Influence of Proteins on Bioaccessibility of α-Tocopherol Encapsulation within High Diacylglycerol-Based Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Protein-Coated α-Tocopherol Emulsions
2.3. In Vitro Simulated Digestion
2.4. Bioaccessibility of α-Tocopherol after Digestion
2.5. Determination of Zeta Potential
2.6. Confocal Laser Scanning Microscopy (CLSM)
2.7. Free Fatty Acid Release Analysis
2.8. Fourier-Transform Infrared Spectroscopy (FTIR)
2.9. Sodium Dodecyl Sulphate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.10. Statistical Analysis
3. Results and Discussion
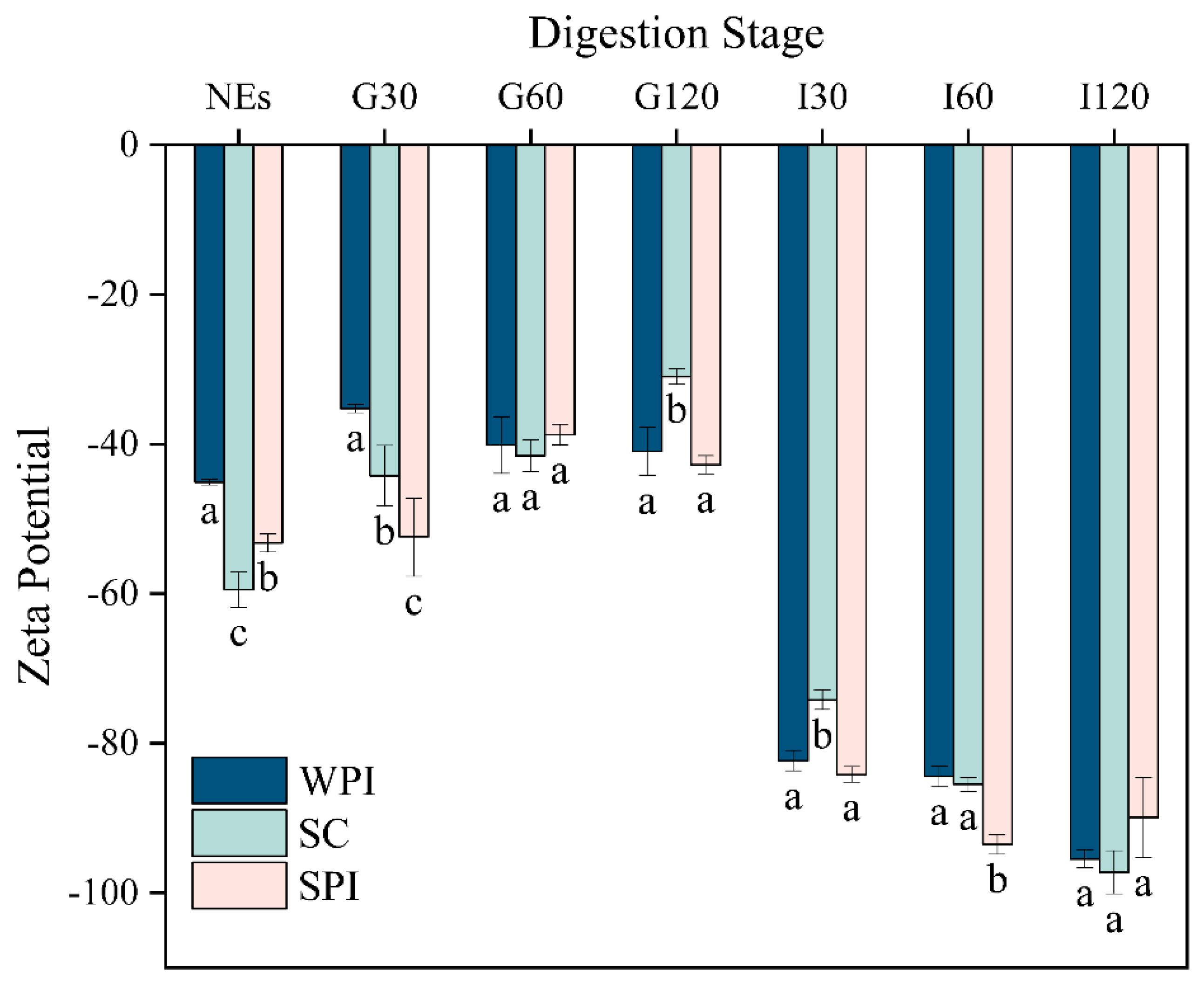
3.1. Changes in Emulsion ζ-Potential during the In Vitro Digestion
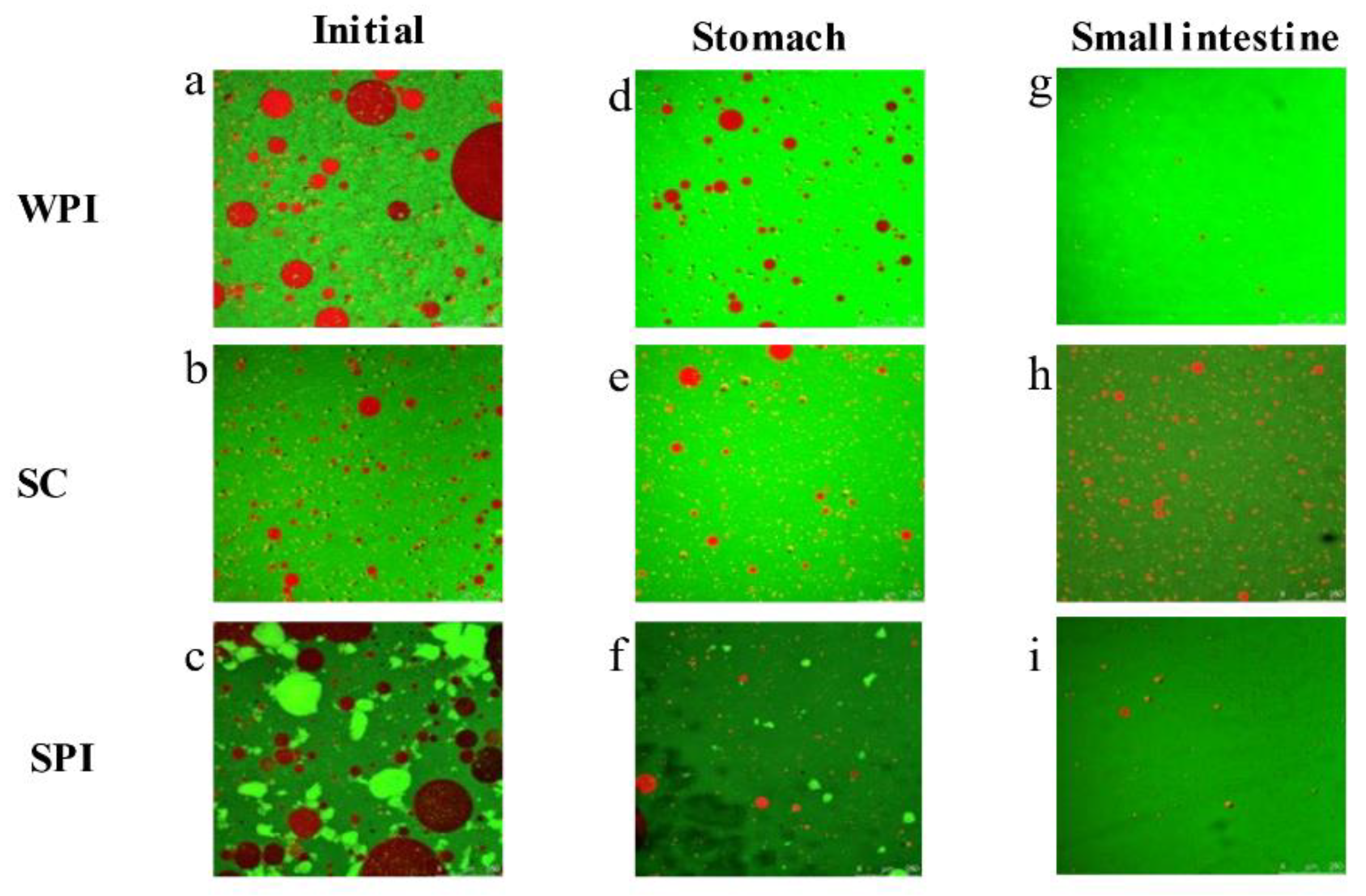
3.2. Microstructure Changes during the In Vitro Digestion
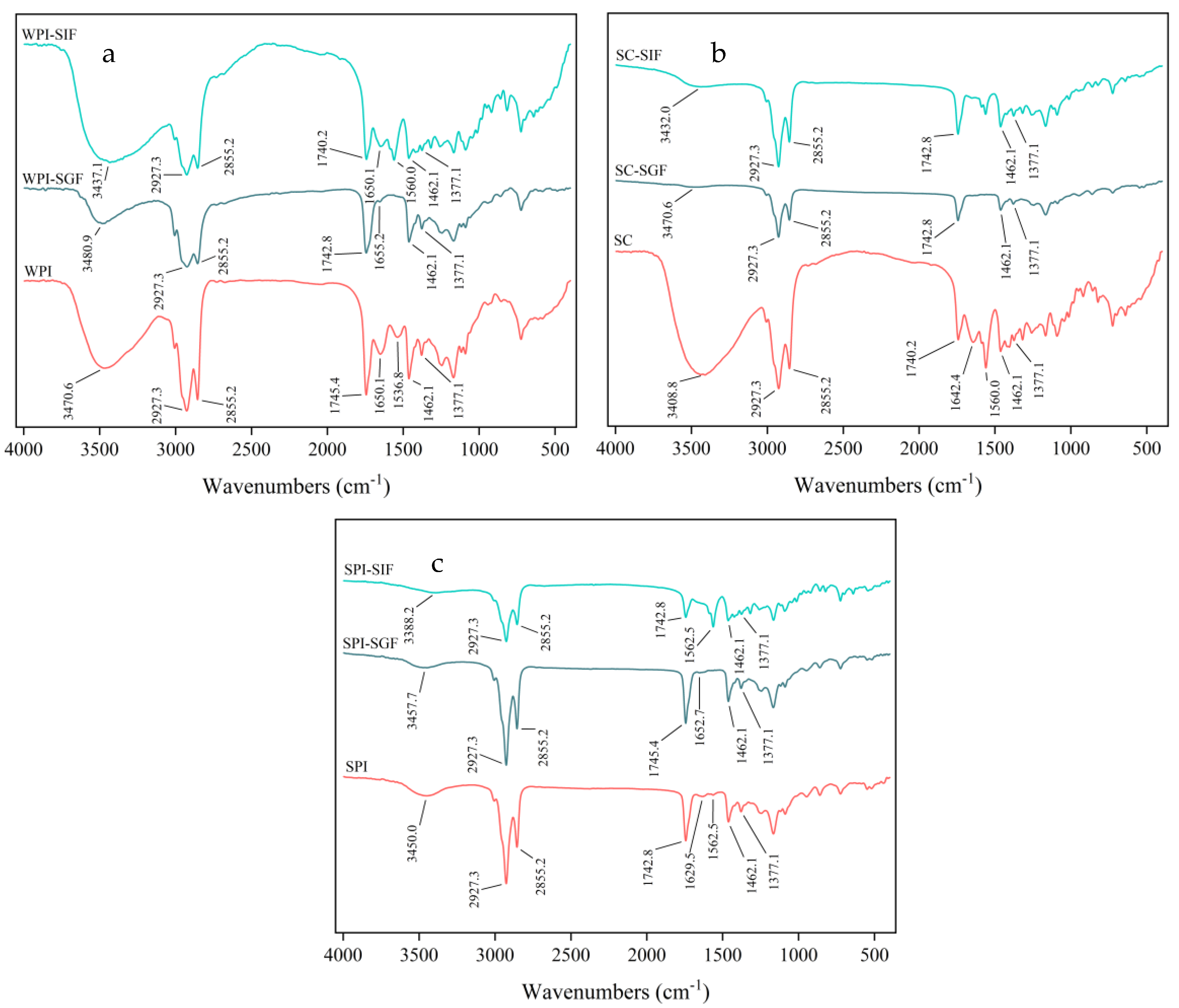
3.3. Fourier Transform Infrared Spectroscopy Analysis
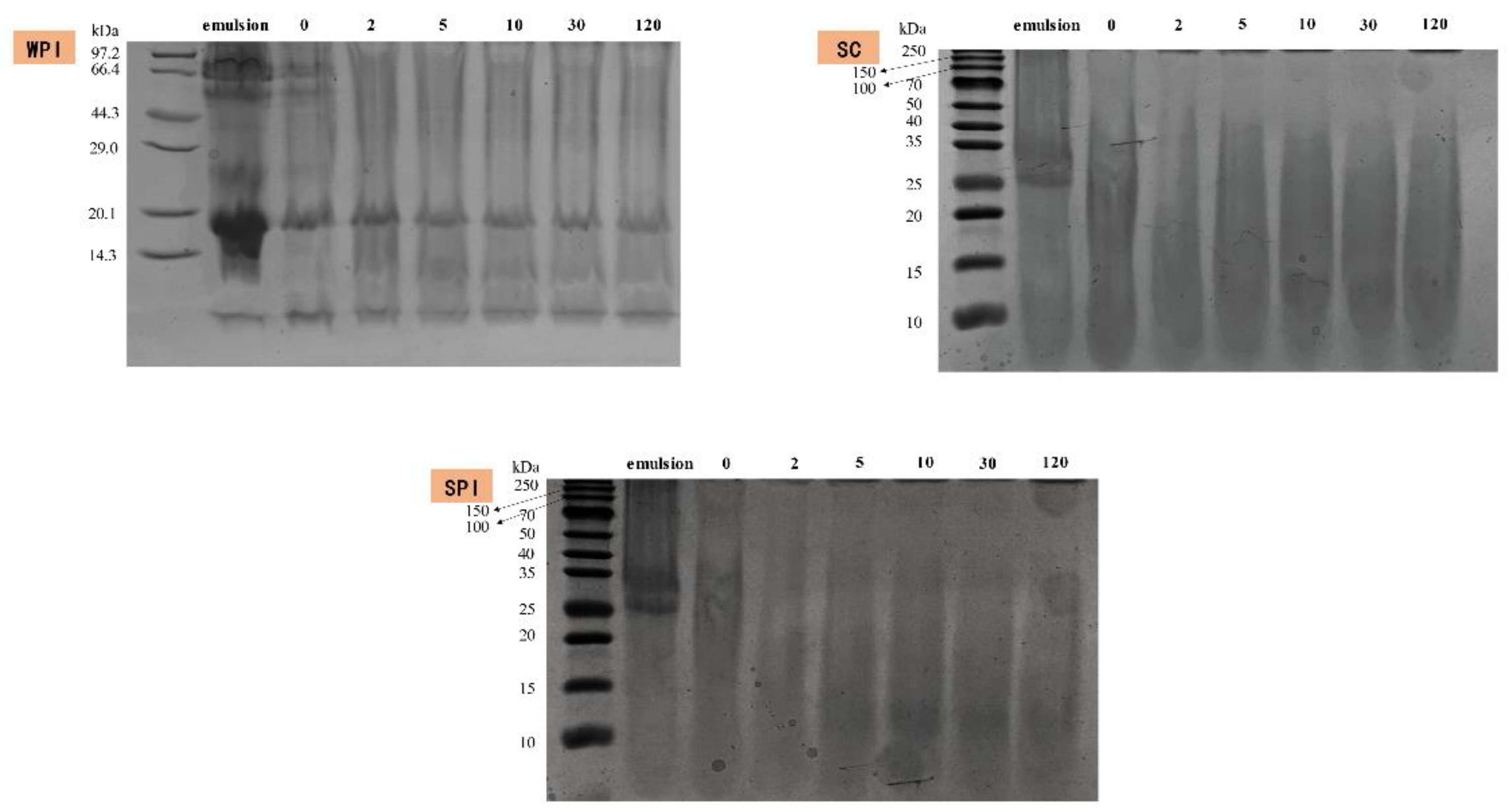
3.4. Proteolytic Hydrolysis during SGF Incubation
3.5. Analysis of FFA Release
3.6. Bioaccessibility of α-Tocopherol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gonnet, M.; Lethuaut, L.; Boury, F. New trends in encapsulation of liposoluble vitamins. J. Control. Release 2010, 146, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, P.W.; Wali, V.B.; Bachawal, S.V.; Shirode, A.B.; Ayoub, N.M.; Akl, M.R. Tocotrienol combination therapy results in synergistic anticancer response. Front. Biosci.-Landmark 2011, 16, 3183–3195. [Google Scholar] [CrossRef] [PubMed]
- Regner-Nelke, L.; Nelke, C.; Schroeter, C.B.; Dziewas, R.; Warnecke, T.; Ruck, T.; Meuth, S.G. Enjoy Carefully: The Multifaceted Role of Vitamin E in Neuro-Nutrition. Int. J. Mol. Sci. 2021, 22, 10087. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Encapsulation of vitamin E in edible emulsions fabricated using a natural surfactant. Food Hydrocoll. 2012, 30, 712–720. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E Inadequacy in Humans: Causes and Consequences. Adv. Nutr. Int. Rev. J. 2014, 5, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Eggersdorfer, M.; Trilok-Kumar, G. Vitamin E status in healthy population in Asia: A review of current literature. Int. J. Vitam. Nutr. Res. 2021, 91, 356–369. [Google Scholar] [CrossRef]
- Noumegni, S.R.; Grangereau, T.; Demir, A.; Bressollette, L.; Couturaud, F.; Hoffmann, C. Cardiovascular Mortality after Venous Thromboembolism: A Meta-Analysis of Prospective Cohort Studies. Semin. Thromb. Hemost. 2021, 48, 481–489. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015, 80, N1602–N1611. [Google Scholar] [CrossRef]
- Yi, J.; Zhang, Y.; Liang, R.; Zhong, F.; Ma, J. Beta-Carotene Chemical Stability in Nanoemulsions Was Improved by Stabilized with Beta-Lactoglobulin–Catechin Conjugates through Free Radical Method. J. Agric. Food Chem. 2014, 63, 297–303. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2014, 45, 175–185. [Google Scholar] [CrossRef]
- Iddir, M.; Vahid, F.; Merten, D.; Larondelle, Y.; Bohn, T. Influence of Proteins on the Absorption of Lipophilic Vitamins, Carotenoids and Curcumin—A Review. Mol. Nutr. Food Res. 2022, 66, 2200076. [Google Scholar] [CrossRef]
- Chen, L.; Yokoyama, W.; Liang, R.; Zhong, F. Enzymatic degradation and bioaccessibility of protein encapsulated β-carotene nano-emulsions during in vitro gastro-intestinal digestion. Food Hydrocoll. 2019, 100, 105177. [Google Scholar] [CrossRef]
- Park, S.; Mun, S.; Kim, Y.-R. Emulsifier Dependent in vitro Digestion and Bioaccessibility of β-Carotene Loaded in Oil-in-Water Emulsions. Food Biophys. 2018, 13, 147–154. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, Y.; Tan, H.; Zhang, R.; McClements, D.J. Vitamin E Encapsulation within Oil-in-Water Emulsions: Impact of Emulsifier Type on Physicochemical Stability and Bioaccessibility. J. Agric. Food Chem. 2019, 67, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef]
- Zhu, P.; Du, X.; Liu, C.; Zhao, G.; Wang, M. Effects of pH during dry-heat preparation on the physicochemical and emulsifying properties of rice starch and whey protein isolate mixtures. Food Hydrocoll. 2023, 140, 108614. [Google Scholar] [CrossRef]
- Deng, L. Current Progress in the Utilization of Soy-Based Emulsifiers in Food Applications—A Review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef]
- de Toledo, A.M.N.; Picone, C.S.F.; Sato, A.C.K. Lecithin-sodium caseinate self-assembled complexes as emulsifying agents in oil-in-water emulsion: Acidic medium approach. Curr. Res. Food Sci. 2022, 5, 958–963. [Google Scholar] [CrossRef]
- Iacobini, C.; Pugliese, G.; Blasetti Fantauzzi, C.; Federici, M.; Menini, S. Metabolically healthy versus metabolically unhealthy obesity. Metabolism 2019, 92, 51–60. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, W.; Sun-Waterhouse, D.; Yan, M.; Zou, Q.; Liu, X.; Lan, D.; Wang, Y. Exploring the fates and molecular changes of different diacylglycerol-rich lipids during in vitro digestion. Food Chem. 2023, 416, 135677. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Luo, R.; Sun-Waterhouse, D.; Liu, Z.; Xu, Q.; Yang, B.; Lan, D.; Wang, W.; Wang, Y. Thermal properties, oxidative stability, and frying applicability of highly pure soybean-based diacylglycerol oil. J. Food Process. Preserv. 2022, 46, e16528. [Google Scholar] [CrossRef]
- Qiu, C.; Lei, M.; Lee, W.J.; Zhang, N.; Wang, Y. Fabrication and characterization of stable oleofoam based on medium-long chain diacylglycerol and β-sitosterol. Food Chem. 2021, 350, 129275. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Li, N.; Faiza, M.; Li, D.; Yao, X.; Zhao, M. Stability and in vitro digestion of high purity diacylglycerol oil-in-water emulsions. LWT 2021, 148, 111744. [Google Scholar] [CrossRef]
- Melnikov, S.M.; Popp, A.K.; Miao, S.; Patel, A.R.; Flendrig, L.M.; Velikov, K.P. Colloidal emulsion based delivery systems for steroid glycosides. J. Funct. Foods 2017, 28, 90–95. [Google Scholar] [CrossRef]
- Xu, Q.; Qin, X.; Lan, D.; Liu, X.; Yang, B.; Liao, S.; Wang, W.; Wang, Y. Water-in-oil emulsions enriched with alpha-linolenic acid in diacylglycerol form: Stability, formation mechanism and in vitro digestion analysis. Food Chem. 2022, 391, 133201. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Marze, S. Bioaccessibility of Nutrients and Micronutrients from Dispersed Food Systems: Impact of the Multiscale Bulk and Interfacial Structures. Crit. Rev. Food Sci. Nutr. 2013, 53, 76–108. [Google Scholar] [CrossRef]
- Lv, S.; Gu, J.; Zhang, R.; Zhang, Y.; Tan, H.; McClements, D.J. Vitamin E Encapsulation in Plant-Based Nanoemulsions Fabricated Using Dual-Channel Microfluidization: Formation, Stability, and Bioaccessibility. J. Agric. Food Chem. 2018, 66, 10532–10542. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Degerli, C.; Dingeo, G.; Desmarchelier, C.; Schleeh, T.; Borel, P.; Larondelle, Y.; Bohn, T. Whey protein isolate modulates beta-carotene bioaccessibility depending on gastro-intestinal digestion conditions. Food Chem. 2019, 291, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, X.; Qi, Q.; Liu, W. Interaction of DPPC liposomes with cholesterol and food protein during in vitro digestion using Dynamic Light Scattering and FTIR spectroscopy analysis. Food Chem. 2022, 375, 129632. [Google Scholar] [CrossRef] [PubMed]
- Ershadi, A.; Eskandari, M.H.; Yousefi, G.H.; Aminlari, M.; Hadian, M.; Esteghlal, S.; Sadeghi, R.; Hosseini, S.M.H. Interaction Between Soy Protein Isolate (SPI) and Water-Soluble Persian Gum to Form Stable Colloidal Complexes. J. Polym. Environ. 2022, 31, 81–89. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2018, 242, 141–152. [Google Scholar] [CrossRef]
- Ma, Q.; Ma, S.; Zhao, Y.; Sun, M.; Li, X.; Liu, L.; Zhang, X.; Sun, Y.; Bora, A.F.M.; Tian, S.; et al. Interaction between whey protein and soy lecithin and its influence on physicochemical properties and in vitro digestibility of emulsion: A consideration for mimicking milk fat globule. Food Res. Int. 2023, 163, 112181. [Google Scholar] [CrossRef] [PubMed]
- Sakuno, M.M.; Matsumoto, S.; Kawai, S.; Taihei, K.; Matsumura, Y. Adsorption and Structural Change of β-Lactoglobulin at the Diacylglycerol−Water Interface. Langmuir 2008, 24, 11483–11488. [Google Scholar] [CrossRef]
- Pool, H.; Mendoza, S.; Xiao, H.; McClements, D.J. Encapsulation and release of hydrophobic bioactive components in nanoemulsion-based delivery systems: Impact of physical form on quercetin bioaccessibility. Food Funct. 2012, 4, 162–174. [Google Scholar] [CrossRef]
- Chen, L.; Liang, R.; Yokoyama, W.; Alves, P.; Pan, J.; Zhong, F. Effect of the co-existing and excipient oil on the bioaccessibility of β-carotene loaded oil-free nanoparticles. Food Hydrocoll. 2020, 106, 105847. [Google Scholar] [CrossRef]
- Xiang, X.; Wen, L.; Wang, Z.; Yang, G.; Mao, J.; An, X.; Kan, J. A comprehensive study on physicochemical properties, bioactive compounds, and emulsified lipid digestion characteristics of Idesia polycarpa var. Vestita Diels fruits oil. Food Chem. 2023, 404, 134634. [Google Scholar] [CrossRef]
- Nik, A.M.; Wright, A.J.; Corredig, M. Impact of interfacial composition on emulsion digestion and rate of lipid hydrolysis using different in vitro digestion models. Colloids Surf. B Biointerfaces 2011, 83, 321–330. [Google Scholar] [CrossRef]
- Jiao, W.; Li, L.; Yu, A.; Zhao, D.; Sheng, B.; Aikelamu, M.; Li, B.; Zhang, X. In Vitro Gastrointestinal Digestibility of Crystalline Oil-in-Water Emulsions: Influence of Fat Crystal Structure. J. Agric. Food Chem. 2019, 67, 927–934. [Google Scholar] [CrossRef]
- Delahaije, R.J.B.M.; Wierenga, P.A.; van Nieuwenhuijzen, N.H.; Giuseppin, M.L.F.; Gruppen, H. Protein Concentration and Protein-Exposed Hydrophobicity as Dominant Parameters Determining the Flocculation of Protein-Stabilized Oil-in-Water Emulsions. Langmuir 2013, 29, 11567–11574. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of structuring emulsion gels by whey or soy protein isolate on the structure, mechanical properties, and in-vitro digestion of alginate-based emulsion gel beads. Food Hydrocoll. 2020, 110, 106165. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of lipid type on gastrointestinal fate of oil-in-water emulsions: In vitro digestion study. Food Res. Int. 2015, 75, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Decker, E.A.; McClements, D.J. Impact of Protein Surface Denaturation on Droplet Flocculation in Hexadecane Oil-in-Water Emulsions Stabilized by β-Lactoglobulin. J. Agric. Food Chem. 2002, 50, 7131–7137. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Dingeo, G.; Yaruro, J.F.P.; Hammaz, F.; Borel, P.; Schleeh, T.; Desmarchelier, C.; Larondelle, Y.; Bohn, T. Influence of soy and whey protein, gelatin and sodium caseinate on carotenoid bioaccessibility. Food Funct. 2020, 11, 5446–5459. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.; Singh, R.P.; Singh, H. Behaviour of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009, 23, 1563–1569. [Google Scholar] [CrossRef]
- Jin, H.; Liu, C.; Zhang, S.; Guo, Z.; Li, J.; Zhao, Q.; Zhang, Y.; Xu, J. Comparison of protein hydrolysates against their native counterparts in terms of structural and antioxidant properties, and when used as emulsifiers for curcumin nanoemulsions. Food Funct. 2020, 11, 10205–10218. [Google Scholar] [CrossRef]
- Li, J.; Ye, A.; Lee, S.J.; Singh, H. Influence of gastric digestive reaction on subsequent in vitro intestinal digestion of sodium caseinate-stabilized emulsions. Food Funct. 2012, 3, 320–326. [Google Scholar] [CrossRef]
- Sassi, P.; Caponi, S.; Ricci, M.; Morresi, A.; Oldenhof, H.; Wolkers, W.F.; Fioretto, D. Infrared versus light scattering techniques to monitor the gel to liquid crystal phase transition in lipid membranes. J. Raman Spectrosc. 2015, 46, 644–651. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Deng, C.; Xie, C.; Huang, M.; Zheng, F. Improving Stability and Accessibility of Quercetin in Olive Oil-in-Soy Protein Isolate/Pectin Stabilized O/W Emulsion. Foods 2020, 9, 123. [Google Scholar] [CrossRef]
- Tarhan, İ. A comparative study of ATR-FTIR, UV–visible and fluorescence spectroscopy combined with chemometrics for quantification of squalene in extra virgin olive oils. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 241, 118714. [Google Scholar] [CrossRef] [PubMed]
- Olale, K.; Walyambillah, W.; Mohammed, S.A.; Sila, A.; Shepherd, K. FTIR-DRIFTS-based prediction of β-carotene, α-tocopherol and l-ascorbic acid in mango (Mangifera indica L.) fruit pulp. SN Appl. Sci. 2019, 1, 279. [Google Scholar] [CrossRef]
- Helal, H.M.; Samy, W.M.; El-Fakharany, E.M.; Kamoun, E.A.; Mortada, S.M.; Sallam, M.A. Maltodextrin-α-tocopherol conjugates of vitamin E: Influence of degree of derivatization on physicochemical properties and biological evaluation. J. Drug Deliv. Sci. Technol. 2020, 60, 102097. [Google Scholar] [CrossRef]
- Golea, C.M.; Codină, G.G.; Oroian, M. Prediction of wheat flours composition using fourier transform infrared spectrometry (FT-IR). Food Control 2023, 143, 109318. [Google Scholar] [CrossRef]
- Li, J.; Xi, Y.; Wu, L.; Zhang, H. Preparation, characterization and in vitro digestion of bamboo shoot protein/soybean protein isolate based-oleogels by emulsion-templated approach. Food Hydrocoll. 2023, 136, 108310. [Google Scholar] [CrossRef]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochemistry 2018, 41, 382–388. [Google Scholar] [CrossRef]
- Stan, M.S.; Cinteza, L.O.; Petrescu, L.; Mernea, M.A.; Calborean, O.; Mihailescu, D.F.; Sima, C.; Dinischiotu, A. Dynamic analysis of the interactions between Si/SiO2 quantum dots and biomolecules for improving applications based on nano-bio interfaces. Sci. Rep. 2018, 8, 5289. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Wang, J.; Dong, M.; Li, L.; Bai, F.; Xu, K.; Wang, L. Sodium caseinate decorating on shellac nanoparticles as a stabilizer for the encapsulation of quercetin. Food Chem. 2022, 395, 133580. [Google Scholar] [CrossRef]
- Furtado, G.D.F.; Silva, K.C.G.; de Andrade, C.C.P.; Cunha, R.L. In vitro digestibility of heteroaggregated droplets coated with sodium caseinate and lactoferrin. J. Food Eng. 2018, 229, 86–92. [Google Scholar] [CrossRef]
- Yan, S.; Xu, J.; Liu, G.; Du, X.; Hu, M.; Zhang, S.; Jiang, L.; Zhu, H.; Qi, B.; Li, Y. Emulsions co-stabilized by soy protein nanoparticles and tea saponin: Physical stability, rheological properties, oxidative stability, and lipid digestion. Food Chem. 2022, 387, 132891. [Google Scholar] [CrossRef]
- Vandemoortele, A.; Babat, P.; Yakubu, M.; De Meulenaer, B. Behavior of Malondialdehyde and Its Whey Protein Adducts during In Vitro Simulated Gastrointestinal Digestion. J. Agric. Food Chem. 2020, 68, 11846–11854. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, J.; Mulvihill, D.M. Preparation, characterisation and selected functional properties of sodium caseinate–maltodextrin conjugates. Food Chem. 2009, 115, 1257–1267. [Google Scholar] [CrossRef]
- McClements, D.J.; Li, Y. Review of in vitro digestion models for rapid screening of emulsion-based systems. Food Funct. 2010, 1, 32–59. [Google Scholar] [CrossRef]
- Diao, X.; Guan, H.; Kong, B.; Liu, D.; Zhang, Y. In vitro digestion of emulsified lard-based diacylglycerols. J. Sci. Food Agric. 2021, 101, 3386–3393. [Google Scholar] [CrossRef] [PubMed]
- Naso, J.N.; Bellesi, F.A.; Pilosof, A.M. β-lactoglobulin peptides originating during in vitro digestion improve the bioaccesibility of healthy oils emulsions by forming mixed bile salts micelles with enhanced capacity to solubilize lipolysis products. Food Hydrocoll. Health 2023, 3, 100121. [Google Scholar] [CrossRef]
- Shen, P.; Zhao, M.; Zhou, F. Design of soy protein/peptide-based colloidal particles and their role in controlling the lipid digestion of emulsions. Curr. Opin. Food Sci. 2021, 43, 61–70. [Google Scholar] [CrossRef]
- Sarkar, A.; Ye, A.; Singh, H. On the role of bile salts in the digestion of emulsified lipids. Food Hydrocoll. 2016, 60, 77–84. [Google Scholar] [CrossRef]
- Dickinson, E.; Golding, M. Influence of calcium ions on creaming and rheology of emulsions containing sodium caseinate. Colloids Surfaces A: Physicochem. Eng. Asp. 1998, 144, 167–177. [Google Scholar] [CrossRef]
- Li, Y.; Hu, M.; McClements, D.J. Factors affecting lipase digestibility of emulsified lipids using an in vitro digestion model: Proposal for a standardised pH-stat method. Food Chem. 2011, 126, 498–505. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of Lipid-Based Encapsulation on the Bioaccessibility and Bioavailability of Phenolic Compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Jiang, T.; Charcosset, C. Encapsulation of curcumin within oil-in-water emulsions prepared by premix membrane emulsification: Impact of droplet size and carrier oil type on physicochemical stability and in vitro bioaccessibility. Food Chem. 2022, 375, 111475. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ye, F.; Zhu, Y.; Miao, M. Development of dendrimer-like glucan-stabilized Pickering emulsions incorporated with β-carotene. Food Chem. 2022, 385, 132626. [Google Scholar] [CrossRef] [PubMed]
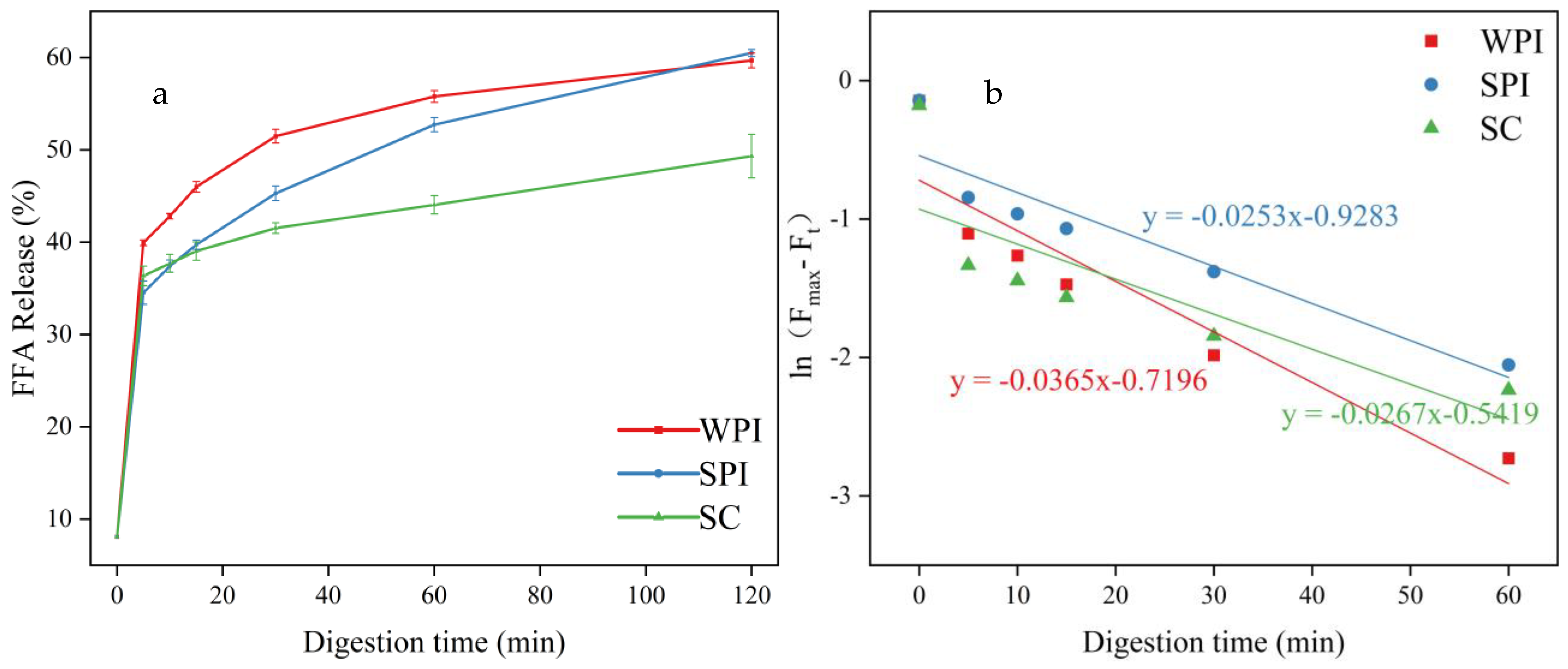
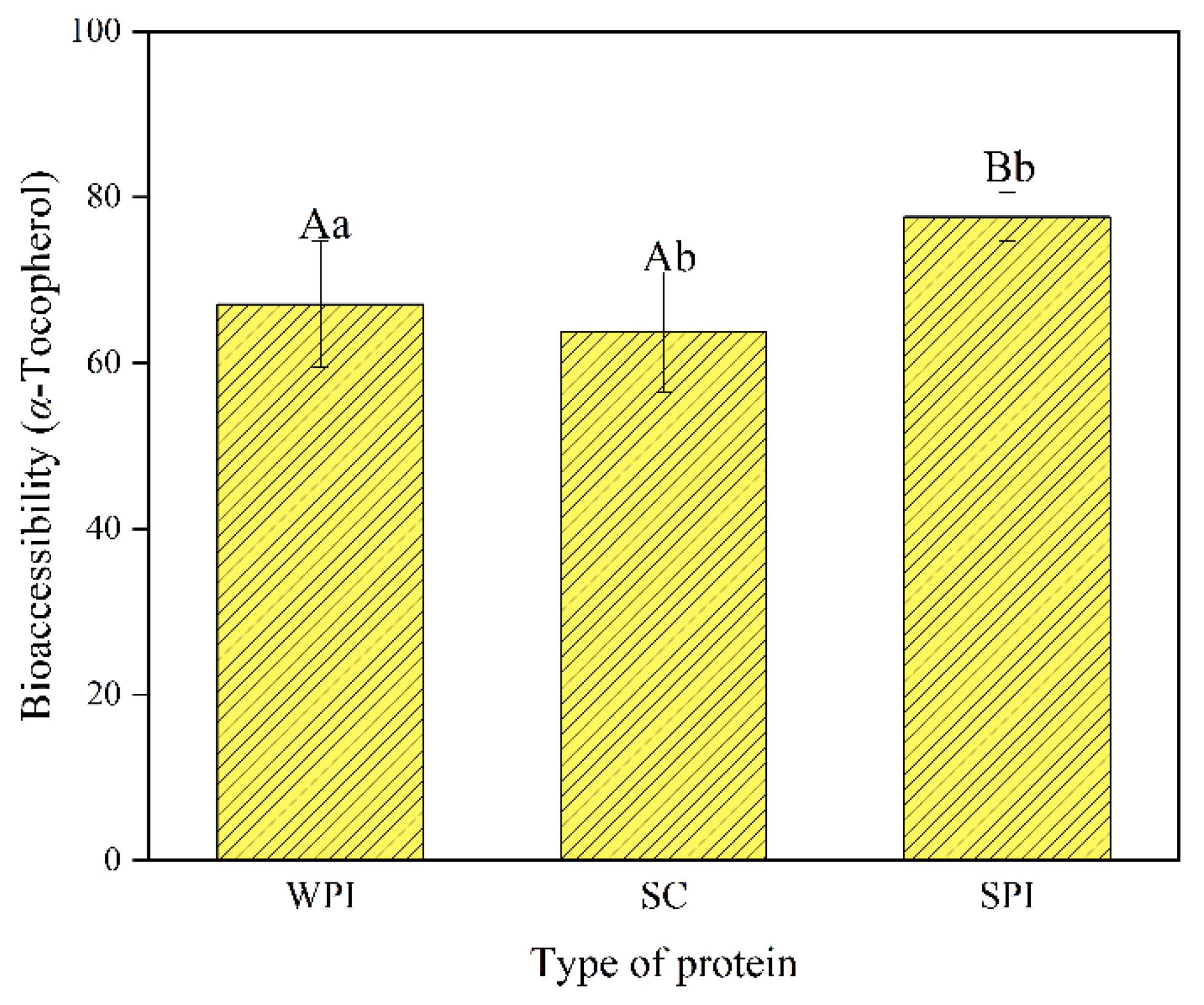
| TAG | FFAs | 1,3-DAG | 1,2-DAG | 1-MAG | 2-MAG | |
|---|---|---|---|---|---|---|
| WPI | 7.21 ± 0.72 a | 59.67 ± 0.80 a | 12.99 ± 0.56 a | 6.12 ± 0.60 a | 9.55 ± 0.24 a | 4.44 ± 0.04 a |
| SC | 10.02 ± 0.55 b | 49.32 ± 2.36 b | 23.10 ± 2.14 b | 9.59 ± 0.93 b | 4.24 ± 0.64 b | 3.75 ± 1.06 a |
| SPI | 6.55 ± 1.19 a | 60.48 ± 0.38 a | 11.06 ± 1.14 a | 5.39 ± 0.60 a | 9.50 ± 0.22 a | 7.02 ± 0.15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Q.; Wang, W.; Xu, Q.; Yan, M.; Lan, D.; Wang, Y. Influence of Proteins on Bioaccessibility of α-Tocopherol Encapsulation within High Diacylglycerol-Based Emulsions. Foods 2023, 12, 2483. https://doi.org/10.3390/foods12132483
Zou Q, Wang W, Xu Q, Yan M, Lan D, Wang Y. Influence of Proteins on Bioaccessibility of α-Tocopherol Encapsulation within High Diacylglycerol-Based Emulsions. Foods. 2023; 12(13):2483. https://doi.org/10.3390/foods12132483
Chicago/Turabian StyleZou, Qian, Weifei Wang, Qingqing Xu, Menglei Yan, Dongming Lan, and Yonghua Wang. 2023. "Influence of Proteins on Bioaccessibility of α-Tocopherol Encapsulation within High Diacylglycerol-Based Emulsions" Foods 12, no. 13: 2483. https://doi.org/10.3390/foods12132483
APA StyleZou, Q., Wang, W., Xu, Q., Yan, M., Lan, D., & Wang, Y. (2023). Influence of Proteins on Bioaccessibility of α-Tocopherol Encapsulation within High Diacylglycerol-Based Emulsions. Foods, 12(13), 2483. https://doi.org/10.3390/foods12132483







