Flavor Characterization of Native Xinjiang Flat Peaches Based on Constructing Aroma Fingerprinting and Stoichiometry Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Determination of Soluble Solid Content (SSC) and Titratable Acidity (TA)
2.4. Determination of Total Phenol Content (TPC) and Total Flavonoid Content (TFC)
2.5. Determination of Soluble Sugars and Organic Acids by HPLC
2.6. Determination of Phenolic Compounds by HPLC
2.7. Determination of Antioxidant Activity In Vitro
2.8. Determination of Volatile Aroma Compounds by HS-SPME-GC-MS
2.9. Date Analysis
3. Results
3.1. The Assessment of Taste of Flat Peaches from Xinjiang
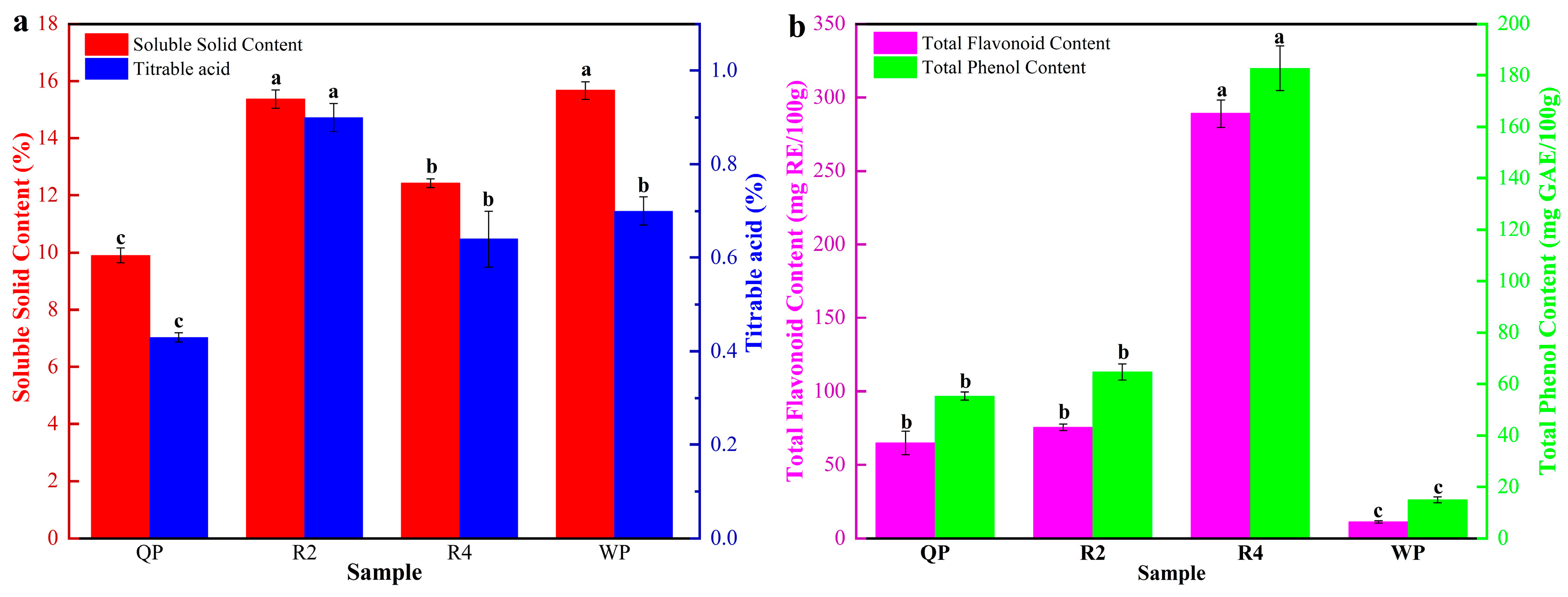
3.1.1. The SSC and TA of Flat Peaches from Xinjiang
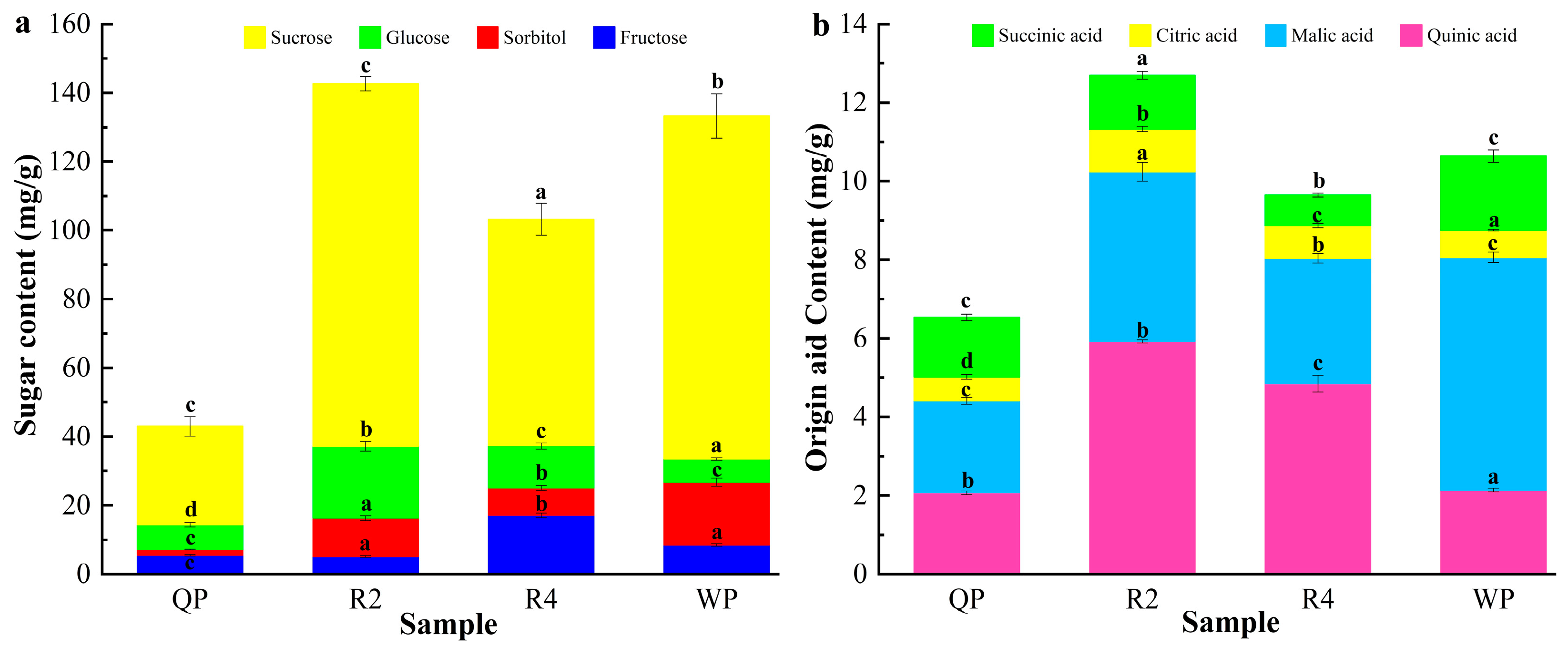
3.1.2. The Composition and Content of Soluble Sugars and Organic Acids of Flat Peaches from Xinjiang
3.2. Assessment of Phenolic Compounds and Antioxidant Capacity of Flat Peaches from Xinjiang
3.2.1. Analysis of Phenolic Compounds, TFC, and TPC in Flat Peaches from Xinjiang
3.2.2. Antioxidant Activity In Vitro in Flat Peaches from Xinjiang
3.2.3. Correlation Analysis of Phenolic Compounds and Antioxidant Capacity of Flat Peaches from Xinjiang
3.3. Construction of Aroma Fingerprinting of Flat Peaches from Xinjiang
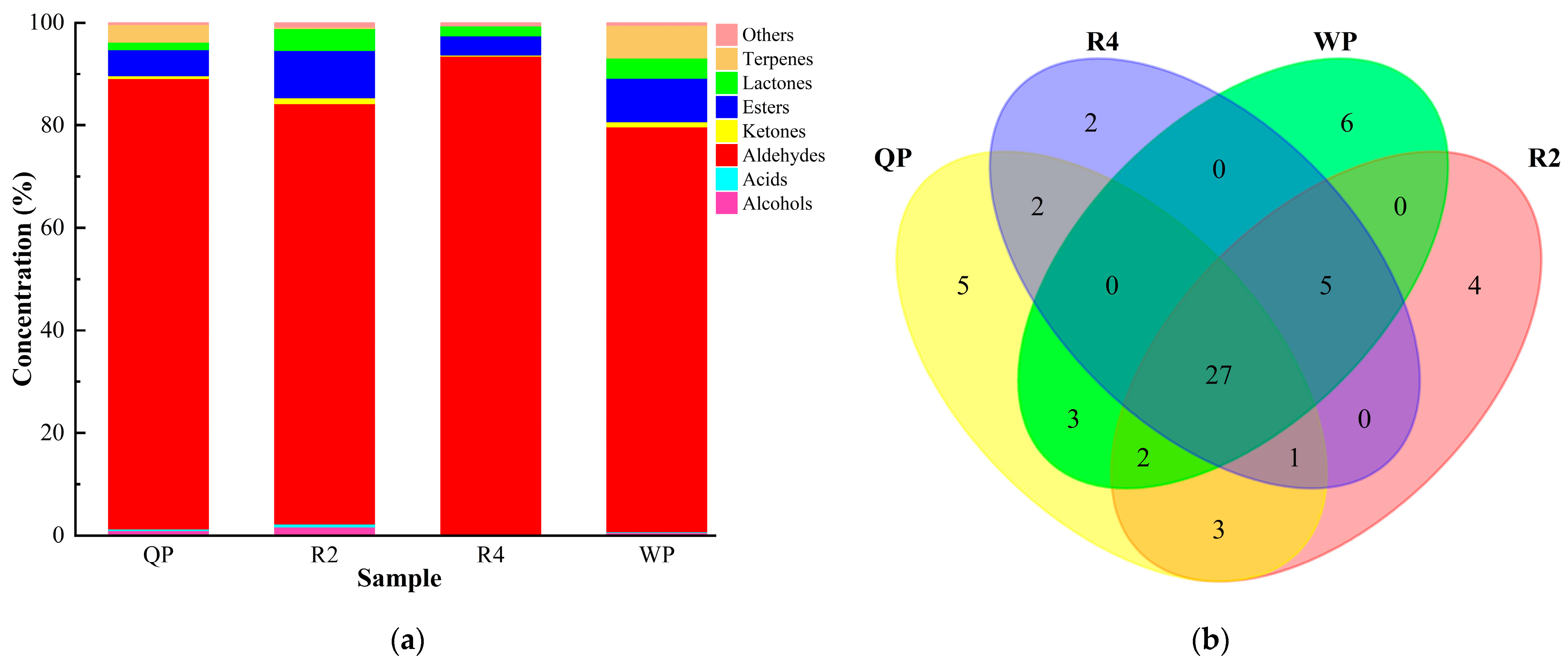
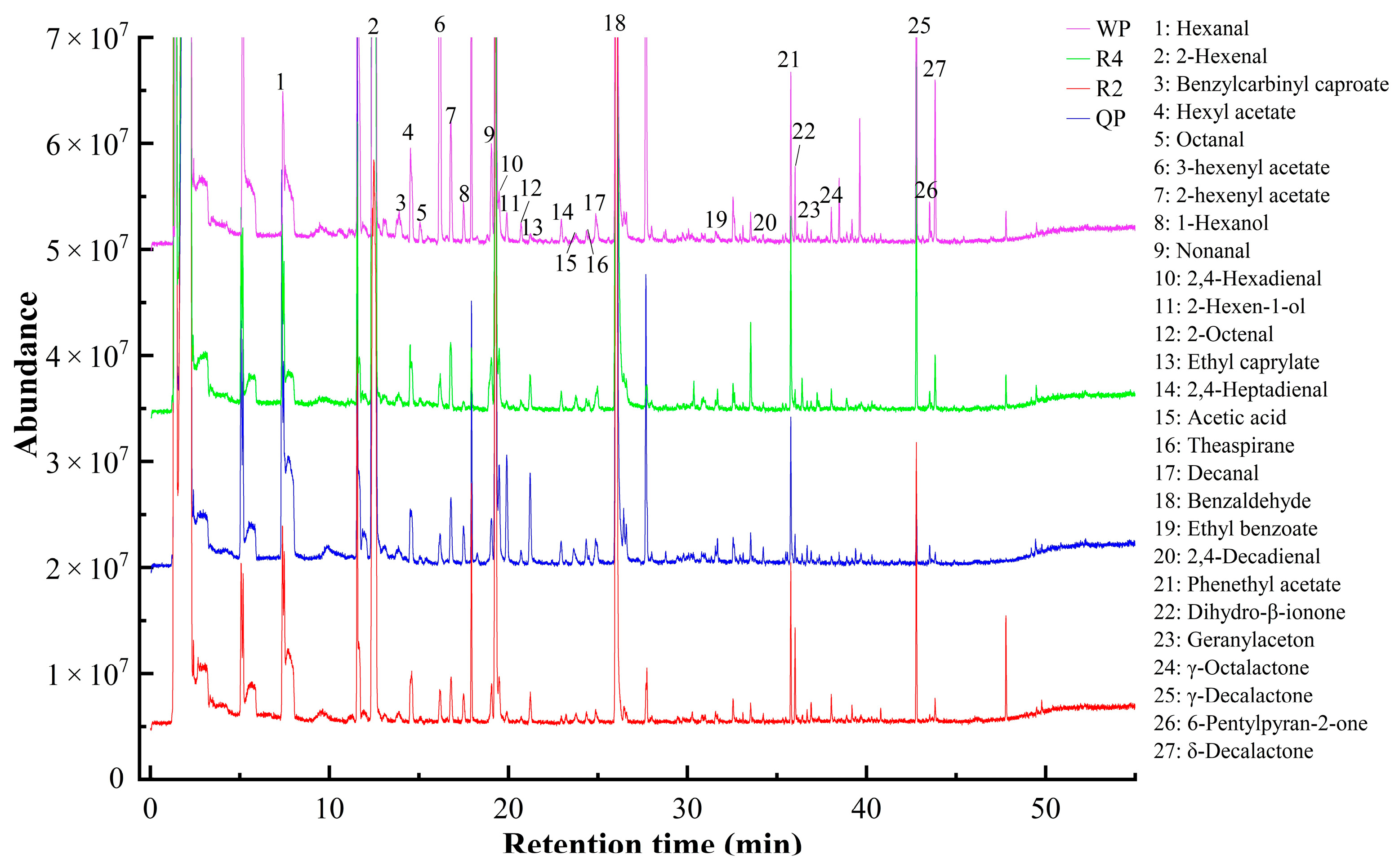
3.3.1. The Composition and Content of Volatile Compounds of Flat Peaches from Xinjiang
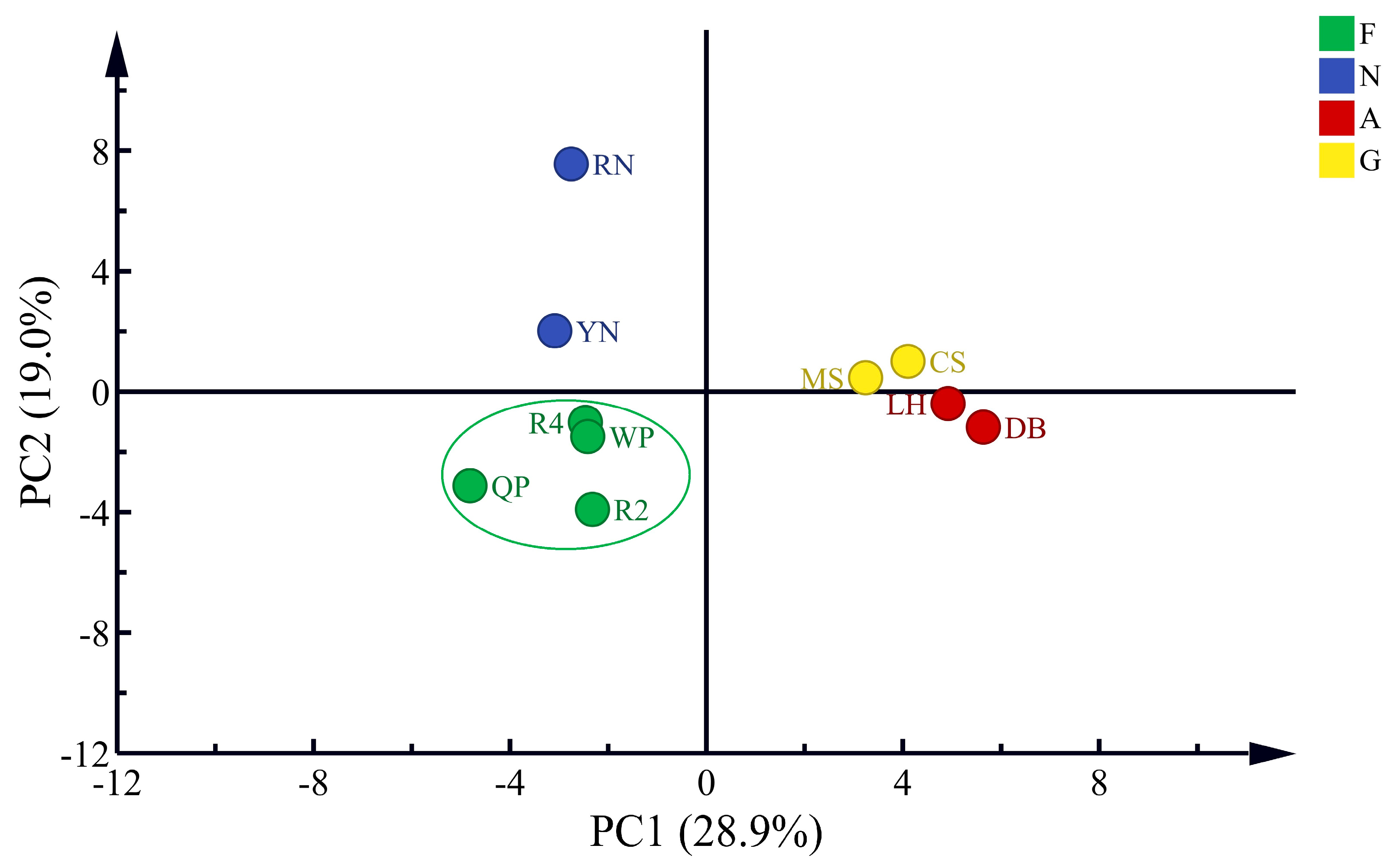
3.3.2. PCA of Volatile Compounds of Flat Peaches from Xinjiang
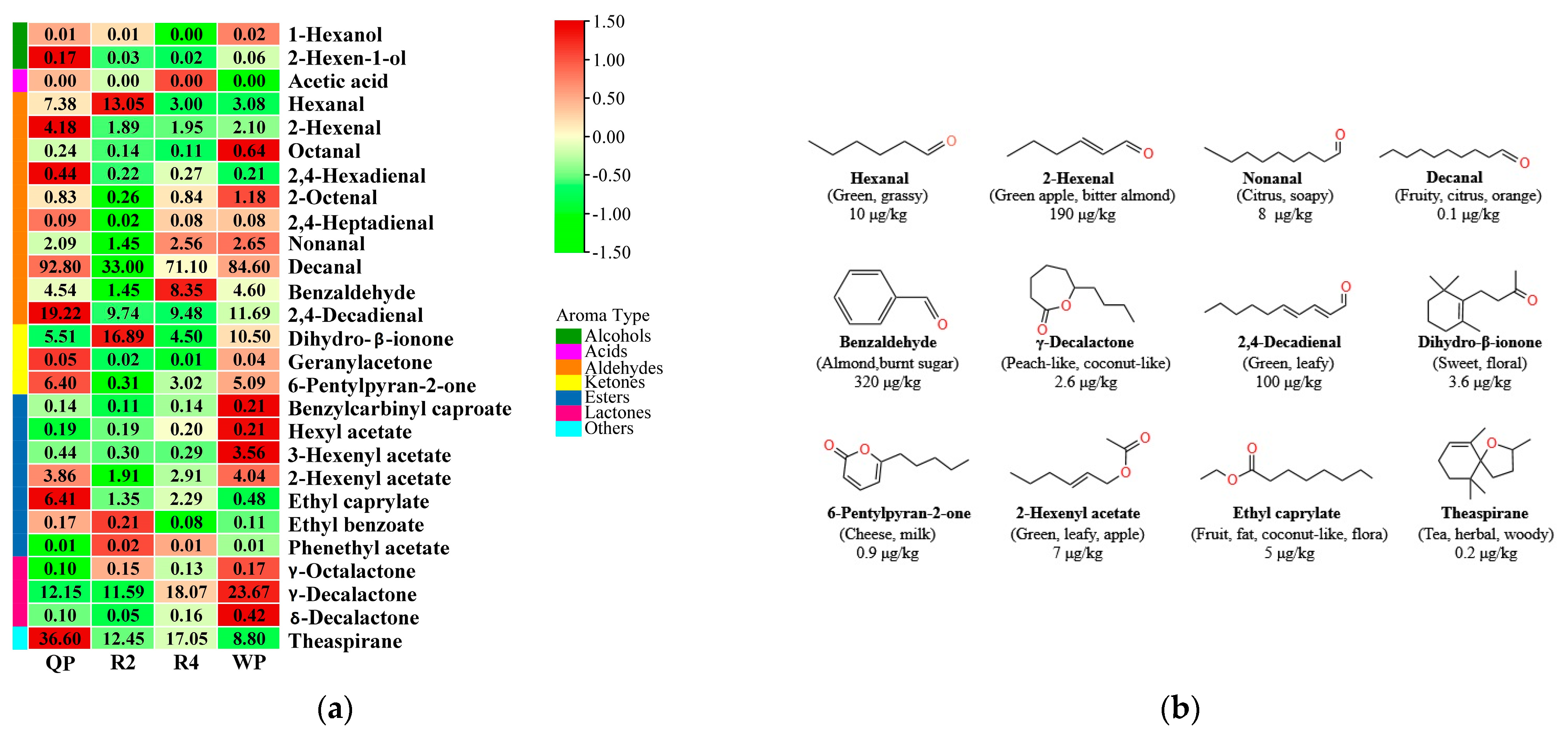
3.3.3. Screening of Common Aromatic Substances in Flat Peaches from Xinjiang
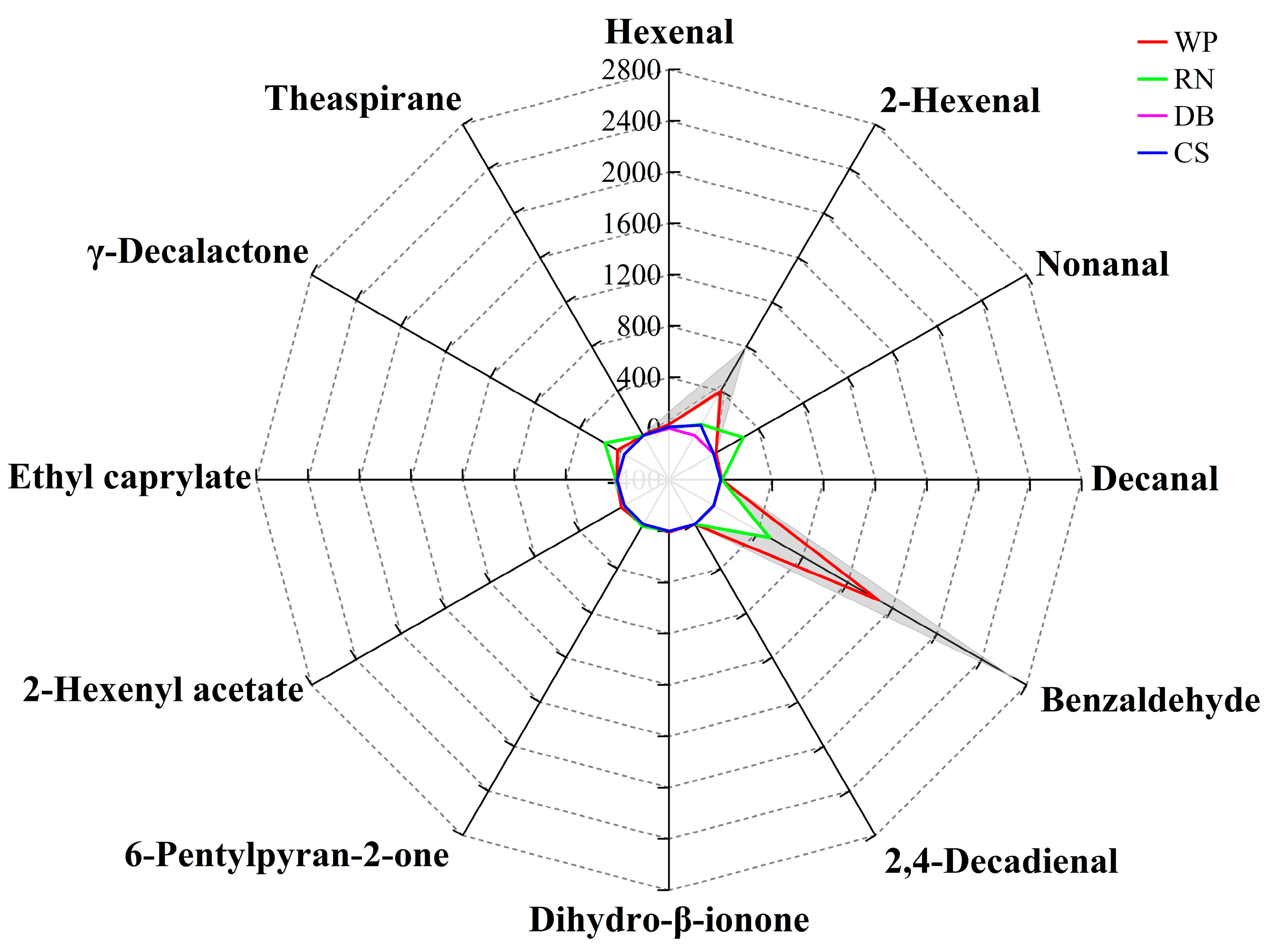
3.3.4. Identification of Characteristic Aroma Fingerprint of Flat Peaches from Xinjiang
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, L. Genetic resources, breeding programs in China, and gene mining of peach: A review. Hortic. Plant J. 2020, 6, 205–215. [Google Scholar] [CrossRef]
- Legua, P.; Hernández, F.; Díaz-Mula, H.M.; Valero, D.; Serrano, M. Quality, bioactive compounds, and antioxidant activity of new flat-type peach and nectarine cultivars: A comparative study. J. Food Sci. 2011, 76, C729–C735. [Google Scholar] [CrossRef]
- Tian, H.; Wang, P.; Zhan, P.; Yan, H.; Zhou, W.; Zhang, F. Effects of β-glucosidase on the aroma characteristics of flat peach juice as assessed by descriptive sensory analysis and gas chromatography and compared by partial least squares regression. LWT Food Sci. Technol. 2017, 82, 113–120. [Google Scholar] [CrossRef]
- Cao, K.; Wang, L.; Zhu, G.; Fang, W.; Chen, C.; Wang, X. High-throughput sequencing of Prunus ferganensis indicates that it is a geographical population of P. persica. Tree Genet. Genomes 2018, 14, 92. [Google Scholar] [CrossRef]
- Ma, R.; Yu, M.; Du, P.; Guo, H.; Song, H. Evaluation of germplasm resources and breeding of flat peach. In Proceedings of the XXVI International Horticultural Congress: Asian Plants with Unique Horticultural Potential: Genetic Resources, Cultural, Toronto, ON, Canada, 11–17 August 2002. [Google Scholar]
- Conte, L.; Moser, L.; Fantechi, P.; Nicotra, A. New types of high quality peaches: Flat peaches (P. persica var. Platicarpa) and Ghiaccio peach series with long on tree fruit life. In Proceedings of the Fifth International Peach Symposium, Davis, CA, USA, 8–11 July 2001. [Google Scholar]
- López-Gómez, A.; Navarro-Martínez, A.; Martínez-Hernández, G.B. Active paper sheets including nanoencapsulated essential oils: A green packaging technique to control ethylene production and maintain quality in fresh horticultural products—A case study on flat peaches. Foods 2020, 9, 1904. [Google Scholar] [CrossRef]
- Batlle, I.; Fontich, C.; Lozano, L.; Iglesias, I.; Reig, G.; Alegre, S.; Echeverría, G.; de Herralde, F.; Claveria, E.; Dolcet-Sanjuan, R. The peach breeding programme IRTA-ASF: Aiming for high fruit quality. In Proceedings of the International Symposium on the Challenge for a Sustainable Production, Protection and Consumption of Mediterranean Fruits and Nuts, Lisbon, Portugal, 22–27 August 2010. [Google Scholar]
- Wu, B.; Zhao, J.; Chen, J.; Xi, H.; Jiang, Q.; Li, S. Maternal inheritance of sugars and acids in peach (P. persica (L.) Batsch) fruit. Euphytica 2012, 188, 333–345. [Google Scholar] [CrossRef]
- Aubert, C.; Chalot, G.; Lurol, S.; Ronjon, A.; Cottet, V. Relationship between fruit density and quality parameters, levels of sugars, organic acids, bioactive compounds and volatiles of two nectarine cultivars, at harvest and after ripening. Food Chem. 2019, 297, 124954. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Desseva, I.; Manolov, I.; Petkova, N.; Vrancheva, R.; Peltekov, A.; Slavov, A.; Zhivondov, A. Comprehensive evaluation of late season peach varieties (Prunus persica L.): Fruit nutritional quality and phytochemicals. Molecules 2021, 26, 2818. [Google Scholar] [CrossRef]
- Redondo, D.; Gimeno, D.; Calvo, H.; Venturini, M.E.; Oria, R.; Arias, E. Antioxidant activity and phenol content in different tissues of stone fruits at thinning and at commercial maturity stages. Waste Biomass Valorization 2021, 12, 1861–1875. [Google Scholar] [CrossRef]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Bompadre, S.; Mezzetti, B.; Quiles, J.L.; Giampieri, F.; Battino, M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016, 56, S46–S59. [Google Scholar] [CrossRef]
- Ceccarelli, D.; Simeone, A.M.; Nota, P.; Piazza, M.G.; Fideghelli, C.; Caboni, E. Phenolic compounds (hydroxycinnamic acids, flavan-3-ols, flavonols) profile in fruit of Italian peach varieties. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 1370–1375. [Google Scholar] [CrossRef]
- Ding, T.; Cao, K.; Fang, W.; Zhu, G.; Chen, C.; Wang, X.; Wang, L. Evaluation of phenolic components (anthocyanins, flavanols, phenolic acids, and flavonols) and their antioxidant properties of peach fruits. Sci. Hortic. 2020, 268, 109365. [Google Scholar] [CrossRef]
- Di Vaio, C.; Marallo, N.; Graziani, G.; Ritieni, A.; Petriccione, M. Phenolic compounds, carotenoids and antioxidant activity of flat and standard peach cultivars [Prunus persica (L.) Batsch]. In Proceedings of the VIII International Peach Symposium, Matera, Italy, 17–20 June 2013. [Google Scholar]
- Wang, L.; Li, M.; Jin, W.; Li, S.; Zhang, S.; Yu, L. Variations in the components of Osmanthus fragrans Lour. essential oil at different stages of flowering. Food Chem. 2009, 114, 233–236. [Google Scholar] [CrossRef]
- Biniecka, M.; Caroli, S. Analytical methods for the quantification of volatile aromatic compounds. Trends Anal. Chem. 2011, 30, 1756–1770. [Google Scholar] [CrossRef]
- Leinen, L.J.; Swenson, V.A.; Juntunen, H.L.; McKay, S.E.; O’Hanlon, S.M.; Videau, P.; Gaylor, M.O. Profiling Volatile constituents of homemade preserved foods prepared in early 1950s South Dakota (USA) using solid-phase microextraction (SPME) with gas chromatography–mass spectrometry (GC–MS) determination. Molecules 2019, 24, 660. [Google Scholar] [CrossRef]
- Bianchi, T.; Weesepoel, Y.; Koot, A.; Iglesias, I.; Eduardo, I.; Gratacós-Cubarsí, M.; Guerrero, L.; Hortós, M.; van Ruth, S. Investigation of the aroma of commercial peach (Prunus persica L. Batsch) types by Proton Transfer Reaction–Mass Spectrometry (PTR–MS) and sensory analysis. Food Res. Int. 2017, 99, 133–146. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.; Wei, W.; Xi, W.; Xu, C.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- An, K.; Liu, H.; Fu, M.; Qian, M.C.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Identification of the cooked off-flavor in heat-sterilized lychee (Litchi chinensis Sonn.) juice by means of molecular sensory science. Food Chem. 2019, 301, 125282. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Vrancheva, R.; Dincheva, I. HS-SPME-GC–MS volatile profile characterization of peach (Prunus persica L. Batsch) varieties grown in the Eastern Balkan Peninsula. Plants 2022, 11, 166. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of aroma trait of the white-fleshed peach ‘Hu Jing Mi Lu’ and the yellow-fleshed peach ‘Jin Yuan’ based on odor activity value and odor characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Li, Q.; Yang, S.; Zhang, R.; Liu, S.; Zhang, C.; Li, Y.; Li, J. Characterization of honey peach (Prunus persica (L.) Batsch) aroma variation and unraveling the potential aroma metabolism mechanism through proteomics analysis under abiotic stress. Food Chem. 2022, 386, 132720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Q.; Granato, D.; Xu, Y.; Ho, C. Association between chemistry and taste of tea: A review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Universität München: Garching, Germany, 1998; pp. 1–63. [Google Scholar]
- Tan, F.; Wang, P.; Zhan, P.; Tian, H. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chem. 2022, 366, 130604. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Bakar, M.F.A.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3, 15067. [Google Scholar] [CrossRef]
- Orazem, P.; Stampar, F.; Hudina, M. Fruit quality of Redhaven and Royal Glory peach cultivars on seven different rootstocks. J. Agric. Food Chem. 2011, 59, 9394–9401. [Google Scholar] [CrossRef]
- Bae, H.; Yun, S.K.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H.; Jun, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food Qual. 2014, 87, 24–29. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Tan, C.; Frankel, E.N.; Barrett, D.M. Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars. J. Agric. Food Chem. 2000, 48, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Juániz, I.; Ludwig, I.A.; Huarte, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.; De Peña, M.-P. Influence of heat treatment on antioxidant capacity and (poly) phenolic compounds of selected vegetables. Food Chem. 2016, 197, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, Z.; Ye, Z.; Su, M. Multiplex analyses of the changes of aromatic compounds during the development of peach fruit using GC–MS and iTRAQ proteomic techniques. Sci. Hortic. 2018, 236, 96–105. [Google Scholar] [CrossRef]
- Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the key aroma volatile compounds in cranberry (Vaccinium macrocarpon Ait.) using gas chromatography–olfactometry (GC–O) and odor activity value (OAV). Eur. Food Res. Technol. 2016, 64, 4990–4999. [Google Scholar] [CrossRef]
- Pino, J.A.; Quijano, C.E. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Sci. Technol. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Yu, H.; Guo, W.; Xie, T.; Ai, L.; Tian, H.; Chen, C. Aroma characteristics of traditional Huangjiu produced around Winter Solstice revealed by sensory evaluation, gas chromatography–mass spectrometry and gas chromatography–ion mobility spectrometry. Food Res. Int. 2021, 145, 110421. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Oszmiański, J.; Golis, T. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Crisosto, G.M. Relationship between ripe soluble solids concentration (RSSC) and consumer acceptance of high and low acid melting flesh peach and nectarine (Prunus persica (L.) Batsch) cultivars. Postharvest Biol. Technol. 2005, 38, 239–246. [Google Scholar] [CrossRef]
- Hilaire, C. The peach industry in France: State of art, research and development. In Proceedings of the First Mediterranean Peach Symposium, Agrigento, Italy, 10 September 2003. [Google Scholar]
- Crisosto, C.; Garner, D.; Crisosto, G.; Bowerman, E. Increasing ‘Blackamber’ plum (Prunus salicina Lindell) consumer acceptance. Postharvest Biol. Technol. 2004, 34, 237–244. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.E.; Goulas, V.; Manganaris, G.A.; Ziogas, V.; Manganaris, A. The appraisal of qualitative parameters and antioxidant contents during postharvest peach fruit ripening underlines the genotype significance. Postharvest Biol. Technol. 2016, 115, 142–150. [Google Scholar] [CrossRef]
- Yuan, L.; You, L.; Yang, X.; Chen, X.; Huang, G.; Chen, X.; Shi, W.; Sun, Y. Consensual regression of soluble solids content in peach by near infrared spectrocopy. Foods 2022, 11, 1095. [Google Scholar] [CrossRef]
- Reig, G.; Iglesias, I.; Gatius, F.; Alegre, S. Antioxidant capacity, quality, and anthocyanin and nutrient contents of several peach cultivars [Prunus persica (L.) Batsch] grown in Spain. J. Agric. Food Chem. 2013, 61, 6344–6357. [Google Scholar] [CrossRef]
- Belisle, C.; Phan, U.T.X.; Adhikari, K.; Chavez, D.J. A fruit quality survey of peach cultivars grown in the southeastern United States. Hort Technol. Hortte 2018, 28, 189–201. [Google Scholar] [CrossRef]
- Orazem, P.; Stampar, F.; Hudina, M. Quality analysis of ‘Redhaven’ peach fruit grafted on 11 rootstocks of different genetic origin in a replant soil. Food Chem. 2011, 124, 1691–1698. [Google Scholar] [CrossRef]
- Meredith, F.I.; Robertson, J.A.; Horvat, R.J. Changes in physical and chemical parameters associated with quality and postharvest ripening of harvester peaches. J. Agric. Food Chem. 1989, 37, 1210–1214. [Google Scholar] [CrossRef]
- Wills, R.B.; Scriven, F.M.; Greenfield, H. Nutrient composition of stone fruit (Prunus spp.) cultivars: Apricot, cherry, nectarine, peach and plum. J. Sci. Food Agric. 1983, 34, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Vimolmangkang, S.; Zheng, H.; Peng, Q.; Jiang, Q.; Wang, H.; Fang, T.; Liao, L.; Wang, L.; He, H.; Han, Y. Assessment of sugar components and genes involved in the regulation of sucrose accumulation in peach fruit. J. Agric. Food Chem. 2016, 64, 6723–6729. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Principal component analysis (PCA) of physicochemical compounds’ content in different cultivars of peach fruits, including qualification and quantification of sugars and organic acids by HPLC. Eur. Food Res. Technol. 2019, 245, 929–938. [Google Scholar] [CrossRef]
- Kroger, M.; Meister, K.; Kava, R. Low-calorie sweeteners and other sugar substitutes: A review of the safety issues. Compr. Rev. Food Sci. Food Saf. 2006, 5, 35–47. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Robertson, J.; Horvat, R.; Lyon, B.; Meredith, F.; Senter, S.; Okie, W. Comparison of quality characteristics of selected yellow-and white-fleshed peach cultivars. J. Food Sci. 1990, 55, 1308–1311. [Google Scholar] [CrossRef]
- Bordonaba, J.G.; Terry, L.A. Manipulating the taste-related composition of strawberry fruits (Fragaria × ananassa) from different cultivars using deficit irrigation. Food Chem. 2010, 122, 1020–1026. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, J.; Wu, X.; Liu, R. Antioxidant and antiproliferative activities of common vegetables. J. Agric. Food Chem. 2002, 50, 6910–6916. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Xu, Y.; Hu, J. Investigation on the phenolic composition, related oxidation and antioxidant activity of thinned peach dried by different methods. LWT Food Sci. Technol. 2021, 147, 111573. [Google Scholar] [CrossRef]
- Liu, H.; Cao, J.; Jiang, W. Evaluation and comparison of vitamin C, phenolic compounds, antioxidant properties and metal chelating activity of pulp and peel from selected peach cultivars. LWT Food Sci. Technol. 2015, 63, 1042–1048. [Google Scholar] [CrossRef]
- Mrázová, M.; Rampáčková, E.; Šnurkovič, P.; Ondrášek, I.; Nečas, T.; Ercisli, S. Determination of selected beneficial substances in peach fruits. Sustainability 2021, 13, 14028. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Gil, M.; Tomás-Barberán, F.; Hess-Pierce, B.; Holcroft, D.; Kader, A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Bonamassa, B.; Canistro, D.; Sapone, A.; Vivarelli, F.; Vornoli, A.; Longo, V.; Paolini, M. Harmful effects behind the daily supplementation of a fixed vegetarian blend in the rat model. Food Chem. Toxicol. 2016, 97, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Cianciosi, D.; Forbes-Hernández, T.; Regolo, L.; Alvarez-Suarez, J.; Navarro-Hortal, M.; Xiao, J.; Quiles, J.; Battino, M.; Giampieri, F. The reciprocal interaction between polyphenols and other dietary compounds: Impact on bioavailability, antioxidant capacity and other physico-chemical and nutritional parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef]
- Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int. J. Mol. Sci. 2016, 17, 998. [Google Scholar] [CrossRef]
- García-Gómez, B.E.; Salazar, J.A.; Nicolás-Almansa, M.; Razi, M.; Rubio, M.; Ruiz, D.; Martínez-Gómez, P. Molecular bases of fruit quality in Prunus species: An integrated genomic, transcriptomic, and metabolic review with a breeding perspective. Int. J. Mol. Sci. 2020, 22, 333. [Google Scholar] [CrossRef]
- Wu, H.; Xu, Y.; Wang, H.; Miao, Y.; Li, C.; Zhao, R.; Shi, X.; Wang, B. Physicochemical characteristics, antioxidant activities, and aroma compound analysis of seven peach cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods 2022, 11, 2944. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, J.; Pu, X.; Shi, X.; Cheng, W.; Wang, B. Volatile compounds analysis and biomarkers identification of four native apricot (Prunus armeniaca L.) cultivars grown in Xinjiang region of China. Foods 2022, 11, 2297. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Wang, H.; Xu, X.; Ye, P.; Wu, H.; Zhao, R.; Shi, X.; Cai, F. Chemical and sensory characteristics of different red grapes grown in Xinjiang, China: Insights into wines composition. Fermentation 2022, 8, 689. [Google Scholar] [CrossRef]
- Niu, Y.; Deng, J.; Xiao, Z.; Zhu, J. Characterization of the major aroma-active compounds in peach (Prunus persica L. Batsch) by gas chromatography–olfactometry, flame photometric detection and molecular sensory science approaches. Food Res. Int. 2021, 147, 110457. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. Exogenous nitric oxide fumigation promoted the emission of volatile organic compounds in peach fruit during shelf life after long-term cold storage. Food Res. Int. 2020, 133, 109135. [Google Scholar] [CrossRef]
- Mohammed, J.; Belisle, C.; Wang, S.; Itle, R.; Adhikari, K.; Chavez, D. Volatile profile characterization of commercial peach (Prunus persica) cultivars grown in Georgia, USA. Horticulturae 2021, 7, 516. [Google Scholar] [CrossRef]
- Sanchez, G.; Besada, C.; Badenes, M.; Monforte, A.; Granell, A. A non-targeted approach unravels the volatile network in peach fruit. PLoS ONE 2012, 7, e38992. [Google Scholar] [CrossRef]
- Xi, W.; Zheng, Q.; Lu, J.; Quan, J. Comparative analysis of three types of peaches: Identification of the key individual characteristic flavor compounds by integrating consumers’ acceptability with flavor quality. Hortic. Plant J. 2017, 3, 1–12. [Google Scholar] [CrossRef]


| Variety | QP | R2 | R4 | WP |
|---|---|---|---|---|
| Coumaric acid | 0.19 ± 0.01 b | 0.39 ± 0.03 a | 0.43 ± 0.03 a | 0.13 ± 0.01 c |
| Proanthocyanidin B1 | 2.77 ± 0.22 b | 2.05 ± 0.16 c | 18.19 ± 0.54 a | 0.75 ± 0.05 d |
| Neochlorogenic acid | 1.12 ± 0.06 c | 1.81 ± 0.07 b | 7.36 ± 0.15 a | 0.17 ± 0.02 d |
| Catechin | 3.14 ± 0.05 b | 1.46 ± 0.04 c | 13.11 ± 0.48 a | 0.40 ± 0.02 d |
| Vanillic acid | 0.08 ± 0.01 c | 0.49 ± 0.02 a | 0.14 ± 0.01 b | 0.08 ± 0.01 c |
| Chlorogenic acid | 1.58 ± 0.02 b | 1.82 ± 0.16 b | 29.49 ± 1.79 a | 0.20 ± 0.02 b |
| Epicatechin | 0.13 ± 0.01 c | 0.44 ± 0.04 b | 1.49 ± 0.11 a | n. d. |
| Rutin | 0.26 ± 0.01 b | 0.12 ± 0.02 d | 0.43 ± 0.02 a | 0.17 ± 0.02 c |
| Quercetin | 0.14 ± 0.01 c | 0.49 ± 0.01 a | 0.11 ± 0.01 d | 0.19 ± 0.03 b |
| Variety | Radical Scavenging Capacity | Reducing Capacity | ||
|---|---|---|---|---|
| ABTS + | DPPH | CUPRAC | FRAP | |
| QP | 324.38 ± 14.31 c | 464.54 ± 12.87 b | 278.50 ± 13.91 b | 297.56 ± 3.85 b |
| R2 | 404.41 ± 24.33 b | 398.04 ± 17.27 c | 274.93 ± 8.78 b | 279.78 ± 8.39 b |
| R4 | 717.61 ± 3.29 a | 799.16 ± 5.57 a | 1145.90 ± 26.32 a | 1252.00 ± 67.66 a |
| WP | 165.40 ± 13.71 d | 207.93 ± 12.81 b | 44.69 ± 3.52 c | 57.56 ± 5.09 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Xu, Y.; Wu, H.; Zhao, R.; Wang, X.; Wang, F.; Fu, Q.; Tang, T.; Shi, X.; Wang, B. Flavor Characterization of Native Xinjiang Flat Peaches Based on Constructing Aroma Fingerprinting and Stoichiometry Analysis. Foods 2023, 12, 2554. https://doi.org/10.3390/foods12132554
Li C, Xu Y, Wu H, Zhao R, Wang X, Wang F, Fu Q, Tang T, Shi X, Wang B. Flavor Characterization of Native Xinjiang Flat Peaches Based on Constructing Aroma Fingerprinting and Stoichiometry Analysis. Foods. 2023; 12(13):2554. https://doi.org/10.3390/foods12132554
Chicago/Turabian StyleLi, Chunyan, Youyou Xu, Huimin Wu, Ruirui Zhao, Xinwei Wang, Fangfang Wang, Qingquan Fu, Tiantian Tang, Xuewei Shi, and Bin Wang. 2023. "Flavor Characterization of Native Xinjiang Flat Peaches Based on Constructing Aroma Fingerprinting and Stoichiometry Analysis" Foods 12, no. 13: 2554. https://doi.org/10.3390/foods12132554
APA StyleLi, C., Xu, Y., Wu, H., Zhao, R., Wang, X., Wang, F., Fu, Q., Tang, T., Shi, X., & Wang, B. (2023). Flavor Characterization of Native Xinjiang Flat Peaches Based on Constructing Aroma Fingerprinting and Stoichiometry Analysis. Foods, 12(13), 2554. https://doi.org/10.3390/foods12132554