Food Allergies and Parasites in Children
Abstract
1. Introduction
2. Epidemiology of Food Allergy
3. Epidemiology of Parasitic Diseases
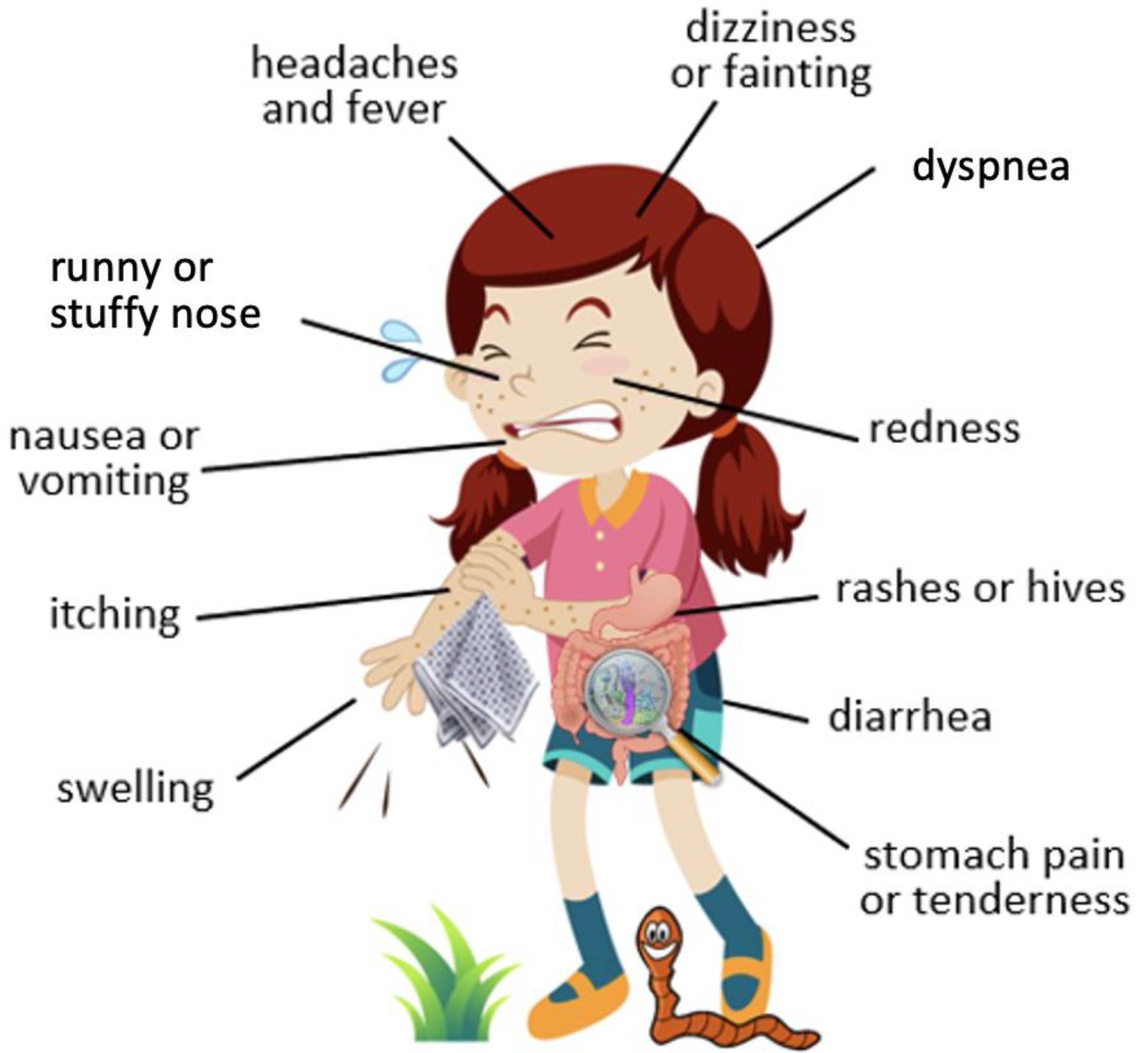
4. Common Symptoms of Food Allergy and Parasitic Infestation
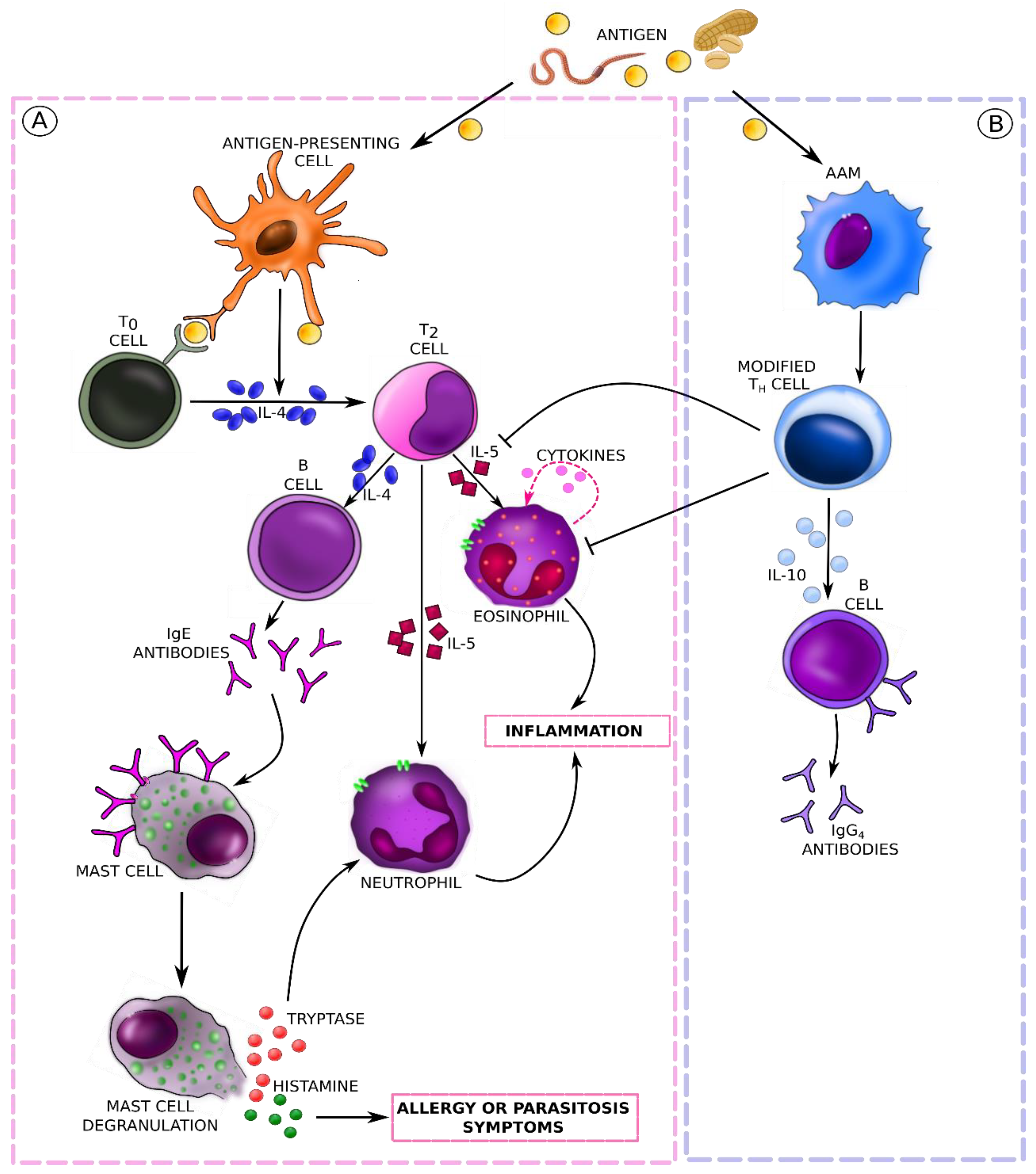
5. Molecular Mechanisms and Immune Response Pathways in Food Allergies (Mainly IgE-Mediated) and Parasitic Infestations
6. Diagnosis of Food Allergy
7. Diagnosis of Parasitoses
| Method Name | Method Description | Ref |
|---|---|---|
| DIRECT SMEAR | A small amount of fresh stool is emulsified in one drop of saline on a glass slide. The emulsified stool is then covered with a coverslip to obtain a thin smear preparation in which eggs/larvae or trophozoites of various parasite species are looked for under a light microscope. | [93] |
| KATO–KATZ TECHNIQUE | A stool sample of approximately 50 mg is placed on a glass slide and then covered with a piece of cellophane soaked in glycerol. Then, the slide is inverted and gently pressed to obtain a thin smear. Glycerol “cleanses” the fecal material. The preparation can be examined under a microscope after 1 to 24 h. Eggs detected in the sample are counted and expressed per gram of feces. | [94] |
| FORMOL-ETHER CONCENTRATION TECHNIQUE | The stool sample is emulsified in water with formol. The resulting suspension is strained to remove large, redundant fecal particles. Then, ether or ethyl acetate is added, and the resulting suspension is centrifuged. Parasitic forms, i.e., cysts, oocysts, eggs, or larvae, are fixed and sedimented, while the fecal remains are suspended in the layer between ether and formol water. The sediment is examined under a light microscope to detect and count the forms of the parasite. | [95] |
| AGAR PLATE CULTURE | The technique requires agar medium (1.5% agar, 0.5% bovine extract, 1.0% peptone, and 0.5% NaCl). The medium (10 mL) is transferred to a Petri dish and left to cool at room temperature. Then, 2 g of fresh feces is placed in the center of the agar plate and incubated at 26–33 °C. The plates are inspected for characteristic patterns of larval movement every 24 h for up to 72 h. The presence of motile larvae indicates a positive test result. | [96] |
| ZINC SULFATE FLOTATION | First, 1 g of feces is emulsified in water and strained to eliminate unnecessary residues. The filtrate is centrifuged, and the precipitate is suspended in 4 mL of ZnSO4 solution (density: 1.180–1.200). The suspension is left for 30–45 min for the eggs and cysts to float to the surface. A coverslip is placed on top of the test tube to collect parasite eggs/larvae, which are subsequently transferred to a glass slide to verify their presence under the microscope. | [97] |
| FLOTAC TECHNIQUE | An accurately weighed sample of 1 g or more of feces is collected after thorough homogenization of a large amount of fecal material in water. The homogenized suspension is filtered through a wire mesh into a tube; then, the tube is centrifuged for 3 min at 1500× g rpm. The obtained supernatant is discarded, and the selected flotation solution is added to the test tube again. The sample is homogenized to form a suspension that can fill the 2 flotation chambers of the FLOTAC apparatus. The FLOTAC apparatus is then closed, and the samples are centrifuged again for 5 min at 1000× g rpm. After centrifugation and transfer of the upper parts of the flotation chambers, they can be read under a microscope. | [98] |
| MCMASTER EGG COUNTING TECHNIQUE | Two grams of feces is transferred to a beaker containing 60 mL of ZnSO4 solution. To homogenize the feces, the resulting suspension is filtered through a gauze or wire mesh into another container. The filtrate is placed in a clean 15 mL tube. A coverslip is placed on its top, and it is left for 15 min. Then, the coverslip is carefully transferred to a slide and examined under a microscope (10× magnification). The suspension is re-homogenized, and both chambers of the McMaster slide are filled using a pipette. The chambers are fixed for up to 3 min so that the eggs float to the top. Debris sediments to the bottom of the chamber. Under the microscope (10×), parasite eggs located in the grid area defined on both sides of the chamber are counted. The number of eggs detected is multiplied by 100 to get the number of eggs per gram of feces. | [96] |
| POLYMERASE CHAIN REACTION (PCR) | The multiplex qPCR method enables the quantification and detection of several target DNA sequences simultaneously. DNA amplification occurs in real time, and a combination of multiple primer sets is used. Studies with species-specific primers/probes have shown increased detection sensitivity for up to eight gastrointestinal parasitic pathogens. | [99] |
8. Allergy to Parasites and Cross-Reactivity
9. Tropomyosin—A Common Allergen Found in Animal Foods and Parasites
10. Anisakis—A Parasite Triggering IgE-Mediated Host Response
- −
- −
- Ani s 2 (100 kDa): paramyosin detected in 88% of people with an allergic reaction.
- −
- Ani s 3 (41 kDa): tropomyosin present in the larval muscles, a somatic antigen playing a crucial role in cross-reactivity due to considerable homology with myosines of other organisms, detected in about 13% of patients with allergic symptoms [126]. Some researchers underline the importance of Ani s 3 as an allergen present in food [127].
- −
- Ani s 4 (9 kDa): thermostable inhibitor of cysteine proteinase, secreted by the excretory glands and present in the basal layer of the epidermis in the third-stage larvae [116]. There are two isoforms of Ani s 4 containing either leucine or proline at the third position of the mature protein. The leucine-containing isoform was shown to be more allergenic than the proline-containing one. Moreover, the first isoform plays an important role as a trigger of anaphylactic reactions [116]. As many as 27% of allergic people respond to Ani s 4 [128].
- −
- Ani s 5 (15 kDa): a weak, thermostable allergen responsible for cross-reactivity, produced by the secretory glands, stomach, and luminal surface of the larval intestinal epithelium. A response to this allergen was confirmed in 49% of people allergic to Anisakis simplex [129].
- −
- Ani s 6 (7 kDa): a serine protease inhibitor showing considerable homology with other serine protease inhibitors, including those from Boophilus microplus, Anopheles stephensi, Glossina morsitans, and Apis mellifera [116]. A reaction to the recombinant rAni s 6 allergen was confirmed in 18% of patients [130].
- −
- Ani s 7 (139 kDa): a serine protease inhibitor. A reaction to this allergen was detected in 83–100% of people allergic to Anisakis simplex [131]. A high titer of sIgE antibodies against Ani s 7 is an indicator of a new infection.
- −
- Ani s 5 (15 kDa), Ani s 8 (15 kDa), and Ani s 9 (14 kDa) are members of the SPX/RAL-2 family sharing amino acid sequence homology. Ani s 9 is structurally similar to the antigens of Ascaris suum or Acanthocheilonema viteae [132]. Cross-reactivity has been reported between Ani s 9 and wasp venom allergens. A response to Ani s 8 was confirmed in 25% of allergic patients, while a response to Ani s 9 was confirmed in 13.8% of allergic individuals [133,134].
- −
- Ani s 10 (22 kDa): probably a somatic antigen of unknown function. It has seven amino acid repeats, each expressing a cleavage site for trypsin and pepsin. It is putatively cleaved in the alimentary tract into seven active peptides [122]. An allergy to it was diagnosed in 39% of tested patients [122].
- −
- Ani s 11 (55 kDa) and Ani s 12 (31 kDa): proteins of unknown function that were identified by the chemiluminescence screening of the Anisakis simplex cDNA expression library. The proteins were described by Kobayashi et al., who reported that Ani s 11 has five or six short repetitive sequences containing from 6 to 15 amino acids, and Ani s 12 has a tandem motif with four cysteine residues [124]. According to the researchers, the reaction to Ani s 11 and Ani s 12 appears in about 50% of people [124].
- −
- −
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Piekarska, B.; Furmańczyk, K.; Walkiewicz, A.; Raciborski, F.; Smoliński, B. Stan środowiska przyrodniczego a choroby alergiczne. Kształcenie Podyplomowe 2011, 90, 216–321. [Google Scholar]
- Noverr, M.C.; Huffnagle, G.B. The “microflora hypothesis” of allergic diseases. Clin. Exp. Allergy 2005, 35, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- New Aspects of Probiotics—A Novel Approach in the Management of Food Allergy—Kirjavainen—1999—Allergy—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1034/j.1398-9995.1999.00103.x (accessed on 26 January 2023).
- Siniscalco, D.; Brigida, A.L.; Antonucci, N.; Siniscalco, D.; Brigida, A.L.; Antonucci, N. Autism and neuro-immune-gut link. AIMS Mol. Sci. 2018, 5, 166–172. [Google Scholar] [CrossRef]
- Cruz, A.A.; Cooper, P.J.; Figueiredo, C.A.; Alcantara-Neves, N.M.; Rodrigues, L.C.; Barreto, M.L. Global issues in allergy and immunology: Parasitic infections and allergy. J. Allergy Clin. Immunol. 2017, 140, 1217–1228. [Google Scholar] [CrossRef]
- de Andrade, C.M.; Carneiro, V.L.; Cerqueira, J.V.; Fonseca, H.F.; Queiroz, G.A.; Costa, R.S.; Alcantara-Neves, N.M.; Cooper, P.; Figueiredo, C.A. Parasites and allergy: Observations from Brazil. Parasite Immunol. 2019, 41, e12588. [Google Scholar] [CrossRef]
- Mpairwe, H.; Amoah, A.S. Parasites and allergy: Observations from Africa. Parasite Immunol. 2019, 41, e12589. [Google Scholar] [CrossRef]
- Maizels, R.M. Regulation of immunity and allergy by helminth parasites. Allergy 2020, 75, 524–534. [Google Scholar] [CrossRef]
- User, S. Food Labelling. Available online: https://www.efanet.org/prevent/food-labelling (accessed on 17 May 2023).
- Pawankar, R.; Canonica, W.G.; Holgate, T.S.; Lockey, R.F.; Blaiss, M.S. WAO White Book on Allergy: Update 2013; World Allergy Organization: Milwaukee, WI, USA, 2013; ISBN 978-0-615-92915-6. [Google Scholar]
- Kaczmarski, M.; Wasilewska, J.; Jarocka-Cyrta, E.; Cudowska, B.; Żur, E.; Matuszewska, E.; Stańczyk-Przyłuska, A.; Zeman, K.; Kamer, B.; Korotkiewicz-Kaczmarska, E.; et al. Food allergy in children and adolescents. Pol. Statement 2011, 9, 31–56. [Google Scholar]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. Pediatr. Allergy Immunol. 2010, 21 (Suppl. 21), 1–125. [Google Scholar] [CrossRef]
- Kalinowski, P.; Mirosław, K. Wiedza rodziców na temat alergii pokarmowej występującej u ich dzieci. Med. Ogólna I Nauk. O Zdrowiu 2014, 20, 88–91. [Google Scholar]
- Alergia Pokarmowa—Alergia. Available online: http://alergia.org.pl/index.php/2022/12/20/alergia-pokarmowa/ (accessed on 21 February 2023).
- Heine, R.G.; AlRefaee, F.; Bachina, P.; De Leon, J.C.; Geng, L.; Gong, S.; Madrazo, J.A.; Ngamphaiboon, J.; Ong, C.; Rogacion, J.M. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children—Common misconceptions revisited. World Allergy Organ. J. 2017, 10, 41. [Google Scholar] [CrossRef]
- Dor-Wojnarowska, A.; Liebhart, J.; Miecielica, J.; Rabski, M.; Fal, A.; Samoliński, B.; Nittner-Marszalska, M. The Impact of Sex and Age on the Prevalence of Clinically Relevant Sensitization and Asymptomatic Sensitization in the General Population. Arch. Immunol. Ther. Exp. 2017, 65, 253–261. [Google Scholar] [CrossRef]
- Gugała, A.; Kurowski, M.; Kowalski, M.L. Alergia na pokarmy w Polsce na tle innych krajów Europy—Wyniki projektu EuroPrevall. Alerg. Astma Immunol. 2021, 26, 18–26. [Google Scholar]
- Łoś-Rycharska, E.; Czerwionka-Szaflarska, M. Nowe podejście do leczenia alergii na pokarmy u niemowląt i dzieci. Pediatr. Pol. 2016, 91, 111–117. [Google Scholar] [CrossRef]
- Kaczmarski, M.; Wasilewska, J.; Cudowska, B.; Semeniuk, J.; Klukowski, M.; Matuszewska, E. The natural history of cow’s milk allergy in north-eastern Poland. Adv. Med. Sci. 2013, 58, 22–30. [Google Scholar] [CrossRef]
- Pyziak, K.; Kamer, B. Natural history of IgE-dependent food allergy diagnosed in children during the first three years of life. Adv. Med. Sci. 2011, 56, 48–55. [Google Scholar] [CrossRef]
- Pelc, J.; Czarnecka-Operacz, M.; Adamski, Z. Structure and function of the epidermal barrier in patients with atopic dermatitis—Treatment options. Part one. Adv. Dermatol. Allergol. 2018, 35, 1–5. [Google Scholar] [CrossRef]
- Góra, D.; Figura, N.; Gregor, M. Epidemiology of allergic diseases among children and adolescents in the Silesian Voivodeship in 2010–2019. Nowa Pediatr. 2020. Available online: https://www.czytelniamedyczna.pl/7034,epidemiologia-chorlb-alergicznych-wrld-dzieci-i-modziezy-w-wojewldztwie-lskim-w.html (accessed on 21 February 2023). [CrossRef]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčíř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell. Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef]
- de Silva, N.R.; Chan, M.S.; Bundy, D.A. Morbidity and mortality due to ascariasis: Re-estimation and sensitivity analysis of global numbers at risk. Trop. Med. Int. Health 1997, 2, 519–528. [Google Scholar] [CrossRef]
- Marshall, M.M.; Naumovitz, D.; Ortega, Y.; Sterling, C.R. Waterborne protozoan pathogens. Clin. Microbiol. Rev. 1997, 10, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Lamblioza (Giardioza): Przyczyny, Objawy i Leczenie. Available online: http://www.mp.pl/social/article/74653 (accessed on 10 February 2023).
- Savioli, L.; Smith, H.; Thompson, A. Giardia and Cryptosporidium join the “Neglected Diseases Initiative”. Trends Parasitol. 2006, 22, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Veesenmeyer, A.F. Important Nematodes in Children. Pediatr. Clin. N. Am. 2022, 69, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Tchakounté, B.N.; Nkouayep, V.R.; Poné, J.W. Soil Contamination Rate, Prevalence, Intensity of Infection of Geohelminths and Associated Risk Factors among Residents in Bazou (West Cameroon). Ethiop. J. Health Sci. 2018, 28, 63–72. [Google Scholar] [CrossRef]
- Zavala, G.A.; van Dulm, E.; Doak, C.M.; García, O.P.; Polman, K.; Campos-Ponce, M. Ascariasis, Amebiasis and Giardiasis in Mexican children: Distribution and geographical, environmental and socioeconomic risk factors. J. Parasit. Dis. 2020, 44, 829–836. [Google Scholar] [CrossRef]
- Pone, J.W.; Mbida, M.; Alango, P.N.E.; Bilong, C.B. Prevalence and intensity of infections of three neglected tropical diseases in patients consulted at a Traditional Health Care Centre in Dschang West Cameroon. Trop. Parasitol. 2012, 2, 24–28. [Google Scholar] [CrossRef]
- Anderson, T.J.C. Ascaris infections in humans from North America: Molecular evidence for cross-infection. Parasitology 1995, 110, 215–219. [Google Scholar] [CrossRef]
- Magnaval, J.F. Treatment of cosmopolitan parasitic diseases. Med. Trop. 2006, 66, 193–198. [Google Scholar]
- Korzeniewski, K.; Augustynowicz, A.; Lass, A. Intestinal parasites in Polish community on the example of military environment. Int. Marit. Health 2014, 65, 216–222. [Google Scholar] [CrossRef]
- Within-Host Mechanisms of Immune Regulation Explain the Contrasting Dynamics of Two Helminth Species in Both Single and Dual Infections | PLOS Computational Biology. Available online: https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1008438 (accessed on 21 February 2023).
- Watts, M.M.; Grammer, L.C. Hypersensitivity pneumonitis. Allergy Asthma Proc. 2019, 40, 425–428. [Google Scholar] [CrossRef]
- Maruszewska-Cheruiyot, M.; Donskow-Łysoniewska, K.; Doligalska, M.; Maruszewska-Cheruiyot, M.; Donskow-Łysoniewska, K.; Doligalska, M. Helminty mistrzami modulacji układu odpornościowego żywiciela. Eduk. Biol. I Sr. 2018, 2, 32–38. [Google Scholar]
- Własienko, A.; Kuchar, E. Diagnosis, treatment and prevention of the most common parasitic diseases in children: Current problems of the pediatrician and family doctor. Lek. POZ 2017, 3, 154–160. [Google Scholar]
- Protasiewicz, M.; Iwaniak, A. Alergie Pokarmowe i Alergeny Żywności. BROMAT. CHEM. TOKSYKOL-XLVII 2014, 2, 237–242. [Google Scholar]
- Knap, J.P. Epidemiologia Chorób Pasożytniczych w Polsce. W: Parazytologia Kliniczna w Ujęciu Wielodyscyplinarnym.; PZWL: Warszawa, Poland, 2004. [Google Scholar]
- Maizels, R.M.; McSorley, H.J. Regulation of the host immune system by helminth parasites. J. Allergy Clin. Immunol. 2016, 138, 666–675. [Google Scholar] [CrossRef]
- Jak Pasożyty Modulują Układ Odpornościowy Chroniąc w Ten Sposób Gospodarza od Alergii? Available online: https://weterynarianews.pl/pasozyty-moduluja-uklad-odpornosciowy/ (accessed on 21 February 2023).
- Gazzinelli-Guimaraes, P.H.; Nutman, T.B. Helminth parasites and immune regulation. Fac. Rev. 2018, 7, F1000–F1685. [Google Scholar] [CrossRef]
- Lifschitz, C.; Szajewska, H. Cow’s milk allergy: Evidence-based diagnosis and management for the practitioner. Eur. J. Pediatr. 2015, 174, 141–150. [Google Scholar] [CrossRef]
- Luyt, D.; Ball, H.; Makwana, N.; Green, M.R.; Bravin, K.; Nasser, S.M.; Clark, A.T. Standards of Care Committee (SOCC) of the British Society for Allergy and Clinical Immunology (BSACI) BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin. Exp. Allergy 2014, 44, 642–672. [Google Scholar] [CrossRef]
- Mao, X.-Q.; Sun, D.-J.; Miyoshi, A.; Feng, Z.; Handzel, Z.T.; Hopkin, J.M.; Shirakawa, T. The Link between Helminthic Infection and Atopy. Parasitol. Today 2000, 16, 186–188. [Google Scholar] [CrossRef]
- NIAID-Sponsored Expert Panel; Boyce, J.A.; Assa’ad, A.; Burks, A.W.; Jones, S.M.; Sampson, H.A.; Wood, R.A.; Plaut, M.; Cooper, S.F.; Fenton, M.J.; et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J. Allergy Clin. Immunol. 2010, 126, S1–S58. [Google Scholar] [CrossRef]
- Zając, K.; Strategie Pasożytów Umożliwiające Unikanie Odpowiedzi Immunologicznej Żywiciela. The Strategies of Parasites Allowing to Avoid the Immune Response of Hosts. 2015. Available online: https://ruj.uj.edu.pl/xmlui/handle/item/194203 (accessed on 21 February 2023).
- Hussain, M.; Borcard, L.; Walsh, K.P.; Rodriguez, M.P.; Mueller, C.; Kim, B.S.; Kubo, M.; Artis, D.; Noti, M. Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J. Allergy Clin. Immunol. 2018, 141, 223–234.e5. [Google Scholar] [CrossRef]
- Doligalska, M. Regulacja reakcji obronnej i alergicznej w inwazjach pasozytow. Wiadomości Parazytol. 2000, 46, 3–20. [Google Scholar]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P.; et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Prusak, A.; Schlegel-Zawadzka, M. Consumer perceptions of peanuts and peanut allergy: The EuroPrevall results of focus groups in Poland. Public Health 2017, 2, 11–20. [Google Scholar] [CrossRef]
- Sutton, B.J.; Davies, A.M.; Bax, H.J.; Karagiannis, S.N. IgE Antibodies: From Structure to Function and Clinical Translation. Antibodies 2019, 8, 19. [Google Scholar] [CrossRef]
- Stone, K.D.; Prussin, C.; Metcalfe, D.D. IgE, Mast Cells, Basophils, and Eosinophils. J. Allergy Clin. Immunol. 2010, 125, S73–S80. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of food allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef]
- Kargulewicz, A. Alergie pokarmowe u dzieci. Lek. POZ 2021, 6, 352–355. [Google Scholar]
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229. [Google Scholar] [CrossRef]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef]
- Institute for Quality and Efficiency in Health Care (IQWiG). Allergies: Overview. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK447112/ (accessed on 21 February 2023).
- Berin, M.C.; Li, H.; Sperber, K. Antibody-mediated antigen sampling across intestinal epithelial barriers. Ann. N. Y. Acad. Sci. 2006, 1072, 253–261. [Google Scholar] [CrossRef]
- Niewiem, M.; Grzybowska-Chlebowczyk, U. Intestinal Barrier Permeability in Allergic Diseases. Nutrients 2022, 14, 1893. [Google Scholar] [CrossRef]
- Chulanetra, M.; Chaicumpa, W. Revisiting the Mechanisms of Immune Evasion Employed by Human Parasites. Front. Cell. Infect. Microbiol. 2021, 11, 639. [Google Scholar] [CrossRef]
- Parkhouse, R.M.; Clark, N.W. Stage specific secreted and somatic antigens of Trichinella spiralis. Mol. Biochem. Parasitol. 1983, 9, 319–327. [Google Scholar] [CrossRef]
- Garside, P.; Sands, W.A.; Kusel, J.R.; Hagan, P. Is the induction of apoptosis the mechanism of the protective effects of TNF alpha in helminth infection? Parasite Immunol. 1996, 18, 111–113. [Google Scholar] [CrossRef]
- Wing, K.; Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 2010, 11, 7–13. [Google Scholar] [CrossRef]
- Doligalska, M.; Brodaczewska, K.; Donskow-Łysoniewska, K. The antiapoptotic activity of Heligmosomoides polygyrus antigen fractions. Parasite Immunol. 2012, 34, 589–603. [Google Scholar] [CrossRef]
- Mishra, R.; Panda, S.K.; Sahoo, P.K.; Bal, M.S.; Satapathy, A.K. Increased Fas ligand expression of peripheral B-1 cells correlated with CD4+ T-cell apoptosis in filarial-infected patients. Parasite Immunol. 2017, 39, e12421. [Google Scholar] [CrossRef]
- Adjobimey, T.; Hoerauf, A. Induction of immunoglobulin G4 in human filariasis: An indicator of immunoregulation. Ann. Trop. Med. Parasitol. 2010, 104, 455–464. [Google Scholar] [CrossRef]
- Helmintoterapia—Działanie Lokalne i Obwodowe na Przykładzie Nieswoistych. Available online: https://phmd.pl/resources/html/article/details?id=196654&language=pl (accessed on 27 January 2023).
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organ. J. 2020, 13, 100080. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Gushken, A.K.F.; Castro, A.P.M.; Yonamine, G.H.; Corradi, G.A.; Pastorino, A.C.; Jacob, C.M.A. Double-blind, placebo-controlled food challenges in Brazilian children: Adaptation to clinical practice. Allergol. Immunopathol. 2013, 41, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Cox, A.L.; Eigenmann, P.A.; Sicherer, S.H. Clinical Relevance of Cross-Reactivity in Food Allergy. J. Allergy Clin. Immunol. Pract. 2021, 9, 82–99. [Google Scholar] [CrossRef] [PubMed]
- Bartuzi, Z.; Kaczmarski, M.; Czerwionka-Szaflarska, M.; Małaczyńska, T.; Krogulska, A. The diagnosis and management of food allergies. Position paper of the Food Allergy Section the Polish Society of Allergology. Postep. Dermatol. Alergol. 2017, 34, 391–404. [Google Scholar] [CrossRef]
- Chinthrajah, R.S.; Tupa, D.; Prince, B.T.; Block, W.M.; Rosa, J.S.; Singh, A.M.; Nadeau, K. Diagnosis of Food Allergy. Pediatr. Clin. N. Am. 2015, 62, 1393–1408. [Google Scholar] [CrossRef]
- Khan, F.M.; Ueno-Yamanouchi, A.; Serushago, B.; Bowen, T.; Lyon, A.W.; Lu, C.; Storek, J. Basophil activation test compared to skin prick test and fluorescence enzyme immunoassay for aeroallergen-specific Immunoglobulin-E. Allergy Asthma Clin. Immunol. 2012, 8, 1. [Google Scholar] [CrossRef]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 3. [Google Scholar] [CrossRef]
- Niggemann, B.; Beyer, K. Diagnosis of Food Allergy in Children: Toward a Standardization of Food Challenge. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 399. [Google Scholar] [CrossRef]
- Component-Resolved Diagnostics in the Clinical and Laboratory Investigation of Allergy—Emma L Callery, Catherine Keymer, Nicholas A Barnes, Anthony W Rowbottom. 2020. Available online: https://journals.sagepub.com/doi/10.1177/0004563219877434 (accessed on 15 February 2023).
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. 23), 1–250. [Google Scholar] [CrossRef]
- Matricardi, P.M.; Dramburg, S.; Potapova, E.; Skevaki, C.; Renz, H. Molecular Diagnosis for Allergen Immunotherapy. J. Allergy Clin. Immunol. 2019, 143, 831–843. Available online: https://www.jacionline.org/article/S0091-6749(19)30110-1/fulltext (accessed on 15 February 2023). [CrossRef]
- Hamilton, R.G. Microarray Technology Applied to Human Allergic Disease. Microarrays 2017, 6, 3. [Google Scholar] [CrossRef]
- Jakob, T.; Matricardi, P.M.; Luengo, O.; Kleine-Tebbe, J. Molecular allergy diagnostics in clinical practice. In Molecular Allergology User’s Guide 2.0; John Wiley: Hoboken, NJ, USA, 2022; pp. 35–52. [Google Scholar]
- Suprun, M.; Getts, R.; Raghunathan, R.; Grishina, G.; Witmer, M.; Gimenez, G.; Sampson, H.A.; Suárez-Fariñas, M. Novel Bead-Based Epitope Assay is a sensitive and reliable tool for profiling epitope-specific antibody repertoire in food allergy. Sci. Rep. 2019, 9, 18425. [Google Scholar] [CrossRef]
- Santos, A.F.; Kulis, M.D.; Sampson, H.A. Bringing the Next Generation of Food Allergy Diagnostics Into the Clinic. J. Allergy Clin. Immunol. 2022, 10, 1–9. [Google Scholar] [CrossRef]
- Sackesen, C.; Suárez-Fariñas, M.; Silva, R.; Lin, J.; Schmidt, S.; Getts, R.; Gimenez, G.; Yilmaz, E.A.; Cavkaytar, O.; Buyuktiryaki, B.; et al. A new Luminex-based peptide assay to identify reactivity to baked, fermented, and whole milk. Allergy 2019, 74, 327–336. [Google Scholar] [CrossRef]
- Soares, F.A.; do Nascimento Benitez, A.; dos Santos, B.M.; Loiola, S.H.N.; Rosa, S.L.; Nagata, W.B.; Inácio, S.V.; Suzuki, C.T.N.; Bresciani, K.D.S.; Falcão, A.X.; et al. A historical review of the techniques of recovery of parasites for their detection in human stools. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190535. [Google Scholar] [CrossRef]
- Barda, B.; Cajal, P.; Villagran, E.; Cimino, R.; Juarez, M.; Krolewiecki, A.; Rinaldi, L.; Cringoli, G.; Burioni, R.; Albonico, M. Mini-FLOTAC, Kato-Katz and McMaster: Three methods, one goal; highlights from north Argentina. Parasites Vectors 2014, 7, 271. [Google Scholar] [CrossRef]
- Ngongeh, L.A. Variation in faecal worm egg counts of experimentally infected goats and mice with time of day and its implications in diagnosis of helminthosis. J. Parasit. Dis. 2017, 41, 997–1000. [Google Scholar] [CrossRef]
- Salimi-Bejestani, M.R.; McGarry, J.W.; Felstead, S.; Ortiz, P.; Akca, A.; Williams, D.J.L. Development of an antibody-detection ELISA for Fasciola hepatica and its evaluation against a commercially available test. Res. Vet. Sci. 2005, 78, 177–181. [Google Scholar] [CrossRef]
- Verweij, J.J. Molecular Diagnostics of Parasitic Infections. In Molecular Diagnostics: Part 2: Clinical, Veterinary, Agrobotanical and Food Safety Applications; van Pelt-Verkuil, E., van Leeuwen, W.B., te Witt, R., Eds.; Springer: Singapore, 2017; pp. 21–31. ISBN 978-981-10-4511-0. [Google Scholar]
- Barker, D.C. Molecular approaches to DNA diagnosis. Parasitology 1989, 99, S125–S146. [Google Scholar] [CrossRef]
- Detection of Giardia Intestinalis DNA in Environme…—Biblioteka Nauki. Available online: https://bibliotekanauki.pl/articles/6172 (accessed on 3 March 2023).
- Mbong Ngwese, M.; Prince Manouana, G.; Nguema Moure, P.A.; Ramharter, M.; Esen, M.; Adégnika, A.A. Diagnostic Techniques of Soil-Transmitted Helminths: Impact on Control Measures. Trop. Med. Infect. Dis. 2020, 5, 93. [Google Scholar] [CrossRef]
- Jayakody, N.K.; Kumbukgahadeniya, P.L.; Silva, A.; Wickramasinghe, N.D.; Wickramasinghe, S.; McManus, D.P.; Weerakoon, K.G. The accuracy of nucleic acid amplification tests (NAATs) in detecting human intestinal nematode infections: A protocol for a systematic review and meta-analysis. PLoS ONE 2022, 17, e0278920. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Bench Aids for the Diagnosis of Intestinal Parasites, 2nd ed.; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151534-4. [Google Scholar]
- Kongs, A.; Marks, G.; Verlé, P.; Van der Stuyft, P. The unreliability of the Kato-Katz technique limits its usefulness for evaluating S. mansoni infections. Trop. Med. Int. Health 2001, 6, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.J.; Gross, J.; Smith, M.C. Comparison of Kato–Katz Direct Smear and Sodium Nitrate Flotation for Detection of Geohelminth Infections. Copa 2008, 75, 339–341. [Google Scholar] [CrossRef]
- Arakaki, T.; Hasegawa, H.; Asato, R.; Ikeshiro, T.; Kinjo, F.; Saito, A.; Iwanaga, M. A New Method to Detect strongyloides Stercoralis from Human Stool. Jpn. J. Trop. Med. Hyg. 1988, 16, 11–17. [Google Scholar] [CrossRef]
- Faust, E.C.; Sawitz, W.; Tobie, J.; Odom, V.; Peres, C.; Lincicome, D.R. Comparative Efficiency of Various Technics for the Diagnosis of Protozoa and Helminths in Feces. J. Parasitol. 1939, 25, 241–262. [Google Scholar] [CrossRef]
- FLOTAC: A Promising Technique for Detecting Helminth Eggs in Human Faeces☆ | Transactions of The Royal Society of Tropical Medicine and Hygiene | Oxford Academic. Available online: https://academic.oup.com/trstmh/article-abstract/103/12/1190/1931659 (accessed on 27 January 2023).
- Taniuchi, M.; Verweij, J.J.; Noor, Z.; Sobuz, S.U.; van Lieshout, L.; Petri, W.A.; Haque, R.; Houpt, E.R. High Throughput Multiplex PCR and Probe-based Detection with Luminex Beads for Seven Intestinal Parasites. Am. J. Trop. Med. Hyg. 2011, 84, 332–337. [Google Scholar] [CrossRef]
- Amoah, A.S.; Boakye, D.A.; Yazdanbakhsh, M.; van Ree, R. Influence of Parasitic Worm Infections on Allergy Diagnosis in Sub-Saharan Africa. Curr. Allergy Asthma Rep. 2017, 17, 65. [Google Scholar] [CrossRef]
- Cooper, P.J. Interactions between helminth parasites and allergy. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 29–37. [Google Scholar] [CrossRef]
- Beldomenico, P.M.; Begon, M. Stress-host-parasite interactions: A vicious triangle? FAVE Sección Cienc. Vet. 2015, 14, 6–19. [Google Scholar] [CrossRef]
- Kunst, H.; Mack, D.; Kon, O.M.; Banerjee, A.K.; Chiodini, P.; Grant, A. Parasitic infections of the lung: A guide for the respiratory physician. Thorax 2011, 66, 528–536. [Google Scholar] [CrossRef]
- Calvert, J.; Burney, P. Ascaris, atopy, and exercise-induced bronchoconstriction in rural and urban South African children. J. Allergy Clin. Immunol. 2010, 125, 100–105.e5. [Google Scholar] [CrossRef]
- Webb, E.L.; Nampijja, M.; Kaweesa, J.; Kizindo, R.; Namutebi, M.; Nakazibwe, E.; Oduru, G.; Kabubi, P.; Kabagenyi, J.; Nkurunungi, G.; et al. Helminths are positively associated with atopy and wheeze in Ugandan fishing communities: Results from a cross-sectional survey. Allergy 2016, 71, 1156–1169. [Google Scholar] [CrossRef]
- Johansson, S.G.; Mellbin, T.; Vahlquist, B. Immunoglobulin levels in Ethiopian preschool children with special reference to high concentrations of immunoglobulin E (IgND). Lancet 1968, 1, 1118–1121. [Google Scholar] [CrossRef]
- Obeng, B.B.; Amoah, A.S.; Larbi, I.A.; de Souza, D.K.; Uh, H.-W.; Fernández-Rivas, M.; van Ree, R.; Rodrigues, L.C.; Boakye, D.A.; Yazdanbakhsh, M.; et al. Schistosome infection is negatively associated with mite atopy, but not wheeze and asthma in Ghanaian schoolchildren. Clin. Exp. Allergy 2014, 44, 965–975. [Google Scholar] [CrossRef]
- Acevedo, N.; Caraballo, L. IgE cross-reactivity between Ascaris lumbricoides and mite allergens: Possible influences on allergic sensitization and asthma. Parasite Immunol. 2011, 33, 309–321. [Google Scholar] [CrossRef]
- Popescu, F.-D. Cross-reactivity between aeroallergens and food allergens. World J. Methodol. 2015, 5, 31–50. [Google Scholar] [CrossRef]
- Amoah, A.S.; Obeng, B.B.; Larbi, I.A.; Versteeg, S.A.; Aryeetey, Y.; Akkerdaas, J.H.; Zuidmeer, L.; Lidholm, J.; Fernández-Rivas, M.; Hartgers, F.C.; et al. Peanut-specific IgE antibodies in asymptomatic Ghanaian children possibly caused by carbohydrate determinant cross-reactivity. J. Allergy Clin. Immunol. 2013, 132, 639–647. [Google Scholar] [CrossRef]
- Wollmann, E.; Hamsten, C.; Sibanda, E.; Ochome, M.; Focke-Tejkl, M.; Asarnoj, A.; Önell, A.; Lilja, G.; Gallerano, D.; Lupinek, C.; et al. Natural clinical tolerance to peanut in African patients is caused by poor allergenic activity of peanut IgE. Allergy 2015, 70, 638–652. [Google Scholar] [CrossRef]
- Downs, M.; Johnson, P.; Zeece, M. Chapter 9—Insects and Their Connection to Food Allergy. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 255–272. ISBN 978-0-12-802856-8. [Google Scholar]
- Broekman, H.; Knulst, A.; den Hartog Jager, S.; Monteleone, F.; Gaspari, M.; De Jong, G.; Houben, G.; Verhoeckx, K. Effect of thermal processing on mealworm allergenicity. Mol. Nutr. Food Res. 2015, 59, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Acevedo, N. New Allergens of Relevance in Tropical Regions: The Impact of Ascaris lumbricoides Infections. World Allergy Organ. J. 2011, 4, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Caraballo, L.; Coronado, S. Parasite allergens. Mol. Immunol. 2018, 100, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Pontone, M.; Giovannini, M.; Barni, S.; Mori, F.; Venturini, E.; Galli, L.; Valleriani, C.; Vecillas, L.D.L.; Sackesen, C.; Lopata, A.L.; et al. IgE-mediated Anisakis allergy in children. Allergol. Immunopathol. 2023, 51, 98–109. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E. Anisakis—Immunology of a foodborne parasitosis. Parasite Immunol. 2016, 38, 548–557. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E.; Lopata, A.L. Allergic reactions to Anisakis found in fish. Curr. Allergy Asthma Rep. 2014, 14, 455. [Google Scholar] [CrossRef]
- Adroher-Auroux, F.J.; Benítez-Rodríguez, R. Anisakiasis and Anisakis: An underdiagnosed emerging disease and its main etiological agents. Res. Vet. Sci. 2020, 132, 535–545. [Google Scholar] [CrossRef]
- Daschner, A.; Cuéllar, C.; Rodero, M. The Anisakis allergy debate: Does an evolutionary approach help? Trends Parasitol. 2012, 28, 9–15. [Google Scholar] [CrossRef]
- Baird, F.J.; Gasser, R.B.; Jabbar, A.; Lopata, A.L. Foodborne anisakiasis and allergy. Mol. Cell. Probes 2014, 28, 167–174. [Google Scholar] [CrossRef]
- Moneo, I.; Caballero, M.L.; Gómez-Aguado, F.; Ortega-Paino, E.; Alonso, M. Isolation and characterization of a major allergen from the fish parasite Anisakis simplex. J. Allergy Clin. Immunol. 2000, 106, 177–182. [Google Scholar] [CrossRef]
- Sereda, M.J.; Hartmann, S.; Lucius, R. Helminths and allergy: The example of tropomyosin. Trends Parasitol. 2008, 24, 272–278. [Google Scholar] [CrossRef]
- Asturias, J.A.; Eraso, E.; Moneo, I.; Martínez, A. Is tropomyosin an allergen in Anisakis? Allergy 2000, 55, 898–899. [Google Scholar] [CrossRef]
- Rodriguez-Mahillo, A.I.; Gonzalez-Muñoz, M.; Gomez-Aguado, F.; Rodriguez-Perez, R.; Corcuera, M.T.; Caballero, M.L.; Moneo, I. Cloning and characterisation of the Anisakis simplex allergen Ani s 4 as a cysteine-protease inhibitor. Int. J. Parasitol. 2007, 37, 907–917. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kakemoto, S.; Shimakura, K.; Shiomi, K. Molecular Cloning and Expression of a New Major Allergen, Ani s 14, from Anisakis simplex. Food Hyg. Saf. Sci. 2015, 56, 194–199. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Shimakura, K.; Ishizaki, S.; Nagashima, Y.; Shiomi, K. Purification and cDNA cloning of a new heat-stable allergen from Anisakis simplex. Mol. Biochem. Parasitol. 2007, 155, 138–145. [Google Scholar] [CrossRef]
- Anadón, A.M.; Romarís, F.; Escalante, M.; Rodríguez, E.; Gárate, T.; Cuéllar, C.; Ubeira, F.M. The Anisakis simplex Ani s 7 major allergen as an indicator of true Anisakis infections. Clin. Exp. Immunol. 2009, 156, 471–478. [Google Scholar] [CrossRef]
- González-Fernández, J.; Rivas, L.; Luque-Ortega, J.R.; Núñez-Ramírez, R.; Campioli, P.; Gárate, T.; Perteguer, M.J.; Daschner, A.; Cuéllar, C. Recombinant vs native Anisakis haemoglobin (Ani s 13): Its appraisal as a new gold standard for the diagnosis of allergy. Experimental. Parasitol. 2017, 181, 119–129. [Google Scholar] [CrossRef]
- Ani s 14 Allergen Details. Available online: http://www.allergen.org/viewallergen.php?aid=824 (accessed on 28 January 2023).

| Allergen Characteristics | ||||
|---|---|---|---|---|
| Biochemical Name | Tropomyosin | |||
| Allergen source | Major taxonomic group | Animalia Nematoda | Animalia Arthropoda | Animalia Arthropoda |
| Order | Ascaridida | Astigmata | Decapoda | |
| Species | Anisakis simplex | Dermatophagoides pteronyssinus | Penaeus aztecus (brown shrimp) | |
| Allergen name | Ani s 3 | Der p 10 | Pen a 1 | |
| Biological function | Tropomyosin, together with the troponin complex, plays a central role in the calcium-dependent regulation of muscle contraction. | |||
| Structural model predicted by AlphaFold |  |  | 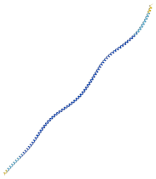 | |
| Molecular weight | 33,203 Da | 32,553 Da | 32,849 Da | |
| Length | 284 aa | 281 aa | 284 aa | |
| Route of allergen exposure | food | airway | food | |
| UniProt | Q9NAS5 | Q304Y3 | Q3Y8M6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Packi, K.; Rudek, A.; Matysiak, J.; Klimczak, S.; Matuszewska, E.; Rzetecka, N.; Matysiak, J. Food Allergies and Parasites in Children. Foods 2023, 12, 2465. https://doi.org/10.3390/foods12132465
Packi K, Rudek A, Matysiak J, Klimczak S, Matuszewska E, Rzetecka N, Matysiak J. Food Allergies and Parasites in Children. Foods. 2023; 12(13):2465. https://doi.org/10.3390/foods12132465
Chicago/Turabian StylePacki, Kacper, Alicja Rudek, Joanna Matysiak, Sylwia Klimczak, Eliza Matuszewska, Natalia Rzetecka, and Jan Matysiak. 2023. "Food Allergies and Parasites in Children" Foods 12, no. 13: 2465. https://doi.org/10.3390/foods12132465
APA StylePacki, K., Rudek, A., Matysiak, J., Klimczak, S., Matuszewska, E., Rzetecka, N., & Matysiak, J. (2023). Food Allergies and Parasites in Children. Foods, 12(13), 2465. https://doi.org/10.3390/foods12132465





