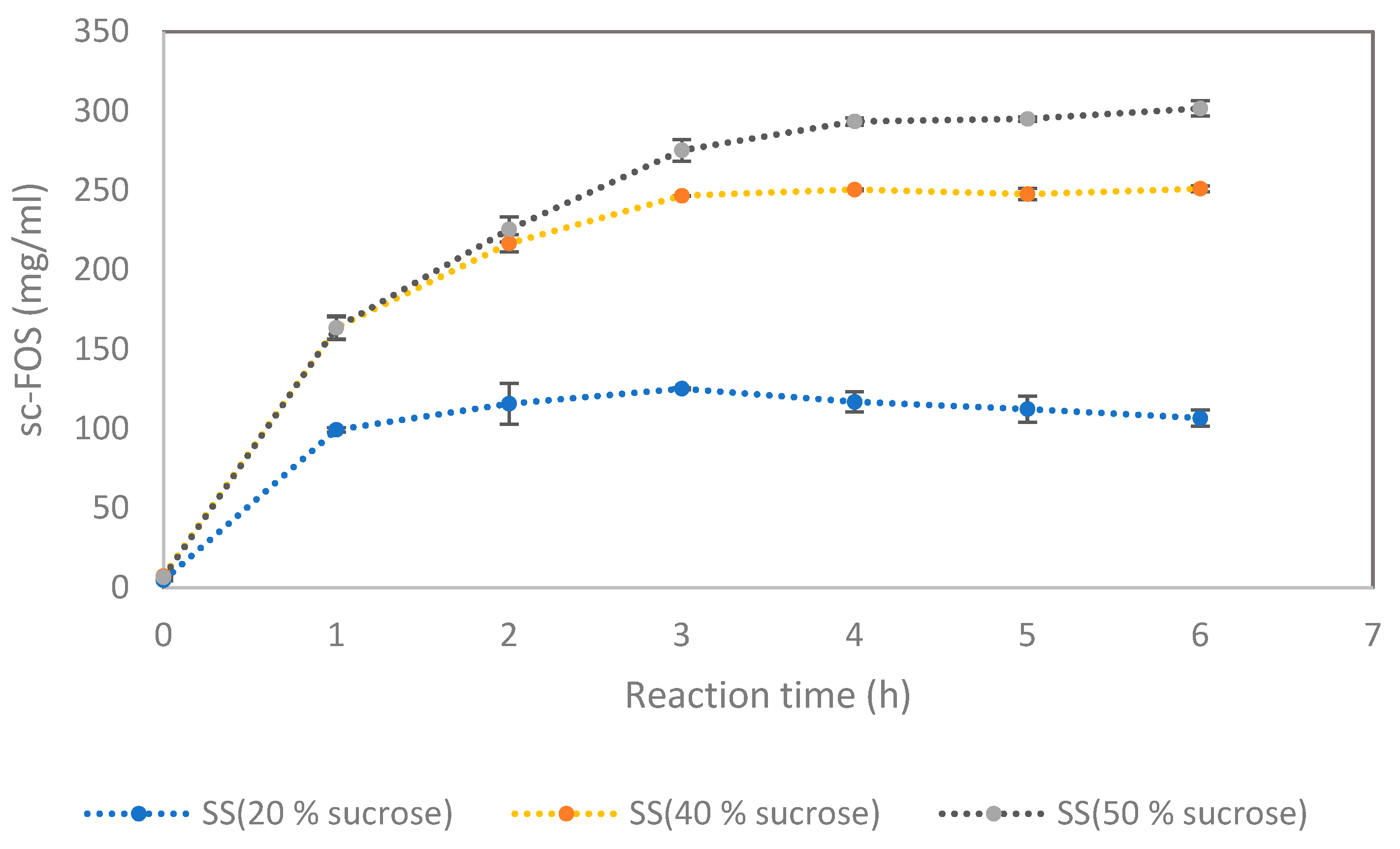
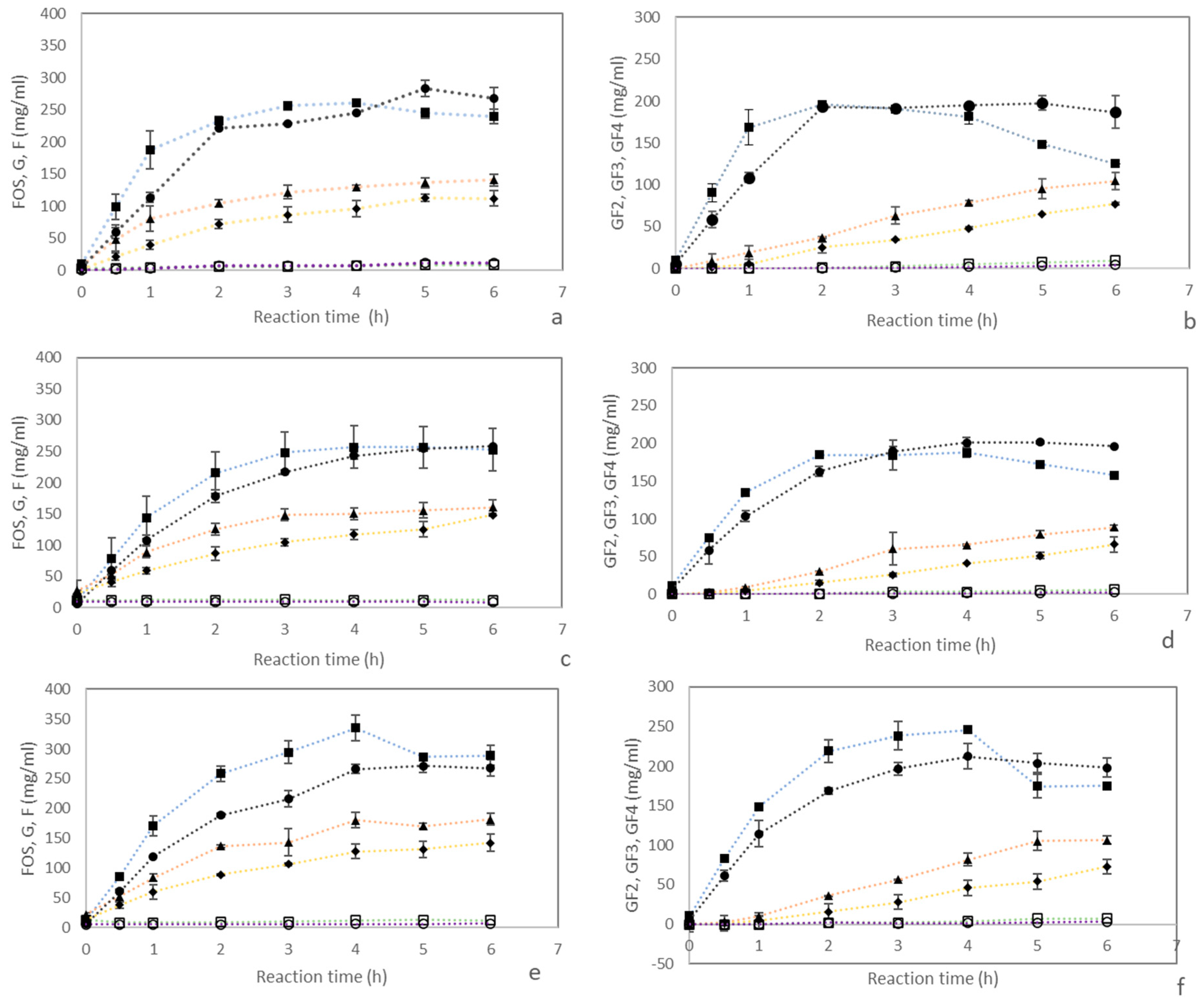
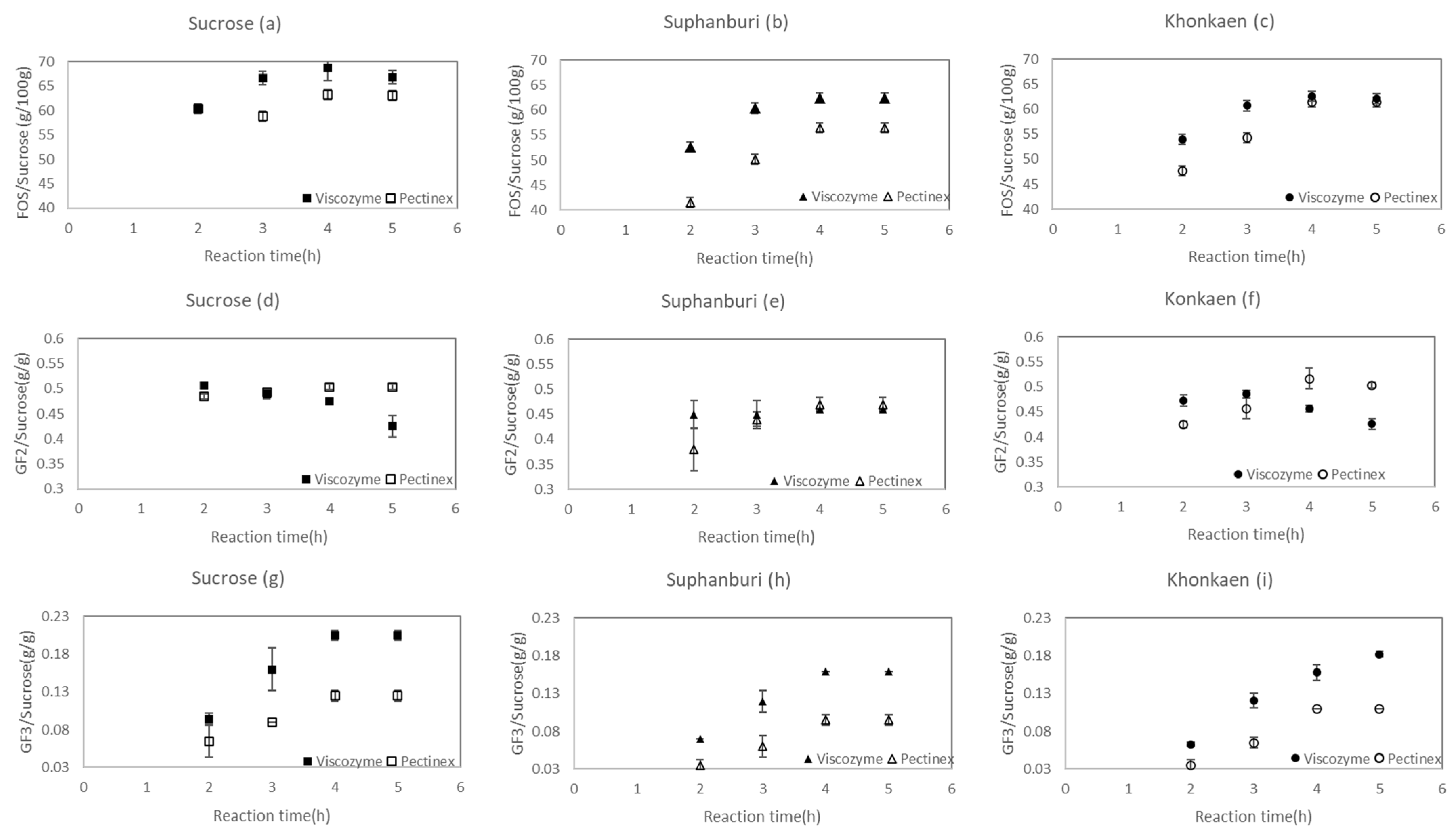
| Sugarcane Variety | Sugar Content (mg/mL) | ||
|---|---|---|---|
| Fructose | Glucose | Sucrose | |
| Khon Kaen 3 | 11.32 ± 0.20 b | 13.81 ± 0.24 b | 759.14 ± 0.36 |
| Suphanburi 50 | 20.75 ± 0.42 a | 28.51 ± 0.18 a | 755.36 ± 0.53 |
| Enzyme | Uf | Ut | Uh |
|---|---|---|---|
| Viscozyme L | 26.89 ± 1.509 | 20.62 ± 1.24 | 1.07 ± 0.19 |
| Pectinex Ultra SP-L | 14.32 ± 0.61 | 12.59 ± 0.43 | nd |
| Sample | °Brix | ICUMSA Color | Viscosity (mPa.S) | Total Phenolic (mgGAE/100 mL) | EC50 Values for DPPH Assay (mg/mL) | EC50 Values for ABTS Assay (mg/mL) | Caloric Value (Kcal/100 mL) |
|---|---|---|---|---|---|---|---|
| SS (Khon Kaen) | 68 ± 0.12 c | 6676.41 ± 84.41 e | 128.53 ± 0.55 f | 196.70 ± 5.93 d | 7.03 ± 0.25 c | 4.34 ± 0.01 d | 313.71 ± 0.03 b |
| SS (Suphanburi) | 68 ± 0.11 c | 6505.85 ± 73.10 f | 131.93 ± 0.60 e | 179.08 ± 1.66 e | 6.30 ± 0.17 d | 4.07 ± 0.02 e | 321.85 ± 0.03 a |
| FOS SS: 20% # | 70 ± 0.11 b | 7285.58 ± 42.20 d | 132.33 ± 0.55 e | 233.31 ± 2.92 c | 8.39 ± 0.05 b | 5.80 ± 0.06 c | 137.64 ± 2.92 e |
| FOS SS: 40% # | 70 ± 0.06 b | 7602.34 ± 73.10 c | 135.83 ± 0.60 c | 237.64 ± 0.88 b | 8.66 ± 0.11 ab | 6.04 ± 0.10 b | 144.82 ± 2.20 e |
| Commercial FOS syrup | 77 ± 0.06 a | 603.86 ± 41.84 g | 158.60 ± 0.46 a | 36.32 ± 0.76 f | 0.41 ± 0.01 e | 1.91 ± 0.05 f | 289.52 ± 7.25 c |
| Sample | Moisture Content (%) | Sugar Composition (g/g, db) | Antioxidant Capacity | Total Phenolic (mg GAE/g db) | Calories (cal/g db) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Sucrose (GF) | GF2 | GF3 | GF4 | DPPH Assay (mg eq Trolox/g db) | ABTS Assay (mg eq Trolox/ g db) | ||||
| SS | 2.00 ± 0.19 b | 0.80 ± 0.00 | nd | nd | nd | 3.39 ± 0.22 a | 6.45 ± 0.22 a | 1.76 ± 0.04 a | 3.40 ± 0.00 b |
| FOS SS | 2.24 ± 0.39 b | 0.11 ± 0.00 | 0.37 ± 0.00 | 0.11 ± 0.00 | Trace | 3.36 ± 0.12 a | 6.33 ± 0.17 a | 1.77 ± 0.03 a | 2.21 ± 0.01 c |
| Sucrose | 3.58 ± 0.25 a | 0.87 ± 0.00 | nd | nd | nd | 1.86 ± 0.10 b | 1.85 ± 0.18 b | 0.25 ± 0.02 b | 3.46 ± 0.00 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamchonemenukool, S.; Buasum, W.; Weerawatanakorn, M.; Thongsook, T. Short-Chain Fructooligosaccharide Synthesis from Sugarcane Syrup with Commercial Enzyme Preparations and Some Physical and Antioxidation Properties of the Syrup and Syrup Powder. Foods 2023, 12, 2895. https://doi.org/10.3390/foods12152895
Kamchonemenukool S, Buasum W, Weerawatanakorn M, Thongsook T. Short-Chain Fructooligosaccharide Synthesis from Sugarcane Syrup with Commercial Enzyme Preparations and Some Physical and Antioxidation Properties of the Syrup and Syrup Powder. Foods. 2023; 12(15):2895. https://doi.org/10.3390/foods12152895
Chicago/Turabian StyleKamchonemenukool, Sudthida, Warathep Buasum, Monthana Weerawatanakorn, and Tipawan Thongsook. 2023. "Short-Chain Fructooligosaccharide Synthesis from Sugarcane Syrup with Commercial Enzyme Preparations and Some Physical and Antioxidation Properties of the Syrup and Syrup Powder" Foods 12, no. 15: 2895. https://doi.org/10.3390/foods12152895
APA StyleKamchonemenukool, S., Buasum, W., Weerawatanakorn, M., & Thongsook, T. (2023). Short-Chain Fructooligosaccharide Synthesis from Sugarcane Syrup with Commercial Enzyme Preparations and Some Physical and Antioxidation Properties of the Syrup and Syrup Powder. Foods, 12(15), 2895. https://doi.org/10.3390/foods12152895






