Antioxidants and Phenolic Acid Composition of Wholemeal and Refined-Flour, and Related Biscuits in Old and Modern Cultivars Belonging to Three Cereal Species
Abstract
1. Introduction
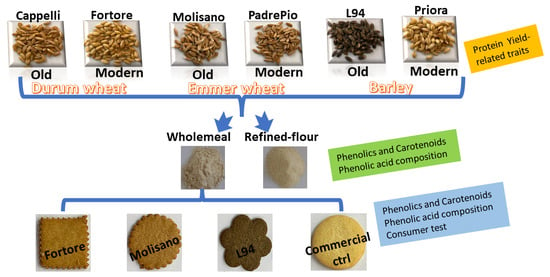
2. Materials and Methods
2.1. Plant Materials
2.2. Wholegrain Analysis
2.3. Processing
2.3.1. Flours Production
2.3.2. Biscuit Production
2.4. Chemical Compounds
2.4.1. Carotenoids
2.4.2. Phenolics
2.4.3. Phenolic Acid and Flavonoid Composition
2.4.4. Antioxidant Activities
2.5. Consumer Acceptance
2.6. Statistical Analysis
3. Results and Discussion
3.1. Whole Grain Quality and Yield-Related Traits
3.2. Effects of Species, Genotype and Crop Year on the Content of Phenolic Compounds and Carotenoids, and Antioxidant Activities in Wholemeal
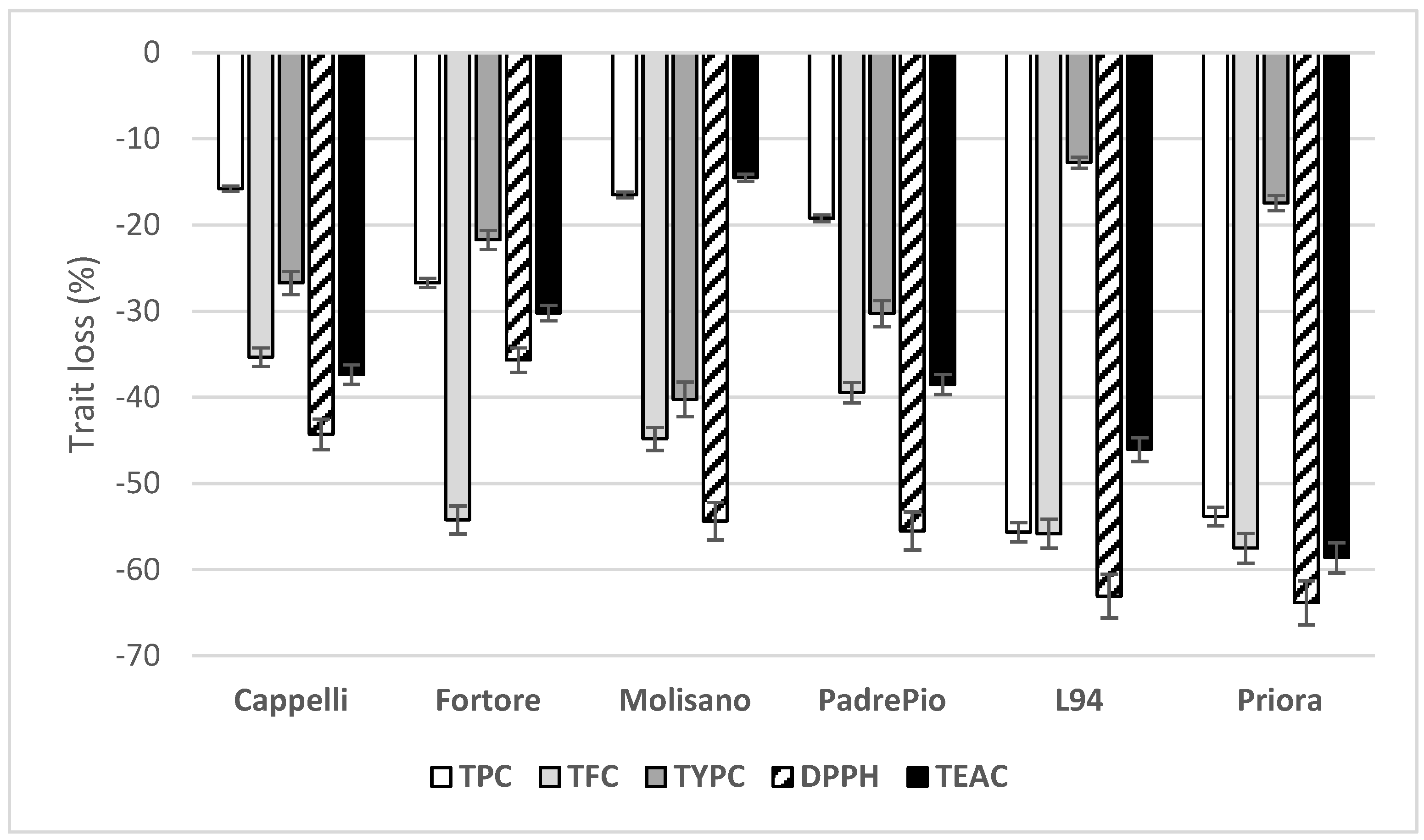
3.3. Effects of Species, Genotype and Crop Year on the Content of Some Phenolic Compounds and Carotenoids and Antioxidant Activities in Refined-Flours
3.4. Phenolic Acid Composition in Wholemeals and Refined-Flours
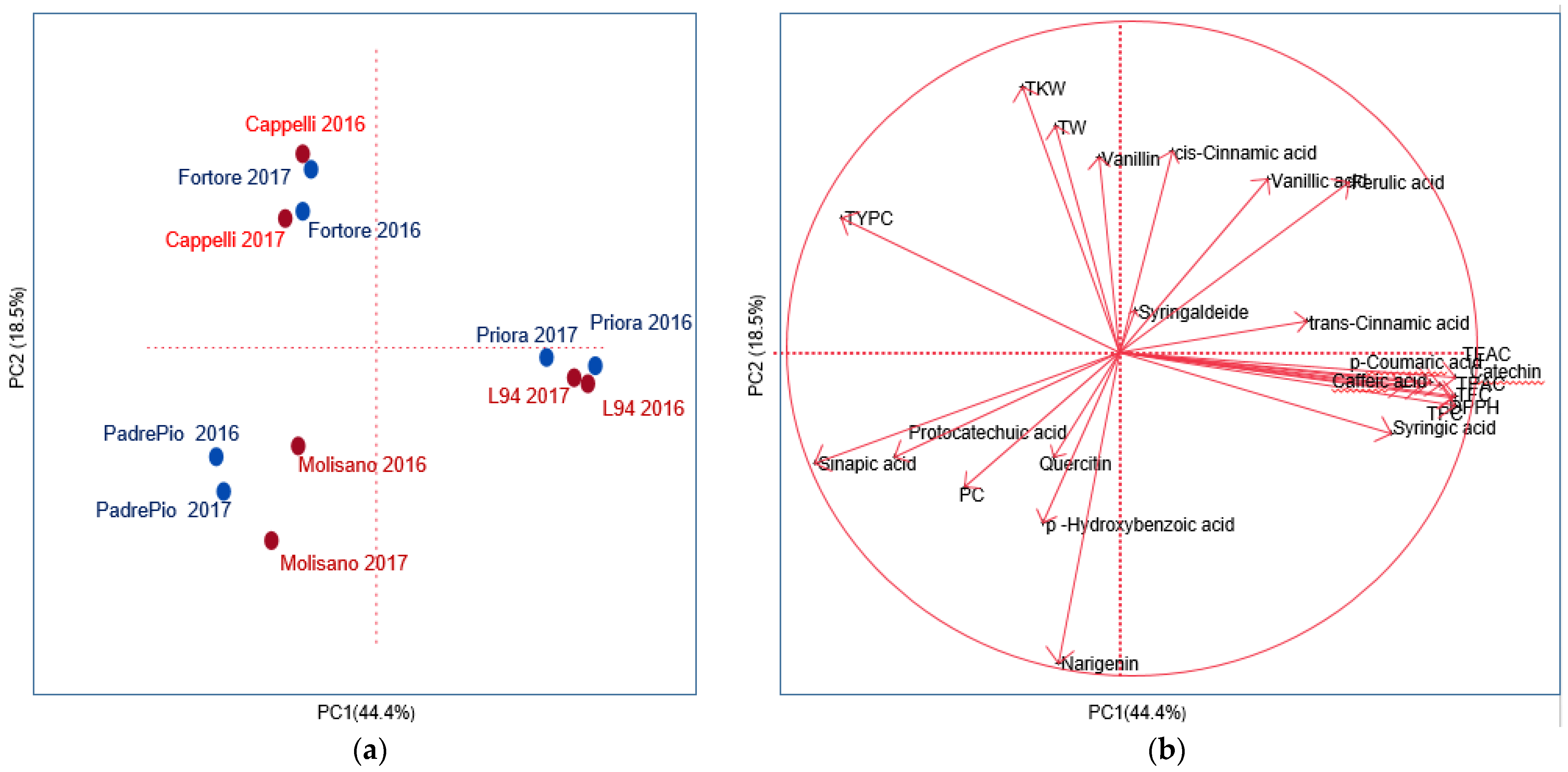
3.5. Principal Component Analysis of Grain and Wholemeal between Phenolics, Phenolic Acids, Quality and Yield-Related Traits
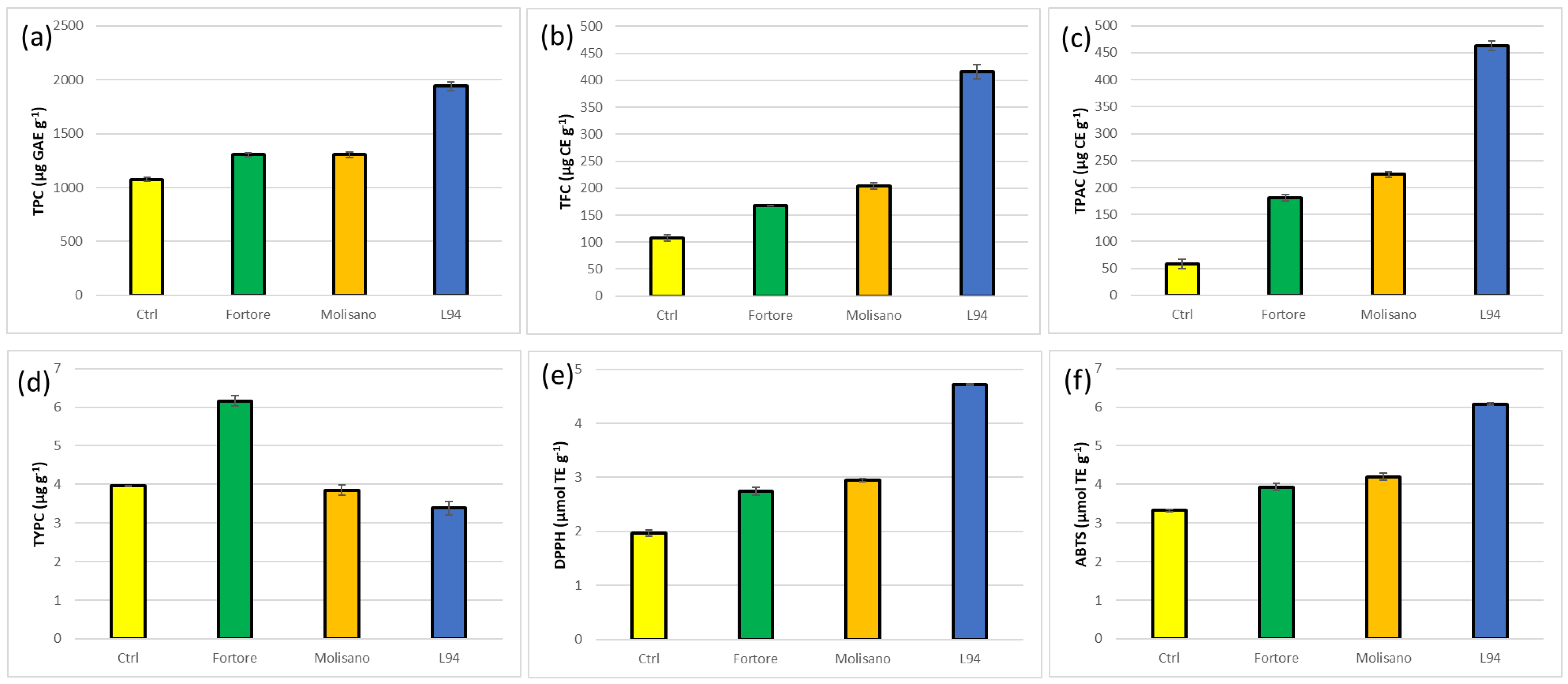
3.6. Effect of Biscuit Processing in Phenolic Compound Level and Composition
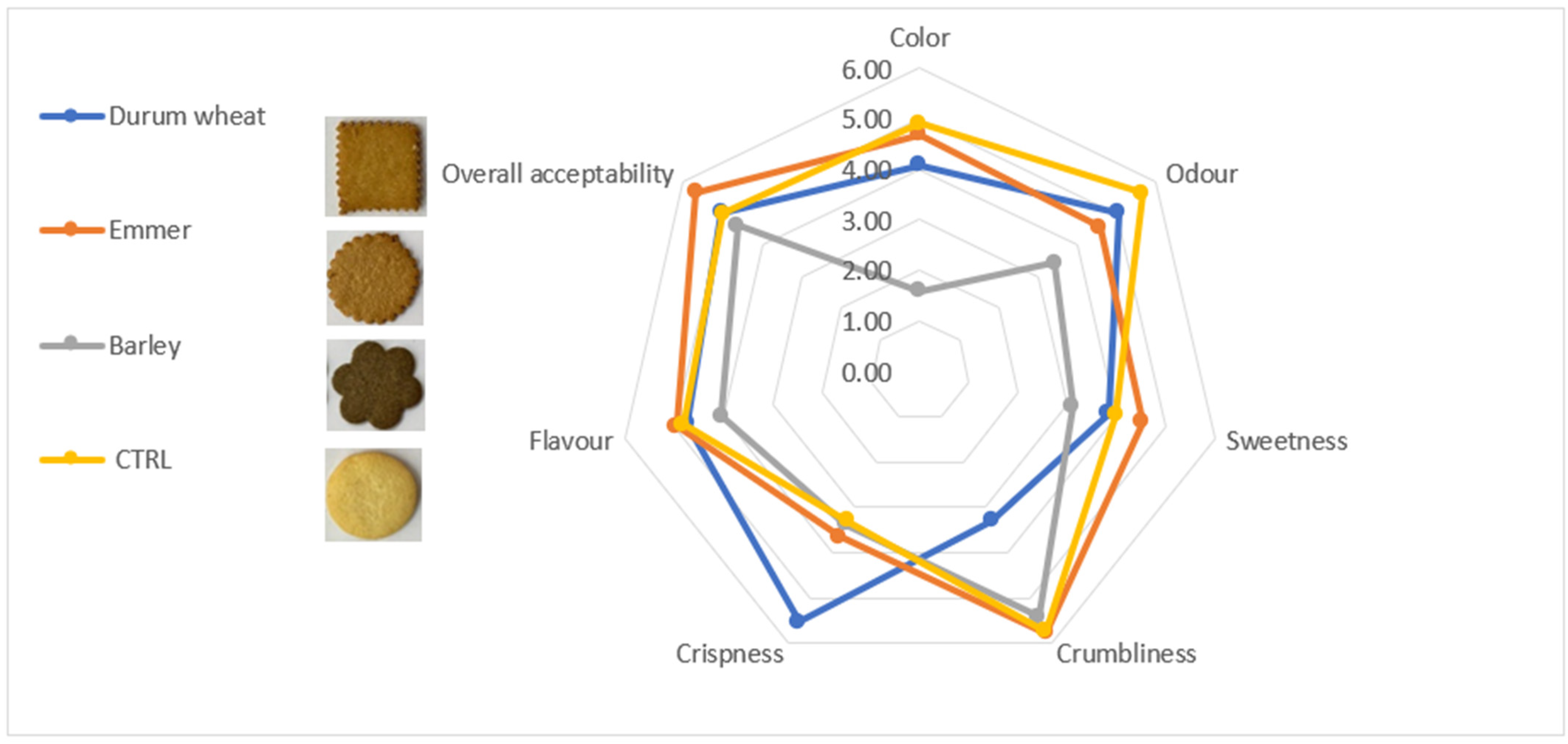
3.7. Sensory Biscuits Profile
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasulu, N.; Fernie, A.R. Diversity: Current and prospective secondary metabolites for nutrition and medicine. Curr. Opin. Biotechnol. 2022, 74, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Van Hung, P. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Barron, C.; Surget, A.; Rouau, X. Relative amounts of tissues in mature wheat grain and their carbohydrate and phenolic acid composition. J. Cereal Sci. 2007, 45, 88–96. [Google Scholar] [CrossRef]
- Fu, B.X.; Chiremba, C.; Pozniak, C.J.; Wang, K.; Nam, S. Total Phenolic and Yellow Pigment Contents and Antioxidant Activities of Durum Wheat Milling Fractions. Antioxidants 2017, 6, 78. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Borrelli, G.M.; Miedico, O.; Giovanniello, V.; Tarallo, M.; Pompa, C.; De Vita, P.; Chiaravalle, A.E. Effects of grain debranning on bioactive compounds, antioxidant capacity and essential and toxic trace elements in purple durum wheats. LWT—Food Sci. Technol. 2020, 118, 108734. [Google Scholar] [CrossRef]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemicals and Antioxidant Activity of Milled Fractions of Different Wheat Varieties. J. Agric. Food Chem. 2005, 53, 2297–2306. [Google Scholar] [CrossRef]
- Bhasin, T. Sensory characteristics of wholegrain. Adv. Obesity Weight Manag. Control 2020, 10, 191–194. [Google Scholar] [CrossRef]
- Laddomada, B.; Durante, M.; Mangini, G.; D’amico, L.; Lenucci, M.S.; Simeone, R.; Piarulli, L.; Mita, G.; Blanco, A. Genetic variation for phenolic acids concentration and composition in a tetraploid wheat (Triticum turgidum L.) collection. Genet. Resour. Crop Evol. 2017, 64, 587–597. [Google Scholar] [CrossRef]
- Li, L.; Shewry, R.; Ward, J.L. Phenolic Acids in Wheat Varieties in the Healthgrain Diversity Screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef]
- Menga, V.; Giovanniello, V.; Savino, M.; Gallo, A.; Colecchia, S.A.; De Simone, V.; Zingale, S.; Ficco, D.B.M. Comparative Analysis of Qualitative and Bioactive Compounds of Whole and Refined Flours in Durum Wheat Grains with Different Year of Release and Yield Potential. Plants 2023, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Napolitano, A.; Fogliano, V. Cereal dietary fibre: A natural functional ingredient to deliver phenolic compounds into the gut. Trends Food Sci. Technol. 2008, 19, 451–463. [Google Scholar] [CrossRef]
- Xavier, A.A.; Pérez-Gálvez, A. Carotenoids as a Source of Antioxidants in the Diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar] [PubMed]
- Panfili, G.; Fratianni, A.; Irano, M. Improved Normal-Phase High-Performance Liquid Chromatography Procedure for the Determination of Carotenoids in Cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, G.; De Leonardis, A.; Platani, C.; Troccoli, A. Distribution along durum wheat kernel of the components involved in semolina colour. J. Cereal Sci. 2008, 48, 494–502. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013, 139, 663–671. [Google Scholar] [CrossRef]
- Troccoli, A.; Borrelli, G.M.; De Vita, P.; Fares, C.; Di Fonzo, N. Durum Wheat Quality: A Multidisciplinary Concept (mini review). J. Cereal Sci. 2000, 32, 99–113. [Google Scholar] [CrossRef]
- Ficco, D.B.M.; Mastrangelo, A.M.; Trono, D.; Borrelli, G.M.; De Vita, P.; Fares, C.; Beleggia, R.; Platani, C.; Papa, R. The colours of durum wheat: A review. Crop Pasture Sci. 2014, 65, 1–15. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Rabalski, I. Bioactive Compounds and their Antioxidant Capacity in Selected Primitive and Modern Wheat Species. Open Agric. J. 2008, 2, 7–14. [Google Scholar] [CrossRef]
- Žilić, S.; Šukalović, V.; Dodig, D.; Maksimović, V.; Maksimović, M.; Basić, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Zrcková, M.; Capouchová, I.; Paznocht, L.; Eliášová, M.; Dvořák, P.; Konvalina, P.; Janovská, D.; Orsák, M.; Bečková, L. Variation of the total content of polyphenols and phenolic acids in einkorn, emmer, spelt and common wheat grain as a function of genotype, wheat species and crop year. Plant Soil Environ. 2019, 65, 260–266. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Di Loreto, A.; Bosi, S.; Montero, L.; Bregola, V.; Marotti, I.; Sferrazza, R.E.; Dinelli, G.; Herrero, M.; Cifuentes, A. Determination of phenolic compounds in ancient and modern durum wheat genotypes. Electrophoresis 2018, 39, 2001–2010. [Google Scholar] [CrossRef]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of Environment and Genotype on Phenolic Acids in Wheat in the HEALTHGRAIN Diversity Screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef]
- Pasqualone, A.; DelVecchio, L.N.; Mangini, G.; Taranto, F.; Blanco, A. Variability of total soluble phenolic compounds and antioxidant activity in a collection of tetraploid wheat. Agric. Food Sci. 2014, 23, 307–316. [Google Scholar] [CrossRef]
- Blanco, A.; Colasuonno, P.; Gadaleta, A.; Mangini, G.; Schiavulli, A.; Simeone, R.; Digesù, A.M.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L. Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat. J. Cereal Sci. 2011, 54, 255–264. [Google Scholar] [CrossRef]
- Giacosa, A.; Peroni, G.; Rondanelli, M. Phytochemical Components and Human Health Effects of Old versus Modern Italian Wheat Varieties: The Case of Durum Wheat Senatore Cappelli. Nutrients 2022, 14, 2779. [Google Scholar] [CrossRef]
- Giunta, F.; Bassu, S.; Mefleh, M.; Motzo, R. Is the Technological Quality of Old Durum Wheat Cultivars Superior to That of Modern Ones When Exposed to Moderately High Temperatures during Grain Filling? Foods 2020, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Dinelli, G.; Segura Carretero, A.; Di Silvestro, R.; Marotti, I.; Fu, S.; Benedettelli, S.; Ghiselli, L.; Fernandez Gutierrez, A. Determination of phenolic compounds in modern and old varieties of durum wheat using liquid chromatography coupled with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 7229–7240. [Google Scholar] [CrossRef] [PubMed]
- Mac Key, J. Wheat: Its concept, evolution and taxonomy. In Durum Wheat Breeding: Current Approaches and Future Strategies; Royo, C., Nachit, M.M., Di Fonzo, N., Araus, J.L., Pfeiffer, W.H., Slafer, G.A., Eds.; Food Products Press: Binghamton, NY, USA, 2005; Chapter 1; pp. 3–61. ISBN 1-56022-966-7. [Google Scholar]
- Dhanavath, S.; Rao, U.P. Nutritional and Nutraceutical Properties of Triticum dicoccum Wheat and Its Health Benefits: An Overview. J. Food Sci. 2017, 82, 2243–2250. [Google Scholar] [CrossRef] [PubMed]
- Lahouar, L.; El Arem, A.; Ghrairi, F.; Chahdoura, H.; Ben Salem, H.; El Felah, M.; Achour, L. Phytochemical content and antioxidant properties of diverse varieties of whole barley (Hordeum vulgare L.) grown in Tunisia. Food Chem. 2014, 145, 578–583. [Google Scholar] [CrossRef]
- Zhu, F. Proanthocyanidins in cereals and pseudocereals. Crit. Rev. Food Sci. Nutr. 2019, 59, 1521–1533. [Google Scholar] [CrossRef]
- Iannucci, A.; Suriano, S.; Codianni, P. Genetic Diversity for Agronomic Traits and Phytochemical Compounds in Coloured Naked Barley Lines. Plants 2021, 10, 1575. [Google Scholar] [CrossRef]
- Dickin, E.; Steele, K.; Edwards-Jones, G.; Wright, D. Agronomic diversity of naked barley (Hordeum vulgare L.): A potential resource for breeding new food barley for Europe. Euphytica 2012, 184, 85–99. [Google Scholar] [CrossRef]
- Beleggia, R.; Platani, C.; Nigro, F.; Cattivelli, L. A micro-method for the determination of Yellow Pigment Content in durum wheat. J. Cereal Sci. 2010, 52, 106–110. [Google Scholar] [CrossRef]
- Suriano, S.; Iannucci, A.; Codianni, P.; Fares, C.; Russo, M.; Pecchioni, N.; Marciello, U.; Savino, M. Phenolic acids profile, nutritional and phytochemical compounds, antioxidant properties in colored barley grown in southern Italy. Food Res. Int. 2018, 113, 221–233. [Google Scholar] [CrossRef]
- Kim, D.-O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Sun, B.; Ricardo-Da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Fares, C.; Platani, C.; Baiano, A.; Menga, V. Effect of processing and cooking on phenolic acid profile and antioxidant capacity of durum wheat pasta enriched with debranning fractions of wheat. Food Chem. 2010, 119, 1023–1029. [Google Scholar] [CrossRef]
- Pereira, D.; Correia, P.M.R.; Guiné, R.P.F. Analysis of the physical-chemical and sensorial properties of Maria type cookies. Acta Chim. Slovaca 2013, 6, 269–280. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy. Eur. J. Agron. 2017, 87, 19–29. [Google Scholar] [CrossRef]
- De Vita, P.; Nicosia, O.L.D.; Nigro, F.; Platani, C.; Riefolo, C.; Di Fonzo, N.; Cattivelli, L. Breeding progress in morpho-physiological, agronomical and qualitative traits of durum wheat cultivars released in Italy during the 20th century. Eur. J. Agron. 2007, 26, 39–53. [Google Scholar] [CrossRef]
- Lawlor, D.W. Carbon and nitrogen assimilation in relation to yield: Mechanisms are the key to understanding production systems. J. Exp. Bot. 2002, 53, 773–787. [Google Scholar] [CrossRef]
- Geisslitz, S.; Longin, C.F.H.; Scherf, K.A.; Koehler, P. Comparative Study on Gluten Protein Composition of Ancient (Einkorn, Emmer and Spelt) and Modern Wheat Species (Durum and Common Wheat). Foods 2019, 8, 409. [Google Scholar] [CrossRef]
- Abeledo, L.G.; Calderini, D.; Slafer, G. Genetic improvement of barley yield potential and its physiological determinants in Argentina (1944–1998). Euphytica 2003, 130, 325–334. [Google Scholar] [CrossRef]
- De Vita, P.; Riefolo, C.; Codianni, P.; Cattivelli, L.; Fares, C. Agronomic and qualitative traits of T. turgidum ssp. dicoccum genotypes cultivated in Italy. Euphytica 2006, 150, 195–205. [Google Scholar] [CrossRef]
- Pagnotta, M.A.; Mondini, L.; Codianni, P.; Fares, C. Agronomical, quality, and molecular characterization of twenty Italian emmer wheat (Triticum dicoccon) accessions. Genet. Resour. Crop Evol. 2009, 56, 299–310. [Google Scholar] [CrossRef]
- Rodrigues, O.; Minella, E.; Costenaro, E.R. Genetic Improvement of Barley (Hordeum vulgare L.) in Brazil: Yield Increase and Associated Traits. Agric. Sci. 2020, 11, 417–430. [Google Scholar] [CrossRef]
- De Vita, P.; Mastrangelo, A.; Matteu, L.; Mazzucotelli, E.; Virzì, N.; Palumbo, M.; Storto, M.L.; Rizza, F.; Cattivelli, L. Genetic improvement effects on yield stability in durum wheat genotypes grown in Italy. Field Crops Res. 2010, 119, 68–77. [Google Scholar] [CrossRef]
- Zieliński, H.; Kozłowska, H. Antioxidant Activity and Total Phenolics in Selected Cereal Grains and Their Different Morphological Fractions. J. Agric. Food Chem. 2000, 48, 2008–2016. [Google Scholar] [CrossRef] [PubMed]
- Menga, V.; Fares, C.; Troccoli, A.; Cattivelli, L.; Baiano, A. Effects of genotype, location and baking on the phenolic content and some antioxidant properties of cereal species. Int. J. Food Sci. Technol. 2010, 45, 7–16. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, W.; Dong, J.; Lu, J.; Chen, J.; Shan, L.; Lin, Y.; Kong, W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008, 107, 296–304. [Google Scholar] [CrossRef]
- Mareček, V.; Mikyška, A.; Hampel, D.; Čejka, P.; Neuwirthová, J.; Malachová, A.; Cerkal, R. ABTS and DPPH methods as a tool for studying antioxidant capacity of spring barley and malt. J. Cereal Sci. 2017, 73, 40–45. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 2007, 71, 1741–1751. [Google Scholar] [CrossRef]
- Kaneda, H.; Kobayashi, N.; Furusho, S.; Sahara, H.; Koshino, S. Reducing activity and flavor stability of beer. MBAA TQ 1995, 32, 90–94. [Google Scholar]
- Laddomada, B.; Blanco, A.; Mita, G.; D’amico, L.; Singh, R.P.; Ammar, K.; Crossa, J.; Guzmán, C. Drought and Heat Stress Impacts on Phenolic Acids Accumulation in Durum Wheat Cultivars. Foods 2021, 10, 2142. [Google Scholar] [CrossRef]
- Irakli, M.; Lazaridou, A.; Mylonas, I.; Biliaderis, C.G. Bioactive Components and Antioxidant Activity Distribution in Pearling Fractions of Different Greek Barley Cultivars. Foods 2020, 9, 783. [Google Scholar] [CrossRef]
- Skendi, A. Alternatives to increase the antioxidant capacity of bread with phenolics. In Trends in Wheat and Bread Making; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; Chapter 11; pp. 311–341. [Google Scholar] [CrossRef]
- Andersson, A.A.; Dimberg, L.; Åman, P.; Landberg, R. Recent findings on certain bioactive components in whole grain wheat and rye. J. Cereal Sci. 2014, 59, 294–311. [Google Scholar] [CrossRef]
- Brandolini, A.; Castoldi, P.; Plizzari, L.; Hidalgo, A. Phenolic acids composition, total polyphenols content and antioxidant activity of Triticum monococcum, Triticum turgidum and Triticum aestivum: A two-years evaluation. J. Cereal Sci. 2013, 58, 123–131. [Google Scholar] [CrossRef]
- Wojakowska, A.; Perkowski, J.; Góral, T.; Stobiecki, M. Structural characterization of flavonoid glycosides from leaves of wheat (Triticum aestivum L.) using LC/MS/MS profiling of the target compounds. J. Mass Spectrom. 2013, 48, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Turkan, I. Flavonoid Naringenin Alleviates Short-Term Osmotic and Salinity Stresses Through Regulating Photosynthetic Machinery and Chloroplastic Antioxidant Metabolism in Phaseolus vulgaris. Front. Plant Sci. 2020, 11, 682. [Google Scholar] [CrossRef] [PubMed]
- Bollina, V.; Kumaraswamy, G.K.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Faubert, D.; Hamzehzarghani, H. Mass spectrometry-based metabolomics application to identify quantitative resistance-related metabolites in barley against Fusarium head blight. Mol. Plant Pathol. 2010, 11, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Kumaraswamy, G.K.; Bollina, V.; Kushalappa, A.C.; Choo, T.M.; Dion, Y.; Rioux, S.; Mamer, O.; Faubert, D. Metabolomics technology to phenotype resistance in barley against Gibberella zeae. Eur. J. Plant Pathol. 2011, 130, 29–43. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, D.; Tan, B. Effect of Layered Debranning Processing on the Proximate Composition, Polyphenol Content, and Antioxidant Activity of Whole Grain Wheat. J. Food Process. Preserv. 2023, 2023, 1083867. [Google Scholar] [CrossRef]
- Giordano, D.; Locatelli, M.; Travaglia, F.; Bordiga, M.; Reyneri, A.; Coïsson, J.D.; Blandino, M. Bioactive compound and antioxidant activity distribution in roller-milled and pearled fractions of conventional and pigmented wheat varieties. Food Chem. 2017, 233, 483–491. [Google Scholar] [CrossRef]
- Suriano, S.; Savino, M.; Codianni, P.; Iannucci, A.; Caternolo, G.; Russo, M.; Pecchioni, N.; Troccoli, A. Anthocyanin profile and antioxidant capacity in coloured barley. Int. J. Food Sci. Technol. 2019, 54, 2478–2486. [Google Scholar] [CrossRef]
- Li, W.; Pickard, M.D.; Beta, T. Effect of thermal processing on antioxidant properties of purple wheat bran. Food Chem. 2007, 104, 1080–1086. [Google Scholar] [CrossRef]
- Duodu, K.G. Effects of processing on antioxidant phenolics of cereal and legume grains. In Advances in Cereal Science: Implications to Food Processing and Health Promotion; Awika, J.M., Piironen, V., Bean, S., Eds.; American Chemical Society: Washington, DC, USA, 2011; pp. 31–54. [Google Scholar]
- Shahidi, F.; Yeo, J.-D. Insoluble-Bound Phenolics in Food. Molecules 2016, 21, 1216. [Google Scholar] [CrossRef] [PubMed]
- Imeneo, V.; Romeo, R.; Gattuso, A.; De Bruno, A.; Piscopo, A. Functionalized Biscuits with Bioactive Ingredients Obtained by Citrus Lemon Pomace. Foods 2021, 10, 2460. [Google Scholar] [CrossRef] [PubMed]
- Biguzzi, C.; Schlich, P.; Lange, C. The impact of sugar and fat reduction on perception and liking of biscuits. Food Qual. Prefer. 2014, 35, 41–47. [Google Scholar] [CrossRef]
| Taxonomic Classification | Accession | Cultivar/Landrace-Origin | Year of Release | Genotype | |
|---|---|---|---|---|---|
| Durum wheat | Cappelli | Cultivar-Selection from Tunisian population ‘Jean Retifah’ | 1915 | Old |  |
| Fortore | Cultivar–Capeiti-8/Valforte | 1995 | Modern |  | |
| Emmer wheat | Molisano | Landrace-Molise region, Central Italy | // | Old |  |
| PadrePio | Cultivar–Simeto/Molise | 2016 | Modern |  | |
| Barley | L94 | Ethiopian landrace line (black and naked grains) | // | Old |  |
| Priora | Cultivar–Arda/Mondo (white and naked grains) | 2000 | Modern |  | |
| Species | Genotype | Type | Year | PC (g kg−1, DM) | TW (Kg hL−1) | TKW (g) |
|---|---|---|---|---|---|---|
| Interaction of Species x Genotype x Year effects (SxGxY) | ||||||
| Durum wheat | Cappelli | Old | 2015/16 | 15.90 | 81.43 a,b | 54.60 a,b |
| Durum wheat | Cappelli | Old | 2016/17 | 16.50 | 81.18 a,b | 52.43 b,c |
| Durum wheat | Fortore | Modern | 2015/16 | 14.30 | 78.16 b,c | 48.33 c,d |
| Durum wheat | Fortore | Modern | 2016/17 | 13.83 | 82.51 a | 57.73 a |
| Emmer wheat | Molisano | Old | 2015/16 | 14.83 | 73.06 e,f | 42.30 e,f |
| Emmer wheat | Molisano | Old | 2016/17 | 16.70 | 69.37 f | 40.13 f |
| Emmer wheat | PadrePio | Modern | 2015/16 | 16.63 | 80.98 a,b | 45.60 d,e |
| Emmer wheat | PadrePio | Modern | 2016/17 | 18.40 | 77.96 b,c | 46.87 d |
| Barley | L94 | Old | 2015/16 | 13.87 | 74.60 c–e | 40.67 f |
| Barley | L94 | Old | 2016/17 | 14.87 | 75.21 c–e | 40.07 f |
| Barley | Priora | Modern | 2015/16 | 14.93 | 80.15 a,b | 47.07 d |
| Barley | Priora | Modern | 2016/17 | 16.23 | 78.93 a–c | 49.03 c,d |
| F(2.24) | 0.99 | 4.0 | 8.56 | |||
| p value | n.s. | * | ** | |||
| Interaction of Species x Genotype effects (SxG) | ||||||
| Durum wheat | Cappelli | Old | 16.20 a,b | 81.31 a | 53.52 a | |
| Durum wheat | Fortore | Modern | 14.07 d | 80.34 a | 53.03 a | |
| Emmer wheat | Molisano | Old | 15.77 b,c | 71.21 c | 41.22 c | |
| Emmer wheat | PadrePio | Modern | 17.52 a | 79.47 a | 46.23 b | |
| Barley | L94 | Old | 14.37 c,d | 74.90 b | 40.37 c | |
| Barley | Priora | Modern | 15.58 b–d | 79.54 a | 48.05 b | |
| F(2.30) | 16.67 | 17.24 | 7.53 | |||
| p value | *** | *** | ** | |||
| Single effect (Species) (S) | ||||||
| Durum wheat | 15.13 b | 80.82 a | 53.28 a | |||
| Emmer wheat | 16.64 a | 75.34 b | 43.73 b | |||
| Barley | 14.98 b | 77.22 b | 44.21 b | |||
| F(2.33) | 6.56 | 7.98 | 24.89 | |||
| p value | ** | *** | *** | |||
| Single effect (Genotype) (G) | ||||||
| Old genotypes | 15.44 | 75.81 b | 45.03 b | |||
| Modern genotypes | 15.72 | 79.78 a | 49.11 a | |||
| F(1.34) | 0.33 | 11.28 | 5.05 | |||
| p value | n.s. | ** | * | |||
| Single effect (Year) (Y) | ||||||
| 2015/16 | 15.08 b | 78.06 | 46.43 | |||
| 2016/17 | 16.09 a | 77.53 | 47.71 | |||
| F(1.34) | 5.03 | 0.15 | 0.44 | |||
| p value | * | n.s. | n.s. | |||
| Species | Genotype | Type | Year | TPC (µg GAE g−1) | TFC (µg CE g−1) | TPAC (µg CE g−1) | TYPC (µg g−1) | DPPH (µmol TE g−1) | TEAC (µmol TE g−1) |
|---|---|---|---|---|---|---|---|---|---|
| Interaction of Species x Genotype x Year effects (SxGxY) | |||||||||
| Durum wheat | Cappelli | Old | 2015/16 | 1065.09 | 288.82 | 118.52 d | 5.66 c | 1.98 c,d | 2.52 e–g |
| Durum wheat | Cappelli | Old | 2016/17 | 1173.80 | 276.07 | 115.25 d | 5.75 c | 2.09 c,d | 2.77 d |
| Durum wheat | Fortore | Modern | 2015/16 | 896.52 | 313.82 | 145.16 d | 7.58 a | 1.74 d | 2.71 d,e |
| Durum wheat | Fortore | Modern | 2016/17 | 925.88 | 301.71 | 128.32 d | 6.79 b | 1.81 c,d | 2.57 e,f |
| Emmer wheat | Molisano | Old | 2015/16 | 1067.93 | 289.47 | 113.09 d | 4.49 e | 1.99 c,d | 2.34 f,g |
| Emmer wheat | Molisano | Old | 2016/17 | 1167.83 | 314.84 | 125.53 d | 4.99 d | 2.20 c,d | 2.28 g |
| Emmer wheat | PadrePio | Modern | 2015/16 | 1037.73 | 295.60 | 132.68 d | 5.71 c | 2.16 c,d | 2.31 g |
| Emmer wheat | PadrePio | Modern | 2016/17 | 1066.12 | 325.12 | 134.86 d | 6.08 c | 2.27 c | 2.37 f,g |
| Barley | L94 | Old | 2015/16 | 2565.04 | 717.66 | 824.41 c | 3.78 f | 8.85 b | 8.10 c |
| Barley | L94 | Old | 2016/17 | 2668.70 | 731.91 | 822.78 c | 3.87 f | 8.38 b | 8.16 c |
| Barley | Priora | Modern | 2015/16 | 2704.27 | 871.41 | 1639.18 a | 2.92 g | 11.43 a | 10.95 a |
| Barley | Priora | Modern | 2016/17 | 2823.60 | 863.26 | 1314.95 b | 3.10 g | 11.06 a | 10.06 b |
| F(2.24) | 1.79 | 1.2 | 73.39 | 7.32 | 0.23 | 10.89 | |||
| p value | n.s. | n.s. | *** | *** | ** | *** | |||
| Interaction of Species x Genotype (SxG) | n.s. | ||||||||
| Durum wheat | Cappelli | Old | 1119.44 c | 282.45 d | 116.88 c | 5.70 b | 2.04 c,d | 2.64 c | |
| Durum wheat | Fortore | Modern | 911.20 e | 307.76 c | 136.74 c | 7.19 a | 1.78 d | 2.64 c | |
| Emmer wheat | Molisano | Old | 1117.88 c | 302.15 c | 119.31 c | 4.74 c | 2.09 c,d | 2.31 d | |
| Emmer wheat | PadrePio | Modern | 1051.93 d | 310.36 c | 133.77 c | 5.89 b | 2.22 c | 2.34 d | |
| Barley | L94 | Old | 2616.87 b | 724.78 b | 823.59 b | 3.83 d | 8.62 b | 8.13 b | |
| Barley | Priora | Modern | 2763.93 a | 867.34 a | 1477.06 a | 3.01 e | 11.24 a | 10.51 a | |
| F(2.30) | 82.11 | 122.99 | 1231.45 | 176.87 | 210.67 | 283.97 | |||
| p value | *** | *** | *** | *** | *** | *** | |||
| Single effect (Species) (S) | |||||||||
| Durum wheat | 1015.32 c | 295.10 c | 126.81 b | 6.45 a | 1.91 c | 2.46 b | |||
| Emmer wheat | 1084.90 b | 306.26 b | 126.54 b | 5.32 b | 2.16 b | 2.33 b | |||
| Barley | 2690.40 a | 796.06 a | 1150.33 a | 3.42 c | 9.93 a | 9.32 a | |||
| F(2.33) | 9224.26 | 7527.75 | 12,747.46 | 1068.10 | 7155.16 | 9508.79 | |||
| p value | *** | *** | *** | *** | *** | *** | |||
| Single effect (Genotype) (G) | |||||||||
| Old genotypes | 1618.06 a | 436.46 b | 353.26 b | 4.76 b | 4.25 b | 4.36 b | |||
| Modern genotypes | 1575.69 b | 495.15 a | 582.52 a | 5.36 a | 5.08 a | 5.16 a | |||
| F(1.34) | 13.83 | 237.67 | 1438.67 | 126.27 | 177.7 | 294.06 | |||
| p value | *** | *** | *** | *** | *** | *** | |||
| Single effect (Year) (Y) | |||||||||
| 2015/16 | 1556.10 b | 462.80 | 495.50 a | 5.02 | 6.49 | 4.82 a | |||
| 2016/17 | 1637.66 a | 468.82 | 440.28 b | 5.10 | 4.64 | 4.70 b | |||
| F(1.34) | 51.24 | 2.5 | 83.47 | 1.90 | 0.81 | 6.55 | |||
| p value | *** | n.s. | *** | n.s. | n.s. | * | |||
| Species | Genotype | Crop Years | TSF Phenolic Acids | TSF Flavonoids | TSC Phenolic Acids | TSC Flavonoids | TIB Phenolic Acids | TIB Flavonoids |
|---|---|---|---|---|---|---|---|---|
| Durum wheat | Cappelli | 2015/16 | 20.11 c,d | n.d. | 26.83 de | n.d. | 465.87 a | n.d. |
| 2016/17 | 20.58 c,d | n.d. | 24.72 e | n.d. | 389.53 b | n.d. | ||
| Fortore | 2015/16 | 13.01 g | n.d. | 29.06 d,e | 2.19 a | 384.25 b | n.d. | |
| 2016/17 | 14.12 f,g | n.d. | 38.11 b | 1.71 b | 314.66 b | n.d. | ||
| Emmer wheat | Molisano | 2015/16 | 23.17 c | 5.37 d | 36.18 b,c | 0.44 d | 238.06 c | n.d. |
| 2016/17 | 19.11 c–f | 5.57 d | 31.44 d | 0.59 c | 266.97 c | n.d. | ||
| PadrePio | 2015/16 | 72.73 a | 2.47 e | 52.24 a | n.d. | 242.67 c | 0.74 c,d | |
| 2016/17 | 43.32 b | 2.37 e | 42.07 b | n.d. | 276.30 c | 1.12 c | ||
| Barley | L94 | 2015/16 | 19.20 c–e | 18.90 c | 27.26 d,e | 0.55 c,d | 502.04 a | 5.19 a,b |
| 2016/17 | 43.03 b | 25.80 b | 30.06 d,e | 0.51 c,d | 423.87 a,b | 4.49 b | ||
| Priora | 2015/16 | 14.70 e–g | 52.76 a | 27.72 d,e | n.d. | 482.55 a | 5.17 a,b | |
| 2016/17 | 15.78 d–g | 53.89 a | 25.45 e | n.d. | 375.15 b | 5.65 a | ||
| F(2.24) (SxGxY) | 48.96 | 11.96 | 14.85 | 4.49 | 1.00 | 3.84 | ||
| p value | *** | *** | *** | * | * | * |
| Durum Wheat | Emmer Wheat | Barley | Commercial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fortore | Molisano | L94 | Control | |||||||||
| Soluble Free | Soluble Conjugated | Insoluble Bound | Soluble Free | Soluble Conjugated | Insoluble Bound | Soluble Free | Soluble Conjugated | Insoluble Bound | Soluble Free | Soluble Conjugated | Insoluble Bound | |
| Vanillic acid | 38.24 ± 0.94 | 37.87 ± 1.43 | 9.75 ± 0.47 | 12.96 ± 0.01 | 19.17 ± 1.24 | 5.16 ± 0.43 | 15.20 ± 1.02 | 19.17 ± 1.24 | 6.4 ± 0.49 | 4.20 ± 0.4 | 52.91 ± 0.15 | 1.23 ± 0.11 |
| Vanillin | 1127.44 ± 8.49 | 661.12 ± 21.87 | 40.13 ± 1.06 | 1237.92 ± 2.26 | 876.22 ± 28.42 | 29.89 ± 1.77 | 1149.56 ± 5.83 | 871.23 ± 35.49 | 29.61 ± 1.19 | 1400.84 ± 12.16 | 868.12 ± 29.77 | 38.59 ± 0.41 |
| Ferulic acid | 6.52 ± 0.17 | 11.23 ± 0.49 | 327.04 ± 22.02 | 5.36 ± 0.57 | 9.17 ± 0.38 | 265.41 ± 5.2 | 5.72 ± 0.41 | 9.20 ± 0.34 | 347.09 ± 0.07 | 1.12 ± 0.11 | 0.88 ± 0.04 | 10.91 ± 1.13 |
| Sinapic acid | 1.40 ± 0.06 | 5.84 ± 0.34 | 10.03 ± 0.49 | 4.08 ± 0.82 | 5.47 ± 0.64 | 9.25 ± 0.15 | n.d. | 12.16 ± 0.30 | 11.29 ± 0.09 | n.d. | n.d. | n.d. |
| Catechin | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| p-Hydroxybenzoic acid | n.d. | n.d. | 1 ± 0.05 | n.d. | n.d. | 0.91 ± 0.03 | n.d. | n.d. | 0.75 ± 0.07 | n.d. | n.d. | n.d. |
| Syringic acid | n.d. | n.d. | 1.04 ± 0.15 | n.d. | n.d. | 0.8 ± 0.07 | n.d. | n.d. | 1.16 ± 0.39 | n.d. | n.d. | n.d. |
| p-Coumaric acid | n.d. | 1.39 ± 0.01 | 9.36 ± 0.72 | n.d. | 0.83 ± 0.04 | 16.61 ± 0.19 | n.d. | 8.27 ± 0.15 | 12.49 ± 1.22 | n.d. | n.d. | 9.27 ± 0.04 |
| Syringaldeide | n.d. | n.d. | 2.89 ± 0.32 | n.d. | n.d. | 3.59 ± 0.05 | n.d. | n.d. | 3.87 ± 0.07 | n.d. | n.d. | n.d. |
| Caffeic acid | n.d. | n.d. | 0.87 ± 0.13 | n.d. | n.d. | 1.87 ± 0.07 | n.d. | n.d. | 0.87 ± 0.13 | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrelli, G.M.; Menga, V.; Giovanniello, V.; Ficco, D.B.M. Antioxidants and Phenolic Acid Composition of Wholemeal and Refined-Flour, and Related Biscuits in Old and Modern Cultivars Belonging to Three Cereal Species. Foods 2023, 12, 2551. https://doi.org/10.3390/foods12132551
Borrelli GM, Menga V, Giovanniello V, Ficco DBM. Antioxidants and Phenolic Acid Composition of Wholemeal and Refined-Flour, and Related Biscuits in Old and Modern Cultivars Belonging to Three Cereal Species. Foods. 2023; 12(13):2551. https://doi.org/10.3390/foods12132551
Chicago/Turabian StyleBorrelli, Grazia Maria, Valeria Menga, Valentina Giovanniello, and Donatella Bianca Maria Ficco. 2023. "Antioxidants and Phenolic Acid Composition of Wholemeal and Refined-Flour, and Related Biscuits in Old and Modern Cultivars Belonging to Three Cereal Species" Foods 12, no. 13: 2551. https://doi.org/10.3390/foods12132551
APA StyleBorrelli, G. M., Menga, V., Giovanniello, V., & Ficco, D. B. M. (2023). Antioxidants and Phenolic Acid Composition of Wholemeal and Refined-Flour, and Related Biscuits in Old and Modern Cultivars Belonging to Three Cereal Species. Foods, 12(13), 2551. https://doi.org/10.3390/foods12132551