Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals
2.3. Measurement of the Total Antioxidant Capacity
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Determination of Total Phenolic Content (TPC)
2.6. Mineral Concentration Measurement
2.7. Soils Physico-Chemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Phenols and Antioxidant Activity
3.2. Mineral Nutrients
3.3. Physico-Chemical Analysis of Soil
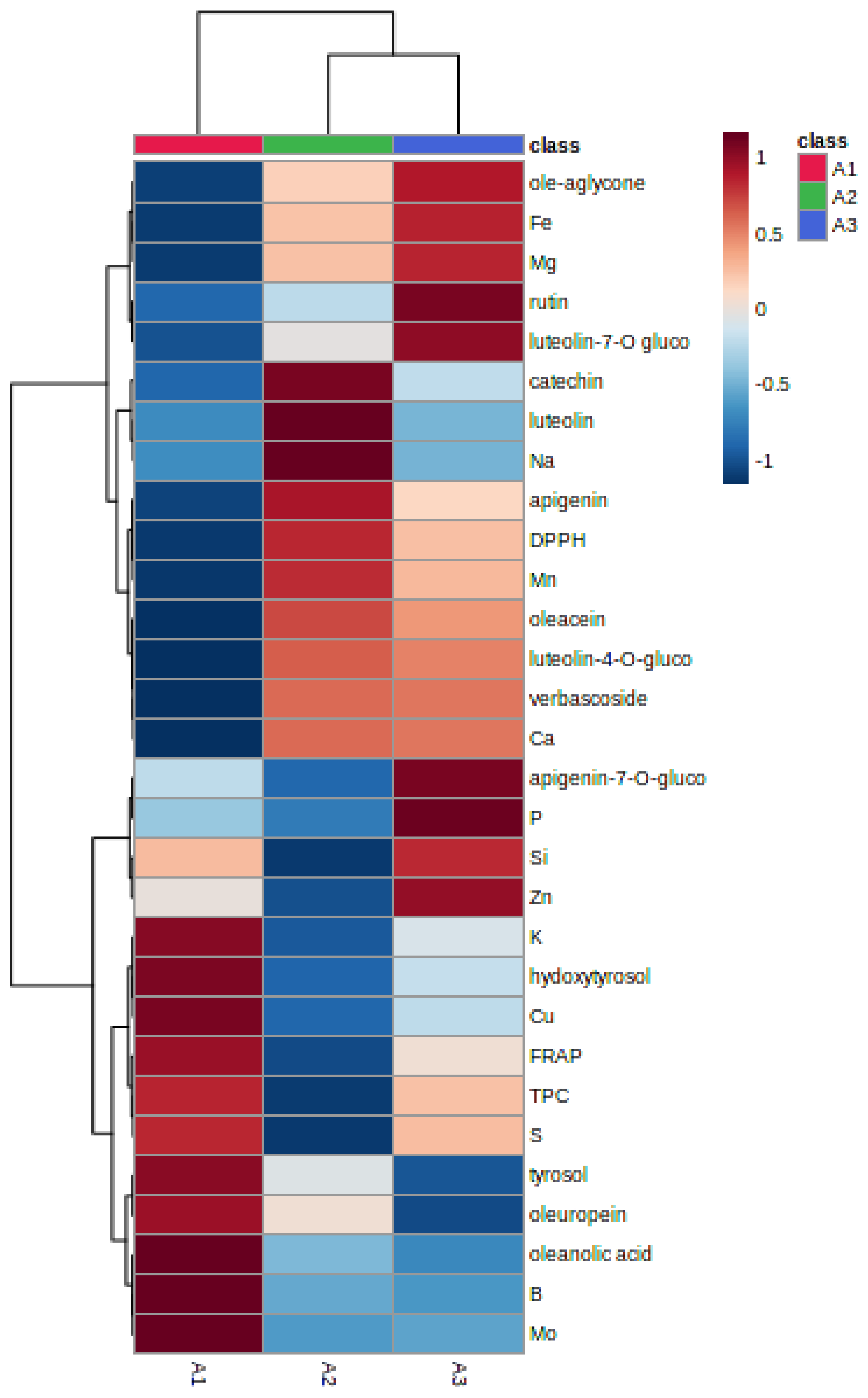
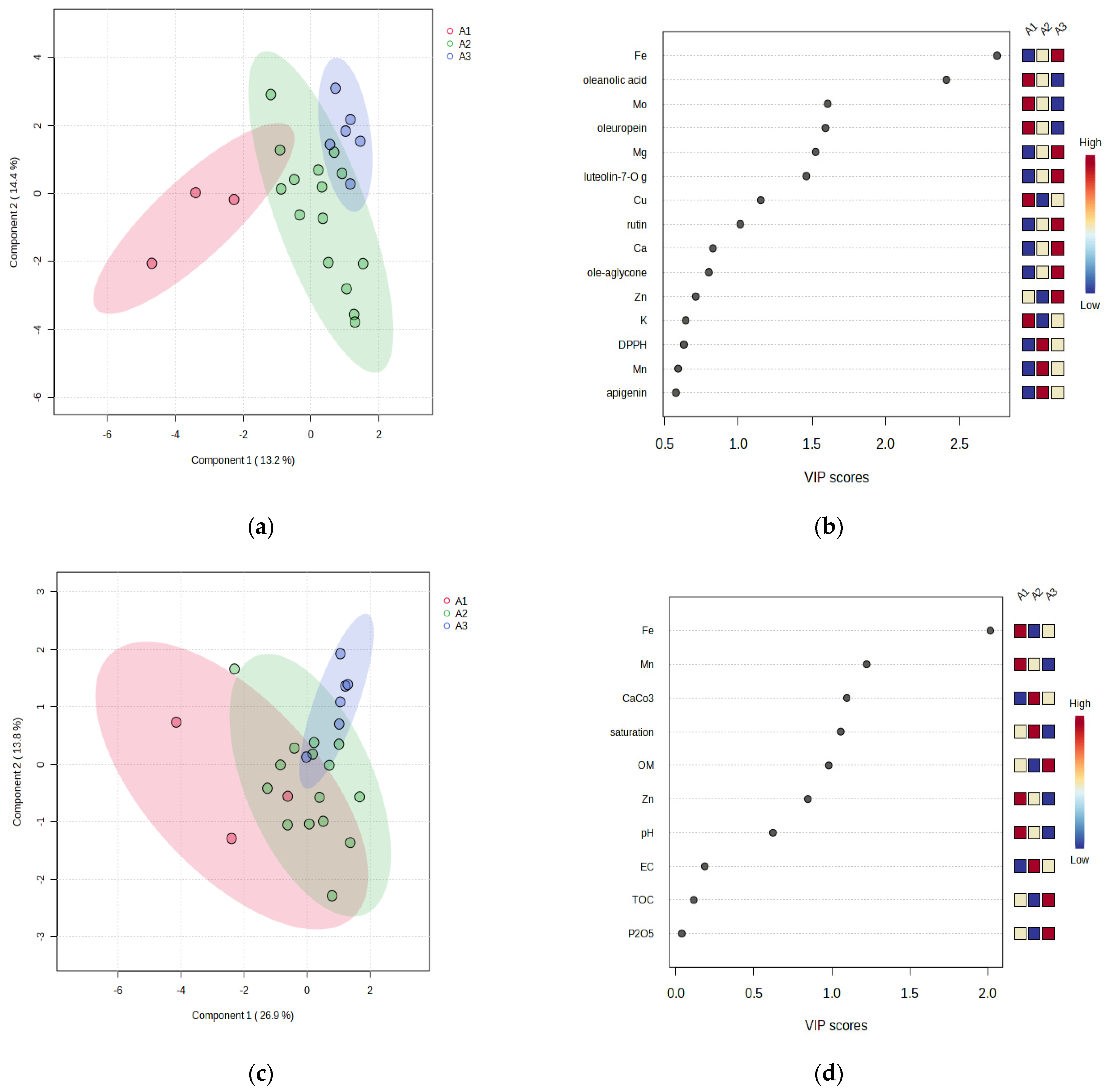
3.4. Classification of Olive Leaves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E.J.F.C. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Abdeljelil, Z.B.; Tekaya, M.; Mechri, B.; Flamini, G.; Hammami, M.J.I.J.A.B. Changes in volatiles of olive tree Olea europaea according to season and foliar fertilization. Int. J. Agric. Biol. 2017, 19, 1633–1639. [Google Scholar]
- González-Hedström, D.; Priego, T.; Amor, S.; de la Fuente-Fernández, M.; Martín, A.I.; López-Calderón, A.; Inarejos-García, A.M.; García-Villalón, Á.L.; Granado, M.J.A. Olive leaf extract supplementation to old wistar rats attenuates aging-induced sarcopenia and increases insulin sensitivity in adipose tissue and skeletal muscle. Antioxidants 2021, 10, 737. [Google Scholar] [CrossRef]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J.J.F. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm. J. 2010, 18, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Antiatherogenic components of olive oil. Curr. Atheroscler. Rep. 2001, 3, 64–67. [Google Scholar] [CrossRef]
- Torić, J.; Karković Marković, A.; Jakobušić Brala, C.; Barbarić, M.J.A.P. Anticancer effects of olive oil polyphenols and their combinations with anticancer drugs. Acta Pharm. 2019, 69, 461–482. [Google Scholar] [CrossRef]
- Han, J.; Talorete, T.P.; Yamada, P.; Isoda, H.J.C. Anti-proliferative and apoptotic effects of oleuropein and hydroxytyrosol on human breast cancer MCF-7 cells. Cytotechnology 2009, 59, 45–53. [Google Scholar] [CrossRef]
- Cifá, D.; Skrt, M.; Pittia, P.; Di Mattia, C.; Ulrih, N.P. Enhanced yield of oleuropein from olive leaves using ultrasound-assisted extraction. Food Sci. Nutr. 2018, 6, 1128–1137. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Phenolic compounds and antioxidant activity of olive leaf extracts. Nat. Prod. Res. 2012, 26, 186–189. [Google Scholar] [CrossRef]
- Temime, S.B.; Wael, T.; Bechir, B.; Leila, A.; Douja, D.; Mokhtar, Z. Changes in olive oil quality of Chétoui variety according to origin of plantation. J. Food Lipids 2006, 13, 88–99. [Google Scholar] [CrossRef]
- Abdallah, M.B.; Trupiano, D.; Polzella, A.; De Zio, E.; Sassi, M.; Scaloni, A.; Zarrouk, M.; Youssef, N.B.; Scippa, G.S. Unraveling physiological, biochemical and molecular mechanisms involved in olive (Olea europaea L. cv. Chétoui) tolerance to drought and salt stresses. J. Plant Physiol. 2018, 220, 83–95. [Google Scholar] [CrossRef]
- Edziri, H.; Jaziri, R.; Chehab, H.; Verschaeve, L.; Flamini, G.; Boujnah, D.; Hammami, M.; Aouni, M.; Mastouri, M. A comparative study on chemical composition, antibiofilm and biological activities of leaves extracts of four Tunisian olive cultivars. Heliyon 2019, 5, e01604. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez Román, D.; Gómez Caravaca, A.M.; Zarrouk, M.; Segura Carretero, A. Geographical characterization of Tunisian olive tree leaves (cv. Chemlali) using HPLC-ESI-TOF and IT/MS fingerprinting with hierarchical cluster analysis. J. Anal. Methods Chem. 2018, 2018, 6789704. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, M.; Şahin, S. Effects of geographical origin and extraction methods on total phenolic yield of olive tree (Olea europaea) leaves. J. Taiwan Inst. Chem. Eng. 2013, 44, 8–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Xu, C.; Deng, Y.; Wen, B.; Xie, P.; Huang, L. Effect of geographical location and soil fertility on main phenolic compounds and fatty acids compositions of virgin olive oil from Leccino cultivar in China. Food Res. Int. 2022, 157, 111207. [Google Scholar] [CrossRef]
- Lukić, I.; Pasković, I.; Žurga, P.; Majetić Germek, V.; Brkljača, M.; Marcelić, Š.; Ban, D.; Grozić, K.; Lukić, M.; Užila, Z. Determination of the variability of biophenols and mineral nutrients in olive leaves with respect to cultivar, collection period and geographical location for their targeted and well-timed exploitation. Plants 2020, 9, 1667. [Google Scholar] [CrossRef]
- Polić Pasković, M.; Vidović, N.; Lukić, I.; Žurga, P.; Majetić Germek, V.; Goreta Ban, S.; Kos, T.; Čoga, L.; Tomljanović, T.; Simonić-Kocijan, S.J.H. Phenolic Potential of Olive Leaves from Different Istrian Cultivars in Croatia. Horticulturae 2023, 9, 594. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Major, N.; Perković, J.; Palčić, I.; Bažon, I.; Horvat, I.; Ban, D.; Goreta Ban, S. The Phytochemical and Nutritional Composition of Shallot Species (Allium × cornutum, Allium × proliferum and A. cepa Aggregatum) Is Genetically and Environmentally Dependent. Antioxidants 2022, 11, 1547. [Google Scholar] [CrossRef] [PubMed]
- Vidović, N.; Pasković, I.; Marcelić, Š.; Lukić, I.; Brkić Bubola, K.; Klisović, D.; Novoselić, A.; Palčić, I.; Polić Pasković, M.; Herak Ćustić, M.; et al. Effect of Combined Sulfur and Nitrogen Foliar Supply on Olive Oil Volatile Compounds and Sensory Attributes. Horticulturae 2022, 8, 912. [Google Scholar] [CrossRef]
- Paredes, M.J.; Moreno, E.; Ramos-Cormenzana, A.; Martinez, J. Characteristics of soil after pollution with waste waters from olive oil extraction plants. Chemosphere 1987, 16, 1557–1564. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Magdich, S.; Ahmed, C.B.; Jarboui, R.; Rouina, B.B.; Boukhris, M.; Ammar, E. Dose and frequency dependent effects of olive mill wastewater treatment on the chemical and microbial properties of soil. Chemosphere 2013, 93, 1896–1903. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Ruiz-Jiménez, J.; de Castro, M.D.L. Discrimination and classification of olive tree varieties and cultivation zones by biophenol contents. J. Agric. Food Chem. 2006, 54, 9706–9712. [Google Scholar] [CrossRef]
- Mansour-Gueddes, S.B.; Saidana-Naija, D.; Flamini, G.; Cheraief, I.; Braham, M. Assessment of the Climatic Condition’s Impact on Volatiles, Polyphenols and Mineral Contents in Tunisian Olive Tree (Olea europaea L.). Pol. J. Environ. Stud. 2022, 31, 219–230. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Pucci, E.; Palumbo, D.; Puiu, A.; Lai, A.; Fiorani, L.; Zoani, C. Characterization and Discrimination of Italian Olive (Olea europaea sativa) Cultivars by Production Area Using Different Analytical Methods Combined with Chemometric Analysis. Foods 2022, 11, 1085. [Google Scholar] [CrossRef]
- Cetinkaya, H.; Koc, M.; Kulak, M. Monitoring of mineral and polyphenol content in olive leaves under drought conditions: Application chemometric techniques. Ind. Crop. Prod. 2016, 88, 78–84. [Google Scholar] [CrossRef]
- Erel, R.; Kerem, Z.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Zipori, I.; Basheer, L.; Yermiyahu, U. Olive (Olea europaea L.) tree nitrogen status is a key factor for olive oil quality. J. Agric. Food Chem. 2013, 61, 11261–11272. [Google Scholar] [CrossRef]
- Tekaya, M.; El-Gharbi, S.; Mechri, B.; Chehab, H.; Bchir, A.; Chraief, I.; Ayachi, M.; Boujnah, D.; Attia, F.; Hammami, M. Improving performance of olive trees by the enhancement of key physiological parameters of olive leaves in response to foliar fertilization. Int. J. Food Prop. 2016, 38, 101. [Google Scholar] [CrossRef]
- Penel, C.; Van Cutsem, P.; Greppin, H. Interactions of a plant peroxidase with oligogalacturonides in the presence of calcium ions. Phytochemistry 1999, 51, 193–198. [Google Scholar] [CrossRef]
- Stateras, D.C.; Moustakas, N.K. Seasonal changes of macro-and micro-nutrients concentration in olive leaves. J. Plant Nutr. 2018, 41, 186–196. [Google Scholar] [CrossRef]
- Hartmann, H.; Uriu, K.; Lilleland, O. Olive nutrition. In Fruit Nutrition; Childers, N.F., Ed.; Horticultural Publications: New Brunswick, NJ, USA, 1966; pp. 252–268. [Google Scholar]
- Pasković, I.; Soldo, B.; Talhaoui, N.; Palčić, I.; Brkljača, M.; Koprivnjak, O.; Germek, V.M.; Ban, D.; Klanjac, J.; Franić, M. Boron foliar application enhances oleuropein level and modulates volatile compound composition in olive leaves. Sci. Hortic. 2019, 257, 108688. [Google Scholar] [CrossRef]
- Suleiman, M.H.; ALaerjani, W.M.A.; Mohammed, M.E.A. Influence of altitudinal variation on the total phenolic and flavonoid content of Acacia and Ziziphus honey. Int. J. Food Prop. 2020, 23, 2077–2086. [Google Scholar] [CrossRef]
- Baccouri, B.; Sieren, T.; Rajhi, I.; Willenberg, I. Characterization of the fingerprint profile of bioactive constituents of extra virgin olive oils from Peninsula Tunisian Cap Bon with regard to altitude. Eur. Food Res. Technol. 2023, 249, 497–509. [Google Scholar] [CrossRef]
- Mousa, Y.M.; Gerasopoulos, D.; Metzidakis, I.; Kiritsakis, A. Effect of altitude on fruit and oil quality characteristics of ‘Mastoides’ olives. J. Sci. Food Agric. 1996, 71, 345–350. [Google Scholar] [CrossRef]
- Zidorn, C. Altitudinal variation of secondary metabolites in flowering heads of the Asteraceae: Trends and causes. Phytochem. Rev. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Saviranta, N.M.; Julkunen-Tiitto, R.; Oksanen, E.; Karjalainen, R.O. Leaf phenolic compounds in red clover (Trifolium pratense L.) induced by exposure to moderately elevated ozone. Environ. Pollut. 2010, 158, 440–446. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I.J.A. A reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
| Location | Antioxidant Activity | TPC | |
|---|---|---|---|
| FRAP | DPPH | ||
| Jendouba | 269.75 ± 5.04 a,b | 256.26 ± 15.87 a,b | 3968.33 ± 241.14 a,b |
| Beja 1 | 280.01 ± 4.99 a | 273.88 ± 2.61 a | 2940.33 ± 341.52 c,d |
| Beja 2 | 247.88 ± 23.56 a,b | 243.37 ± 13.21 a,b | 3485 ± 639.26 b,c |
| Bouarada 1 | 245.61 ± 20.94 a,b | 236.15 ± 27.39 a,b | 3313 ± 59.10 b,c |
| Bouarada 2 | 269.24 ± 31.13 a,b | 270.28 ± 11.77 a,b | 4308.33 ± 441.51 a |
| Bouarada 3 | 223.97 ± 15.45 b | 266.72 ± 0.54 b | 2378 ± 286.71 d |
| Zaghouan | 263.65 ± 16.36 a,b | 276.27 ± 3.57 a | 3448.67 ± 219.51 b,c |
| Nabeul | 260.64 ± 10.86 a,b | 278.86 ± 2.67 a | 4060.67 ± 83.06 a,b |
| p-value | * | ** | *** |
| Location | Triterpenes | Simple Phenols | Phenolics Acids | Secoiridoids | |||
|---|---|---|---|---|---|---|---|
| Oleanolic Acid | Tyrosol | Hydroxytyrosol | Verbascoside | Oleuropein | Oleacein | Ole-Aglycone | |
| Jendouba | 198.28 ± 14.41 b | 22.6 ± 5.80 b,c | 24.27 ± 2.16 b | 58.5 ± 4.01 a | 8604.27 ± 319.94 a,b | 158.95 ± 3.50 f | 18.33 ± 2.17 c |
| Beja 1 | 158.08 ± 7.01 b,c | 34.85 ± 2.25 a | 5.38 ± 0.75 d,e | 40.92 ± 1.74 b,c | 8998.17 ± 428.63 a | 260.43 ± 10.10 c,d | 26.53 ± 3.92 b |
| Beja 2 | 144.13 ± 37.98 b,c | 21.32 ± 3.14 b,c | 9.07 ± 1.60 c,d | 23.72 ± 2.79 d | 8152.73 ± 286.82 a,b | 226.92 ± 4.47 d,e | 21.62 ± 1.76 b,c |
| Bouarada 1 | 118.93 ± 24.55 c | 29.6 ± 2.01 a,b | 9.88 ± 2.01 c,d | 31.92 ± 3.48 c,d | 7141.4 ± 200.40 c | 380.9 ± 18.48 a | 27.88 ± 1.72 b |
| Bouarada 2 | 172.7 ± 10.59 b,c | 19.87 ± 0.88 c | 32.02 ± 3.11 a | 27.32 ± 4.19 d | 7801.34 ± 581.64 b,c | 323.38 ± 28.46 b | 21.72 ± 4,23 b,c |
| Bouarada 3 | 275.07 ± 10.65 a | 23.95 ± 4.37 b,c | 0.13 ± 0.23 e | 49.85 ± 5.33 a,b | 7120.48 ± 173.17 c | 237.32 ± 7.80 c,d | 36 ± 0.31 a |
| Zaghouan | 141.12 ± 22.84 b,c | 21.43 ± 1.71 b,c | 13.42 ± 2.17 c | 45.07 ± 1.02 b | 8988.98 ± 407.75 a | 195.25 ± 15.94 e,f | 36.9 ± 3.99 a |
| Nabeul | 175.33 ± 25.90 b,c | 25.55 ± 1.72 b,c | 25.52 ± 2.72 b | 58.02 ± 4.60 a | 7760.5 ± 199.28 b,c | 273.67 ± 7.43 c | 21.73 ± 2.38 b,c |
| p-value | *** | *** | *** | *** | *** | *** | *** |
| RT (min) | 29.64 | 8.49 | 13.63 | 11.86 | 14.75 | 15.25 | 20.56 |
| Source of Variation | Flavonoids | ||||||
|---|---|---|---|---|---|---|---|
| Catechin | Rutin | Luteolin-7-O-Glucoside | Apigenin-7-O-Gluccoside | Luteolin | Luteolin-4-O-Glucoside | Apigenin | |
| Jendouba | 36.18 ± 4.93 d | 237.47 ± 23.67 c | 771.18 ± 38.91 c | 76.92 ± 9.57 a | 5.58 ± 0.62 d | 34.75 ± 2.17 a,b | 0 ± 0 e |
| Beja 1 | 41.3 ± 4.82 b,c,d | 328.25 ± 19.84 b | 1210.22 ± 116.93 b | 36.72 ± 4.50 e | 16.7 ± 0.87 b | 39.37 ± 4.82 a | 0.96 ± 0.24 d |
| Beja 2 | 50.8 ± 5.01 a,b | 370.85 ± 10.83 b | 1095,58 ± 35.92 b | 45.62 ± 2.36 d,e | 14.33 ± 0.97 b,c | 27.25 ± 4.15 b,c | 2.35 ± 0.17 c |
| Bouarada 1 | 44.83 ± 4.83 a,b,c,d | 240.67 ± 32.69 c | 769.68 ± 26.34 c | 50.28 ± 4.43 c,d | 24.18 ± 1.36 a | 23.33 ± 2.65 c | 3.88 ± 0.35 a |
| Bouarada 2 | 56.17 ± 6.08 a | 357.5 ± 41.73 c | 899.6 ± 52.16 c | 65.37 ± 3.71 a,b | 11.23 ± 2.5 c | 41.6 ± 3.38 a | 0 ± 0 e |
| Bouarada 3 | 55.42 ± 2.97 a | 117.43 ± 6.05 a | 1424.6 ± 74.94 a | 21.82 ± 1.33 f | 27.46 ± 2.77 a | 41.85 ± 2.96 a | 3.25 ± 0.55 a,b |
| Zaghouan | 38.25 ± 3.04 c,d | 393.32 ± 15.90 b | 1103 ± 24.14 b | 36.57 ± 1.53 e | 14.03 ± 1.29 b,c | 40.52 ± 1.14 a | 2.48 ± 0.40 b,c |
| Nabeul | 49.25 ± 1.59 a,b,c | 324.72 ± 9.27 c | 811.68 ± 50.17 c | 61.77 ± 2.95 b,c | 11.93 ± 2.05 b,c | 34.5 ± 4.85 a,b | 0 ± 0 e |
| p-value | *** | *** | *** | *** | *** | *** | *** |
| RT (min) | 7.47 | 11.13 | 12.25 | 13.87 | 16.79 | 3.59 | 20.06 |
| Macronutrients (g/kg DW) | Micronutrients (mg/kg DW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | P | K | Ca | S | Na | Mg | Fe | Zn | Mn | Cu | B | Si | Mo |
| Jendouba | 1.01 ± 0.01 a | 12.2 ± 0.51 a | 20.47 ± 1.38 e | 2.2 ± 0.03 a | 4.08 ± 0.19 d | 0.92 ± 0.02 d | 69.49 ± 4.47 c | 21.25 ± 0.78 a | 14.29 ± 0.52 d | 7.6 ± 0.21 a | 7.68 ± 0.19 a,b | 96.42 ± 9.06 b,c | 0.29 ± 0.04 a |
| Beja 1 | 0.64 ± 0.02 d,e | 9.85 ± 0.43 b,c | 15.45 ± 0.48 f | 1.44 ± 0.06 e | 2.23 ± 0.29 f | 0.61 ± 0.01 f | 62.74 ± 3.93 c | 14.41 ± 0.51 c | 31.63 ± 0.66 b | 3.58 ± 0.23 b,c | 7.53 ± 0.28 a,b | 103.58 ± 8.64 b,c | 0.15 ± 0.01 c |
| Beja 2 | 0.98 ± 0.15 a,b | 9.66 ± 0.1 b,c | 22.86 ± 0.47 d,e | 1.84 ± 0.03 c,d | 6.89 ± 0.04 b | 0.86 ± 0.005 d,e | 73.18 ± 1.26 c | 16.79 ± 0.34 b | 24.04 ± 0.14 c | 4.57 ± 0.24 b | 6.78 ± 2.07 b,c | 80.91 ± 5.52 c | 0.16 ± 0.005 c |
| Bouarada 1 | 0.62 ± 0.15 d,e | 7.64 ± 0.05 d | 26.61 ± 0.24 b | 1.72 ± 0.04 d | 8.38 ± 0.19 a | 0.89 ± 0.0 d,e | 102.08 ± 3.13 a,b | 7.96 ± 0.20 d | 28.83 ± 0.68 b | 2.55 ± 0.09 c | 7.66 ± 0.49 a,b | 235.83 ± 5.57 a | 0.17 ± 0.01 b,c |
| Bouarada 2 | 0.6 ± 0.04 e | 9.36 ± 0.11 c | 23.02 ± 0.88 c,d,e | 1.73 ± 0.1 d | 5.38 ± 0.07 c | 0.8 ± 0.02 e | 100.3 ± 4.48 a,b | 14.8 ± 1.13 c | 24.75 ± 0.12 c | 4.22 ± 0.36 b,c | 8.23 ± 0.8 a,b | 199.25 ± 14.17 a | 0.24 ± 0.03 a,b |
| Bouarada 3 | 0.73 ± 0.03 e | 5.78 ± 0.22 e | 35.46 ± 1.84 a | 2.01 ± 0.09 b,c | 2.35 ± 0.03 e,f | 2.25 ± 0.10 a | 83.21 ± 2.21 b,c | 14.25 ± 0.91 c | 50 ± 2.39 a | 3.97 ± 1.52 b,c | 4.98 ± 0.33 c | 167.08 ± 5.63 a,b | 0.27 ± 0.02 a |
| Zaghouan | 0.93 ± 0.2 c | 9.21 ± 0.13 c | 23.54 ± 0.47 c,d | 2.07 ± 0.06 a,b | 4.09 ± 0.05 d | 1.45 ± 0.01 b | 80.65 ± 6.10 b,c | 18.38 ± 0.60 b | 51.13 ± 0.78 a | 4.95 ± 0.53 b | 6.43 ± 0.23 b,c | 134.38 ± 10.05 a,b,c | 0.19 ± 0.01 b,c |
| Nabeul | 0.68 ± 0.2 c,d | 10.29 ± 0.19 d | 25.63 ± 0.41 b,c | 1.92 ± 0.04 b,c | 2.68 ± 0.07 e | 1.1 ± 0.02 c | 121.13 ± 11.23 a | 13.45 ± 0.45 c | 25.33 ± 0.57 c | 4.38 ± 0.13 b | 9.79 ± 0.51 a | 238.75 ± 4.50 a | 0.24 ± 0.02 a,b |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Location | CaCO3 | pH | OM | Saturation | TOC | P2O5 | EC | Mn | Zn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Jendouba | 4.93 ± 1 c | 7.61 ± 7.60 b | 1.06 ± 0.23 a,b | 44 ± 5.29 a,b | 0.93 ± 0.17 a | 15.3 ± 0.06 b,c,d,e | 1.07 ± 0.12 a | 107.5 ± 0.1 a | 7.34 ± 0.14 b | 570.63 ± 0.33 a |
| Beja 1 | 23.33 ± 1.52 b | 8.05 ± 8.04 a,b | 0.76 ± 0.48 b | 47 ± 4.35 a | 0.42 ± 0.04 b,c | 18.23 ± 0.03 a,b,c,d | 0.85 ± 0.95 a | 34.53 ± 0.20 c | 10.25 ± 0.13 a | 385.18 ± 0.46 b |
| Beja 2 | 61 ± 4.35 a | 8.12 ± 8.11 a | 0.93 ± 0.05 a,b | 51.67 ± 2.88 a | 0.62 ± 0.09 b | 21.17 ± 8.07 a,b,c | 0.98 ± 0.03 a | 45.17 ± 0.20 b | 4.26 ± 0.06 c | 128.3 ± 0.33 d |
| Bouarada 1 | 65.67 ± 2.08 a | 8.13 ± 8.13 a | 1.22 ± 0.33 a,b | 47.33 ± 5.13 a | 0.98 ± 0.13 a | 26.45 ± 0.36 a | 1.02 ± 0.11 a | 7.08 ± 5.25 f | 0.29 ± 0.03 g | 27.68 ± 0.24 h |
| Bouarada 2 | 57.33 ± 2.51 a | 7.95 ± 7.95 a,b | 0.97 ± 0.02 a,b | 48.67 ± 2.51 a | 0.6 ± 0.14 b | 9.94 ± 0.52 d,e | 1.03 ± 0.07 a | 24.53 ± 0.20 d | 4.06 ± 0.06 c | 117.88 ± 0.82 e |
| Bouarada 3 | 60 ± 13.22 a | 7.75 ± 7.75 a,b | 0.82 ± 0.18 b | 51 ± 6.55 a | 0.58 ± 0.07 b,c | 12.41 ± 0.40 c,d,e | 1.03 ± 0.24 a | 12.38 ± 0.17 e | 1.62 ± 0.11 e | 48.27 ± 0.06 g |
| Zaghouan | 38 ± 5 b | 7.87 ± 7.87 a,b | 1.57 ± 0.09 a | 48.33 ± 5.77 a | 0.95 ± 0.03 a | 21.93 ± 3.63 a,b | 0.86 ± 0.18 a | 19.43 ± 0.15 d | 2.4 ± 0.45 d | 77.49 ± 0.36 f |
| Nabeul | 5.8 ± 0.36 c | 7.93 ± 7.93 a,b | 0.91 ± 0.12 a,b | 31.67 ± 1.52 b | 0.29 ± 0.06 c | 8.67 ± 0.01 e | 0.79 ± 0.6 a | 3.07 ± 0.06 f | 1.01 ± 0.13 f | 185.98 ± 10.11 c |
| p-value | *** | * | * | ** | *** | *** | n.s | *** | *** | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakraoui, M.; Hannachi, H.; Pasković, I.; Vidović, N.; Polić Pasković, M.; Palčić, I.; Major, N.; Goreta Ban, S.; Hamrouni, L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods 2023, 12, 2565. https://doi.org/10.3390/foods12132565
Zakraoui M, Hannachi H, Pasković I, Vidović N, Polić Pasković M, Palčić I, Major N, Goreta Ban S, Hamrouni L. Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods. 2023; 12(13):2565. https://doi.org/10.3390/foods12132565
Chicago/Turabian StyleZakraoui, Mariem, Hédia Hannachi, Igor Pasković, Nikolina Vidović, Marija Polić Pasković, Igor Palčić, Nikola Major, Smiljana Goreta Ban, and Lamia Hamrouni. 2023. "Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves" Foods 12, no. 13: 2565. https://doi.org/10.3390/foods12132565
APA StyleZakraoui, M., Hannachi, H., Pasković, I., Vidović, N., Polić Pasković, M., Palčić, I., Major, N., Goreta Ban, S., & Hamrouni, L. (2023). Effect of Geographical Location on the Phenolic and Mineral Composition of Chetoui Olive Leaves. Foods, 12(13), 2565. https://doi.org/10.3390/foods12132565











