Determination of Multimycotoxin in Cereal-Based Products Sold in Open-Air Markets
Abstract
1. Introduction
| Mycotoxins | Food Type | |||
|---|---|---|---|---|
| Cornmeal | Extruded Maize Snack | Maize-Based Breakfast Cereal | Oatmeal | |
| TAF (μg·kg−1) | 4 | 4 | 4 | 4 |
| AFB1 (μg·kg−1) | 2 | 2 | 2 | 2 |
| OTA (μg·kg−1) | 3 | 3 | 3 | 3 |
| DON (μg·kg−1) | 750 | 500 | 500 | 750 |
| ZEN (μg·kg−1) | 200 | 50 | 50 | 75 |
| FUM (μg·kg−1) | 1000 | 400 | 400 | - |
2. Materials and Methods
2.1. Sample Collection
2.2. Preparation of Mobile Phases
2.3. Sample Preparation and Extraction
2.4. LC-MS/MS Equipment
2.5. Recovery Rate
- LOD: limit of detection;
- LOQ: limit of quantification;
- SD: standard deviation;
- xmean: the arithmetic mean of the data;
- xi: each value of the dataset;
- n: the total number of data.
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clark, W.A.V. Human Migration; Thrall, G.I., Ed.; Reprint; WVU Research Repository: Morgantown, WV, USA, 2020. [Google Scholar]
- Sik, E.; Wallace, C. The development of open-air markets in East-Central Europe. Int. J. Urban Reg. Res. 1999, 23, 697–714. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Jacxsens, L.; De Saeger, S.; De Meulenaer, B. Co-occurrence of multiple mycotoxins in dry chilli (Capsicum annum L.) samples from the markets of Sri Lanka and Belgium. Food Control 2014, 46, 26–34. [Google Scholar] [CrossRef]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Manu, N.; Opit, G.P.; Osekre, E.A.; Arthur, F.H.; Mbata, G.; Armstrong, P.; Danso, J.K.; McNeill, S.G.; Campbell, J.F. Moisture content, insect pest infestation and mycotoxin levels of maize in markets in the northern region of Ghana. J. Stored Prod. Res. 2019, 80, 10–20. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Niaz, B.; Rasheed, A.; Umar, M.; Hussain, M.; Ahmad Nayik, G.; Ansari, M.J. Quality and Safety Aspects of Cereal Grains. In Cereal Grains: Composition, Nutritional Attributes, and Potential Applications; Nayik, G.A., Tufail, T., Anjum, F.M., Ansari, M.J., Eds.; CRC Press: Boca Raton, FL, USA, 2023; p. 41. [Google Scholar]
- Majeed, S.; De Boevre, M.; De Saeger, S.; Rauf, W.; Tawab, A.; Fazal-e-Habib; Rahman, M.; Iqbal, M. Multiple Mycotoxins in Rice: Occurrence and Health Risk Assessment in Children and Adults of Punjab, Pakistan. Toxins 2018, 10, 77. [Google Scholar] [CrossRef]
- Palumbo, R.; Crisci, A.; Venâncio, A.; Cortiñas Abrahantes, J.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and Co-Occurrence of Mycotoxins in Cereal-Based Feed and Food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef]
- Kendra, D.F.; Dyer, R.B. Opportunities for biotechnology and policy regarding mycotoxin issues in international trade. Int. J. Food Microbiol. 2007, 119, 147–151. [Google Scholar] [CrossRef]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Müller, J.; Vanlauwe, B.; Bandyopadhyay, R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application–A review. Food Control 2017, 76, 127–138. [Google Scholar] [CrossRef]
- Jindal, N.; Mahipal, S.K.; Rottinghaus, G.E. Occurrence of fumonisin B1 in maize and poultry feeds in Haryana, India. Mycopathologia 1999, 148, 37–40. [Google Scholar] [CrossRef]
- Belli, N.; Marin, S.; Sanchis, V.; Ramos, A.J. Ochratoxin A (OTA) in wines, musts and grape juices: Occurrence, regulations and methods of analysis. FSTI 2002, 8, 325–335. [Google Scholar] [CrossRef]
- Scudamore, K.A.; Patel, S. Occurrence of Fusarium mycotoxins in maize imported into the UK, 2004–2007. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2009, 26, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Scussel, V.M.; Savi, G.D.; Costas, L.L.F.; Xavier, J.J.M.; Manfio, D.; Bittencourt, K.O.; Agular, K.; Stein, S.M. Fumonisins in corn (Zea mays L.) from Southern Brazil. Food Addit. Contam. Part B Surveill. 2014, 7, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Burlakoti, R.R.; Tamburic-Ilincic, L.; Limay-Rios, V.; Burlakoti, P. Comparative population structure and trichothecene mycotoxin profiling of Fusarium graminearum from corn and wheat in Ontario, central Canada. Plant Pathol. 2017, 66, 14–27. [Google Scholar] [CrossRef]
- Turkish Food Codex Regulation (TFC). Communiqué on Determination of Maximum Levels of Certain Contaminants in Foodstuffs. Off. Gaz. 2011, 28157. [Google Scholar]
- Kim, D.-H.; Hong, S.-Y.; Kang, J.W.; Cho, S.M.; Lee, K.R.; An, T.K.; Lee, C.; Chung, S.H. Simultaneous Determination of Multi-Mycotoxins in Cereal Grains Collected from South Korea by LC/MS/MS. Toxins 2017, 9, 106. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Gregori, E.; Russo, S.; Marchegiani, F.; Moracci, G.; Brera, C. Development of a LC-MS/MS Method for the Multi-Mycotoxin Determination in Composite Cereal-Based Samples. Toxins 2017, 9, 169. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Gambacorta, L.; Bibi, R.; Ciriaci, M.; Paoloni, A.; Pecorelli, I. Multimycotoxin analysis by LC-MS/MS in cereal food and feed: Comparison of different approaches for extraction, purification, and calibration. J. AOAC Int. 2018, 101, 647–657. [Google Scholar] [CrossRef]
- EC No 657/2002 Commission Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results (Text with EEA Relevance) (Notified under Document Number C (2002) 3044). Off. J. Eur. Union L 2002, 221, 8. Available online: http://data.europa.eu/eli/dec/2002/657/oj (accessed on 18 May 2023).
- ISO/TS 21748; Guidance for the Use of Repeatability, Reproducibility and Trueness Estimates in Measurement Uncertainty Estimation. ISO, International Organization for Standardization: Geneva, Switzerland, 2010.
- Ellison, S.L.; Williams, A. Quantifying Uncertainty in Analytical Measurement, 3rd ed.; EURACHEM/CITAC Guide, CG4; Eurachem: Gembloux, Belgium; CITAC: London, UK, 2012. [Google Scholar]
- Amirahmadi, M.; Shoeibi, S.; Rastegar, H.; Elmi, M.; Mousavi Khaneghah, A. Simultaneous analysis of mycotoxins in corn flour using LC/MS-MS combined with a modified QuEChERS procedure. Toxin Rev. 2018, 37, 187–195. [Google Scholar] [CrossRef]
- EC No 401/2006 of 23 February 2006 Laying Down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs (Text with EEA Relevance). Off. J. Eur. Union L 2006, 70, 12–34.
- European Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union L 2006, 364, 5–24.
- European Commission EC No 165/2010 of 26 February 2010, Amending Regulation (EC) no 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs as Regards Aflatoxin. Off. J. Eur. Union L 2010, 50, 8–12.
- Alborch, L.; Bragulat, M.R.; Castella, G.; Abarca, M.L.; Cabanes, F.J. Mycobiota and mycotoxin contamination of maize flours and popcorn kernels for human consumption commercialized in Spain. Food Microbiol. 2012, 32, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, J.-J.; Huang, B.-F.; Cai, Z.-X.; Ren, Y.-P. High-performance liquid chromatographic determination of multi-mycotoxin in cereals and bean foodstuffs using interference-removal solid-phase extraction combined with optimized dispersive liquid–liquid microextraction. J. Sep. Sci. 2017, 40, 2081–2314. [Google Scholar] [CrossRef]
- Manizan, A.M.; Oplatowska-Stachowiak, M.; Piro-Metayer, I.; Campbell, K.; Koffi-Nevry, R.; Elliott, C.; Akaki, K.D.; Montet, D.; Brabet, C. Multi-mycotoxin determination in rice, maize and peanut products most consumed in Côte d’Ivoire by UHPLC-MS/MS. Food Control 2018, 87, 22–23. [Google Scholar] [CrossRef]
- Warth, B.; Parich, A.; Atehnkeng, J.; Bandyopadhyay, R.; Schuhmacher, R.; Sulyok, M.; Krska, R. Quantitation of Mycotoxins in Food and Feed from Burkina Faso and Mozambique Using a Modern LC-MS/MS Multitoxin Method. J. Agric. Food Chem. 2012, 60, 9352–9363. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Wang, F.; Ma, X.; Li, Z.; Guo, D.; Wang, Y.; Wan, F.; Deng, L.; Zhang, S. Determination of 16 Mycotoxins in Maize by Ultrahigh-Performance Liquid Chromatography–Tandem Mass Spectrometry. Anal. Lett. 2018, 51, 702–716. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Romera, D.; Mateo, E.M. Study on mycotoxin contamination of maize kernels in Spain. Food Control 2020, 118, 107370. [Google Scholar] [CrossRef]
- Ediage, E.N.; Di Mavungu, J.D.; Monbaliu, S.; Peteghem, C.V.; De Saeger, S. A Validated Multianalyte LC-MS/MS Method for Quantification of 25 Mycotoxins in Cassava Flour, Peanut Cake and Maize Samples. J. Agric. Food Chem. 2011, 59, 5173–5180. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Faridah, A.; Khatib, A. A UPLC–MS/MS for simultaneous determination of aflatoxins, ochratoxin A, zearalenone, DON, fumonisins, T-2 toxin and HT-2 toxin, in cereals. Food Control 2012, 25, 647–653. [Google Scholar] [CrossRef]
- Paschoal, F.N.; De Azevedo Silva, D.; De Souza, R.V.S.; de Oliveira, M.S.; Pereira, D.A.A.; de Souza, S.V.C. A Rapid Single-Extraction Method for the Simultaneous Determination of Aflatoxins B1, B2, G1, G2, Fumonisin B1, and Zearalenone in Corn Meal by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 1631–1644. [Google Scholar] [CrossRef]
- Ortiz, J.; Camp, J.V.; Mestdagh, F.; Donoso, S.; De Meulenaer, B. Mycotoxin co-occurrence in rice, oat flakes and wheat noodles used as staple foods in Ecuador. Food Addit. Contam. Part A 2013, 30, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Mañes, J.; Juan-García, A.; Moltó, J.C. Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry. Appl. Sci. 2022, 12, 3942. [Google Scholar] [CrossRef]
- Vidal, A.; Marín, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Determination of aflatoxins, deoxynivalenol, ochratoxin A and zearalenone in wheat and oat based bran supplements sold in the Spanish market. Food Chem. Toxicol. 2013, 53, 133–138. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 Mycotoxins in the Grain of Cereals Cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Roscoe, M.; Pleskach, K. A multi-year survey of mycotoxins and ergosterol in Canadian oats. Mycotoxin Res. 2020, 36, 103–114. [Google Scholar] [CrossRef]
- Meyer, J.C.; Hennies, I.; Wessels, D.; Schwarz, K. Survey of mycotoxins in milling oats dedicated for food purposes between 2013 and 2019 by LC–MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 1934–1947. [Google Scholar] [CrossRef]
- Al-Taher, F.; Cappozzo, J.; Zweigenbaum, J.; Lee, H.J.; Jackson, L.; Ryu, D. Detection and quantitation of mycotoxins in infant cereals in the U.S. market by LC-MS/MS using a stable isotope dilution assay. Food Control 2017, 72, 27–35. [Google Scholar] [CrossRef]
- Kuzdraliński, A.; Solarska, E.; Mazurkiewicz, J. Mycotoxin content of organic and conventional oats from southeastern Poland. Food Control 2023, 33, 68–72. [Google Scholar] [CrossRef]
- Martos, P.A.; Thompson, W.; Diaz, G.J. Multiresidue mycotoxin analysis in wheat, barley, oats, rye and maize grain by high performance liquid chromatography-tandem mass spectrometry. World Mycotoxin J. 2010, 3, 205–223. [Google Scholar] [CrossRef]
- Oueslati, S.; Romero-González, R.; Lasram, S.; Frenich, A.G.; Vidal, J.L.M. Multi-mycotoxin determination in cereals and derived products marketed in Tunisia using ultra-high performance liquid chromatography coupled to triple quadrupole mass spectrometry. Food Chem. Toxicol. 2012, 50, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Abia, W.A.; Warth, B.; Sulyok, M.; Krska, R.; Tchana, A.N.; Njobeh, P.B.; Dutton, M.F.; Moundipa, P.F. Determination of multi-mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC-MS/MS). Food Control 2013, 31, 438–453. [Google Scholar] [CrossRef]
- De Boevre, M.; Di Mavungu, J.D.; Maene, P.; Audenaert, K.; Deforce, D.; Haesaert, G.; Eeckhout, M.; Callebaut, A.; Berthiller, F.; Van Peteghem, C.; et al. Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 819–835. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a Changing Climate: Semi-Quantitative Multi-Mycotoxin Analysis of Grain Grown in Exceptional Climatic Conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Tabassum, M.; Banik, M.; Daayf, F.; Fernando, W.G.D.; Harris, L.J.; Sura, S.; Wang, X. Naturally Occurring Fusarium Species and Mycotoxins in Oat Grains from Manitoba, Canada. Toxins 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]

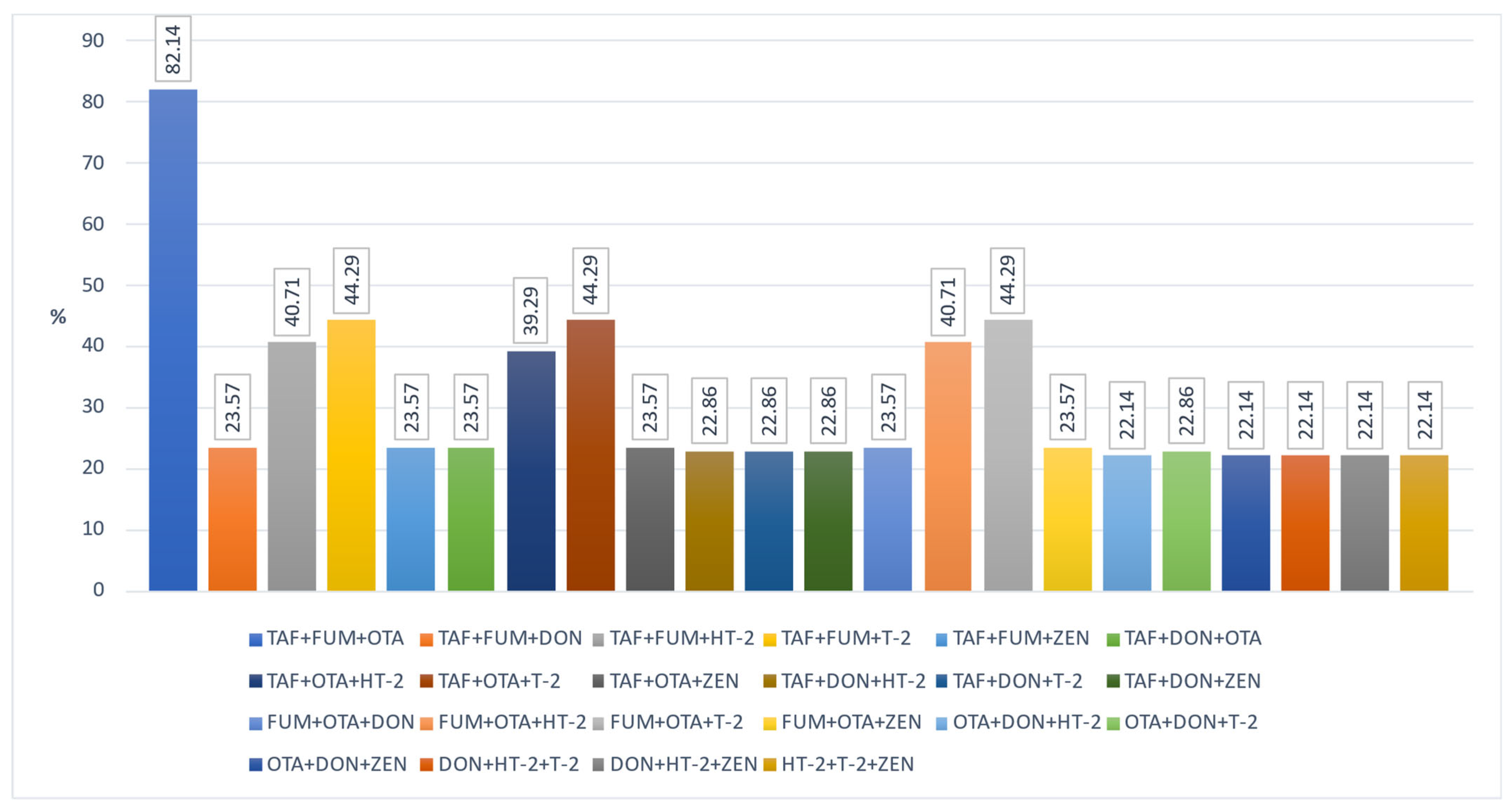
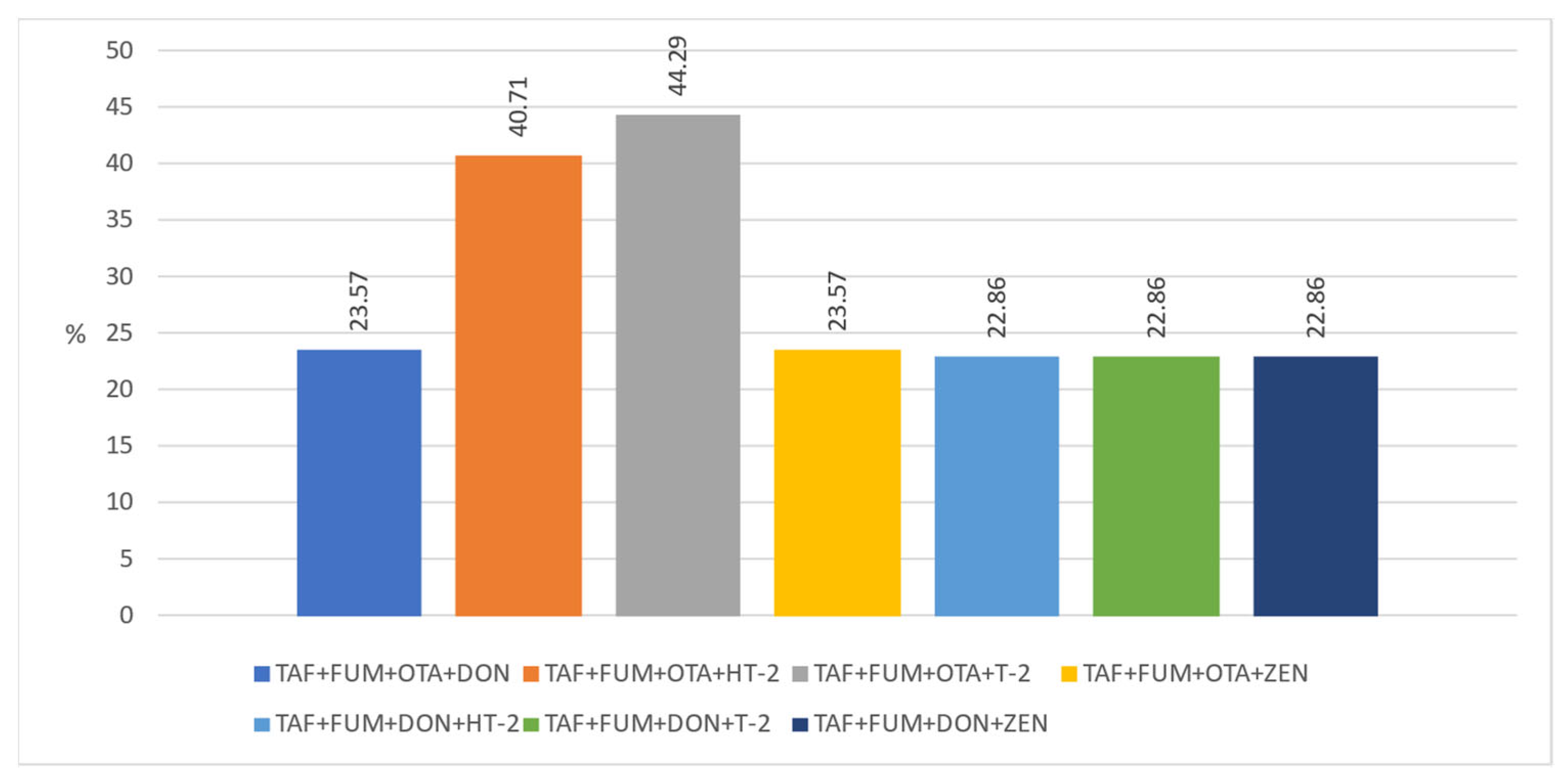
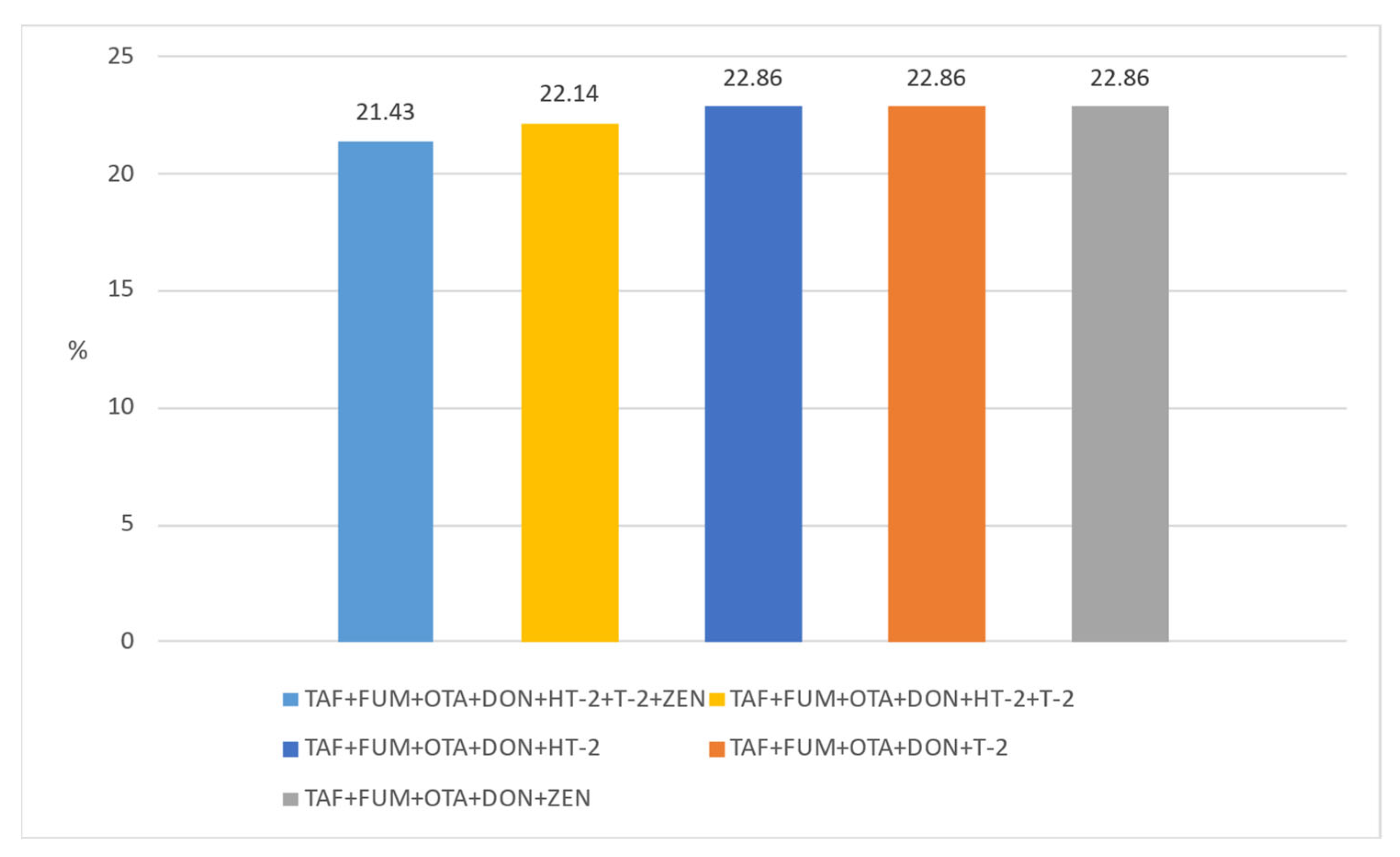
| AFB1 | LOD > (<0.24 μg·kg−1) (n) | LOD-LOQ > (0.24–0.78 > μg·kg−1) (n) | ≥0.78–1 μg·kg−1 (n) | ≥1–2 μg·kg−1 (n) | ≥2 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
|---|---|---|---|---|---|---|---|---|
| Cornmeal | 34 | 4 | 1 | 2 | - | 1.01 | 0.07 | 1.01 |
| Extruded Maize Snack | 2 | 2 | 8 | 19 | 1 | 1.30 | 0.46 | 1.20 |
| Maize Based Breakfast Cereal | - | - | 2 | 24 | 1 | 1.39 | 0.35 | 1.33 |
| Oatmeal | - | - | - | 2 | 38 | 3.00 | 2.11 | 2.55 |
| TOTAL | 36 | 6 | 11 | 47 | 40 | 2.01 | 1.60 | 1.65 |
| AFB2 | LOD> (<0.26 μg·kg−1) (n) | LOD-LOQ > (0.26–0.82 > μg·kg−1) (n) | ≥0.82–1 μg·kg−1 (n) | ≥1–2 μg·kg−1 (n) | ≥2 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 19 | 22 | - | - | - | - | - | - |
| Extruded Maize Snack | 25 | 7 | - | - | - | - | - | - |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | 6 | - | - | - | 34 | 2.39 | 0.18 | 2.35 |
| TOTAL | 77 | 29 | - | - | 34 | 2.39 | 0.18 | 2.35 |
| AFG1 | LOD> (<0.22 μg·kg−1) (n) | LOD-LOQ > (0.22–0.74 > μg·kg−1) (n) | ≥0.74–1 μg·kg−1 (n) | ≥1–2 μg·kg−1 (n) | ≥2 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 30 | 11 | - | - | - | - | - | - |
| Extruded Maize Snack | 25 | 7 | - | - | - | - | - | - |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | - | - | - | 33 | 2.41 | 0.07 | 2.38 | |
| TOTAL | 89 | 18 | - | - | 33 | 2.41 | 0.07 | 2.38 |
| AFG2 | LOD> (<0.24 μg·kg−1) (n) | LOD-LOQ > (0.24–0.78 > μg·kg−1) (n) | ≥0.78–1 μg·kg−1 (n) | ≥1–2 μg·kg−1 (n) | ≥2 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 21 | 6 | 4 | 7 | 3 | 1.58 | 0.74 | 1.43 |
| Extruded Maize Snack | 25 | 2 | - | - | 5 | 4.27 | 4.77 | 2.04 |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | 10 | - | - | 17 | 13 | 2.29 | 1.15 | 1.89 |
| TOTAL | 83 | 8 | 4 | 24 | 21 | 2.29 | 1.84 | 1.82 |
| TAF | LOD> (<0.22 μg·kg−1) (n) | LOD-LOQ> (0.22–0.74 > μg·kg−1) (n) | ≥0.74–2 μg·kg−1 (n) | ≥2–4 μg·kg−1 (n) | ≥4 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 15 | 9 | 14 | 3 | - | 1.58 | 0.74 | 0.43 |
| Extruded Maize Snack | - | - | 24 | 7 | 1 | 1.93 | 2.16 | 1.31 |
| Maize Based Breakfast Cereal | - | - | 26 | 1 | - | 1.39 | 0.35 | 1.33 |
| Oatmeal | - | - | 2 | 2 | 36 | 8.74 | 3.56 | 8.99 |
| TOTAL | 15 | 9 | 66 | 13 | 37 | 3.79 | 4.14 | 1.65 |
| FB1 | LOD> (<31.14 μg·kg−1) (n) | LOD-LOQ > (31.14–103.79 > μg·kg−1) (n) | ≥103.79–150 μg·kg−1 (n) | ≥150–200 μg·kg−1 (n) | ≥200 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 18 | 21 | 2 | - | - | 141.98 | 0.0006 | 141.98 |
| Extruded Maize Snack | 25 | 5 | 1 | 1 | 150.36 | 10.49 | 150.36 | |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | 38 | 2 | - | - | - | - | - | - |
| TOTAL | 108 | 28 | 3 | 1 | - | 146.17 | 7.75 | 142.46 |
| FB2 | LOD> (<17.11 μg·kg−1) (n) | LOD-LOQ> (17.11–57.04 > μg·kg−1) | ≥57.04–100 μg·kg−1 (n) | ≥100–200 μg·kg−1 (n) | ≥200 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 14 | 12 | 15 | - | - | 58.38 | 0.95 | 58.18 |
| Extruded Maize Snack | - | 2 | 30 | - | - | 60.24 | 4.28 | 59.07 |
| Maize Based Breakfast Cereal | - | - | 27 | - | - | 60.34 | 3.15 | 59.07 |
| Oatmeal | - | - | 6 | 34 | - | 166.55 | 45.36 | 182.93 |
| TOTAL | 14 | 14 | 78 | 34 | - | 97.97 | 58.02 | 59.99 |
| FUM | LOD> (<17.11 μg·kg−1) (n) | LOD-LOQ> (17.11–57.04 > μg·kg−1) (n) | ≥57.04–200 μg·kg−1 (n) | ≥200–800 μg·kg−1 (n) | ≥800 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 14 | 12 | 13 | 2 | - | 82.05 | 56.04 | 58.18 |
| Extruded Maize Snack | - | 2 | 28 | 2 | - | 65.87 | 41.57 | 58.92 |
| Maize Based Breakfast Cereal | - | - | 27 | - | - | 60.34 | 3.15 | 59.07 |
| Oatmeal | - | - | 40 | - | - | 166.55 | 45.36 | 182.39 |
| TOTAL | 14 | 14 | 108 | 4 | - | 106.31 | 62.25 | 59.94 |
| OTA | LOD> (<0.26 μg·kg−1) (n) | LOD-LOQ> (0.26–0.85 > μg·kg−1) (n) | ≥0.85–1 μg·kg−1 (n) | ≥1–3 μg·kg−1 (n) | ≥3 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 14 | 27 | - | - | - | - | - | - |
| Extruded Maize Snack | - | 32 | - | - | - | - | - | - |
| Maize Based Breakfast Cereal | 3 | 24 | - | - | - | - | - | - |
| Oatmeal | - | 6 | - | 34 | - | 1.30 | 0.002 | 1.30 |
| TOTAL | 17 | 89 | - | 34 | - | 1.30 | 0.002 | 1.30 |
| DON | LOD> (<28.51 μg·kg−1) (n) | LOD-LOQ > (28.51–95.03 > μg·kg−1) (n) | ≥95.03–150 μg·kg−1 (n) | ≥150–500 μg·kg−1 (n) | ≥500 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 41 | - | - | - | - | - | - | - |
| Extruded Maize Snack | 32 | - | - | - | - | - | - | - |
| Maize Based Breakfast Cereal | 26 | 1 | - | - | - | - | - | - |
| Oatmeal | 7 | - | - | 33 | - | 171.26 | 2.63 | 170.64 |
| TOTAL | 106 | 1 | - | 33 | - | 171.26 | 2.63 | 170.64 |
| ZEN | LOD > (<2.94 μg·kg−1) (n) | LOD-LOQ > (2.94–9.80 > μg·kg−1) (n) | ≥9.80–50 μg·kg−1 (n) | ≥50–100 μg·kg−1 (n) | ≥100 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 41 | - | - | - | - | - | - | - |
| Extruded Maize Snack | 32 | - | - | - | - | - | - | - |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | 7 | - | 33 | - | - | 16.41 | 0.34 | 16.32 |
| TOTAL | 107 | - | 33 | - | - | 16.41 | 0.34 | 16.32 |
| HT-2 | LOD > (<3.32 μg·kg−1) (n) | LOD-LOQ > (3.32–11.08 > μg·kg−1) (n) | ≥11.08–20 μg·kg−1 (n) | ≥20–40 μg·kg−1 (n) | ≥40–50 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 22 | 13 | 5 | 1 | - | - | - | - |
| Extruded Maize Snack | 27 | 3 | 2 | - | 16.07 | 1.63 | 16.07 | |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | 7 | - | - | 27 | 6 | 39.29 | 1.36 | 39.09 |
| TOTAL | 83 | 16 | 7 | 28 | 6 | 35.17 | 8.65 | 38.91 |
| T-2 | LOD > (<3.16 μg·kg−1) (n) | LOD-LOQ > (3.16–10.55 > μg·kg−1) (n) | ≥10.55–50 μg·kg−1 (n) | ≥50–100 μg·kg−1 (n) | ≥100 μg·kg−1 (n) | x μg·kg−1 | SD μg·kg−1 | Med μg·kg−1 |
| Cornmeal | 19 | 22 | - | - | - | - | - | - |
| Extruded Maize Snack | 25 | 7 | - | - | - | - | - | - |
| Maize Based Breakfast Cereal | 27 | - | - | - | - | - | - | - |
| Oatmeal | 7 | 33 | - | - | - | - | - | - |
| TOTAL | 78 | 62 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz Eker, F.; Muratoglu, K.; Ozturk, M.; Cetin, B.; Buyukunal, S.K. Determination of Multimycotoxin in Cereal-Based Products Sold in Open-Air Markets. Foods 2023, 12, 2744. https://doi.org/10.3390/foods12142744
Yilmaz Eker F, Muratoglu K, Ozturk M, Cetin B, Buyukunal SK. Determination of Multimycotoxin in Cereal-Based Products Sold in Open-Air Markets. Foods. 2023; 12(14):2744. https://doi.org/10.3390/foods12142744
Chicago/Turabian StyleYilmaz Eker, Funda, Karlo Muratoglu, Muhsin Ozturk, Bayram Cetin, and Serkan Kemal Buyukunal. 2023. "Determination of Multimycotoxin in Cereal-Based Products Sold in Open-Air Markets" Foods 12, no. 14: 2744. https://doi.org/10.3390/foods12142744
APA StyleYilmaz Eker, F., Muratoglu, K., Ozturk, M., Cetin, B., & Buyukunal, S. K. (2023). Determination of Multimycotoxin in Cereal-Based Products Sold in Open-Air Markets. Foods, 12(14), 2744. https://doi.org/10.3390/foods12142744







