The Influence of (Poly)phenol Intake in Saliva Proteome: Short- and Medium-Term Effects of Apple
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
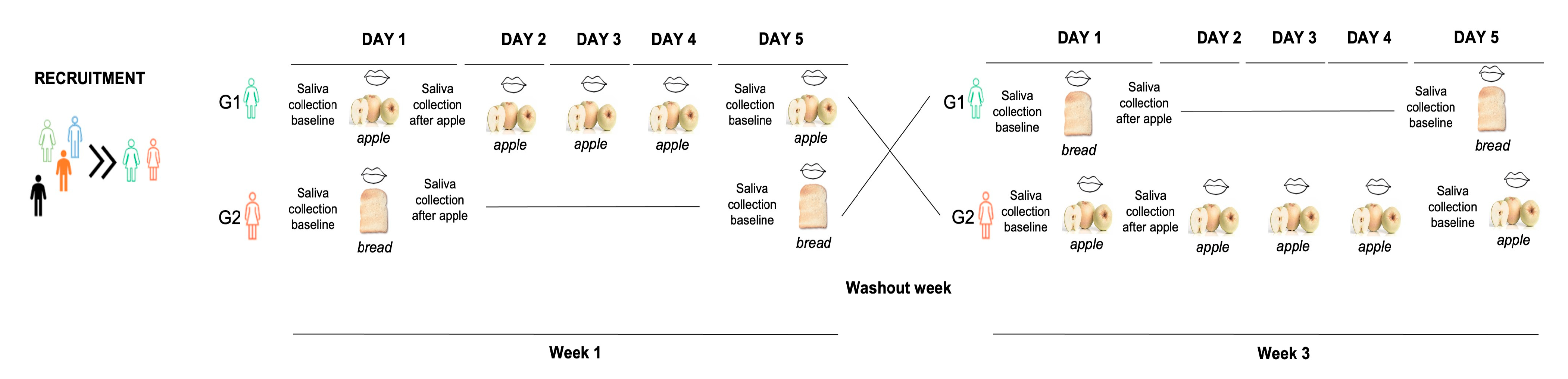
2.2. Experimental Procedures
2.3. Saliva Collection, Cleaning, and Protein Quantification
2.4. Extraction and Quantification of Total Phenols from Bravo de Esmolfe Apples
2.5. SDS PAGE Salivary Protein Profiles
2.6. In Vitro Analysis of Saliva*Polyphenol Extract Interactions
2.7. Two-Dimensional Electrophoretic (2DE) Salivary Protein Profiles
2.8. Statistical Analysis
3. Results
3.1. Effects of Apple Intake on Salivary Total Protein and SDS-PAGE Profile
3.1.1. Short-Term Effects
3.1.2. Medium-Term Effects
3.2. Interaction between Salivary Proteins and Apple Phenolic Extract
3.3. Effect of Apple Intake on Salivary 2-DE Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.J.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in europe: The european prospective investigation into cancer and nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Cieślik, E.; Grȩda, A.; Adamus, W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006, 94, 135–142. [Google Scholar] [CrossRef]
- Tvarijonaviciute, A.; Martinez-Subiela, S.; Lopez-Jornet, P.; Lamy, E. Saliva in Health and Disease: The Present and Future of a Unique Sample for Diagnosis; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Lamy, E.; da Costa, G.; Santos, R.; Capela E Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Sales Baptista, E. Sheep and goat saliva proteome analysis: A useful tool for ingestive behavior research? Physiol. Behav. 2009, 98, 393–401. [Google Scholar] [CrossRef]
- Shimada, T. Salivary proteins as a defense against dietary tannins. J. Chem. Ecol. 2006, 32, 1149–1163. [Google Scholar] [CrossRef]
- Da Costa, G.; Lamy, E.; Capela E Silva, F.; Andersen, J.; Sales Baptista, E.; Coelho, A.V. Salivary amylase induction by tannin-enriched diets as a possible countermeasure against tannins. J. Chem. Ecol. 2008, 34, 376–387. [Google Scholar] [CrossRef]
- Lamy, E.; Graça, G.; da Costa, G.; Franco, C.; E Silva, F.C.; Baptista, E.S.; Coelho, A.V. Changes in mouse whole saliva soluble proteome induced by tannin-enriched diet. Proteome Sci. 2010, 8, 65. [Google Scholar] [CrossRef]
- Martin, L.E.; Nikonova, L.V.; Kay, K.; Paedae, A.B.; Contreras, R.J.; Torregrossa, A.M. Salivary proteins alter taste-guided behaviors and taste nerve signaling in rat. Physiol. Behav. 2018, 184, 150–161. [Google Scholar] [CrossRef]
- Martin, L.E.; Nikonova, L.V.; Kay, K.E.; Torregrossa, A.M. Altering salivary protein profile can increase acceptance of a novel bitter diet. Appetite 2019, 136, 8–17. [Google Scholar] [CrossRef]
- Louro, T.; Simões, C.; Penetra, M.J.; Carreira, L.; Castelo, P.M.; Luis, H.; Moreira, P.; Lamy, E. Relationship between mediterranean diet adherence and saliva composition. Nutrients 2021, 13, 1246. [Google Scholar] [CrossRef]
- Crawford, C.R.; Running, C.A. Addition of chocolate milk to diet corresponds to protein concentration changes in human saliva. Physiol. Behav. 2020, 225, 113080. [Google Scholar] [CrossRef]
- Yousaf, N.Y.; Melis, M.; Mastinu, M.; Contini, C.; Cabras, T.; Barbarossa, I.T.; Tepper, B.J. Time course of salivary protein responses to cranberry-derived polyphenol exposure as a function of prop taster status. Nutrients 2020, 12, 2878. [Google Scholar] [CrossRef]
- Rodrigues, L.; da Costa, G.; Cordeiro, C.; Pinheiro, C.C.; Amado, F.; Lamy, E. Relationship between saliva protein composition and 6-n-Propylthiouracil bitter taste responsiveness in young adults. J. Sens. Stud. 2017, 32, e12275. [Google Scholar] [CrossRef]
- Rodrigues, L.; Costa, G.; Cordeiro, C.; Pinheiro, C.; Amado, F.; Lamy, E. Salivary proteome and glucose levels are related with sweet taste sensitivity in young adults. Food Nutr. Res. 2017, 61, 1389208. [Google Scholar] [CrossRef]
- Rodrigues, L.; Espanca, R.; Costa, A.R.; Antunes, C.M.; Pomar, C.; Capela-Silva, F.; Pinheiro, C.C.; Domingues, P.; Amado, F.; Lamy, E. Comparison of salivary proteome of children with different sensitivities for bitter and sweet tastes: Association with body mass index. Int. J. Obes. 2019, 43, 701–712. [Google Scholar] [CrossRef]
- Orjuela-Palacio, J.M.; Zamora, M.C.; Lanari, M.C. Consumers’ acceptance of a high-polyphenol yerba mate/black currant beverage: Effect of repeated tasting. Food Res. Int. 2014, 57, 26–33. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Bordalo, M.; Seabra, I.J.; Silva, A.B.; Terrasso, A.P.; Brito, C.; Serra, M.; Bronze, M.R.; Duarte, C.M.M.; Braga, M.E.M.; de Sousa, H.C.; et al. Using high-pressure technology to develop antioxidant-rich extracts from bravo de esmolfe apple residues. Antioxidants 2021, 10, 1469. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.N.C.; Madaleno, R.O.; Castro, L.M.M.N. Drying kinetics of two fruits Portuguese cultivars (Bravo de Esmolfe apple and Madeira banana): An experimental study. Heliyon 2022, 8, e09341. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Simões, C.; Rodrigues, L.; Costa, A.R.; Vitorino, R.; Amado, F.; Antunes, C.; do Carmo, I. Changes in the salivary protein profile of morbidly obese women either previously subjected to bariatric surgery or not. J. Physiol. Biochem. 2015, 71, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Huq, N.L.; Myroforidis, H.; Cross, K.J.; Stanton, D.P.; Veith, P.D.; Ward, B.R.; Reynolds, E.C. The interactions of CPP-ACP with saliva. Int. J. Mol. Sci. 2016, 17, 915. [Google Scholar] [CrossRef]
- Beeley, J.A.; Sweeney, D.; Lindsay, J.C.; Buchanan, M.L.; Sarna, L.; Khoo, K.S. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis of human parotid salivary proteins. Electrophoresis 1991, 12, 1032–1041. [Google Scholar] [CrossRef]
- Zallocco, L.; Giusti, L.; Ronci, M.; Mussini, A.; Trerotola, M.; Mazzoni, M.R.; Lucacchini, A.; Sebastiani, L. Salivary proteome changes in response to acute psychological stress due to an oral exam simulation in university students: Effect of an olfactory stimulus. Int. J. Mol. Sci. 2021, 22, 4295. [Google Scholar] [CrossRef]
- Chevalier, F.; Hirtz, C.; Chay, S.; Cuisinier, F.; Sommerer, N.; Rossignol, M.; De Périère, D.D. Proteomic studies of saliva: A proposal for a standardized handling of clinical samples. Clin. Proteom. 2007, 3, 13–21. [Google Scholar] [CrossRef]
- Jessie, K.; Pang, W.W.; Rahim, Z.H.A.; Hashim, O.H. Proteomic analysis of whole human saliva detects enhanced expression of interleukin-1 receptor antagonist, thioredoxin and lipocalin-1 in cigarette smokers compared to non-smokers. Int. J. Mol. Sci. 2010, 11, 4488–4505. [Google Scholar] [CrossRef]
- Castillo-Felipe, C.; Franco-Martínez, L.; Tvarijonaviciute, A.; Lopez-Jornet, P.; Lamy, E. Proteomics-based identification of salivary changes in patients with burning mouth syndrome. Biology 2021, 10, 392. [Google Scholar] [CrossRef]
- Simões, C.; Caeiro, I.; Carreira, L.; Silva, F.C.e.; Lamy, E. How different snacks produce a distinct effect in salivary protein composition. Molecules 2021, 26, 2403. [Google Scholar] [CrossRef]
- Lamy, E.; Mau, M. Saliva proteomics as an emerging, non-invasive tool to study livestock physiology, nutrition and diseases. J. Proteom. 2012, 75, 4251–4258. [Google Scholar] [CrossRef]
- Beverly, A.B.; Zhu, L.; Fish, T.L.; Thannhauser, T.; Rutzke, M.A.; Miller, D.D. Green Tea Ingestion by Rats Does Not Affect Iron Absorption but Does Alter the Composition of the Saliva Proteome. J. Food Sci. 2012, 77, H96–H104. [Google Scholar] [CrossRef]
- Lamy, E.; da Costa, G.; Santos, R.; Capela e Silva, F.; Potes, J.; Pereira, A.; Coelho, A.V.; Baptista, E.S. Effect of condensed tannin ingestion in sheep and goat parotid saliva proteome. J. Anim. Physiol. Anim. Nutr. 2011, 95, 304–312. [Google Scholar] [CrossRef]
- Louro, T.; Simões, C.; Lima, W.; Carreira, L.; Castelo, P.M.; Luis, H.; Moreira, P.; Lamy, E. Salivary Protein Profile and Food Intake: A Dietary Pattern Analysis. J. Nutr. Metab. 2021, 2021, 6629951. [Google Scholar] [CrossRef]
- Brown, F.N.; Mackie, A.R.; He, Q.; Branch, A.; Sarkar, A. Protein-saliva interactions: A systematic review. Food Funct. 2021, 12, 3324–3351. [Google Scholar] [CrossRef]
- Lamy, E.; Torregrossa, A.-M.; Castelo, P.M.; Capela e Silva, F. Saliva in Ingestive Behavior Research: Association with Oral Sensory Perception and Food Intake. In Saliva in Health and Disease: The Present and Future of a Unique Sample for Diagnosis; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Hofmann, T.; Glabasnia, A.; Schwarz, B.; Wisman, K.N.; Gangwer, K.A.; Hagerman, A.E. Protein binding and astringent taste of a polymeric procyanidin, 1,2,3,4,6-penta-O-galloyl-β-D-glucopyranose, castalagin, and grandinin. J. Agric. Food Chem. 2006, 54, 9503–9509. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Carpenter, G.H. Alternative Mechanisms of Astringency—What is the Role of Saliva? J. Texture Stud. 2013, 44, 364–375. [Google Scholar] [CrossRef]
- Hara, K.; Ohara, M.; Hayashi, I.; Hino, T.; Nishimura, R.; Iwasaki, Y.; Ogawa, T.; Ohyama, Y.; Sugiyama, M.; Amano, H. The green tea polyphenol (-)-epigallocatechin gallate precipitates salivary proteins including alpha-amylase: Biochemical implications for oral health. Eur. J. Oral Sci. 2012, 120, 132–139. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; De Freitas, V.; Sutherland, D.S.; Sales, M.G.F. SPR based studies for pentagalloyl glucose binding to α- Amylase. Proc. Procedia Eng. 2012, 47, 498–501. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Guerreiro, C.; Soares, S.; Mateus, N.; De Freitas, V. Tannins in food: Insights into the molecular perception of astringency and bitter taste. Molecules 2020, 25, 2590. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Atzori, E.; Cabras, S.; Zonza, A.; Calò, C.; Muroni, P.; Nieddu, M.; Padiglia, A.; Sogos, V.; Tepper, B.J.; et al. The Gustin (CA6) Gene Polymorphism, rs2274333 (A/G), as a Mechanistic Link between PROP Tasting and Fungiform Taste Papilla Density and Maintenance. PLoS ONE 2013, 8, e74151. [Google Scholar] [CrossRef]
- Carreira, L.; Midori Castelo, P.; Simões, C.; Capela e Silva, F.; Viegas, C.; Lamy, E. Changes in Salivary Proteome in Response to Bread Odour. Nutrients 2020, 12, 1002. [Google Scholar] [CrossRef] [PubMed]
- Melis, M.; Yousaf, N.Y.; Mattes, M.Z.; Cabras, T.; Messana, I.; Crnjar, R.; Tomassini Barbarossa, I.; Tepper, B.J. Sensory perception of and salivary protein response to astringency as a function of the 6-n-propylthioural (PROP) bitter-taste phenotype. Physiol. Behav. 2017, 173, 163–173. [Google Scholar] [CrossRef]
- Jöbstl, E.; O’Connell, J.; Fairclough, J.P.A.; Williamson, M.P. Molecular model for astringency produced by polyphenol/protein interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef]
- Mehansho, H.; Clements, S.; Sheares, B.T.; Smith, S.; Carlson, D.M. Induction of proline-rich glycoprotein synthesis in mouse salivary glands by isoproterenol and by tannins. J. Biol. Chem. 1985, 260, 4418–4423. [Google Scholar] [CrossRef]
- Dsamou, M.; Morzel, M.; Le Corre, L.; Séverin, I.; Chagnon, M.-C. Caffeine increases the expression of cystatin SN in human submandibular acinar-like HSG cells. Arch. Oral Biol. 2013, 58, 1511–1516. [Google Scholar] [CrossRef]
- Santos, J.L.; Saus, E.; Smalley, S.V.; Cataldo, L.R.; Alberti, G.; Parada, J.; Gratacòs, M.; Estivill, X. Copy number polymorphism of the salivary amylase gene: Implications in human nutrition research. J. Nutrigenet. Nutr. 2012, 5, 117–131. [Google Scholar] [CrossRef]
- Delimont, N.M.; Katz, B.B.; Fiorentino, N.M.; Kimmel, K.A.; Haub, M.D.; Rosenkranz, S.K.; Tomich, J.M.; Lindshield, B.L. Salivary cystatin sn binds to phytic acid in vitro and is a predictor of nonheme iron bioavailability with phytic acid supplementation in a proof of concept pilot study. Curr. Dev. Nutr. 2019, 3, nzz057. [Google Scholar] [CrossRef]
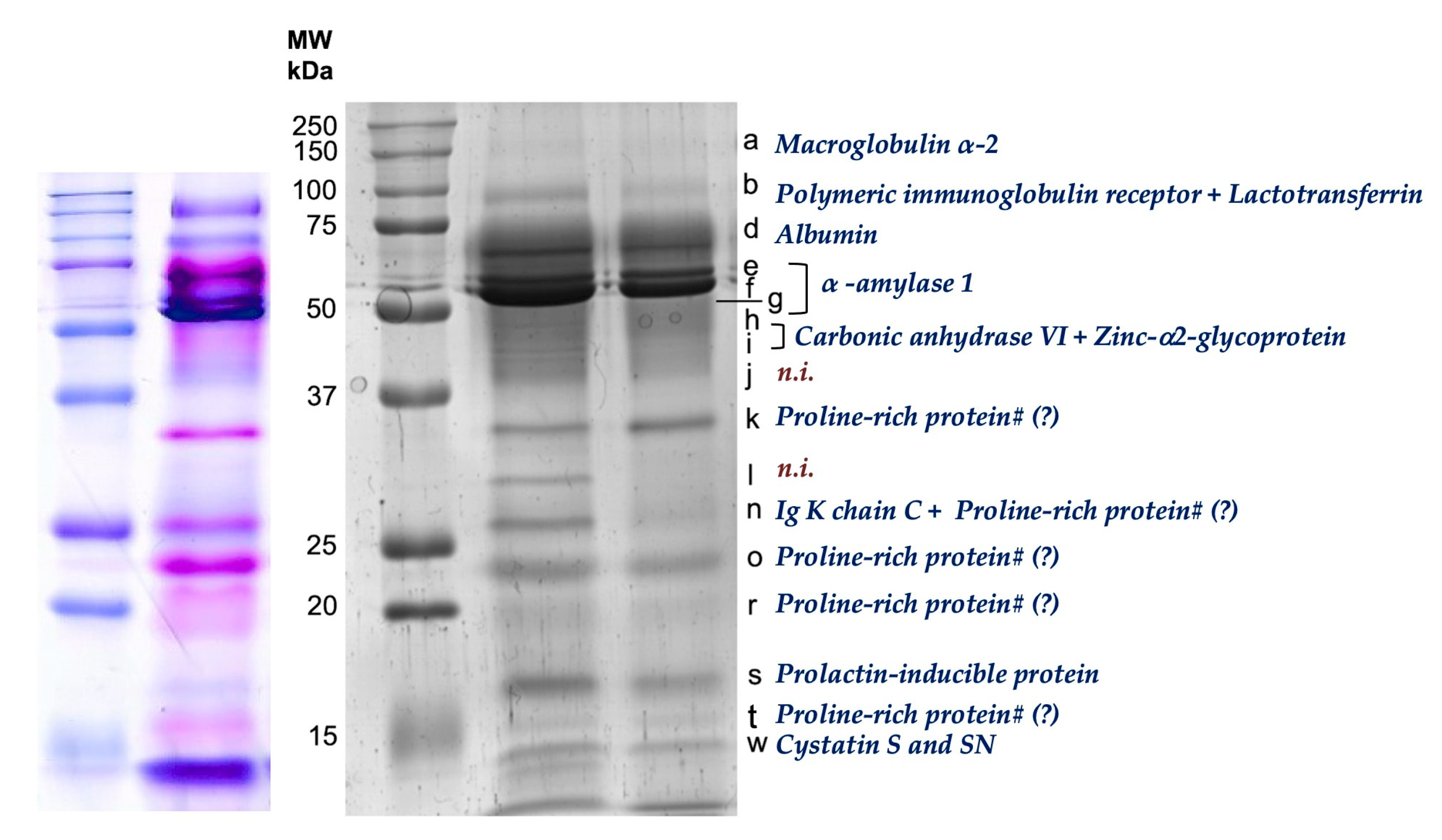


| Salivary Parameter | Apple | Control (Bread) | Treat * Period (p-Value) | ||||
|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | ||
| Flow rate | 0.53 ± 0.28 | 0.71 ± 0.32 | 0.001 * | 0.49 ± 0.30 | 0.50 ± 0.31 | 0.879 | 0.017 * |
| Total protein concentration | 675.9 ± 336.6 | 982.9 ± 377.7 | 0.062 | 370.4 ± 72.4 | 376.1 ± 112.5 | 0.878 | 0.111 |
| Band a | 4.41 ± 1.78 | 3.40 ± 1.28 | 0.155 | 4.64 ± 1.53 | 6.24 ± 2.75 | 0.418 | 0.166 |
| Band b | 6.88 ± 2.26 | 6.19 ± 2.27 | 0.445 | 8.75 ± 1.83 | 8.07 ± 1.44 | 0.491 | 0.992 |
| Band d | 8.71 ± 3.52 | 12.83 ± 5.33 | 0.008 * | 11.25 ± 4.43 | 9.44 ± 4.62 | 0.338 | 0.012 * |
| Band e | 8.29 ± 2.30 | 8.89 ± 3.30 | 0.570 | 11.77 ± 5.80 | 8.09 ± 1.81 | 0.103 | 0.051 |
| Band f | 8.25 ± 2.10 | 12.98 ± 3.63 | 0.001 * | 11.26 ± 3.83 | 9.77 ± 3.38 | 0.371 | 0.003 * |
| Band g | 3.63 ± 1.96 | 5.23 ± 3.58 | 0.261 | 7.57 ± 4.15 | 3.63 ± 3.69 | 0.126 | 0.035 * |
| Band h | 4.22 ± 2.30 | 3.46 ± 2.94 | 0.576 | 3.10 ± 1.54 | 3.48 ± 1.51 | 0.552 | 0.503 |
| Band i | 3.60 ± 1.66 | 3.02 ± 1.15 | 0.496 | 3.98 ± 1.06 | 3.39 ± 1.55 | 0.180 | 0.990 |
| Band j | 3.88 ± 1.37 | 4.76 ± 2.32 | 0.208 | 4.64 ± 1.33 | 6.42 ± 0.48 | 0.129 | 0.471 |
| Band k | 4.68 ± 1.76 | 8.06 ± 4.25 | 0.335 | 3.53 ± 1.59 | 4.91 ± 2.05 | 0.562 | 0.626 |
| Band l | 4.46 ± 1.60 | 3.20 ± 0.58 | 0.140 | 3.43 ± 0.25 | 3.91 ± 0.66 | 0.346 | 0.194 |
| Band n | 8.55 ± 2.30 | 8.09 ± 2.99 | 0.615 | 9.67 ± 2.38 | 6.78 ± 2.07 | 0.032 * | 0.112 |
| Band o | 6.05 ± 1.59 | 8.04 ± 2.11 | 0.012 * | 6.84 ± 1.23 | 9.19 ± 1.89 | 0.006 * | 0.718 |
| Band r | 4.87 ± 1.16 | 5.37 ± 1.36 | 0.469 | 5.26 ± 1.21 | 3.94 ± 0.77 | 0.041 * | 0.044 * |
| Band s | 6.10 ± 3.18 | 5.90 ± 2.18 | 0.881 | 4.36 ± 0.50 | 4.70 ± 0.06 | 0.471 | 0.837 |
| Band t | 5.27 ± 3.30 | 4.14 ± 2.18 | 0.479 | 2.18 ± 0.25 | 2.27 ± 0.76 | 0.916 | 0.691 |
| Band w | 6.83 ± 2.36 | 9.46 ± 2.79 | 0.004 * | 10.97 ± 2.83 | 12.97 ± 2.06 | 0.014 * | 0.568 |
| Salivary Parameter | Apple | Control (Bread) | Treat * Period (p-Value) | ||||
|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | ||
| Flow rate | 0.61 ± 0.23 | 0.59 ± 0.26 | 0.778 | 0.48 ± 0.13 | 0.57 ± 0.35 | 0.479 | 0.400 |
| Total protein concentration | 703.0 ± 332.6 | 555.0 ± 363.2 | 0.111 | 555.3 ± 237.3 | 708.4 ± 389.7 | 0.313 | 0.065 |
| Band a | 3.72 ± 0.97 | 4.73 ± 1.19 | 0.158 | 4.78 ± 2.38 | 5.17 ± 0.69 | 0.704 | 0.609 |
| Band b | 6.21 ± 1.89 | 6.46 ± 1.47 | 0.598 | 8.22 ± 2.58 | 7.23 ± 1.28 | 0.489 | 0.295 |
| Band d | 8.61 ± 3.92 | 7.81 ± 2.20 | 0.577 | 8.52 ± 2.98 | 6.37 ± 1.91 | 0.219 | 0.551 |
| Band e | 7.85 ± 2.07 | 8.01 ± 1.79 | 0.857 | 11.24 ± 3.76 | 9.69 ± 3.53 | 0.525 | 0.419 |
| Band f | 8.09 ± 1.64 | 9.12 ± 2.82 | 0.311 | 8.24 ± 3.93 | 9.23 ± 2.54 | 0.548 | 0.982 |
| Band g | 3.48 ± 1.54 | 3.48 ± 1.03 | 0.997 | 2.57 ± 0.59 | 3.40 ± 0.82 | 0.052 | 0.395 |
| Band h | 4.09 ± 2.34 | 4.57 ± 3.63 | 0.762 | 2.94 ± 0.99 | 2.44 ± 0.46 | 0.337 | 0.621 |
| Band i | 3.47 ± 1.75 | 5.52 ± 3.68 | 0.215 | 3.62 ± 1.16 | 3.20 ± 1.15 | 0.675 | 0.281 |
| Band j | 3.90 ± 1.05 | 4.12 ± 1.75 | 0.777 | 3.27 ± 1.09 | 3.74 ± 0.69 | 0.604 | 0.818 |
| Band k | 4.94 ± 1.55 | 4.26 ± 1.16 | 0.324 | 4.97 ± 1.38 | 2.98 ± 0.84 | 0.059 | 0.228 |
| Band l | 4.43 ± 1.50 | 3.79 ± 1.87 | 0.286 | 3.91 ± 1.53 | 3.08 ± 1.22 | 0.639 | 0.883 |
| Band n | 8.68 ± 2.01 | 8.26 ± 2.91 | 0.724 | 9.06 ± 1.24 | 8.57 ± 1.40 | 0.472 | 0.966 |
| Band o | 6.59 ± 2.07 | 6.91 ± 2.28 | 0.634 | 6.93 ± 3.08 | 7.00 ± 1.19 | 0.963 | 0.861 |
| Band r | 5.24 ± 1.31 | 4.72 ± 1.74 | 0.571 | 5.30 ± 1.59 | 5.30 ± 1.47 | 0.996 | 0.697 |
| Band s | 5.48 ± 1.93 | 3.88 ± 1.85 | 0.314 | 4.39 ± 1.09 | 4.69 ± 1.57 | 0.681 | 0.295 |
| Band t | 4.20 ± 1.69 | 4.48 ± 1.86 | 0.816 | 3.03 ± 1.33 | 3.74 ± 0.43 | 0.289 | 0.799 |
| Band w | 6.39 ± 2.15 | 7.40 ± 2.17 | 0.089 | 9.13 ± 2.98 | 7.14 ± 2.15 | 0.239 | 0.029 * |
| Spot | Apple | Control (Bread) | Interaction T * p (p-Value) | Protein ID | Refs | ||
|---|---|---|---|---|---|---|---|
| Change | p-Value | Change | p-Value | ||||
| Short term | |||||||
| 2 | ↓ | 0.028 * | --- | 0.173 | 0.017 * | α-Amylase | [29,30,31] |
| 4 | ↑ | 0.043 * | ↑ | 0.046 * | 0.023 * | n.i. | |
| 10 | ↑ | 0.046 * | ↑ | 0.028 * | 0.609 | Cystatins S,SN | [21,29] |
| 13 | --- | 0.753 | ↑ | 0.028 * | 0.018 * | α-Amylase | [20,29,30,31] |
| 14 | ↓ | 0.028 * | ↑ | 0.046 * | 0.0005 * | Zinc α-2 glycoprotein | [30,31] |
| 21 | ↑ | 0.028 * | --- | 0.249 | 0.667 | Prolactin inducible protein (PIP) | [30] |
| 24 | ↓ | 0.043 * | --- | 0.249 | 0.038 * | n.i | |
| 25 | ↓ | 0.046 * | --- | 0.753 | 0.165 | n.i. | |
| 26 | ↑ | 0.028 * | --- | 0.116 | 0.782 | Carbonic anhydrase VI (CA-VI) | [30] |
| 37 | ↑ | 0.043 * | ↑ | 0.028 * | 0.146 | SPLUNC | [31] |
| 41 | ↓ | 0.028 * | ↓ | 0.046 * | 0.244 | Ig α chain C | [19] |
| 42 | ↓ | 0.075 | ↑ | 0.028 * | 0.0005 * | n.i | |
| 43 | ↑ | 0.028 * | ↑ | 0.043 * | 0.052 | Cystatins S,SN | [19,20,21] |
| 50 | ↑ | 0.028 * | --- | 0.686 | 0.001 * | Actin cytoplasmic 1 | [21,29,30,31] |
| 51 | ↓ | 0.068 | ↑ | 0.028 * | 0.0005 * | Zinc α-2 glycoprotein | [30] |
| 52 | ↓ | 0.028 * | ↑ | 0.028 * | 0.0005 * | Zinc α-2 glycoprotein | [30] |
| 64 | --- | 0.116 | --- | 0.345 | 0.034 * | α-Enolase | [31] |
| 65 | ↓ | 0.028 * | ↓ | 0.028 * | 0.316 | IgK chain C region | [29,31] |
| 69 | ↓ | 0.028 * | --- | 0.249 | 0.002 * | n.i. | |
| 70 | ↓ | 0.046 * | --- | 0.116 | 0.280 | IgK chain C region | [29,31] |
| 71 | ↑ | 0.028 * | ↓ | 0.028 * | 0.0005 * | IgK chain C region | [29,31] |
| 80 | ↓ | 0.028 * | --- | 0.917 | 0.0005 * | n.i. | |
| 83 | ↓ | 0.046 * | --- | 0.225 | 0.009 * | n.i | |
| 84 | ↓ | 0.043 * | ↓ | 0.028 * | 0.256 | n.i | |
| 85 | --- | 0.500 | ↑ | 0.028 * | 0.022 * | Albumin | [29,30,31] |
| 91 | ↓ | 0.046 * | --- | 0.600 | 0.120 | Ig α chain C | [29] |
| 92 | ↑ | 0.028 * | ↓ | 0.028 * | 0.0005 * | IgK chain C region | [19,29,31] |
| 93 | ↓ | 0.028 * | ↓ | 0.046 * | 0.010 * | Zinc α-2 glycoprotein | [31] |
| Medium term | |||||||
| 7 | ↑ | 0.043 * | --- | 0.109 | 0.002 * | IgK chain C region | [32] |
| 10 | ↑ | 0.173 | ↓ | 0.043 * | 0.009 * | Cystatins S,SN | [21,29] |
| 14 | ↓ | 0.028 * | --- | 0.144 | 0.320 | Zinc α-2 glycoprotein | [30,31] |
| 16 | ↓ | 0.028 * | --- | 0.686 | 0.002 * | α-Amylase | [21,30,31] |
| 24 | ↑ | 0.173 | --- | 0.109 | 0.047 * | n.i | |
| 25 | ↓ | 0.028 * | --- | 0.686 | 0.017 * | n.i | |
| 36 | ↑ | 0.075 | ↓ | 0.043 * | 0.008 * | Prolactin inducible protein (PIP) | [21,30] |
| 40 | ↑ | 0.043 * | --- | 0.500 | 0.097 | α-Amylase/Ig α chain C | [19,30,31] |
| 43 | --- | 0.249 | ↓ | 0.043 * | 0.003 * | Cystatins S,SN | [19,20,21] |
| 45 | ↓ | 0.043 * | --- | 0.893 | 0.121 | Prolactin inducible protein (PIP) | [31] |
| 47 | ↓ | 0.028 * | --- | 0.225 | 0.005 * | n.i | |
| 54 | ↑ | 0.075 | --- | 0.273 | 0.041 * | SPLUNC | [31] |
| 71 | ↓ | 0.046 * | --- | 0.893 | 0.064 | IgK chain C region | [21] |
| 79 | ↑ | 0.028 * | --- | 0.686 | 0.051 | n.i. | |
| 80 | ↓ | 0.028 * | --- | 0.180 | 0.006 * | n.i. | |
| 81 | ↑ | 0.068 | --- | 0.138 | 0.008 * | n.i. | |
| 83 | ↓ | 0.028 * | --- | 0.465 | 0.032 * | n.i. | |
| 85 | ↑ | 0.028 * | --- | 0.225 | 0.036 * | Albumin | [29,30,31] |
| 87 | ↓ | 0.028 * | --- | 0.080 | 0.434 | Albumin | |
| 88 | ↓ | 0.028 * | --- | 0.345 | 0.302 | Albumin | |
| 97 | ↑ | 0.043 * | --- | 0.500 | 0.017 * | Carbonic anhydrase VI (CA-VI) | [31] |
| 101 | --- | 0.463 | ↓ | 0.043 * | 0.024 * | n.i. | |
| 129 | ↓ | 0.028 * | --- | 0.080 | 0.004 * | Albumin | [29,30,31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louro, T.; Carreira, L.; Caeiro, I.; Simões, C.; Ricardo-Rodrigues, S.; Rato, A.E.; Capela e Silva, F.; Luís, H.; Moreira, P.; Lamy, E. The Influence of (Poly)phenol Intake in Saliva Proteome: Short- and Medium-Term Effects of Apple. Foods 2023, 12, 2540. https://doi.org/10.3390/foods12132540
Louro T, Carreira L, Caeiro I, Simões C, Ricardo-Rodrigues S, Rato AE, Capela e Silva F, Luís H, Moreira P, Lamy E. The Influence of (Poly)phenol Intake in Saliva Proteome: Short- and Medium-Term Effects of Apple. Foods. 2023; 12(13):2540. https://doi.org/10.3390/foods12132540
Chicago/Turabian StyleLouro, Teresa, Laura Carreira, Inês Caeiro, Carla Simões, Sara Ricardo-Rodrigues, Ana Elisa Rato, Fernando Capela e Silva, Henrique Luís, Pedro Moreira, and Elsa Lamy. 2023. "The Influence of (Poly)phenol Intake in Saliva Proteome: Short- and Medium-Term Effects of Apple" Foods 12, no. 13: 2540. https://doi.org/10.3390/foods12132540
APA StyleLouro, T., Carreira, L., Caeiro, I., Simões, C., Ricardo-Rodrigues, S., Rato, A. E., Capela e Silva, F., Luís, H., Moreira, P., & Lamy, E. (2023). The Influence of (Poly)phenol Intake in Saliva Proteome: Short- and Medium-Term Effects of Apple. Foods, 12(13), 2540. https://doi.org/10.3390/foods12132540












