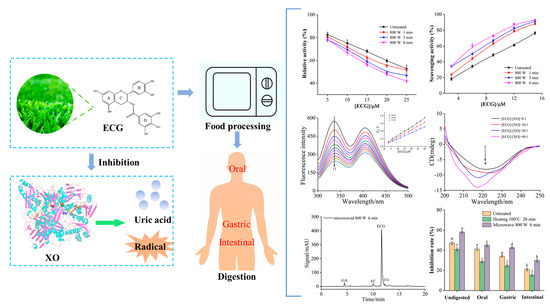
Effects of Processing Conditions and Simulated Digestion In Vitro on the Antioxidant Activity, Inhibition of Xanthine Oxidase and Bioaccessibility of Epicatechin Gallate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Different pH Conditions
2.3. Conventional Heating Treatment
2.4. Microwave Heating Treatment
2.5. Analysis of DPPH Free Radical Scavenging Activity
2.6. Relative Activity Measurement of XO
2.7. Fluorescence Quenching and Thermodynamic Analysis
2.8. Measurements of Circular Dichromatic (CD) Spectra
2.9. HPLC Analysis
2.10. Simulated Digestion Experiments In Vitro
2.10.1. Preparation of Simulated Digestive Solutions
2.10.2. In Vitro Simulated Digestion
2.10.3. Assays of Bioaccessibility and Biologic Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effects of pH on the Activity of ECG
3.1.1. UV–Vis Absorption Spectra of ECG at Different pH Values
3.1.2. Effects of pH Value on the Free Radical Scavenging Activity of ECG
3.1.3. Effects of pH Value on the Inhibitory Activity of ECG against XO
3.2. Effects of Conventional and Microwave Heating Treatments on the Free Radical Scavenging Activity of ECG
3.2.1. Effects of Heating Temperature and Time
3.2.2. Effects of Microwave Power and Time
3.3. Effects of Conventional and Microwave Heating Treatments on the Inhibitory Activity of ECG against XO
3.3.1. Effects of Heating Temperature and Time
3.3.2. Effects of Microwave Power and Time
3.4. Analysis of Binding Properties
3.5. Analysis of Secondary Structure
3.6. Degradation Mechanism of ECG
3.7. Bioaccessibility, Antioxidant and XO Inhibitory Activity of ECG after Simulated Digestion
3.7.1. Bioaccessibility of ECG
3.7.2. Effects of Simulated Digestion on the Antioxidant Activity of ECG
3.7.3. Effects of Simulated Digestion on the Inhibitory Activity of ECG against XO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on alpha-amylase and alpha-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Masuoka, N.; Kubo, I. Suppression of superoxide anion generation catalyzed by xanthine oxidase with alkyl caffeates and the scavenging activity. Int. J. Food Sci. Nutr. 2016, 67, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Prieto, J.M.; Arbones-Mainar, J.M.; Valero, M.S.; Lopez, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef]
- Stamp, L.K.; Chapman, P.T. Allopurinol hypersensitivity: Pathogenesis and prevention. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101501. [Google Scholar] [CrossRef]
- Zhu, M.; Pan, J.; Hu, X.; Zhang, G. Epicatechin gallate as xanthine oxidase inhibitor: Inhibitory kinetics, binding characteristics, synergistic inhibition, and action mechanism. Foods 2021, 10, 2191. [Google Scholar] [CrossRef]
- Guillard, V.; Mauricio-Iglesias, M.; Gontard, N. Effect of novel food processing methods on packaging: Structure, composition, and migration properties. Crit. Rev. Food Sci. Nutr. 2010, 50, 969–988. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Liu, J.; Li, R.; Zhou, J.; Li, M.; Lu, J.; Zhao, G.; Li, X.; Sui, W.; et al. Steam explosion improves extractability, antioxidant activity and α-glucosidase inhibitory activity of the constituents of Java tea (Clerodendranthus spicatus). Innov. Food Sci. Emerg. Technol. 2023, 86, 103350. [Google Scholar] [CrossRef]
- Chacha, J.S.; Zhang, L.; Ofoedu, C.E.; Suleiman, R.A.; Dotto, J.M.; Roobab, U.; Agunbiade, A.O.; Duguma, H.T.; Mkojera, B.T.; Hossaini, S.M.; et al. Revisiting non-thermal food processing and preservation methods-action mechanisms, pros and cons: A technological update (2016–2021). Foods 2021, 10, 1430. [Google Scholar] [CrossRef]
- D’Amelia, V.; Sarais, G.; Fais, G.; Dessi, D.; Giannini, V.; Garramone, R.; Carputo, D.; Melito, S. Biochemical characterization and effects of cooking methods on main phytochemicals of red and purple potato tubers, a natural functional food. Foods 2022, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Mu, H.; Zeng, M.; Gao, D.; Qin, F.; Chen, J.; He, Z. Effects of heating on the total phenolic content, antioxidant activities and main functional components of simulated Chinese herb candy during boiling process. J. Food Meas. Charact. 2018, 13, 476–486. [Google Scholar] [CrossRef]
- Guzik, P.; Szymkowiak, A.; Kulawik, P.; Zając, M.; Migdał, W. The confrontation of consumer beliefs about the impact of microwave-processing on food and human health with existing research. Trends Food Sci. Technol. 2022, 119, 110–121. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Pérez-Cruz, C.; Guzmán-Gerónimo, R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Yu, Q.; Duan, J.; Yu, N.; Fan, L. Enhancing the antityrosinase activity of saponins and polyphenols from Asparagus by hot air coupled with microwave treatments. LWT 2020, 124, 109174. [Google Scholar] [CrossRef]
- Zheng, Y.; Shi, P.; Li, Y.; Zhuang, Y.; You, L.; Liu, L.; Wang, W. A novel ACE-inhibitory hexapeptide from camellia glutelin-2 hydrolysates: Identification, characterization and stability profiles under different food processing conditions. LWT 2021, 147, 111682. [Google Scholar] [CrossRef]
- Kim, E.S.; Liang, Y.R.; Jin, J.; Sun, Q.F.; Lu, J.L.; Du, Y.Y.; Lin, C. Impact of heating on chemical compositions of green tea liquor. Food Chem. 2007, 103, 1263–1267. [Google Scholar] [CrossRef]
- Seczyk, L.; Sugier, D.; Swieca, M.; Gawlik-Dziki, U. The effect of in vitro digestion, food matrix, and hydrothermal treatment on the potential bioaccessibility of selected phenolic compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef]
- Marchese, A.; Coppo, E.; Sobolev, A.P.; Rossi, D.; Mannina, L.; Daglia, M. Influence of in vitro simulated gastroduodenal digestion on the antibacterial activity, metabolic profiling and polyphenols content of green tea (Camellia sinensis). Food Res. Int. 2014, 63, 182–191. [Google Scholar] [CrossRef]
- Chai, T.T.; Xiao, J.; Mohana Dass, S.; Teoh, J.Y.; Ee, K.Y.; Ng, W.J.; Wong, F.C. Identification of antioxidant peptides derived from tropical jackfruit seed and investigation of the stability profiles. Food Chem. 2021, 340, 127876. [Google Scholar] [CrossRef]
- Aalim, H.; Belwal, T.; Jiang, L.; Huang, H.; Meng, X.; Luo, Z. Extraction optimization, antidiabetic and antiglycation potentials of aqueous glycerol extract from rice (Oryza sativa L.) bran. LWT 2019, 103, 147–154. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, M.; Zhang, G.; Hu, X.; Pan, J. Novel insights into the interaction mechanism of 5-hydroxymethyl-2-furaldehyde with β-casein and its effects on the structure and function of β-casein. LWT 2021, 152, 112360. [Google Scholar] [CrossRef]
- Liu, X.; Ling, Z.; Zhou, X.; Ahmad, F.; Zhou, Y. Comprehensive spectroscopic probing the interaction and conformation impairment of bovine serum albumin (BSA) by herbicide butachlor. J. Photochem. Photobiol. B 2016, 162, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hu, X.; Zhang, Y.; Pan, J.; Zhang, G. Revealing the groove binding characteristics of plant growth regulator 3-indoleacetic acid with calf thymus DNA. J. Mol. Liq. 2021, 326, 115265. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Z.; Zhou, Q.; Yan, J.; Zhang, J.; Su, G. Hypouricemic effect in hyperuricemic mice and xanthine oxidase inhibitory mechanism of dietary anthocyanins from purple sweet potato (Ipomoea batatas L.). J. Funct. Foods 2020, 73, 104151. [Google Scholar] [CrossRef]
- Zeng, L.; Ding, H.; Hu, X.; Zhang, G.; Gong, D. Galangin inhibits alpha-glucosidase activity and formation of non-enzymatic glycation products. Food Chem. 2019, 271, 70–79. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Vittori, S.; Sagratini, G. Determination of fourteen polyphenols in pulses by high performance liquid chromatography-diode array detection (HPLC-DAD) and correlation study with antioxidant activity and colour. Food Chem. 2017, 221, 689–697. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Kaur, R.; Rajput, R.; Nag, P.; Kumar, S.; Rachana; Singh, M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 39, 398–407. [Google Scholar] [CrossRef]
- Su, Y.L.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.; Hou, Z.; Ho, C.; Yang, C. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Lee, K.Y.; Gul, K.; Rahman, M.S.; Kim, A.N.; Chun, J.; Kim, H.J.; Choi, S.G. Phenolics and antioxidant activity of aqueous turmeric extracts as affected by heating temperature and time. LWT 2019, 105, 149–155. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, Y.; Li, Z.; Xie, X.; Gong, E.S.; Tian, J.; Si, X.; Wang, Y.; Gao, N.; Shu, C.; et al. Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and beta-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium spp.) puree. Food Chem. 2021, 342, 128564. [Google Scholar] [CrossRef]
- Pineiro, Z.; Palma, M.; Barroso, C.G. Determination of catechins by means of extraction with pressurized liquids. J. Chromatogr. A 2004, 1026, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, W.; Wen, R. Kinetic study of the thermal stability of tea catechins in aqueous systems using a microwave reactor. J. Agric. Food Chem. 2006, 54, 5924–5932. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Cavalcanti, R.N.; Cardozo, T.S.F.; Couto, S.M.; Guimaraes, J.T.; Balthazar, C.F.; Rocha, R.S.; Pimentel, T.C.; Freitas, M.Q.; Raices, R.S.L.; et al. Effects of microwave heating on the chemical composition and bioactivity of orange juice-milk beverages. Food Chem. 2021, 345, 128746. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Q.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef]
- Nadeem, M.; Ghaffar, A.; Hashim, M.M.; Murtaza, M.A.; Ranjha, M.M.A.N.; Mehmood, A.; Riaz, M.N. Sonication and microwave processing of phalsa drink: A synergistic approach. Int. J. Fruit Sci. 2021, 21, 993–1007. [Google Scholar] [CrossRef]
- Tsao, R. Synergistic interactions between antioxidants used in food preservation. In Handbook of Antioxidants for Food Preservation; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2015; pp. 335–347. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.; Kim, Y.; Uyama, H.; Kobayashi, S. Amplification of antioxidant activity and aanthine oxidase inhibition of catechin by enzymatic polymerization. Biomacromolecules 2003, 4, 469–471. [Google Scholar] [CrossRef]
- Hsieh, H.J.; Lin, J.A.; Chen, K.T.; Cheng, K.C.; Hsieh, C.W. Thermal treatment enhances the alpha-glucosidase inhibitory activity of bitter melon (Momordica charantia) by increasing the free form of phenolic compounds and the contents of Maillard reaction products. J. Food Sci. 2021, 86, 3109–3121. [Google Scholar] [CrossRef]
- He, Q.; Lv, Y.; Yao, K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chem. 2007, 101, 1178–1182. [Google Scholar] [CrossRef]
- Alongi, M.; Frias Celayeta, J.M.; Vriz, R.; Kinsella, G.K.; Rulikowska, A.; Anese, M. In vitro digestion nullified the differences triggered by roasting in phenolic composition and alpha-glucosidase inhibitory capacity of coffee. Food Chem. 2021, 342, 128289. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Chen, C.; Fu, X. The effect of ultrasound irradiation on the physicochemical properties and α-glucosidase inhibitory effect of blackberry fruit polysaccharide. Food Hydrocoll. 2019, 96, 568–576. [Google Scholar] [CrossRef]






| Sample | T (K) | Ksv (×104 L mol−1) | Ra | Ka (×104 L mol−1) | Rb | n | ΔH° (kJ mol−1) | ΔG° (kJ mol−1) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|---|---|---|---|---|
| Untreated | 298 | 1.76 ± 0.02 c | 0.9982 | 1.82 ± 0.05 b | 0.9934 | 0.91 ± 0.01 | −25.05 ± 0.20 | −24.32 ± 0.40 | −2.44 ± 0.07 |
| 304 | 1.32 ± 0.03 e | 0.9984 | 1.51 ± 0.02 d | 0.9928 | 0.95 ± 0.02 | −24.31 ± 0.35 | |||
| 310 | 1.15 ± 0.01 g | 0.9964 | 1.23 ± 0.04 f | 0.9985 | 0.87 ± 0.02 | −24.29 ± 0.28 | |||
| Heating (100 °C, 20 min) | 298 | 1.21 ± 0.02 f | 0.9963 | 0.92 ± 0.12 g | 0.9911 | 0.89 ± 0.02 | −37.70 ± 0.32 | −22.69 ± 0.71 | −50.38 ± 1.05 |
| 304 | 1.05 ± 0.04 h | 0.9950 | 0.74 ± 0.17 h | 0.9808 | 0.84 ± 0.03 | −22.38 ± 0.80 | |||
| 310 | 0.86 ± 0.01 i | 0.9984 | 0.51 ± 0.11 i | 0.9897 | 0.79 ± 0.02 | −22.08 ± 0.55 | |||
| Microwave (800 W, 6 min) | 298 | 2.25 ± 0.05 a | 0.9948 | 2.18 ± 0.01 a | 0.9944 | 0.88 ± 0.02 | −34.57 ± 0.11 | −24.78 ± 0.86 | −32.85 ± 0.34 |
| 304 | 1.94 ± 0.03 b | 0.9973 | 1.70 ± 0.02 c | 0.9882 | 0.90 ± 0.03 | −24.58 ± 0.64 | |||
| 310 | 1.56 ± 0.02 d | 0.9984 | 1.27 ± 0.03 e | 0.9870 | 0.87 ± 0.03 | −24.39 ± 0.53 |
| Sample | Molar Ratio [ECG]:[XO] | α−Helix (%) | β−Sheet (%) | β−Turn (%) | Random Coil (%) |
|---|---|---|---|---|---|
| Free XO | 0:1 | 8.41 ± 0.21 j | 41.59 ± 0.36 d | 22.27 ± 0.36 a | 27.73 ± 0.36 g |
| ECG–XO (Untreated) | 10:1 | 10.83 ± 0.51 f | 42.51 ± 0.41 c | 21.65 ± 0.94 d | 25.12 ± 0.69 h |
| 20:1 | 10.96 ± 0.62 e | 43.41 ± 0.74 b | 21.59 ± 0.13 e | 23.03 ± 0.31 i | |
| 40:1 | 11.83 ± 0.93 c | 45.02 ± 0.22 a | 21.39 ± 0.25 g | 22.51 ± 0.24 j | |
| ECG–XO (Heating) (100 °C, 20 min) | 10:1 | 8.66 ± 0.34 i | 40.74 ± 0.24 e | 21.91 ± 0.18 b | 28.69 ± 0.11 e |
| 20:1 | 10.98 ± 0.74 d | 38.08 ± 0.19 h | 21.76 ± 0.14 c | 29.17 ± 0.43 b | |
| 40:1 | 12.63 ± 0.26 b | 37.32 ± 0.55 j | 20.47 ± 0.31 h | 29.58 ± 0.38 a | |
| ECG–XO (Microwave) (800 W, 6 min) | 10:1 | 9.18 ± 0.19 h | 40.52 ± 0.46 f | 21.76 ± 0.47 c | 28.54 ± 0.71 f |
| 20:1 | 10.38 ± 0.65 g | 39.33 ± 0.75 g | 21.54 ± 0.19 f | 28.75 ± 0.82 d | |
| 40:1 | 13.52 ± 0.62 a | 37.57 ± 0.91 i | 20.08 ± 0.54 i | 28.93 ± 0.68 c |
| ECG | Concentration (mM) | Bioaccessibility (%) | |||
|---|---|---|---|---|---|
| Undigested | Oral | Gastric | Intestinal | ||
| Untreated | 1.00 ± 0.03 a | 0.77 ± 0.04 c | 0.68 ± 0.02 d | 0.40 ± 0.04 i | 40.30 ± 2.71 A |
| Heating (100 °C, 20 min) | 0.67 ± 0.02 e | 0.51 ± 0.03 g | 0.44 ± 0.03 h | 0.22 ± 0.01 k | 32.84 ± 0.49 C |
| Microwave (800 W, 6 min) | 0.87 ± 0.02 b | 0.68 ± 0.04 d | 0.59 ± 0.04 f | 0.32 ± 0.02 j | 36.78 ± 1.42 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Fei, X.; Gong, D.; Zhang, G. Effects of Processing Conditions and Simulated Digestion In Vitro on the Antioxidant Activity, Inhibition of Xanthine Oxidase and Bioaccessibility of Epicatechin Gallate. Foods 2023, 12, 2807. https://doi.org/10.3390/foods12142807
Zhu M, Fei X, Gong D, Zhang G. Effects of Processing Conditions and Simulated Digestion In Vitro on the Antioxidant Activity, Inhibition of Xanthine Oxidase and Bioaccessibility of Epicatechin Gallate. Foods. 2023; 12(14):2807. https://doi.org/10.3390/foods12142807
Chicago/Turabian StyleZhu, Miao, Xiaoyun Fei, Deming Gong, and Guowen Zhang. 2023. "Effects of Processing Conditions and Simulated Digestion In Vitro on the Antioxidant Activity, Inhibition of Xanthine Oxidase and Bioaccessibility of Epicatechin Gallate" Foods 12, no. 14: 2807. https://doi.org/10.3390/foods12142807
APA StyleZhu, M., Fei, X., Gong, D., & Zhang, G. (2023). Effects of Processing Conditions and Simulated Digestion In Vitro on the Antioxidant Activity, Inhibition of Xanthine Oxidase and Bioaccessibility of Epicatechin Gallate. Foods, 12(14), 2807. https://doi.org/10.3390/foods12142807