Volatilome Analysis and Evolution in the Headspace of Packed Refrigerated Fish
Abstract
1. Introduction
- (i)
- The packaging systems used to generate typical headspace gas composition evolution during packed fish shelf life and containment of VOCs according to packaging materials barrier properties.
- (ii)
- The fish spoilage mechanisms generating VOCs with emphasis on their chemical structure, properties, and possible origin. VOCs are mainly generated via enzymatic reactions, lipid oxidation, and microbial actions.
- (iii)
- A comparison with the traditional methods to assess fish freshness (e.g., TVB-N or TMA assay), which will be detailed thereafter.
- (iv)
- The analytical methods allowing the isolation, identification, and assay of VOCs present in the headspace of a packaging, listing for each method their advantages and limits.
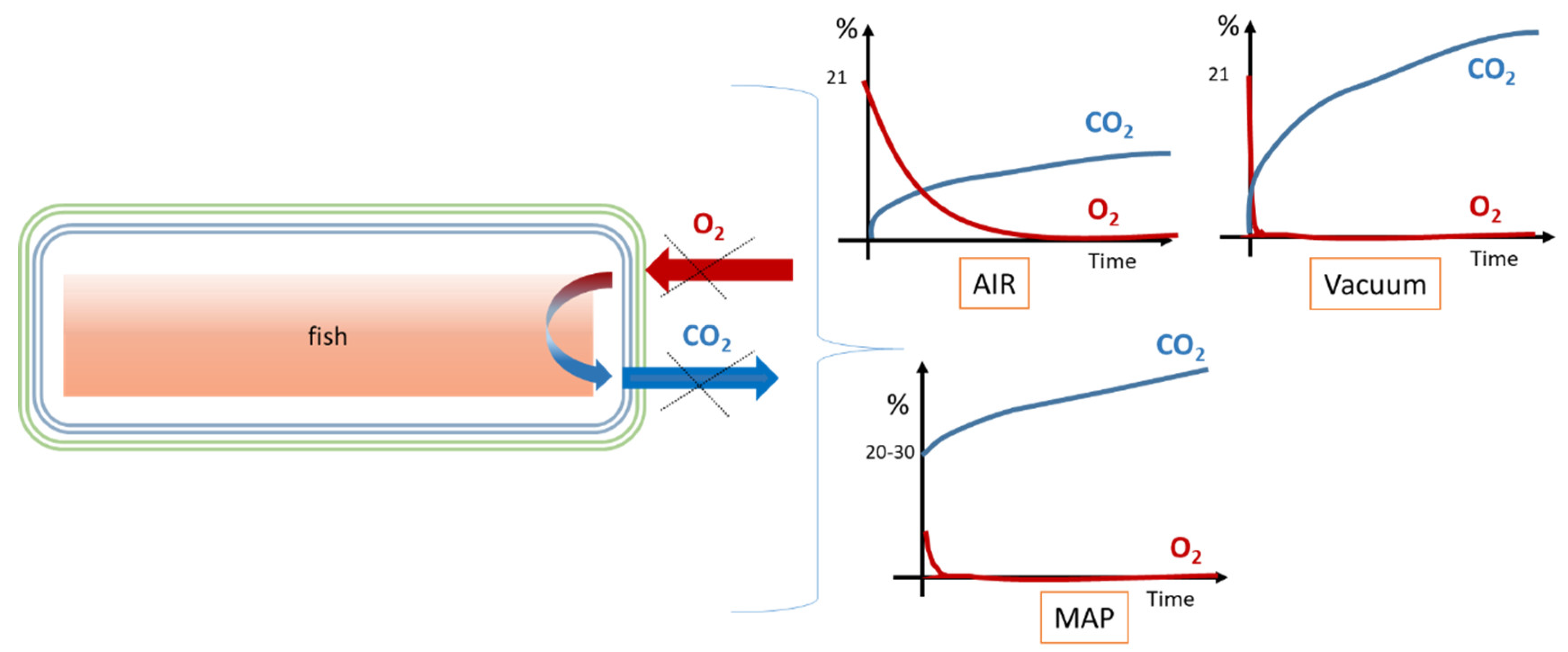
2. Headspace Atmosphere Composition as a Function of Packaging
3. Fishes Alteration Criteria
3.1. Quality Indicators of Fish Protein Degradation
3.2. Quality Indicators Markers of Fish Oxidation
3.2.1. Analytical Methods
3.2.2. Correlation between Volatilome Composition and Fish Rancidity/Oxidation Indicators
3.3. Microbial Spoilage of Fish
4. Volatilome Analysis
4.1. TVB-N and TMA
4.2. Volatilome Analysis and Identification of Specific Markers
| Class | VOC | Extraction and Analytical Method | References |
|---|---|---|---|
| Acids | Acetic acid | SPME/GC/MS | [13,48,51,70,73] |
| Dynamic headspace/GC/MS | [47,75] | ||
| Static headspace/SIFT/MS | [81,86] | ||
| Alcohols | Ethanol | SPME/GC/MS | [46,48,49,70] |
| Dynamic headspace/GC/MS | [75] | ||
| Static headspace/SIFT/MS | [32,81,86] | ||
| 3-methyl-1-butanol | SPME/GC/MS | [49,51,72,76] | |
| Dynamic headspace/GC/MS | [47,75] | ||
| Static headspace/SIFT/MS | [32] | ||
| 2,3-butanediol | SPME/GC/MS | [49,70] | |
| Dynamic headspace/GC/MS | [75] | ||
| Static headspace/SIFT/MS | [68,86] | ||
| 1-penten-3-ol | SPME/GC/MS | [48] | |
| Dynamic headspace/GC/MS | [47,74,75] | ||
| Aldehydes | 2 or 3 methyl-butanal | SPME/GC/MS | [48,49,51] |
| Dynamic headspace/GC/MS | [75] | ||
| Static headspace/SIFT/MS | [32] | ||
| Amines | TMA | SPME/GC/MS | [49,72,76] |
| Dynamic headspace/GC/MS | [47,75] | ||
| Esters | Ethyl acetate | SPME/GC/MS | [49,71] |
| Dynamic headspace/GC/MS | [75] | ||
| Static headspace/SIFT/MS | [32,86] | ||
| Ketones | 3-hydroxy-2-butanone (=acetoin) | SPME/GC/MS | [49,51,70,72,73] |
| Dynamic headspace/GC/MS | [82] | ||
| Static headspace/SIFT/MS | [32,86] | ||
| Sulfur compounds | Hydrogen sulfide, carbon disulfide and dimethyl disulfide, methanethiol (methyl mercaptan) | SPME/GC/MS | [76] |
| Dynamic headspace/GC/MS | [47,75] | ||
| Static headspace/SIFT/MS | [32,81,86] |
4.3. Electronic Nose
5. Effect of Different Factors Affecting Fish Quality on Volatilome Composition
5.1. Effect of Temperature
5.2. Effect of Atmosphere Composition
5.3. Hurdle Technology
5.3.1. Modified Atmosphere or Vacuum Packaging in Combination with Non-Thermal Treatments (High Hydrostatic Pressure, Irradiation, UV-C Treatment, Treatment with Ozonated Water, etc.)
5.3.2. Modified Atmosphere or Vacuum Packaging in Combination with Antimicrobial and/or Antioxidant Compounds
5.3.3. Modified Atmosphere or Vacuum Packaging in Combination with Bioprotective Lactic Acid Bacteria
| Combined Treatment | Seafood/Fish Type | Quality Parameter Related to Volatile Organic Compounds Monitored | Reference |
|---|---|---|---|
| Non-thermal treatments | |||
| HHP treatment after VP of fish fillets or of fish fillets coated in chitosan-based edible films | Rainbow trout (Oncorhynchus mykiss Walbaum) fillets | -TVB-N assay every 4 days up to 44 days storage at 4 °C. -The acceptable limit value of 30 mg TVB-N/100 g has been exceeded in the VP, VP HHP-treated, coated in chitosan-based film VP, and coated in chitosan-based film VP HHP-treated fillets on the 12th, 20th, 24th, and 44th days storage at 4 °C, respectively. Coating in chitosan-based films and to a lesser extent HHP-treatment decreased TVB-N increase during storage. Combination of coating in chitosan-based film and subsequent HHP-treatment have a synergistic effect on the decrease of TVB-N increase during refrigerated storage of trout fillets. | [112] |
| UV-C treatment before MAP (50% CO2-50% N2) | Nile tilapia (Oreochromis niloticus) fillets | -Ammonia concentration in fillets after 10 days storage (AP, VP, MAP, UV-C treated and AP, UV-C treated and MAP). -Ammonia concentration after 10 days storage at 4 °C was lower in MAP and UV-C treated fillets or fillets UV-C treated before being packed in a MAP. | [114] |
| Ozonated water (0.3 mg.L−1) and MAP (50% CO2-50% N2) | Striped red mullet (Mullus surmuletus) | -Assay of TVB-N, TMA-N and odor of raw fish at regular intervals. -TVB-N and TMA-N limit values were reached later when raw fish samples were treated with ozonated water prior to MAP. Nevertheless, the limit of acceptability based on sensory analysis was reached after 10 days storage at 4 °C on both cases (vs. after 7 days for control samples stored in air). | [131] |
| Treatment by cold plasma generated using the mixed gases (oxygen, carbon dioxide argon: 10: 60:30) for 5 min in combination with ethanolic coconut husk extract in either free or liposomal encapsulated form followed by MAP (60% CO2, 30% Ar2, 10% O2) | Asian sea bass slices | -TVB-N and TMA assays every 3 days for 21 days storage at 4 °C -The combination of cold plasma treatment with ethanolic coconut extract extended the shelf life to more than 18 days at 4 °C and reduced both TVB-N and TMA accumulation. | [132] |
| Encapsulated or non encapsulated betel leaf extract and/or in-bag dielectric discharge (80 kV, 300 s) (nonthermal plasma) and/or MAP | Nile tilapia fillets | -TVB-N and TMA assays every 3 days for 15 days storage at 4 °C. Untreated control fillets and nonthermal plasma-treated fillets subsequently stored under a CO2/Ar/O2 (60%/30%/10% v/v) MAP reached the maximal acceptable TVB-N limit (35 mg/100 g according to EU regulation) within 6 days and 9 days, respectively. Addition of 400 ppm unlike addition of 200 ppm of encapsulated or non-encapsulated betel leaf extract combined with non-thermal plasma treatment and MAP allowed to delay to over 12 days this duration of storage. The same trends were observed following TMA assay. | [115] |
| Antimicrobial and/or antioxidant compounds | |||
| Dipping in 1% (w/w) cinnamon essential oil in water emulsion or in marinade containing 1% (w/w) cinnamon essential oil followed by either MAP (60% CO2-40% N2) or VP | Salmon (Salmo salar) | -Evaluation of off-odors by sensory analysis. -Cinnamon essential oil addition did not extend microbial shelf life of salmon. -No significant effects on odor relative hedonic score evolution of salmon during 14 days refrigerated storage of VP or MAP and dipping with cinnamon essential oil or not were observed. | [133] |
| Dipping for 30 min in 0.3% or 0.5% (w/v) Capparis spinosa root extract followed by MAP (5% O2, 20% CO2, 75% N2) | Rainbow trout (Oncorhynchus mykiss) fillets | -TMA assay and sensory evaluation (including odor) after cooking at 98 °C for 20 min every 7 days up to 28 days of refrigerated storage. -Dipping in Capparis spinosa root extract prior to MAP significantly delayed TMA accumulation and cooked fish odor alteration and increased rainbow trout fillets shelf life. | [134] |
| Oregano essential oil and/or nisin combined with MAP (75% CO2-25% N2) | Grass carp (Ctenopharyngodon idellus) | -Assay of TVB-N and monitoring of odor every 4 days during storage at 4 °C. -Shelf life of nisin-treated and oregano essential-oil treated grass carp fillets increased from 16 days to 20 days, while it increased to 28 days following a nisin and oregano essential oil treatment. -TVB-N values evolution during storage was not modified by treatments with nisin and/or oregano essential oil. Nevertheless, TVB-N values always remained below the 25 mg/100 g upper acceptable limit. | [17] |
| Addition of 0.1% (w/v) thyme essential oil prior to MAP (5% O2, 50% CO2, 45% N2) | Fresh Mediterranean swordfish fillets | -TVB-N and TMA assays and odor sensory evaluation for 16 days storage at 4 °C. -Extension of shelf life from approximately 13 days for MAP fillets to 15.5 days for fillets added with thyme essential oil before MAP. | [135] |
| Addition of 2% (v/w) or 4% (v/w) sage essential oil prior to VP | Fresh rainbow trout fillets | -TVB-N and odor sensory evaluation for 34 days storage at 4 °C. -Shelf life extension by 5 and 15 days of VP fillets added with 2% (v/w) and 4% (v/w) sage essential oil, respectively (compared to VP fillets without essential oil). | [136] |
| Grafting of in silico designed antimicrobial peptide 1018K6 on polypropylene (PP) packaging films | Salmon (Salmo salar) fillets | -TVB-N and TMA assays and odor sensory evaluation for 7 days storage at 4 °C. -TVB-N and TMA accumulation as well as odor alteration of salmon fillets were lower when salmon fillets were stored in films grafted with peptide 1018K6 than in control films. | [137] |
| Cellulose acetate films incorporated with bifidocin A | Mackerel (Scomberomorus niphonius) fillets | -TVB-N assay and odor sensory evaluation over 15 days storage at 4 °C. -Addition of bifidocin A in films significantly delayed TVB-N accumulation and odor alteration. | [138] |
| Bioprotective lactic acid bacteria | |||
| Dipping of gutted fishes in a 107 cfu/g Latilactobacillus sakei suspension with or without 0.1% (w/v) glucose for 10 min prior to VP | Fresh gutted sea bass (Dicentrarchus labrax) and sea bream (Sparus aurata) | -TVB-N assay and sensory evaluation after cooking over 14 days storage at 6 °C. -Dipping in Latilactobacillus sakei suspension with or without glucose resulted in an extension of shelf life of both gutted fishes from 12 to 14 days and fishes dipped in Latilactobacillus sakei suspension with glucose had a better sensory score. | [139] |
| Dipping of fresh plaice fillets in a 7 log cfu.mL−1 suspension of Bifidobacterium bifidium with 400 ppm thymol for 2 min prior to air, MAP (65% N2, 30% CO2, 5% O2) or VP and subsequent storage at 4 °C or 12 °C | Plaices (Pleuronectes platessa) fillets | -Sensory analysis including odor evaluation up to 17 days of storage. -Calculated sensory shelf life appeared longer than that estimated based on monitoring of total viable count of microorganisms. -Bifidobacterium bifidium and thymol performed an efficient synergy in controlling hygiene indicator microorganisms. | [140] |
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Brief to The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022; ISBN 978-92-5-136367-6. [Google Scholar]
- Clune, S.; Crossin, E.; Verghese, K. Systematic Review of Greenhouse Gas Emissions for Different Fresh Food Categories. J. Clean. Prod. 2017, 140, 766–783. [Google Scholar] [CrossRef]
- Hussain, M.A.; Sumon, T.A.; Mazumder, S.K.; Ali, M.M.; Jang, W.J.; Abualreesh, M.H.; Sharifuzzaman, S.M.; Brown, C.L.; Lee, H.-T.; Lee, E.-W.; et al. Essential Oils and Chitosan as Alternatives to Chemical Preservatives for Fish and Fisheries Products: A Review. Food Control 2021, 129, 108244. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Jeksrud, W.K.; Rosnes, J.T. A Review of Modified Atmosphere Packaging of Fish and Fishery Products—Significance of Microbial Growth, Activities and Safety. Int. J. Food Sci. Technol. 2002, 37, 107–127. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United. La Qualité et Son Évolution Dans le Poisson Frais; Food & Agriculture Org.: Rome, Italy, 1999; ISBN 978-92-5-203507-7. [Google Scholar]
- Getu, A.; Misganaw, K.; Bazezew, M. Post-Harvesting and Major Related Problems of Fish Production. Fish. Aquac. J. 2015, 6, 10–4172. [Google Scholar] [CrossRef]
- Amaral, R.A.; Pinto, C.A.; Lima, V.; Tavares, J.; Martins, A.P.; Fidalgo, L.G.; Silva, A.M.; Gil, M.M.; Teixeira, P.; Barbosa, J.; et al. Chemical-Based Methodologies to Extend the Shelf Life of Fresh Fish—A Review. Foods 2021, 10, 2300. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, R.; Cheng, L.; Zhu, W.; Li, S.; Chen, X. Mechanisms Underlying the Deterioration of Fish Quality after Harvest and Methods of Preservation. Food Control 2022, 135, 108805. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Kastrup Dalsgaard, T. Relationship between Lipid and Protein Oxidation in Fish. Aquac Res 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Rosnes, J.T.; Kleiberg, G.H. Effect of Modified Atmosphere Packaging and Superchilled Storage on the Microbial and Sensory Quality of Atlantic Salmon (Salmo salar) Fillets. J. Food Sci. 2003, 68, 1467–1472. [Google Scholar] [CrossRef]
- Erikson, U.; Misimi, E.; Gallart-Jornet, L. Superchilling of Rested Atlantic Salmon: Different Chilling Strategies and Effects on Fish and Fillet Quality. Food Chem. 2011, 127, 1427–1437. [Google Scholar] [CrossRef]
- Macé, S.; Cornet, J.; Chevalier, F.; Cardinal, M.; Pilet, M.-F.; Dousset, X.; Joffraud, J.-J. Characterisation of the Spoilage Microbiota in Raw Salmon (Salmo salar) Steaks Stored under Vacuum or Modified Atmosphere Packaging Combining Conventional Methods and PCR–TTGE. Food Microbiol. 2012, 30, 164–172. [Google Scholar] [CrossRef]
- Macé, S.; Joffraud, J.-J.; Cardinal, M.; Malcheva, M.; Cornet, J.; Lalanne, V.; Chevalier, F.; Sérot, T.; Pilet, M.-F.; Dousset, X. Evaluation of the Spoilage Potential of Bacteria Isolated from Spoiled Raw Salmon (Salmo salar) Fillets Stored under Modified Atmosphere Packaging. Int. J. Food Microbiol. 2013, 160, 227–238. [Google Scholar] [CrossRef]
- Lytou, A.E.; Panagou, E.Z.; Nychas, G.-J.E. Volatilomics for Food Quality and Authentication. Curr. Opin. Food Sci. 2019, 28, 88–95. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent Progress in Food Flavor Analysis Using Gas Chromatography–Ion Mobility Spectrometry (GC–IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef]
- Xu, C.-H.; Chen, G.-S.; Xiong, Z.-H.; Fan, Y.-X.; Wang, X.-C.; Liu, Y. Applications of Solid-Phase Microextraction in Food Analysis. TrAC Trends Anal. Chem. 2016, 80, 12–29. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Z.; Wang, Z.; Chen, L.; Ding, Y.; Lyu, F.; Zhou, X. Interactions of Fish Proteins and Volatile Flavor Compounds: Mechanisms, Affecting Factors and Analytical Methods. Mini-Rev. Org. Chem. 2021, 18, 259–269. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A Comprehensive Review on Freshness of Fish and Assessment: Analytical Methods and Recent Innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Starowicz, M. Analysis of Volatiles in Food Products. Separations 2021, 8, 157. [Google Scholar] [CrossRef]
- Tsironi, T.N.; Taoukis, P.S. Current Practice and Innovations in Fish Packaging. J. Aquat. Food Prod. Technol. 2018, 27, 1024–1047. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Smith, J.P.; Simpson, B.K.; Haard, N.F. Spoilage and Shelf-life Extension of Fresh Fish and Shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef]
- Sampels, S. The Effects of Storage and Preservation Technologies on the Quality of Fish Products: A Review. J. Food Process. Preserv. 2015, 39, 1206–1215. [Google Scholar] [CrossRef]
- Bouletis, A.D.; Arvanitoyannis, I.S.; Hadjichristodoulou, C. Application of Modified Atmosphere Packaging on Aquacultured Fish and Fish Products: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2263–2285. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.C.; Ma, L.M.; Doyle, M.P. Clostridium Botulinum Toxin Production in Relation to Spoilage of Atlantic Salmon (Salmo salar) Packaged in Films of Varying Oxygen Permeabilities and with Different Atmospheres. J. Food Prot. 2015, 78, 2006–2018. [Google Scholar] [CrossRef]
- Bugueño, G.; Escriche, I.; Martínez-Navarrete, N.; del Mar Camacho, M.; Chiralt, A. Influence of Storage Conditions on Some Physical and Chemical Properties of Smoked Salmon (Salmo salar) Processed by Vacuum Impregnation Techniques. Food Chem. 2003, 81, 85–90. [Google Scholar] [CrossRef]
- Chan, S.S.; Skare, M.; Rotabakk, B.T.; Sivertsvik, M.; Lerfall, J.; Løvdal, T.; Roth, B. Evaluation of Physical and Instrumentally Determined Sensory Attributes of Atlantic Salmon Portions Packaged in Modified Atmosphere and Vacuum Skin. LWT 2021, 146, 111404. [Google Scholar] [CrossRef]
- Fonseca, S.C.; Oliveira, F.A.R.; Brecht, J.K. Modelling Respiration Rate of Fresh Fruits and Vegetables for Modified Atmosphere Packages: A Review. J. Food Eng. 2002, 52, 99–119. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Stamatiou, A.P.; Drosinos, E.H.; Nychas, G.-J.E. Control of Spoilage Microorganisms in Minced Pork by a Self-Developed Modified Atmosphere Induced by the Respiratory Activity of Meat Microflora. Food Microbiol. 2008, 25, 915–921. [Google Scholar] [CrossRef]
- Đorđević, J.; Pavlicević, N.; Bošković, M.; Janjić, J.; Glišić, M.; Starcević, M.; Baltić, M.Ž. Effect of Vacuum and Modified Atmosphere Packaging on Microbiological Properties of Cold-Smoked Trout. In Proceedings of the 59th International Meat Industry Conference (MEATCON2017), Zlatibor, Serbia, 1–4 October 2017; Volume 85, p. 012084. [Google Scholar] [CrossRef]
- Chaix, E.; Guillaume, C.; Guillard, V. Oxygen and Carbon Dioxide Solubility and Diffusivity in Solid Food Matrices: A Review of Past and Current Knowledge. Compr. Rev. Food Sci. Food Saf. 2014, 13, 261–286. [Google Scholar] [CrossRef]
- Boz, Z.; Welt, B.; Brecht, J.; Pelletier, W.; McLamore, E.; Kiker, G.; Butler, J.E. Review of Challenges and Advances in Modification of Food Package Headspace Gases. J. Appl. Packag. Res. 2018, 10, 5. [Google Scholar]
- Kuuliala, L.; Sader, M.; Solimeo, A.; Pérez-Fernández, R.; Vanderroost, M.; De Baets, B.; De Meulenaer, B.; Ragaert, P.; Devlieghere, F. Spoilage Evaluation of Raw Atlantic Salmon (Salmo salar) Stored under Modified Atmospheres by Multivariate Statistics and Augmented Ordinal Regression. Int. J. Food Microbiol. 2019, 303, 46–57. [Google Scholar] [CrossRef]
- Fernández, K.; Aspe, E.; Roeckel, M. Shelf-Life Extension on Fillets of Atlantic Salmon (Salmo salar) Using Natural Additives, Superchilling and Modified Atmosphere Packaging. Food Control 2009, 20, 1036–1042. [Google Scholar] [CrossRef]
- Hansen, A.Å.; Mørkøre, T.; Rudi, K.; Rødbotten, M.; Bjerke, F.; Eie, T. Quality Changes of Prerigor Filleted Atlantic Salmon (Salmo salar L.) Packaged in Modified Atmosphere Using CO2 Emitter, Traditional MAP, and Vacuum. J. Food Sci. 2009, 74, M242–M249. [Google Scholar] [CrossRef]
- Sivertsvik, M.; Rosnes, J.T.; Bergslien, H. Modified Atmosphere Packaging. In Minimal Processing Technologies in the Food Industry; Woodhead Publishing Ltd: Cambridge, UK, 2002; pp. 61–86. ISBN 1-85573-547-4. [Google Scholar]
- Gavara, R.; Catalá Moragrega, R.; López Carballo, G.; Cerisuelo, J.P.; Domínguez, I.; Muriel Galet, V.; Hernández Muñoz, P. Use of EVOH for Food Packaging Applications; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-08-100596-5. [Google Scholar]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, e302029. [Google Scholar] [CrossRef]
- Kontominas, M.G.; Badeka, A.V.; Kosma, I.S.; Nathanailides, C.I. Recent Developments in Seafood Packaging Technologies. Foods 2021, 10, 940. [Google Scholar] [CrossRef]
- Mendes, R. Technological Processing of Fresh Gilthead Seabream (Sparus aurata): A Review of Quality Changes. Food Rev. Int. 2019, 35, 20–53. [Google Scholar] [CrossRef]
- Alfaro, B.; Hernández, I.; Baliño-Zuazo, L.; Barranco, A. Quality Changes of Atlantic Horse Mackerel Fillets (Trachurus trachurus) Packed in a Modified Atmosphere at Different Storage Temperatures: Effects of Packaging and Temperature on Mackerel. J. Sci. Food Agric. 2013, 93, 2179–2187. [Google Scholar] [CrossRef]
- Sykes, A.V.; Oliveira, A.R.; Domingues, P.M.; Cardoso, C.M.; Andrade, J.P.; Nunes, M.L. Assessment of European Cuttlefish (Sepia officinalis, L.) Nutritional Value and Freshness under Ice Storage Using a Developed Quality Index Method (QIM) and Biochemical Methods. LWT–Food Sci. Technol. 2009, 42, 424–432. [Google Scholar] [CrossRef]
- Kohn, H.I.; Liversedge, M. On a New Aerobic Metabolite Whose Production by Brain Is Inhibited by Apomorphine, Emetine, Ergotamine, Epinephrine, and Menadione. J. Pharmacol. Exp. Ther. 1944, 82, 292–300. [Google Scholar]
- Bernárdez, M.; Pastoriza, L.; Sampedro, G.; Herrera, J.J.R.; Cabo, M.L. Modified Method for the Analysis of Free Fatty Acids in Fish. J. Agric. Food Chem. 2005, 53, 1903–1906. [Google Scholar] [CrossRef]
- Ho, C.-T.; Hartman, T.G. (Eds.) Lipids in Food Flavors; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; Volume 558, ISBN 978-0-8412-2922-8. [Google Scholar]
- Berset, C.; Cuvelier, M.-E. Méthodes d’évaluation de Degré d’oxydation Des Lipides et de Mesure Du Pouvoir Antioxydant. Sci. Aliment. 1996, 16, 219–245. [Google Scholar]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of Antioxidant Activity with the Thiobarbituric Acid Reactive Substances Assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Alasalvar, C.; Taylor, K.D.A.; Shahidi, F. Comparison of Volatiles of Cultured and Wild Sea Bream (Sparus aurata) during Storage in Ice by Dynamic Headspace Analysis/Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 2616–2622. [Google Scholar] [CrossRef] [PubMed]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziaris, I.S. Microbiological Spoilage and Investigation of Volatile Profile during Storage of Sea Bream Fillets under Various Conditions. Int. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar] [CrossRef]
- Duflos, G.; Coin, V.M.; Cornu, M.; Antinelli, J.-F.; Malle, P. Determination of Volatile Compounds to Characterize Fish Spoilage Using Headspace/Mass Spectrometry and Solid-Phase Microextraction/Gas Chromatography/Mass Spectrometry. J. Sci. Food Agric. 2006, 86, 600–611. [Google Scholar] [CrossRef]
- Iglesias, J.; Medina, I. Solid-Phase Microextraction Method for the Determination of Volatile Compounds Associated to Oxidation of Fish Muscle. J. Chromatogr. A 2008, 1192, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Soncin, S.; Chiesa, L.M.; Panseri, S.; Biondi, P.; Cantoni, C. Determination of Volatile Compounds of Precooked Prawn (Penaeus vannamei) and Cultured Gilthead Sea Bream (Sparus aurata) Stored in Ice as Possible Spoilage Markers Using Solid Phase Microextraction and Gas Chromatography/Mass Spectrometry: Volatiles of Ice-Stored Prawn and Sea Bream as Spoilage Markers. J. Sci. Food Agric. 2009, 89, 436–442. [Google Scholar] [CrossRef]
- Jacobsen, C.; Hartvigsen, K.; Lund, P.; Adler-Nissen, J.; Hølmer, G.; Meyer, A.S. Oxidation in Fish-Oil-Enriched Mayonnaise. Eur. Food Res. Technol. 2000, 210, 242–257. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological Spoilage of Fish and Fish Products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Broekaert, K.; Heyndrickx, M.; Herman, L.; Devlieghere, F.; Vlaemynck, G. Seafood Quality Analysis: Molecular Identification of Dominant Microbiota after Ice Storage on Several General Growth Media. Food Microbiol. 2011, 28, 1162–1169. [Google Scholar] [CrossRef]
- Stamatis, N.; Arkoudelos, J.S. Effect of Modified Atmosphere and Vacuum Packaging on Microbial, Chemical and Sensory Quality Indicators of Fresh, Filleted Sardina Pilchardus at 3 °C. J. Sci. Food Agric. 2007, 87, 1164–1171. [Google Scholar] [CrossRef]
- Skjerdal, O.T.; Lorentzen, G.; Tryland, I.; Berg, J.D. New Method for Rapid and Sensitive Quantification of Sulphide-Producing Bacteria in Fish from Arctic and Temperate Waters. Int. J. Food Microbiol. 2004, 93, 325–333. [Google Scholar] [CrossRef]
- López-Caballero, M.E.; Sánchez-Fernández, J.A.; Moral, A. Growth and Metabolic Activity of Shewanella putrefaciens Maintained under Different CO2 and O2 Concentrations. Int. J. Food Microbiol. 2001, 64, 277–287. [Google Scholar] [CrossRef]
- Dalgaard, P. Qualitative and Quantitative Characterization of Spoilage Bacteria from Packed Fish. Int. J. Food Microbiol. 1995, 26, 319–333. [Google Scholar] [CrossRef]
- Yi, Z.; Xie, J. Comparative Proteomics Reveals the Spoilage-Related Factors of Shewanella putrefaciens under Refrigerated Condition. Front. Microbiol. 2021, 12, 740482. [Google Scholar] [CrossRef]
- Zhuang, S.; Tian, L.; Liu, Y.; Wang, L.; Hong, H.; Luo, Y. Amino Acid Degradation and Related Quality Changes Caused by Common Spoilage Bacteria in Chill-Stored Grass Carp (Ctenopharyngodon idella). Food Chem. 2023, 399, 133989. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood Spoilage Microbiota and Associated Volatile Organic Compounds at Different Storage Temperatures and Packaging Conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Malle, P.; Poumeyrol, M. A New Chemical Criterion for the Quality Control of Fish: Trimethylamine/Total Volatile Basic Nitrogen (%). J. Food Prot. 1989, 52, 419–423. [Google Scholar] [CrossRef]
- Taliadourou, D.; Papadopoulos, V.; Domvridou, E.; Savvaidis, I.N.; Kontominas, M.G. Microbiological, Chemical and Sensory Changes of Whole and Filleted Mediterranean Aquacultured Sea Bass (Dicentrarchus labrax) Stored in Ice. J. Sci. Food Agric. 2003, 83, 1373–1379. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Srivastav, P.P.; Pathak, S.S. Kinetics of Total Volatile Basic Nitrogen and Trimethylamine Formation in Stored Rohu (Labeo rohita) Fish. J. Aquat. Food Prod. Technol. 2019, 28, 452–464. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ozyurt, G.; Ozogul, F.; Kuley, E.; Polat, A. Freshness Assessment of European Eel () by Sensory, Chemical and Microbiological Methods. Food Chem. 2005, 92, 745–751. [Google Scholar] [CrossRef]
- Castro, P.; Padrón, J.C.P.; Cansino, M.J.C.; Velázquez, E.S.; Larriva, R.M.D. Total Volatile Base Nitrogen and Its Use to Assess Freshness in European Sea Bass Stored in Ice. Food Control 2006, 17, 245–248. [Google Scholar] [CrossRef]
- Guo, H.; Feng, T.; Qi, W.; Kong, Q.; Yue, L.; Wang, H. Effects of Electron-beam Irradiation on Volatile Flavor Compounds of Salmon Fillets by the Molecular Sensory Science Technique. J. Food Sci. 2021, 86, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L.; Zhang, Y.; Song, H.; Raza, A.; Pan, W.; Gong, L.; Jiang, C. Comparison of Different Volatile Extraction Methods for the Identification of Fishy Off-Odor in Fish By-Products. Molecules 2022, 27, 6177. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, J.; Zhu, Y.; Wang, X.; Shi, W. Volatile Components Present in Different Parts of Grass Carp. J Food Biochem 2018, 42, e12668. [Google Scholar] [CrossRef]
- Kritikos, A.; Aska, I.; Ekonomou, S.; Mallouchos, A.; Parlapani, F.F.; Haroutounian, S.A.; Boziaris, I.S. Volatilome of Chill-Stored European Seabass (Dicentrarchus labrax) Fillets and Atlantic Salmon (Salmo salar) Slices under Modified Atmosphere Packaging. Molecules 2020, 25, 1981. [Google Scholar] [CrossRef]
- Parlapani, F.F.; Verdos, G.I.; Haroutounian, S.A.; Boziaris, I.S. The Dynamics of Pseudomonas and Volatilome during the Spoilage of Gutted Sea Bream Stored at 2 °C. Food Control 2015, 55, 257–265. [Google Scholar] [CrossRef]
- Mikš-Krajnik, M.; Yoon, Y.-J.; Ukuku, D.O.; Yuk, H.-G. Volatile Chemical Spoilage Indexes of Raw Atlantic Salmon (Salmo salar) Stored under Aerobic Condition in Relation to Microbiological and Sensory Shelf Lives. Food Microbiol. 2016, 53, 182–191. [Google Scholar] [CrossRef]
- Wierda, R.L.; Fletcher, G.; Xu, L.; Dufour, J.-P. Analysis of Volatile Compounds as Spoilage Indicators in Fresh King Salmon (Oncorhynchus tshawytscha) During Storage Using SPME−GC−MS. J. Agric. Food Chem. 2006, 54, 8480–8490. [Google Scholar] [CrossRef]
- Aro, T.; Tahvonen, R.; Koskinen, L.; Kallio, H. Volatile Compounds of Baltic Herring Analysed by Dynamic Headspace Sampling–Gas Chromatography–Mass Spectrometry. Eur Food Res Technol 2003, 216, 483–488. [Google Scholar] [CrossRef]
- Olafsdottir, G.; Jonsdottir, R.; Lauzon, H.L.; Luten, J.; Kristbergsson, K. Characterization of Volatile Compounds in Chilled Cod (Gadus morhua) Fillets by Gas Chromatography and Detection of Quality Indicators by an Electronic Nose. J. Agric. Food Chem. 2005, 53, 10140–10147. [Google Scholar] [CrossRef]
- El Barbri, N.; Amari, A.; Vinaixa, M.; Bouchikhi, B.; Correig, X.; Llobet, E. Building of a Metal Oxide Gas Sensor-Based Electronic Nose to Assess the Freshness of Sardines under Cold Storage. Sens. Actuators B Chem. 2007, 128, 235–244. [Google Scholar] [CrossRef]
- Vakinti, M.; Mela, S.-M.; Fernández, E.; Psillakis, E. Room Temperature and Sensitive Determination of Haloanisoles in Wine Using Vacuum-Assisted Headspace Solid-Phase Microextraction. J. Chromatogr. A 2019, 1602, 142–149. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Gaca, A.; Marcinkowska, M. Use of Sorbent-Based Vacuum Extraction for Determination of Volatile Phenols in Beer. Food Anal. Methods 2018, 11, 3089–3094. [Google Scholar] [CrossRef]
- Jeleń, H.H.; Marcinkowska, M.A.; Marek, M. Determination of Volatile Terpenes in Coriander Cold Pressed Oil by Vacuum Assisted Sorbent Extraction (VASE). Molecules 2021, 26, 884. [Google Scholar] [CrossRef]
- Epping, R.; Koch, M. On-Site Detection of Volatile Organic Compounds (VOCs). Molecules 2023, 28, 1598. [Google Scholar] [CrossRef]
- Dalsvåg, H.; Cropotova, J.; Jambrak, A.R.; Janči, T.; Španěl, P.; Dryahina, K.; Smith, D.; Rustad, T. Mass Spectrometric Quantification of Volatile Compounds Released by Fresh Atlantic Salmon Stored at 4 °C under Modified Atmosphere Packaging and Vacuum Packaging for up to 16 Days. ACS Food Sci. Technol. 2021, 2, 400–414. [Google Scholar] [CrossRef]
- Erickson, M.C.; Ma, L.M.; Doyle, M.P. Development of Models To Relate Microbiological and Headspace Volatile Parameters in Stored Atlantic Salmon to Acceptance and Willingness To Prepare the Product by Senior Consumers. J. Food Prot. 2015, 78, 2156–2169. [Google Scholar] [CrossRef]
- Kuuliala, L.; Abatih, E.; Ioannidis, A.-G.; Vanderroost, M.; De Meulenaer, B.; Ragaert, P.; Devlieghere, F. Multivariate Statistical Analysis for the Identification of Potential Seafood Spoilage Indicators. Food Control 2018, 84, 49–60. [Google Scholar] [CrossRef]
- Emborg, J.; Laursen, B.G.; Rathjen, T.; Dalgaard, P. Microbial Spoilage and Formation of Biogenic Amines in Fresh and Thawed Modified Atmosphere-packed Salmon (Salmo salar) at 2 °C. J. Appl. Microbiol. 2002, 92, 790–799. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kondo, R.; Itonaga, S.; Domen, A.; Mabuchi, R. Screening Plant Extracts for Quality Preservation of Dark Muscle Fish Flesh: A Simple Method. J. Food Process. Preserv. 2020, 44, e14315. [Google Scholar] [CrossRef]
- Noseda, B.; Islam, M.T.; Eriksson, M.; Heyndrickx, M.; De Reu, K.; Van Langenhove, H.; Devlieghere, F. Microbiological Spoilage of Vacuum and Modified Atmosphere Packaged Vietnamese Pangasius Hypophthalmus Fillets. Food Microbiol. 2012, 30, 408–419. [Google Scholar] [CrossRef]
- Du, W.-X.; Lin, C.-M.; Huang, T.; Kim, J.; Marshall, M.; Wei, C.-I. Potential Application of the Electronic Nose for Quality Assessment of Salmon Fillets Under Various Storage Conditions. J Food Sci. 2002, 67, 307–313. [Google Scholar] [CrossRef]
- O’Connell, M.; Valdora, G.; Peltzer, G.; Martín Negri, R. A Practical Approach for Fish Freshness Determinations Using a Portable Electronic Nose. Sens. Actuators B Chem. 2001, 80, 149–154. [Google Scholar] [CrossRef]
- Li, X. Electronic Nose to Monitor the Freshness of Red Fish (Sebastes marinus) Stored in Ice and Modified Atmosphere Packaging (MAP). In UNU-Fisheries Training Programme, Final Project; UNU: Tokyo, Japan, 2000. [Google Scholar]
- Hindle, F.; Kuuliala, L.; Mouelhi, M.; Cuisset, A.; Bray, C.; Vanwolleghem, M.; Devlieghere, F.; Mouret, G.; Bocquet, R. Monitoring of Food Spoilage by High Resolution THz Analysis. Analyst 2018, 143, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.D.; Olafsdottir, G.; Einarsson, S.; Martinelli, E.; Paolesse, R.; D’Amico, A. Comparison and Integration of Different Electronic Noses for Freshness Evaluation of Cod-Fish Fillets. Sens. Actuators B Chem. 2001, 77, 572–578. [Google Scholar] [CrossRef]
- Barbri, N.E.; Mirhisse, J.; Ionescu, R.; Bari, N.E.; Correig, X.; Bouchikhi, B.; Llobet, E. An Electronic Nose System Based on a Micro-Machined Gas Sensor Array to Assess the Freshness of Sardines. Sens. Actuators B Chem. 2009, 141, 538–543. [Google Scholar] [CrossRef]
- El Barbri, N.; Llobet, E.; El Bari, N.; Correig, X.; Bouchikhi, B. Application of a Portable Electronic Nose System to Assess the Freshness of Moroccan Sardines. Mater. Sci. Eng. C 2008, 28, 666–670. [Google Scholar] [CrossRef]
- Claus, P.; Cattenoz, T.; Landaud, S.; Chaillou, S.; Peron, A.-C.; Coeuret, G.; Slimani, S.; Livache, T.; Demarigny, Y.; Picque, D. Discrimination of Spoiled Beef and Salmon Stored under Different Atmospheres by an Optoelectronic Nose. Comparison with GC-MS Measurements. Future Foods 2022, 5, 100106. [Google Scholar] [CrossRef]
- Huss, H.H. La Qualité et Son Évolution Dans Le Poisson Frais. In Organisation des Nations Unies Pour l’Alimentation et l’Agriculture; FAO: Rome, Italy, 1999. [Google Scholar]
- Narasimha Rao, D.; Sachindra, N.M. Modified Atmosphere and Vacuum Packaging of Meat and Poultry Products. Food Rev. Int. 2002, 18, 263–293. [Google Scholar] [CrossRef]
- Randell, K.; Hattula, T.; Skyttä, E.; Sivertsvik, M.; Bergslien, H.; Ahvenainen, R. Quality of Filleted Salmon in Various Retail Packages. J. Food Qual. 1999, 22, 483–497. [Google Scholar] [CrossRef]
- Smith, J.P.; Ramaswamy, H.S.; Simpson, B.K. Developments in Food Packaging Technology. Part II. Storage Aspects. Trends Food Sci. Technol. 1990, 1, 111–118. [Google Scholar] [CrossRef]
- Al-Nehlawi, A.; Saldo, J.; Vega, L.F.; Guri, S. Effect of High Carbon Dioxide Atmosphere Packaging and Soluble Gas Stabilization Pre-Treatment on the Shelf-Life and Quality of Chicken Drumsticks. Meat Sci. 2013, 94, 1–8. [Google Scholar] [CrossRef]
- Gokoglu, N. Innovations in Seafood Packaging Technologies: A Review. Food Rev. Int. 2020, 36, 340–366. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, L.; Geeraerd, A.H.; Mast, J.; Briers, Y.; Elst, K.; Van Ginneken, L.; Van Impe, J.F.; Devlieghere, F. Membrane Permeabilization and Cellular Death of Escherichia coli, Listeria monocytogenes and Saccharomyces cerevisiae as Induced by High Pressure Carbon Dioxide Treatment. Food Microbiol. 2010, 27, 541–549. [Google Scholar] [CrossRef]
- Yu, T.; Chen, Y. Effects of Elevated Carbon Dioxide on Environmental Microbes and Its Mechanisms: A Review. Sci. Total Environ. 2019, 655, 865–879. [Google Scholar] [CrossRef]
- Bertoloni, G.; Bertucco, A.; De Cian, V.; Parton, T. A Study on the Inactivation of Micro-Organisms and Enzymes by High Pressure CO2. Biotechnol. Bioeng. 2006, 95, 155–160. [Google Scholar] [CrossRef]
- Oluwole, A.O. Modified Atmosphere Packaging and Quality of Fresh Cape Hake (Merluccius capensis) Fish Fillets. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Morin, R.; Samuel, A.; Boyer, J.; Carbonneau, M.E.; Coulombe, F.; Coulombe, N.; Desbiens, M.; Leclerc, L.; Martin, C.; Morin, R. Transformation. In Elevage des Salmonidés; Québec: Quebec City, QC, Canada, 2002. [Google Scholar]
- Josephson, D.B.; Lindsay, R.C.; Olafsdottir, G. Measurement of Volatile Aroma Constituents as a Means for Following Sensory Deterioration of Fresh Fish and Fishery Products. In Proceedings of the International Symposium on Seafood Quality Determination, Coordinated by the University of Alaska Sea Grant College Program, Anchorage, AK; Kramer, D.E., Liston, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Wu, T.; Wang, M.; Wang, P.; Tian, H.; Zhan, P. Advances in the Formation and Control Methods of Undesirable Flavors in Fish. Foods 2022, 11, 2504. [Google Scholar] [CrossRef]
- Leistner, L. Hurdle Technology Applied to Meat Products of the Shelf Stable Product and Intermediate Moisture Food Types. In Properties of Water in Foods: In Relation to Quality and Stability; Simatos, D., Multon, J.L., Eds.; Springer: Dordrecht, The Netherlands, 1985; pp. 309–329. ISBN 978-94-009-5103-7. [Google Scholar]
- Leistner, L. Basic Aspects of Food Preservation by Hurdle Technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- Rosario, D.K.A.; Rodrigues, B.L.; Bernardes, P.C.; Conte-Junior, C.A. Principles and Applications of Non-Thermal Technologies and Alternative Chemical Compounds in Meat and Fish. Crit. Rev. Food Sci. Nutr. 2021, 61, 1163–1183. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Nonthermal Processes for Shelf-Life Extension of Seafoods: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 892–904. [Google Scholar] [CrossRef]
- Günlü, A.; Sipahioğlu, S.; Alpas, H. The Effect of Chitosan-Based Edible Film and High Hydrostatic Pressure Process on the Microbiological and Chemical Quality of Rainbow Trout (Oncorhynchus mykiss Walbaum) Fillets during Cold Storage (4±1°C). High Press. Res. 2014, 34, 110–121. [Google Scholar] [CrossRef]
- Reale, A.; Sorrentino, E.; Iaffaldano, N.; Rosato, M.P.; Ragni, P.; Coppola, R.; Capitani, D.; Sobolev, A.P.; Tremonte, P.; Succi, M.; et al. Effects of Ionizing Radiation and Modified Atmosphere Packaging on the Shelf Life of Aqua-Cultured Sea Bass (Dicentrarchus labrax). World J. Microbiol. Biotechnol. 2008, 24, 2757–2765. [Google Scholar] [CrossRef]
- Lázaro, C.A.; Monteiro, M.L.G.; Conte-Junior, C.A. Combined Effect of Modified Atmosphere Packaging and UV-C Radiation on Pathogens Reduction, Biogenic Amines, and Shelf Life of Refrigerated Tilapia (Oreochromis niloticus) Fillets. Molecules 2020, 25, 3222. [Google Scholar] [CrossRef] [PubMed]
- Tagrida, M.; Benjakul, S.; Zhang, B. Use of Betel Leaf (Piper betle L.) Ethanolic Extract in Combination with Modified Atmospheric Packaging and Nonthermal Plasma for Shelf-Life Extension of Nile Tilapia (Oreochromis niloticus) Fillets. J. Food Sci. 2021, 86, 5226–5239. [Google Scholar] [CrossRef] [PubMed]
- Olatunde, O.O.; Benjakul, S. Natural Preservatives for Extending the Shelf-Life of Seafood: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Bahmid, N.A.; Heising, J.; Fogliano, V.; Dekker, M. Packaging Design Using Mustard Seeds as a Natural Antimicrobial: A Study on Inhibition of Pseudomonas Fragi in Liquid Medium. Foods 2020, 9, 789. [Google Scholar] [CrossRef]
- Houicher, A.; Bensid, A.; Regenstein, J.M.; Özogul, F. Control of Biogenic Amine Production and Bacterial Growth in Fish and Seafood Products Using Phytochemicals as Biopreservatives: A Review. Food Biosci. 2021, 39, 100807. [Google Scholar] [CrossRef]
- Rathod, N.B.; Nirmal, N.P.; Pagarkar, A.; Özogul, F.; Rocha, J.M. Antimicrobial Impacts of Microbial Metabolites on the Preservation of Fish and Fishery Products: A Review with Current Knowledge. Microorganisms 2022, 10, 773. [Google Scholar] [CrossRef]
- Khodanazary, A. Quality Characteristics of Refrigerated Mackerel Scomberomorus Commerson Using Gelatin-Polycaprolactone Composite Film Incorporated with Lysozyme and Pomegranate Peel Extract. Int. J. Food Prop. 2019, 22, 2057–2071. [Google Scholar] [CrossRef]
- Bechaux, J.; Gatellier, P.; Le Page, J.-F.; Drillet, Y.; Sante-Lhoutellier, V. A Comprehensive Review of Bioactive Peptides Obtained from Animal Byproducts and Their Applications. Food Funct. 2019, 10, 6244–6266. [Google Scholar] [CrossRef]
- Speranza, B.; Bevilacqua, A.; Conte, A.; Del Nobile, M.A.; Sinigaglia, M.; Corbo, M.R. Use of Desirability Approach to Predict the Inhibition of Pseudomonas fluorescens, Shewanella putrefaciens and Photobacterium phosphoreum in Fish Fillets Through Natural Antimicrobials and Modified Atmosphere Packaging. Food Bioprocess Technol. 2013, 6, 2319–2330. [Google Scholar] [CrossRef]
- Calo-Mata, P.; Arlindo, S.; Boehme, K.; de Miguel, T.; Pascoal, A.; Barros-Velazquez, J. Current Applications and Future Trends of Lactic Acid Bacteria and Their Bacteriocins for the Biopreservation of Aquatic Food Products. Food Bioprocess. Technol. 2008, 1, 43–63. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Jaffrès, E.; Dousset, X.; Pillot, G.; Choiset, Y.; Haertlé, T.; H-Kittikun, A.; Chobert, J.-M. Application of a Nisin Z-Producing Lactococcus lactis subsp. lactis KT2W2L Isolated from Brackish Water for Biopreservation in Cooked, Peeled and Ionized Tropical Shrimps during Storage at 8 °C under Modified Atmosphere Packaging. Eur. Food Res. Technol. 2015, 240, 1259–1269. [Google Scholar] [CrossRef]
- Saraoui, T.; Cornet, J.; Guillouet, E.; Pilet, M.F.; Chevalier, F.; Joffraud, J.-J.; Leroi, F. Improving Simultaneously the Quality and Safety of Cooked and Peeled Shrimp Using a Cocktail of Bioprotective Lactic Acid Bacteria. Int. J. Food Microbiol. 2017, 241, 69–77. [Google Scholar] [CrossRef]
- Danza, A.; Lucera, A.; Lavermicocca, P.; Lonigro, S.L.; Bavaro, A.R.; Mentana, A.; Centonze, D.; Conte, A.; Del Nobile, M.A. Tuna Burgers Preserved by the Selected Lactobacillus paracasei IMPC 4.1 Strain. Food Bioprocess. Technol. 2018, 11, 1651–1661. [Google Scholar] [CrossRef]
- Ramaroson, M.; Guillou, S.; Rossero, A.; Rezé, S.; Anthoine, V.; Moriceau, N.; Martin, J.-L.; Duranton, F.; Zagorec, M. Selection Procedure of Bioprotective Cultures for Their Combined Use with High Pressure Processing to Control Spore-Forming Bacteria in Cooked Ham. Int. J. Food Microbiol. 2018, 276, 28–38. [Google Scholar] [CrossRef]
- Lee, H.; Shahbaz, H.M.; Yang, J.; Jo, M.H.; Kim, J.U.; Yoo, S.; Kim, S.H.; Lee, D.-U.; Park, J. Effect of High Pressure Processing Combined with Lactic Acid Bacteria on the Microbial Counts and Physicochemical Properties of Uncooked Beef Patties during Refrigerated Storage. J. Food Process. Preserv. 2021, 45, e15345. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of Phenolic Compounds on the Growth of Selected Probiotic and Pathogenic Bacteria. Lett. Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Sireswar, S.; Dey, G.; Sreesoundarya, T.K.; Sarkar, D. Design of Probiotic-Fortified Food Matrices Influence Their Antipathogenic Potential. Food Biosci. 2017, 20, 28–35. [Google Scholar] [CrossRef]
- Bono, G.; Badalucco, C. Combining Ozone and Modified Atmosphere Packaging (MAP) to Maximize Shelf-Life and Quality of Striped Red Mullet (Mullus surmuletus). LWT Food Sci. Technol. 2012, 47, 500–504. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Vongkamjan, K. Cold Plasma Combined with Liposomal Ethanolic Coconut Husk Extract: A Potential Hurdle Technology for Shelf-Life Extension of Asian Sea Bass Slices Packaged under Modified Atmosphere. Innov. Food Sci. Emerg. Technol. 2020, 65, 102448. [Google Scholar] [CrossRef]
- Haute, S.V.; Raes, K.; Devlieghere, F.; Sampers, I. Combined Use of Cinnamon Essential Oil and MAP/Vacuum Packaging to Increase the Microbial and Sensorial Shelf Life of Lean Pork and Salmon. Food Packag. Shelf Life 2017, 12, 51–58. [Google Scholar] [CrossRef]
- Khadem, P.; Motalebi, A.A.; Rokni, N.; Razavilar, V. Effects of Capparis Spinosa Root Extract and Modified Atmosphere Packaging on the Shelf Life of Rainbow Trout (Oncorhynchus mykiss) Fillets by Measuring of Antioxidant and Antimicrobial Parameters. Iran. J. Fish. Sci. 2020, 19, 272–285. [Google Scholar] [CrossRef]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of Thyme Essential Oil and Packaging Treatments on Fresh Mediterranean Swordfish Fillets during Storage at 4°C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Çoban, O.E.; Patir, B.; Özpolat, E.; Kuzgun, N.K. Improving the Quality of Fresh Rainbow Trout by Sage Essential Oil and Packaging Treatments. J. Food Saf. 2016, 36, 299–307. [Google Scholar] [CrossRef]
- Ambrosio, R.L.; Gogliettino, M.; Agrillo, B.; Proroga, Y.T.R.; Balestrieri, M.; Gratino, L.; Cristiano, D.; Palmieri, G.; Anastasio, A. An Active Peptide-Based Packaging System to Improve the Freshness and Safety of Fish Products: A Case Study. Foods 2022, 11, 338. [Google Scholar] [CrossRef]
- Liu, G.; Li, X.; Hao, X.; Wang, C.; Sun, B. Effects of Bioactive Packaging Films Incorporated with Bifidocin A on Microbial Reduction and Quality Parameters of Chill-Stored Spanish Mackerel (Scomberomorus niphonius) Fillets. J. Food Qual. 2019, 2019, 7108382. [Google Scholar] [CrossRef]
- Iacumin, L.; Jayasinghe, A.S.; Pellegrini, M.; Comi, G. Evaluation of Different Techniques, Including Modified Atmosphere, under Vacuum Packaging, Washing, and Latilactobacillus sakei as a Bioprotective Agent, to Increase the Shelf-Life of Fresh Gutted Sea Bass (Dicentrarchus labrax) and Sea Bream (Sparus aurata) Stored at 6 ± 2 °C. Biology 2022, 11, 217. [Google Scholar] [CrossRef]
- Altieri, C.; Speranza, B.; Del Nobile, M.A.; Sinigaglia, M. Suitability of Bifidobacteria and Thymol as Biopreservatives in Extending the Shelf Life of Fresh Packed Plaice Fillets. J. Appl. Microbiol. 2005, 99, 1294–1302. [Google Scholar] [CrossRef]

| Vacuum Packaging (VP) | Modified Atmosphere Packaging (MAP) | ||
|---|---|---|---|
| Flexible top film | polymer and main layer thicknesses | OPA */PE ** (15 µm/50 µm) | PE/PA ***/EVOH ****/PA/PE (global film thickness 24 µm) |
| WVTR | less than 11 g/m2/day for 90% RH at 38 °C, ASTM E96 | 18 g/m2/day for 100% RH at 38 °C, ASTM F1249 | |
| OTR | less than 40 cm3/(m2.day.bar) for 0% RH at 23 °C, ASTM D3985 | 24 cm3/(m2.day.bar) (permeance) for 0% RH at 23 °C, ASTM D3985 | |
| Semi-rigid tray | polymer and main layer thicknesses | PA/PE (80 µm/30 µm) | PET *****/PE (300 µm/30 µm) |
| WVTR | less than 5 g/m2/day for 90% RH at 38 °C, ASTM F1249 | data unavailable | |
| OTR | less than 50–55 cm3/m2/day for 75% RH at 23 °C, ASTM D3985 | less than 5 cm3/tray.day (test conditions unavailable) | |
| Fish Species | TVB-N Limit (mg/100 g) |
|---|---|
| Sebastes spp. (Helicolenus dactylopterus, Sebastichthys capensis) | 25 |
| Pleuronectidae family (except for halibut: Hippoglossus spp.) | 30 |
| Salmo salar | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, D.; Joly, C.; Dupas-Farrugia, C.; Adt, I.; Oulahal, N.; Degraeve, P. Volatilome Analysis and Evolution in the Headspace of Packed Refrigerated Fish. Foods 2023, 12, 2657. https://doi.org/10.3390/foods12142657
Martin D, Joly C, Dupas-Farrugia C, Adt I, Oulahal N, Degraeve P. Volatilome Analysis and Evolution in the Headspace of Packed Refrigerated Fish. Foods. 2023; 12(14):2657. https://doi.org/10.3390/foods12142657
Chicago/Turabian StyleMartin, Doriane, Catherine Joly, Coralie Dupas-Farrugia, Isabelle Adt, Nadia Oulahal, and Pascal Degraeve. 2023. "Volatilome Analysis and Evolution in the Headspace of Packed Refrigerated Fish" Foods 12, no. 14: 2657. https://doi.org/10.3390/foods12142657
APA StyleMartin, D., Joly, C., Dupas-Farrugia, C., Adt, I., Oulahal, N., & Degraeve, P. (2023). Volatilome Analysis and Evolution in the Headspace of Packed Refrigerated Fish. Foods, 12(14), 2657. https://doi.org/10.3390/foods12142657









