Effect of Peanut Protein Treated with Alkaline Protease and Flavorzyme on BALB/c Mice
Abstract
1. Introduction
2. Materials Section
Materials
3. Methods
3.1. Effect of Fla and Alk Treatment on the Protein Content of Peanut Protein
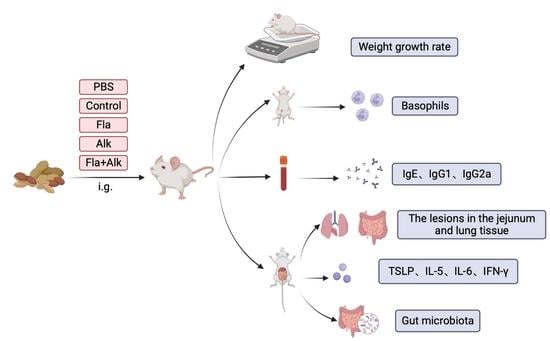
3.2. Effects of Fla and Alk-Treated Peanut Protein in BALB/c Mice
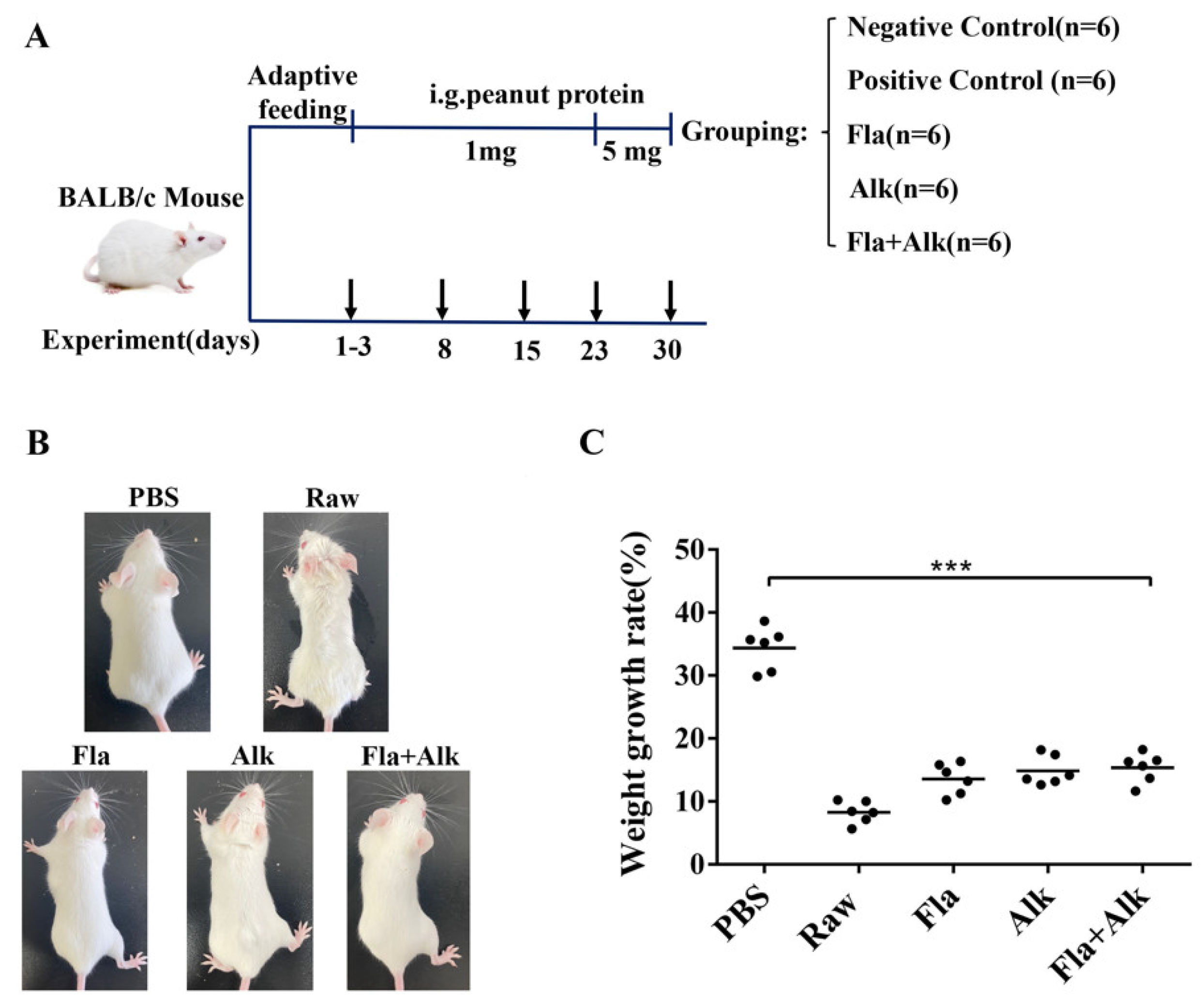
3.2.1. Protocol for Peanut Protein Sensitization of BALB/c Mice
3.2.2. Effect of Fla and Alk-Treated Peanut Protein on Morphology and Weight Growth Rate in Mice
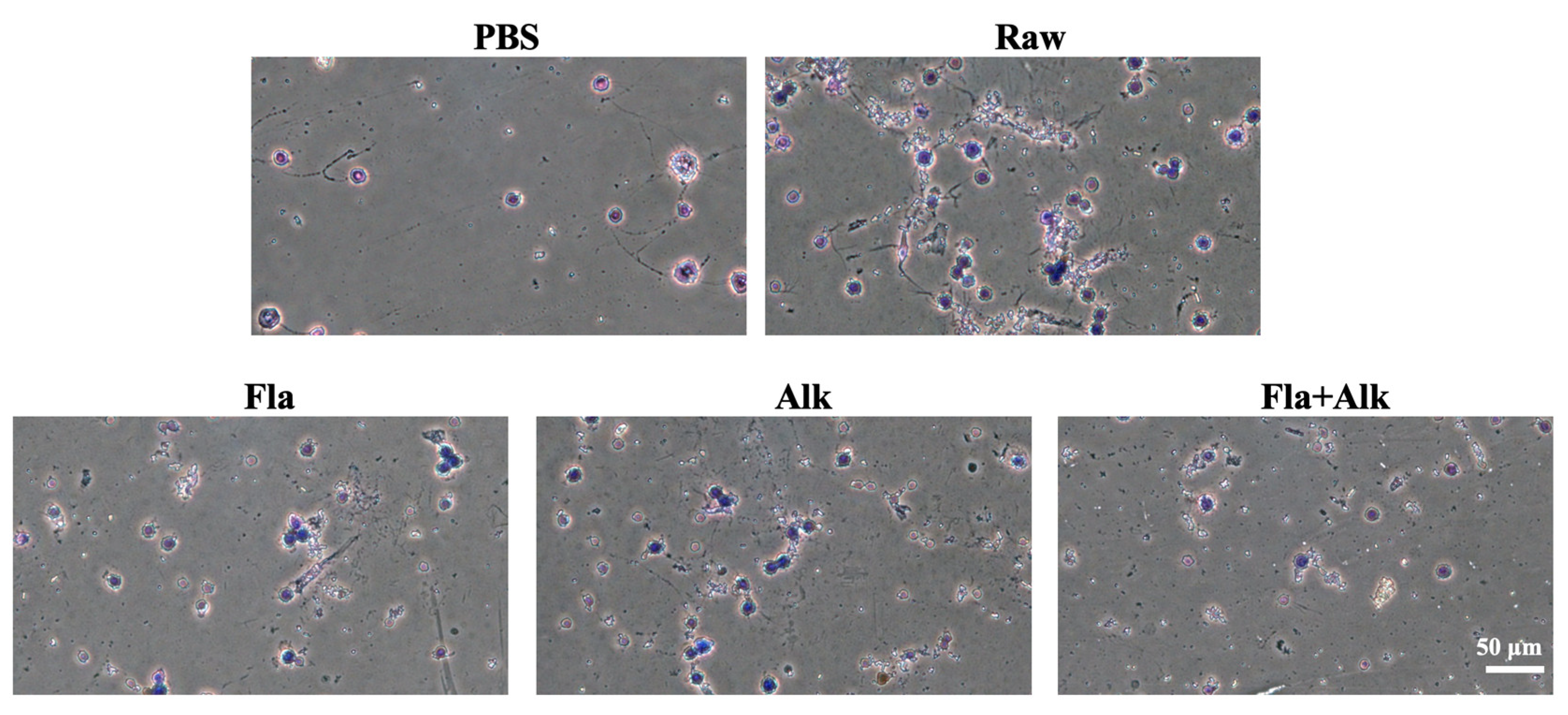
3.2.3. Effects of Fla and Alk-Treated Peanut Protein on Mice Basophils
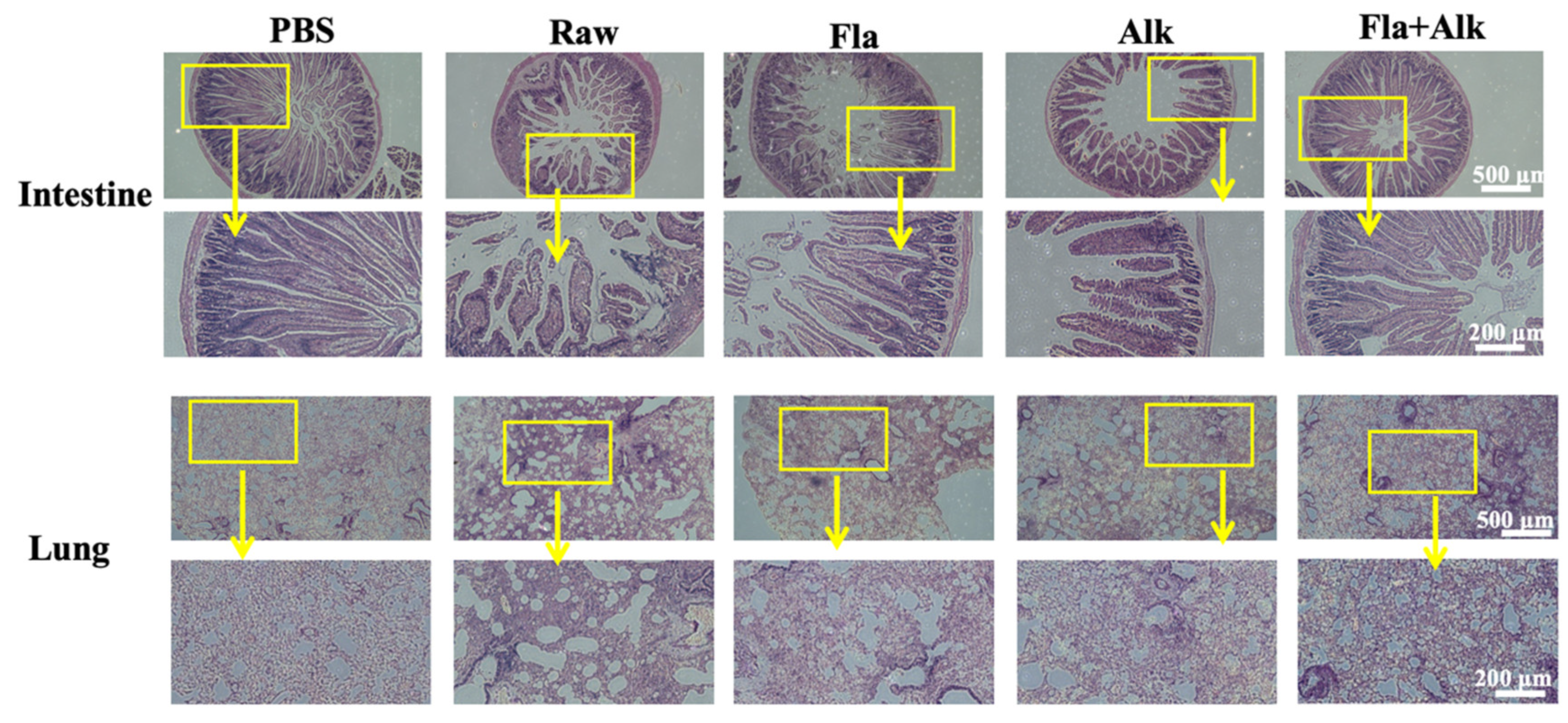
3.2.4. Effects of Fla and Alk-Treated Peanut Protein on Intestine and Lung
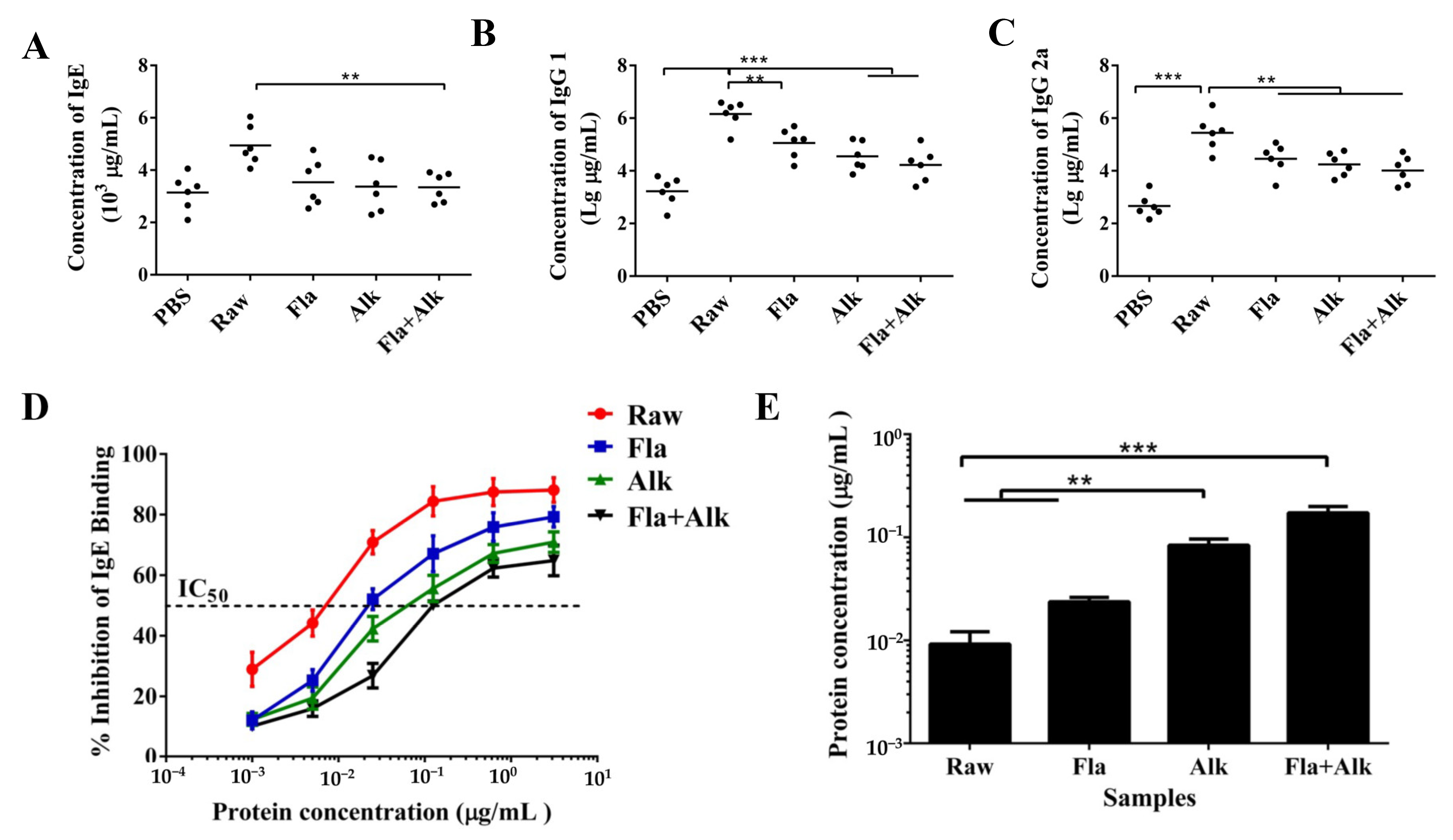
3.2.5. Effect of Fla and Alk-Treated Peanut Protein on Antibody Secretion
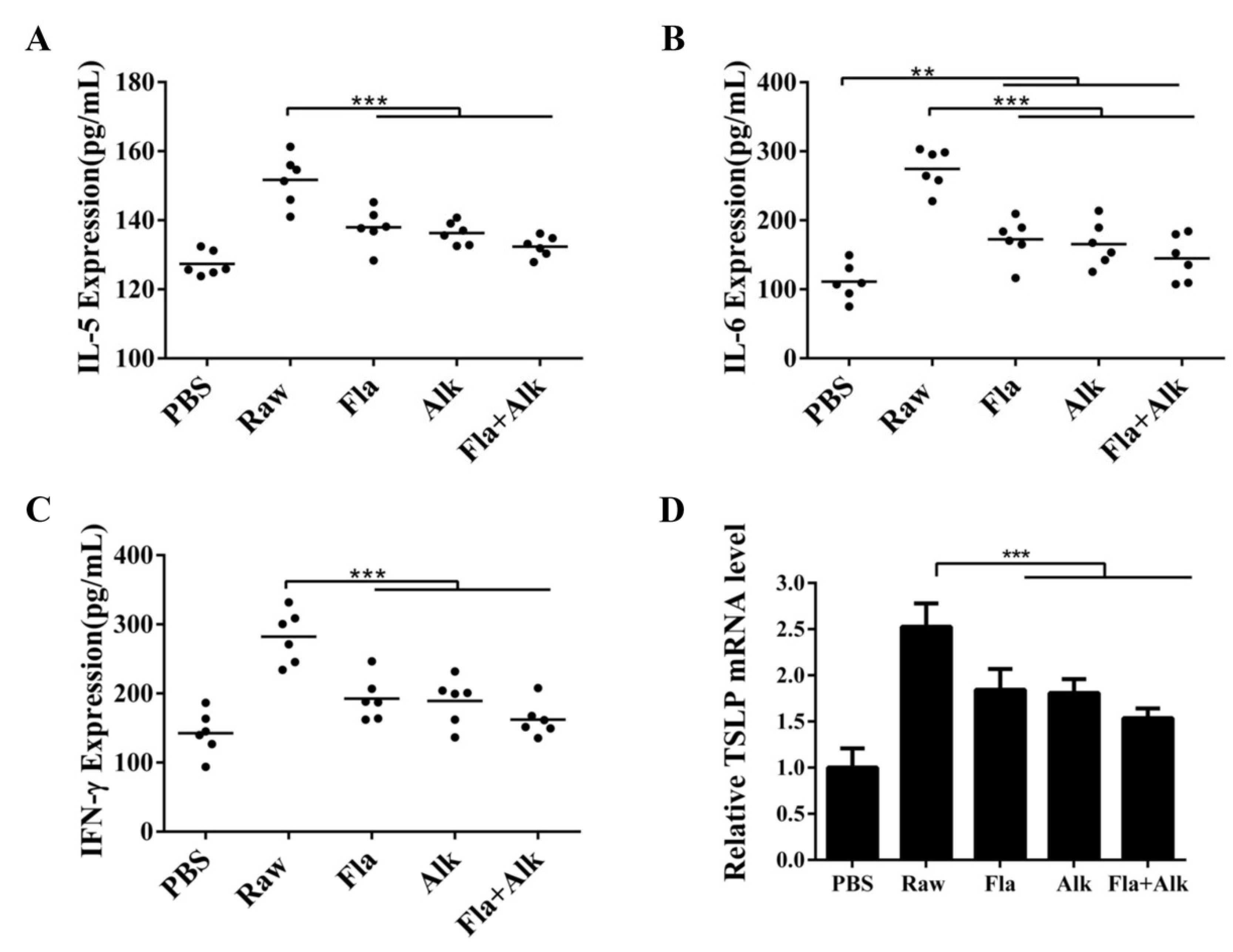
3.2.6. Effects of Fla and Alk-Treated Peanut Protein on the Secretion of Proinflammatory Factors and TSLP Gene Expression
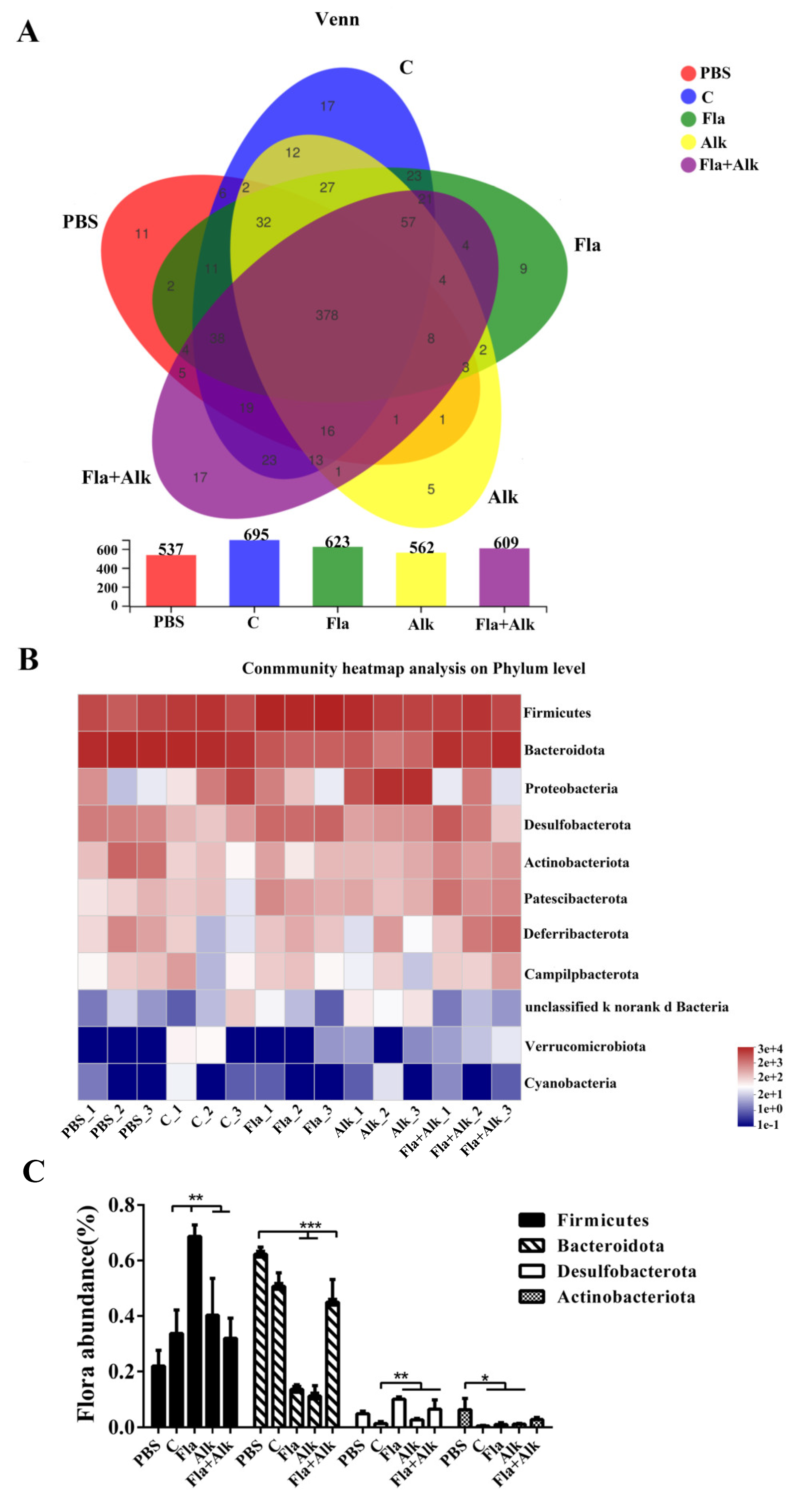
3.2.7. Effects of Fla and Alk-Treated Peanut Protein on the Intestinal Flora of Mice
3.3. Statistical Analysis
4. Results
4.1. Effects of Fla and Alk-Treated Peanut Protein on Morphology and Weight Growth Rate in Mice
4.2. Effects of Fla and Alk-Treated Peanut Protein on Mice Basophils
4.3. Effects of Fla and Alk-Treated Peanut Protein on Intestine and Lung
4.4. Effects of Fla and Alk-Treated Peanut Protein on Antibody Secretion
4.5. Effects of Fla and Alk-Treated Peanut Protein on the Secretion of Proinflammatory Factors and TSLP Gene Expression
4.6. Effects of Fla and Alk-Treated Peanut Protein on the Intestinal Flora of Mice
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ekezie, F.G.C.; Cheng, J.H.; Sun, D.W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Patel, R.; Koterba, A.P. Peanut Allergy; StatPearls: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538526/ (accessed on 28 May 2023).
- Bock, S.A.; Muñoz-Furlong, A.; Sampson, H.A. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J. Allergy Clin. Immunol. 2007, 119, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- Haidar, E.; Lakkis, J.; Karam, M.; Koubaa, M.; Louka, N.; Debs, E. Peanut Allergenicity: An Insight into Its Mitigation Using Thermomechanical Processing. Foods 2023, 12, 1253. [Google Scholar] [CrossRef] [PubMed]
- Loh, W.; Tang, M.L.K. The Epidemiology of Food Allergy in the Global Context. Int. J. Environ. Res. Public. Health 2018, 15, 2043. [Google Scholar] [CrossRef]
- Shah, F.; Shi, A.; Ashley, J.; Kronfel, C.; Wang, Q.; Maleki, S.J.; Adhikari, B.; Zhang, J. Peanut Allergy: Characteristics and Approaches for Mitigation. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1361–1387. [Google Scholar] [CrossRef]
- Pi, X.; Wan, Y.; Yang, Y.; Li, R.; Wu, X.; Xie, M.; Li, X.; Fu, G. Research progress in peanut allergens and their allergenicity reduction. Trends Food Sci. Technol. 2019, 93, 212–220. [Google Scholar] [CrossRef]
- Blanc, F.; Vissers, Y.M.; Adel-Patient, K.; Rigby, N.M.; Mackie, A.R.; Gunning, A.P.; Wellner, N.K.; Skov, P.S.; Przybylski-Nicaise, L.; Ballmer-Weber, B.; et al. Boiling peanut Ara h 1 results in the formation of aggregates with reduced allergenicity. Mol. Nutr. Food Res. 2011, 55, 1887–1894. [Google Scholar] [CrossRef]
- Prodić, I.; Smiljanić, K.; Simović, A.; Radosavljević, J.; Ćirković Veličković, T. Thermal Processing of Peanut Grains Impairs Their Mimicked Gastrointestinal Digestion While Downstream Defatting Treatments Affect Digestomic Profiles. Foods 2019, 8, 463. [Google Scholar] [CrossRef]
- Meng, S.; Li, J.; Chang, S.; Maleki, S.J. Quantitative and kinetic analyses of peanut allergens as affected by food processing. Food Chem. X 2019, 1, 100004. [Google Scholar] [CrossRef]
- Novak, N.; Maleki, S.J.; Cuadrado, C.; Crespo, J.F.; Cabanillas, B. Interaction of Monocyte-Derived Dendritic Cells with Ara h 2 from Raw and Roasted Peanuts. Foods 2020, 9, 863. [Google Scholar] [CrossRef]
- Bavaro, S.L.; Di Stasio, L.; Mamone, G.; De Angelis, E.; Nocerino, R.; Canani, R.B.; Logrieco, A.F.; Montemurro, N.; Monaci, L. Effect of thermal/pressure processing and simulated human digestion on the immunoreactivity of extractable peanut allergens. Food Res. Int. 2018, 109, 126–137. [Google Scholar] [CrossRef]
- Bavaro, S.L.; Orlando, A.; De Angelis, E.; Russo, F.; Monaci, L. Investigation on the allergen profile of the soluble fraction of autoclaved peanuts and its interaction with Caco-2 cells. Food Funct. 2019, 10, 3615–3625. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity assessment on thermally processed peanut influenced by extraction and assessment methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Zhao, Y.; Wang, J.; Wang, M.; Niu, B.; Chen, Q. Different thermal processing effects on peanut allergenicity. J. Sci. Food Agric. 2019, 99, 2321–2328. [Google Scholar] [CrossRef]
- Li, H.; Yu, J.; Ahmedna, M.; Goktepe, I. Reduction of major peanut allergens Ara h 1 and Ara h 2, in roasted peanuts by ultrasound assisted enzymatic treatment. Food Chem. 2013, 141, 762–768. [Google Scholar] [CrossRef]
- Prodic, I.; Stanic-Vucinic, D.; Apostolovic, D.; Mihailovic, J.; Radibratovic, M.; Radosavljevic, J.; Burazer, L.; Milcic, M.; Smiljanic, K.; van Hage, M.; et al. Influence of peanut matrix on stability of allergens in gastric-simulated digesta: 2S albumins are main contributors to the IgE reactivity of short digestion-resistant peptides. Clin. Exp. Allergy 2018, 48, 731–740. [Google Scholar] [CrossRef]
- Di Stasio, L.; Tranquet, O.; Picariello, G.; Ferranti, P.; Morisset, M.; Denery-Papini, S.; Mamone, G. Comparative analysis of eliciting capacity of raw and roasted peanuts: The role of gastrointestinal digestion. Food Res. Int. 2020, 127, 108758. [Google Scholar] [CrossRef]
- Tian, Y.; Rao, H.; Xue, W. Advances in research on the detection of peanut allergens, desensitized process and therapy. Sci. Technol. Food Ind. 2017, 38, 306–311. [Google Scholar]
- Mikiashvili, N.; Yu, J. Changes in immunoreactivity of allergen-reduced peanuts due to post-enzyme treatment roasting. Food Chem. 2018, 256, 188–194. [Google Scholar] [CrossRef]
- Cabanillas, B.; Pedrosa, M.M.; Rodríguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of Enzymatic Hydrolysis on the Allergenicity of Roasted Peanut Protein Extract. Int. Arch. Allergy Immunol. 2012, 157, 41–50. [Google Scholar] [CrossRef]
- Yu, J.; Ahmedna, M.; Goktepe, I.; Cheng, H.; Maleki, S. Enzymatic treatment of peanut kernels to reduce allergen levels. Food Chem. 2011, 127, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.S.; Singh, P.N.; Singh, S.K. Fungal alkaline proteases and their potential applications in different industries. Front. Microbiol. 2023, 14, 1138401. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Butkinaree, C.; Somsong, P.; Duangmal, K. Assessment of the DPP-IV inhibitory potential of mung bean and adzuki bean protein hydrolysates using enzymatic hydrolysis process: Specificity of peptidases and novel peptides. Int. J. Food Sci. Technol. 2023, 58, 2995–3005. [Google Scholar] [CrossRef]
- Dent, T.; Campanella, O.; Maleky, F. Enzymatic hydrolysis of soy and chickpea protein with Alcalase and Flavourzyme and formation of hydrogen bond mediated insoluble aggregates. Curr. Res. Food Sci. 2023, 6, 100487. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Monian, B.; Tu, A.A.; Ruiter, B.; Morgan, D.M.; Petrossian, P.M.; Smith, N.P.; Gierahn, T.M.; Ginder, J.H.; Shreffler, W.G.; Love, J.C. Peanut oral immunotherapy differentially suppresses clonally distinct subsets of T helper cells. J. Clin. Investig. 2022, 132, e150634. [Google Scholar] [CrossRef]
- Yu, J.; Mikiashvili, N. Effectiveness of different proteases in reducing allergen content and IgE-binding of raw peanuts. Food Chem. 2020, 307, 125565. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H.; Liu, H.; Wang, Q. Recent Advances for the Developing of Instant Flavor Peanut Powder: Generation and Challenges. Foods 2022, 11, 1544. [Google Scholar] [CrossRef]
- Chung, S.Y.; Vercellotti, J.R.; Sanders, T.H. Effect of Maturity and Curing on Peanut Proteins. Adv. Exp. Med. Biol. 1998, 434, 35. [Google Scholar]
- Zheng, L.; Zhao, Y.; Xiao, C.; Sun-Waterhouse, D.; Zhao, M.; Su, G. Mechanism of the discrepancy in the enzymatic hydrolysis efficiency between defatted peanut flour and peanut protein isolate by Flavorzyme. Food Chem. 2015, 168, 100–106. [Google Scholar] [CrossRef]
- Shi, X.; Guo, R.; White, B.L.; Yancey, A.; Sanders, T.H.; Davis, J.P.; Burks, A.W.; Kulis, M. Allergenic Properties of Enzymatically Hydrolyzed Peanut Flour Extracts. Int. Arch. Allergy Immunol. 2015, 162, 123–130. [Google Scholar] [CrossRef]
- Liu, X.; Feng, J.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Oral allergy syndrome and anaphylactic reactions in BALB/c mice caused by soybean glycinin and β-conglycinin. Clin. Exp. Allergy 2008, 38, 350–356. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Zhao, Y.; Tang, G.; Niu, B.; Chen, Q. Boiling and roasting treatment affecting the peanut allergenicity. Ann. Transl. Med. 2018, 6, 357. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Q.; Zhang, T.; Cai, Q.; Chen, Q. Thermal processing effects on peanut allergen Ara h 2 allergenicity in mice and its antigenic epitope structure. Food Chem. 2016, 212, 657–662. [Google Scholar] [CrossRef]
- Li, X.M.; Schofield, B.H.; Huang, C.K.; Kleiner, G.I.; Sampson, H.A. A murine model of IgE-mediated cow’s milk hypersensitivity. J. Allergy Clin. Immunol. 1999, 103, 206–214. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Li, T.; Pan, H.; Liu, J.; Fan, M.; Qian, H.; Zhang, H.; Ying, H.; Wang, L. Phosphorylation and Enzymatic Hydrolysis with Alcalase and Papain Effectively Reduce Allergic Reactions to Gliadins in Normal Mice. J. Agric. Food Chem. 2019, 67, 6313–6323. [Google Scholar] [CrossRef]
- Xu, J.Z.; Tang, M.Q.; Liu, Y.N.; Xu, J.H.; Xu, X.X. Safety assessment of monosodium glutamate based on intestinal function and flora in mice. Food Sci. Hum. Well. 2022, 11, 155–164. [Google Scholar] [CrossRef]
- Bunyavanich, S. Food allergy: Could the gut microbiota hold the key? Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 201–202. [Google Scholar] [CrossRef]
- Fu, L.L.; Peng, J.X.; Zhao, S.S.; Zhang, Y.; Su, X.R.; Wang, Y.B. Lactic acid bacteria-specific induction of CD4(+)Foxp3(+)T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci. Rep. 2017, 7, 1987. [Google Scholar] [CrossRef]
- Bjorksten, B.; Sepp, E.; Julge, K.; Voor, T.; Mikelsaar, M. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 2001, 108, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Cahenzli, J.; Koller, Y.; Wyss, M.; Geuking, M.B.; McCoy, K.D. Intestinal Microbial Diversity during Early-Life Colonization Shapes Long-Term IgE Levels. Cell Host Microbe 2013, 14, 559–570. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, E.; Wang, S.; Niu, B.; Chen, Q. Effect of Peanut Protein Treated with Alkaline Protease and Flavorzyme on BALB/c Mice. Foods 2023, 12, 2634. https://doi.org/10.3390/foods12132634
Shu E, Wang S, Niu B, Chen Q. Effect of Peanut Protein Treated with Alkaline Protease and Flavorzyme on BALB/c Mice. Foods. 2023; 12(13):2634. https://doi.org/10.3390/foods12132634
Chicago/Turabian StyleShu, Erlian, Shuo Wang, Bing Niu, and Qin Chen. 2023. "Effect of Peanut Protein Treated with Alkaline Protease and Flavorzyme on BALB/c Mice" Foods 12, no. 13: 2634. https://doi.org/10.3390/foods12132634
APA StyleShu, E., Wang, S., Niu, B., & Chen, Q. (2023). Effect of Peanut Protein Treated with Alkaline Protease and Flavorzyme on BALB/c Mice. Foods, 12(13), 2634. https://doi.org/10.3390/foods12132634