Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications
Abstract
1. Introduction
2. Current Food Packaging Materials and Associated Issues/Challenges
3. Possible Solutions for Current Food Packaging Materials
4. Degradation Chemistry of Biopolymers
5. Important Properties of Biopolymers in Food Packaging
6. Biodegradable Polymers Currently Used in the Food Packaging Industry
6.1. Polysaccharide-Based Biopolymers
6.1.1. Starch

| Packaging Material | Characteristics of Food Packaging System | Mechanical Properties | Thermal Properties | Application | Reference |
|---|---|---|---|---|---|
| Starch | |||||
| Starch-cinnamon essential oil (CEO)—TiO2 NPs |
| TS (MPa): ~18, ~25(respectively for starch, starch—5% TiO2—3% CEO) EB (%): ~26, ~24 (respectively, for starch, starch—5% TiO2—3% CEO) | - | Potential active food packaging material for fresh pistachio packaging | [6] |
| Starch—PBAT |
| TS (MPa): 1.5, 7.4 MPa (respectively, for starch—0% PBAT, starch—50 wt% PBAT) EB (%): ~100, ~450 (respectively, for starch—0% PBAT, starch—50 wt% PBAT) | - | Potential active food packaging material | [9] |
| Starch—cellulose nanofibers (CNF) |
| TS (MPa): 8.9 ± 0.1, 16.5 ± 0.4 (respectively, for starch, starch—10% CNF) EB (%): 83.2 ± 0.7, 13.2 ± 1.2(respectively, for starch, starch—10% CNF | - | Potential active food packaging material | [105] |
| Starch—cellulose nanocrystals (CNC) |
| TS (MPa): ~16, ~24 (respectively, for starch, starch—15% CNC) EB (%): ~13, ~4 (respectively, for starch, starch—15% CNC) | Tonset (°C): 307 ± 3.21, 266 ± 6.03 (respectively, for starch, starch—15% CNC) Tmax (°C): 335 ± 2.65, 328 ± 1.53 (respectively, for starch, starch—15% CNC) | Potential active food packaging material | [7] |
| Starch—ZnO-rosemary polyphenols included in PVA |
| TS (MPa): 2.1 ± 0.2, 3.5 ± 0.2 (respectively, for starch, starch—ZnO-rosemary polyphenols included in PVA) EB (%): 50 ± 16, 76 ± 9 (respectively, for starch, starch–ZnO-rosemary polyphenols included in PVA) | - | Potential active food packaging material | [8] |
| Starch—cellulose nanofiber (CNF)-thymol |
| TS (MPa): ~11, ~6 (respectively, for starch—CNF, starch—CNF-10% thymol) EB (%): ~110, ~122 (respectively, for starch—CNF, starch—CNF-10% thymol) | - | Potential active food packaging material | [106] |
| Cassava starch—red cabbage extract |
| TS (MPa): 5.73 ± 0.12, 10.37 ± 0.22 (respectively, for native cassava starch, oxidized-acetylated starch) EB (%): 102.44 ± 3.2, 60.52 ± 3.39 (respectively, for native cassava starch, oxidized-acetylated starch) | Weight losses of the first phase (30–105 °C), the second phase (106–320 °C), and the third phase (above 320 °C) | Potential intelligent food packaging material. | [107] |
| Chitosan | |||||
| Chitosan—polyvinyl alcohol-anthocyanins |
| - | - | Potential intelligent food packaging material for real-time shrimp freshness monitoring | [109] |
| Chitosan—cellulose acetate phthalate—ZnO NPs |
| TS (MPa): 8.29 ± 0.16, 9.83 ± 0.19 (respectively, for chitosan, chitosan—cellulose acetate phthalate—ZnO NPs) EB (%): 12.67 ± 0.38, 15.44 ± 0.46 (respectively, for chitosan, chitosan—cellulose acetate phthalate—ZnO NPs) | - | Potential active food packaging material for black grapefruits by increasing shelf-life up to 9 days | [110] |
| Chitosan—TiO2 NPs |
| TS (MPa): 10.96 ± 1.57, 16.43 ± 0.46 (respectively, for chitosan, chitosan—TiO2 NPs) EB (%): 57.71 ± 1.28 53.06 ± 2.15 (respectively, for chitosan, chitosan—TiO2 NPs) | - | Potential active food packaging material to maintain quality and extend storage life of climacteric fruit | [111] |
| Chitosan—TiO2 NPs—Cymbopogon citratus essential oil |
| TS (MPa): 37.50 ± 0.00, 62.46 ± 0.13 (respectively, for chitosan, chitosan—1%TiO2 NPs—0.5% Cymbopogon citratus essential oil) EB (%): 4.77 ± 0.03, 4.81 ± 0.01 (respectively, for chitosan, chitosan—1%TiO2 NPs—0.5% Cymbopogon citratus essential oil) | - | Potential active packaging material for prolong shelf-life of minced meat by reducing microbial growth | [112] |
| Chitosan—graphene oxide NPs |
| TS (MPa): 0.063 ± 0.0041, 0.083 ± 0.0034 (respectively, for chitosan, chitosan—graphene oxide NPs) EB (%): 6.45 ± 0.05, 6.95 ± 0.72 (respectively, for chitosan, chitosan—graphene oxide NPs) | - | Potential active food packaging bag for prolonging shelf-life of melon fruits | [12] |
| Chitosan-pullulan—clove-essential-oil-loaded chitosan–ZnO hybrid NPs |
| TS (MPa): 62.0 ± 0.2, 83.7 ± 0.2 (respectively, for chitosan, chitosan-pullulan—clove-essential-oil-loaded chitosan—3% ZnO hybrid NPs) EB (%): 5.0 ± 0.1, 5.1 ± 0.5 (respectively, for chitosan, chitosan-pullulan—clove-essential-oil-loaded chitosan—3% ZnO hybrid NPs) | - | Potential active packaging material for prolonging shelf-life of chicken | [113] |
| Chitosan—modified silica NPs |
| TS (MPa): 101.29 ± 0.57, 125.25 ± 0.36 (respectively, for chitosan, chitosan—4% modified silica NPs) EB (%): 4.78 ± 0.06, 2.26 ± 0.11 (respectively, for chitosan, chitosan—4% modified silica NPs | Potential antioxidant active packaging material | [114] | |
| Chitosan-alginate—TiO2 NPs |
| TS (MPa): 1.82 ± 0.16, 26.86 ± 0.28 (respectively, for chitosan—alginate, chitosan-alginate—0.2% TiO2 NPs) EB (%): 2.05 ± 0.64, 3.66 ± 0.63 (respectively, for chitosan –alginate, chitosan-alginate—0.2% TiO2 NPs) | The first stage of weight loss is the temperature range of 60–180 °C The second stage of weight loss takes place in the temperature range of 210–400 °C | Potential active food packaging bag for prolonging shelf-life of cherry tomatoes | [29] |
| Carrageenan | |||||
| Carrageenan—CuS NPs |
| TS (MPa): ~60, ~62 (respectively, for carrageenan, carrageenan—0.15% CuS NPs EB (%): ~35, ~45(respectively, for carrageenan, carrageenan—0.15% CuS NPs | First step occurred at about 60–110 °C The second stage from 120 to 220 °C The third stage, from 230 to 290 °C | Potential antimicrobial active food packaging material for beef packaging | [115] |
| κ-carrageenan—Zataria multiflora extract—nanoclay |
| TS (MPa): 17.0 ± 2.0, 33.7 ± 3.9 (respectively, for κ-carrageenan-nanoclay, κ-carrageenan-Zataria multiflora extract—nanoclay) EB (%): 63.8 ± 16.8, 20.9 ± 5.7 (respectively, for κ-carrageenan-nanoclay, κ-carrageenan- Zataria multiflora extract—nanoclay) | Initial step of weight loss around 40–120 °C The second step around 120–260 °C The third weight loss stage from 260 to 500 °C | Potential active food packaging material | [16] |
| κ-carrageenan—pomegranate flesh and peel extracts |
| TS (MPa): 24.73 ± 1.25, 30.94 ± 0.85 (respectively, for κ-carrageenan, κ-carrageenan—pomegranate flesh and peel extracts) EB (%): 13.82 ± 2.45, 22.29 ± 1.54 (respectively, for κ-carrageenan, κ-carrageenan—pomegranate flesh and peel extracts) | - | Potential active intelligent food packaging material | [116] |
| κ-carrageenan—cassava starch |
| TS (MPa): 19.23 ± 3.58, 25.88 ± 2.55 (respectively, for 100% κ-carrageenan, 75% κ-carrageenan—cassava starch) EB (%): 4.36 ± 0.90, 8.41 ± 1.71 (respectively, for 100% κ-carrageenan, 75% κ-carrageenan—cassava starch) | Inflection points in DTG curves for the κ-carrageenan (210 °C) and starch (334 °C) films | Potential active food packaging material | [13] |
| κ-carrageenan—cellulose nanocrystals |
| TS (MPa): 38.33 ± 3.79, 52.73 ± 0.70 (respectively, for κ-carrageenan, κ-carrageenan—7% cellulose nanocrystals) EB (%): 21.50 ± 3.72, 25.83 ± 2.61 (respectively, for κ-carrageenan, κ-carrageenan—7% cellulose nanocrystals) | The first degradation, which occurred at 30–200 °C The second degradation at 230–400 °C | Potential active food packaging material | [117] |
| κ-carrageenan-gelatin—TiO2 NPs—anthocyanin |
| TS (MPa): 53.9 ± 0.6, 23.6 ± 2.2 (respectively for 3% κ-carrageenan-gelatin, 3% κ-carrageenan-gelatin—3% TiO2 NPs—anthocyanin) EB (%): 1.47 ± 0.05, 30.4 ± 0.2 (respectively, for 3% κ-carrageenan-gelatin, 3% κ-carrageenan-gelatin- 3% TiO2 NPs—anthocyanin) | The first degradation 170–200 °C Second degradation around 230–250 °C Third degradation around 460–480 °C | Potential smart and active packaging material | [118] |
| κ-carrageenan—honey bee pollen phenolic compounds |
| TS (MPa): 24.60 ± 1.65, 35.97 ± 0.95 (respectively, for κ-carrageenan, κ-carrageenan—honey bee pollen phenolic compounds) EB (%): 69.91 ± 1.75, 78.64 ± 2.08 (respectively, for κ-carrageenan, κ-carrageenan—honey bee pollen phenolic compounds) | TOnset: 348 °C, 348 °C (respectively, for κ-carrageenan, κ-carrageenan—honey bee pollen phenolic compounds) | Potential edible films for beef | [119] |
| Cellulose | |||||
| Carboxymethyl cellulose—chitosan—ZnO NPs |
| - | - | Potential active food packaging material for bread by reducing microbial growth | [18] |
| Carboxymethyl cellulose—guanidinylated chitosan enriched with TiO2 NPs |
| TS (MPa): 25.12 ± 1.43, 29.36 ± 1.88 (respectively, for carboxymethyl cellulose, carboxymethyl cellulose—guanidinylated chitosan enriched with 5% TiO2 NPs) EB (%): (respectively, for carboxymethyl cellulose, carboxymethyl cellulose—guanidinylated chitosan enriched with 5% TiO2 NPs) | The first mass loss around 100 °C The second mass loss of ca. occurred at 216–326 °C The third mass loss around 600 °C | Potential active food packaging material for excellent resistance to mass loss and spoilage of green bell pepper | [20] |
| Methylcellulose—jambolão (Syzygium cumini) skins extract |
| TS (MPa): 16.10 ± 1.52, 21.4 ± 1.55 (respectively, for methylcellulose, methylcellulose film—50% jambolão extract) EB (%): 14.2 ± 2.0, 37.5 ± 2.0 (respectively, for methylcellulose, methylcellulose film—50% jambolão extract) | Tg (°C): 166.07, 135.97 (respectively, for methylcellulose, methyl-cellulose film—50% jambolão extract) Tm (°C): 174.37, 161.46 (respectively, for methylcelllose, methyl-cellulose film—50% jambolão extract) | Potential active intelligent food packaging for meat and aquatic products, where lipid oxidation occurs, and the pH modification is associated with food spoilage | [120] |
| Carboxymethyl cellulose (CMC)-starch |
| TS (MPa): 50.2 ± 6.9, 32.6 ± 2.1 (respectively, for CMC, 80% CMC-20% starch) EB (%): 7.6 ± 2.2, 21.2 ± 4.3 (respectively, for CMC, 80% CMC-20% starch) | The first degradation at approximately 95 °C The second step of the thermal occurs between 145 °C and 160 °C. The third stage occurred in the range of 250–350 °C | Potential active food packaging material | [121] |
| Cellulose—ZnO NPs |
| TS (MPa): 141.70 ± 3.70, 126.61 ± 15.34 (respectively, for cellulose, cellulose—1% ZnO NPs) EB (%): 3.05 ± 0.34, 2.58 ± 0.73 (respectively, for cellulose, cellulose—1% ZnO NPs) | Minor weight loss of cellulose films at 50–55 °C Depending on the concentration of ZnONP, the thermal degradation was observed in the range of 270–330 °C | Potential antimicrobial food packaging material | [122] |
| Bacterial cellulose (BC)-carboxymethyl cellulose (CMC)-yeast |
| TS (MPa): 17.02 ± 1.19, 2.23 ± 0.33 (respectively, for BC, BC-CNC-yeast) EB (%): 4.77 ± 0.56, 15.53 ± 0.84 (respectively, for BC, BC-CNC-yeast) | BC-CNC-yeast first degradation step at 90 °C BC first degradation step at 100 °C Second degradation step for BC-CNC-yeast started between 240 °C to 260 °C and continued until 330 °C BC cellulose skeleton degrades up to 300 °C | Potential edible food packaging materials | [123] |
| Agar | |||||
| Agar—melanin NPs |
| TS (MPa): 34.8 ± 0.7, 46.7 ± 1.7 (respectively, for agar, agar—0.5% melanin NPs) EB (%): 11.8 ± 0.7, 12.2 ± 0.9 (respectively, for agar, agar—0.5% melanin NPs) | Initial weight loss at 60–110 °C The next weight loss started at around 200 °C The maximum weight loss at 250 °C | Potential antioxidant active food packaging material | [21] |
| Agar—thermoplastic corn starch—glycerol |
| TS (MPa): 1.8 ± 0.2, 10.7 ± 2.1 (respectively, for thermoplastic corn starch, 60% agar—thermoplastic corn starch) | - | Potential active food packaging material | [22] |
| Agar—grey triggerfish skin gelatin—vine leaves ethanolic extract |
| TS (MPa): 68.15 ± 1.20, 62.50 ± 1.10 (respectively, for gelatin-agar bilayer and gelatin-agar bilayer—5 mg/mL vine leaves) EB (%): 21.20 ± 1.91, 25.20 ± 1.10 (respectively, for gelatin-agar bilayer and gelatin-agar bilayer—5 mg/mL vine leaves) | Tg (°C): 65.15, 65.24 (respectively, for gelatin-agar bilayer and gelatin-agar bilayer—5 mg/mL vine leaves) | Potential active food packaging material | [23] |
| Agar—sodium alginate-SiO2 NPs |
| TS (MPa): 45.18 ± 1.34, 74.68 ± 2.23 (respectively, for agar—sodium alginate, agar—sodium alginate-10 wt% SiO2 NPs) EB (%): 33.04 ± 0.40, 52.99 ± 1.65 (respectively, for agar—sodium alginate, agar—sodium alginate-10 wt% SiO2 NPs) | The first step of weight loss occurred at 50–150 °C The second stage of weight loss was 160–310 °C The third step, for temperature higher than 310 °C | Potential active food packaging material | [3] |
| Agar—maltodextrin bees wax |
| TS (MPa): ~20, ~40 (respectively, for agar—maltodextrin bees wax, agar—maltodextrin bees wax-tween 80) | The first endothermic peak centered at 65 °C, the second melting peaks around 110 °C | Potential active food packaging material for higher water vapor resistance material | [124] |
| Agar—AgNPs |
| - | - | Potential active food packaging material. | [125] |
| Agar—sugarcane Wax—butterfly pea flower extract |
| TS (MPa): 0.412 ± 0.016, 1.140 ± 0.172 (respectively, for agar—butterfly pea flower extract, agar—sugarcane wax—butterfly pea flower extract) EB (%): 69.000 ± 0.091, 46.000 ± 0.175 (respectively, for agar—butterfly pea flower ex-tract, agar—sugarcane wax—butterfly pea flower ex-tract) | Potential intelligent food packaging material for optical tracking of shrimp freshness | [126] | |
| Pectin | |||||
| Pectin–polycaprolactone |
| EB (%): ~1, ~20 (respectively, for pectin, pectin–polycaprolactone) | The first one, centered around 100 °C, is due to the loss of water; the second between 200 and 400 °C is attributed to the pyrolytic decomposition of macromolecular chains; and the third one is between 500 and 700 °C | Potential active food packaging material. | [24] |
| Pectin—copaiba oil nanoemulsions |
| TS (MPa): 41.8 ± 6.5, 12.4 ± 4.7 (respectively, for pectin, pectin—6% copaiba oil nanoemulsions) EB (%): 1.7 ± 0.1, 2.4 ± 0.5 (respectively, for pectin, pectin—6% copaiba oil nanoemulsions) | Tonset (°C): 215, 200 (respectively, for pectin, pectin—6% copaiba oil nanoemulsions) | Potential active food packaging material | [27] |
| Pectin—cocoa bean shell waste extract—ZnO-Zn-NPs |
| - | Tmax (°C): 231 ± 1, 229 ± 1 (respectively, for pectin, pectin—5% cocoa bean shell waste extract—3% ZnO-Zn-NPs) | Potential active food packaging material | [25] |
| Pectin—pullulan |
| TS (MPa): 19.5 ± 2.8, 19.1 ± 2.6, 23.2 ± 2.4 (respectively, for pectin, pullulan, 30% pectin-70% pullulan) EB (%): 1.8 ± 0.3, 4.7 ± 0.3, 2.9 ± 0.9 (respectively, for pectin, pullulan, 30% pectin–70% pullulan) | The first step weight loss between 60 and 120 °C The second degradation step in the temperature range 150–240 °C The third step of degradation between 240 and 370 °C | Potential active food packaging material | [40] |
| Pectin-starch—TiO2 NPs |
| TS (MPa): 22.34 ± 0.89, 26.16 ± 0.16 (respectively, for pectin-starch, pectin-starch—TiO2 NPs) EB (%): 12.96 ± 0.43, 8.12 ± 0.94 (respectively, for pectin-starch, pectin-starch—TiO2 NPs) | Tg (°C): 63.05 ± 1.2, 79.63 ± 0.42 (respectively, for pectin- starch, pectin-starch—TiO2 NPs) Tm (°C): 156.41 ± 0.30, 172.33 ± 0.65(respectively for pectin-starch, pectin-starch—TiO2 NPs) | Potential edible film | [127] |
| Pectin—kiwifruit (Actinidia chinensis) peel extract |
| TS (MPa): 42.30 ± 0.82, 21.65 ± 0.97 (respectively, for pectin, pectin—1.5% kiwifruit peel extract) EB (%): 10.77 ± 0.70, 20.32 ± 1.32 (respectively, for pectin, pectin-1.5% kiwifruit peel extract) | Potential active food packaging material | [128] | |
| Pectin-agar—zinc sulfide NPs |
| TS (MPa): 50.3 ± 2.8, 47.4 ± 3.2 (respectively, for pectin-agar, pectin-agar—zinc sulfide NPs) EB (%): 4.7 ± 1.5, 9.9 ± 2.6 (respectively, for pec-tin-agar, pectin-agar—zinc sulfide NPs) | The first weight loss occurred at 50–110 °C with a maximum decomposition temperature of 55–60 °C The second weight loss was observed at 115–250 °C with a maximum decomposition temperature of ~220 °C The third weight loss appeared 250–340 °C with a maximum degradation around 300 °C | Potential active food packaging material | [129] |
| Alginate | |||||
| Sodium alginate—oregano essential oil |
| - | - | Potential edible film for prolong the shelf-life of ham slices by reducing microbial growth | [32] |
| Alginate—sepiolite modified with myrtle berries extract |
| TS (MPa): 38 ± 4, 87 ± 8 (respectively, for alginate, alginate—sepiolite modified with myrtle berries extract) EB (%): 3.8 ± 0.9, 5.6 ± 0.9 (respectively, for alginate, alginate—sepiolite modified with myrtle berries extract) | The first stage of weight loss 100 °C The second stage occurs in the temperature range of 110–160 °C The third stage occurs in the temperature range of 160–366 °C The fourth stage at temperatures above 366 °C | Potential active food packaging material | [28] |
| Sodium alginate-carboxymethyl cellulose—epigallocatechin gallate |
| TS (MPa): 4.28 ± 0.69, 10.78 ± 2.15 (respectively, for sodium alginate, sodium alginate—carboxymethyl cellulose—1.6% epigallocatechin gallate) EB (%): 27.50 ± 2.08, 11.20 ± 1.57 (respectively, for sodium alginate, sodium alginate—carboxymethyl cellulose—1.6% epigallocatechin gallate) | - | Edible coatings for prolong the shelf-life of fresh pork by reducing lipid oxidation and microbial growth | [31] |
| Sodium alginate-pectin-citric acid—tartaric acid |
| TS (MPa): 18.38, 17.20 (respectively, for sodium alginate-citric acid, pectin—citric acid) | Tonset (°C): 99.8, 99.9 | Potential edible packing film for food wrapping | [130] |
| Alginate—Zn-MgO NPs |
| - | - | Extend the shelf-life of Cold-Smoked Salmon by controlling L. monocytogenes growth Potential antimicrobial active food packaging material | [131] |
| Sodium alginate-cellulose nano whisker—copper oxide NPs |
| - | - | Prevent microbial contamination in fresh cut pepper Potential active food packaging material | [132] |
| Alginate—aloe vera–garlic oil |
| TS (MPa): 17 ± 0.71, 21.85 ± 1.22 (respectively, for alginate, alginate—2% aloe vera–5% garlic oil) EB (%): 10 ± 0.91, 41.55 ± 0.64 (respectively, for alginate, alginate—2% aloe vera–5% garlic oil) | The first stage of mass loss around 100 °C The second step of mass loss at 218 °C The 3rd stage of mass loss at 266 °C | Edible coating for tomato | [133] |
| Alginate—sulfur NPs |
| TS (MPa): 58.5 ± 0.8, 63.8 ± 1.2 (respectively, for alginate, alginate—3% sulfur NPs) EB (%): 7.5 ± 0.1, 6.8 ± 0.9 (respectively, for alginate, alginate—3% sulfur NPs) | The initial weight loss of up to 100 °C The second step degradation occurred between 200 and 300 °C | Potential active food packaging material | [134] |
| Gums | |||||
| Gellan gum—xanthan gum-zinc oxide NPs |
| TS (MPa): 22.1 ± 0.9, 35.5 ± 1.2 (respectively, for gellan gum—xanthan gum, gellan gum—xanthan gum-5 wt% zinc oxide NPs) EB (%): 30.0 ± 1.5, 25.1 ± 1.1 (respectively, for gellan gum—xanthan gum, gellan gum—xanthan gum-5 wt% zinc oxide NPs) | Tg (°C): 69.9 ± 0.4, 74.8 ± 0.9 (respectively, for gellan gum—xanthan gum, gellan gum—xanthan gum-5 wt% zinc oxide NPs) Tm (°C): 217.0 ± 0.3, 219.3 ± 0.4 (respectively for gellan gum—xanthan gum, gellan gum—xanthan gum-5 wt% zinc oxide NPs) | Potential active food packaging material | [34] |
| Xanthan gum—PVA-red grape pomace |
| - | - | Potential active food packaging material | [33] |
| Xanthan—curdlan |
| TS (MPa): ~16, ~14, ~28 (respectively, for curdlan, xanthan, 50% xanthan-50% curdlan) EB (%): ~25, ~7, ~17 (respectively, for curdlan, xanthan, 50% xanthan-50% curdlan) | The first weight loss observed between 86.5 and 104.33 °C The maximum weight loss was observed between 294.3 and 319.04 °C | Potential active food packaging material | [135] |
| Gellan gum—purple sweet potato anthocyanins |
| TS (MPa): 1.2 ± 0.2, 8.9 ± 1.1 (respectively, for gellan gum, gellan gum—purple sweet potato anthocyanins) EB (%): 1.5 ± 0.9, 4.3 ± 1.2 (respectively, for gellan gum, gellan gum—purple sweet potato anthocyanins) | - | Potential intelligent food packaging material to detect the spoilage of protein-rich foods caused by bacteria growth | [136] |
| Gellan gum—agar-montmorillonite |
| TS (MPa): 29.9 ± 1.2, 44.0 ± 1.4 (respectively, for gellan gum— agar, gellan gum—agar-10% montmorillonite) EB (%): 29.5 ± 0.9, 19.9 ± 0.8 (respectively, for gellan gum-—agar, gellan gum—agar-10% montmorillonite) | Tg (°C): 70.2 ± 0.4, 77.1 ± 0.8 (respectively, for gellan gum—agar, gellan gum—agar-10% montmorillonite) Tm (°C): 198.4 ± 0.3, 214.2 ± 0.5 (respectively, for gellan gum—agar, gellan gum—agar-10% montmorillonite) | Potential active food packaging material | [87] |
| Tragacanth gum—PVA gallic acid |
| TS (MPa): 15.3 ± 2.1, 45.7 ± 1.4 (respectively for PVA, tragacanth gum—PVA gallic acid) EB (%): 149.3 ± 16.2, 69.4 ± 25.1 (respectively, for PVA, tragacanth gum—PVA gallic acid) | Tg (°C): 43.3, 70.5 (respectively, for PVA, tragacanth gum—PVA gallic acid) Tm (°C): 192.7, 216.3 (respectively, for PVA, tragacanth gum—PVA gallic acid) | Potential active food packaging material | [137] |
| Lignin | |||||
| Lignin—gellan gum-hydroxyethyl cellulose |
| TS (MPa): 23.0 ± 1.1, 39.0 ± 0.8 (respectively, for gellan gum, lignin—gellan gum-hydroxyethyl cellulose) EB (%): 20.3 ± 0.4, 32.5 ± 0.4 (respectively, for gellan gum, lignin—gellan gum-hydroxyethyl cellulose) | Tg (°C): 149.2 ± 0.5, 156.9 ± 0.3 (respectively, for gellan gum, lignin—gellan gum-hydroxyethyl cellulose) Tm (°C): 205.6 ± 0.6, 216.0 ± 0.3 (respectively, for gellan gum, lignin—gellan gum-hydroxyethyl cellulose) | Potential active food packaging material | [93] |
| Alkali lignin-lignosulfonate—soy protein isolate |
| TS (MPa): 4.74 ± 0.34, 8.01 ± 0.89, 10.98 ± 1.02 (respectively, for soy protein, 10% lignosulfonate–soy protein, 10% alkali lignin—soy protein) EB (%): 126.33 ± 17.9, 79.95 ± 5.32, 7.45 ± 1.24 (respectively, for soy protein, 10% lignosulfonate—soy protein, 10% alkali lignin—soy protein) | The first weight loss 50–100 °C. The second weight loss occurred at around 300 °C | Potential active food packaging material | [36] |
| Lignin—nanocellulose |
| - | - | Potential active food packaging material | [37] |
| Lignin—poly(lactic acid) |
| TS (MPa): ~40, ~30 (respectively, for PLA, PLA—40% lignin) EB (%): ~15, ~2 (respectively, for PLA, PLA—40% lignin) | Tonset (°C): 323.6, 306.1 (respectively, for PLA, PLA—40% lignin) Tmax (°C): 330.2, 320.7 (respectively, for PLA, PLA—40% lignin) | Potential active food packaging material | [38] |
| Pullulan | |||||
| Pullulan-tempo cellulose nanofibrils—montmorillonite clay |
| TS (MPa): ~35, ~5 (respectively, for pullulan, pullulan-tempo cellulose nanofibrils—5% montmorillonite clay) | Maximum decomposition temperature for pullulan and pullulan-tempo cellulose nanofibrils—montmorillonite clay film were around 98 °C and 308.27 °C | Potential active food packaging material | [39] |
| Pullulan—lysozyme nanofibers |
| TS (MPa): 35.0 ± 4.4, 37.6 ± 2.2 (respectively, pullulan, pullulan—5% lysozyme nanofibers) EB (%): 6.63 ± 1.11, 1.84 ± 0.29 (respectively, pullulan, pullulan—5% lysozyme nanofibers) | Pullulan has a single weight loss step with initial and maximum decomposition temperatures of 250 and 300 °C Lysozyme nanofibers has a single-step degradation profile with maximum degradation temperature of 308 °C | Potential edible films for active packaging | [138] |
| Pullulan—egg white |
| TS (MPa): 60.65, 329.48 (respectively, for pullulan, pullulan—egg white) EB (%): 1.43, 10.33 (respectively, for pullulan, pullulan—egg white) | Initial loss at 100 °C Final weight loss step at 270–450 °C | Potential edible films for active packaging | [139] |
| Pullulan-graphene—nanocellulose |
| TS (MPa): ~7, ~20 (respectively, for pullulan—nanocellulose, pullulan-graphene—nanocellulose | - | Potential active food packaging material | [140] |
| Pullulan-curcumin—Ag NPs |
| - | - | Potential active food packaging material | [141] |
| Pullulan-carboxylated cellulose nanocrystal-tea polyphenol |
| TS (MPa): 25.28 ± 1.21, 34.49 ± 1.32 (respectively, for pullulan-carboxylated cellulose nanocrystal, pullulan-carboxylated cellulose nanocrystal-5% tea polyphenol) EB (%): 8.67 ± 0.54, 5.76 ± 0.25 (respectively, for pullulan-carboxylated cellulose nanocrystal, pullulan-carboxylated cellulose nanocrystal-5% tea polyphenol) | The first step of thermal degradation was 80–150 °C Maximum decomposition temperature at around 230–400 °C | Potential active food packaging material | [142] |
| Pullulan-chitin nanofbers-curcumin—anthocyanins |
| TS (MPa): 23.95 ± 5.57, 10.18 ± 4.37 (respectively, for pullulan, pullulan-chitin nanofibers-curcumin—anthocyanins) EB (%): 7.45 ± 2.66, 10.05 ± 6.83 (respectively, for pullulan, pullulan-chitin nanofibers-curcumin—anthocyanins) | Significant weight loss at temperatures between 250 and 400 °C | Potential active and intelligent food packaging material | [143] |
| Pullulan—propolis extract |
| TS (MPa): 24.62 ± 2.12, 14.42 ± 1.99 (respectively, for pullulan, pullulan—propolis extract) EB (%): 21.00 ± 0.92, 15.92 ± 1.51 (respectively, for pullulan, pullulan—propolis extract) | - | Potential active food packaging material | [144] |
| Curdlan | |||||
| Curdlan—PVA-thyme essential oil |
| TS (MPa): ~9, ~12 (respectively, for curdlan, 4curdlan—1PVA-thyme essential oil) EB (%): ~90, ~180 (respectively, for curdlan, 4curdlan—1PVA-thyme essential oil) | The heat absorption peak of curdlan film is 309 °C When PVA is added, the heat absorption peak conversion temperature of the film is up to 342 °C | Increased shelf-life of chilled meat up to 10 days Potential active food packaging material. | [41] |
| Curdlan-Xanthan |
| TS (MPa): ~16, ~14, ~28 (respectively, for curdlan, xanthan, 50% xanthan-50% curdlan) EB (%): preparation of a novel curdlan/bacterial cellulose/cinnamon essential oil blending film for food packaging application 25, ~7, ~17 (respectively, for curdlan, xanthan, 50% xanthan-50% curdlan) | The first weight loss observed between 86.5 and 104.33 °C The maximum weight loss was observed between 294.3 and 319.04 °C | Potential active food packaging material | [135] |
| Curdlan-bacterial cellulose-cinnamon essential oil |
| TS (MPa): ~5, ~7 (respectively, for curdlan, curdlan-2% bacterial celllose-15% cinnamon essential oil) EB (%): ~70, ~80 (respectively, for curdlan, curdlan-2% bacterial celllose-15% cinnamon essential oil) | The first heat absorption peak of the films was observed around 40–110 °C Exothermic peak of curdlan films are around 270–300 °C Exothermic peaks of blending film were around 285 °C and 282 °C | Potential active food packaging material | [42] |
6.1.2. Chitosan
6.1.3. Carrageenan
6.1.4. Cellulose
6.1.5. Agar
6.1.6. Pectin
6.1.7. Alginate
6.1.8. Gums
6.1.9. Lignin
6.1.10. Pullulan
6.1.11. Curdlan
6.2. Protein-Based Biopolymers
6.2.1. Whey Protein
6.2.2. Gelatin
6.2.3. Soy Proteins
6.2.4. Zein
6.2.5. Keratin
6.2.6. Collagen
6.3. Aliphatic Polyesters
6.3.1. Poly Lactic Acid (PLA)
| Packaging Material | Characteristics of Food Packaging System | Mechanical Properties | Thermal Properties | Application | Reference |
|---|---|---|---|---|---|
| Gelatin | |||||
| Gelatin-grapefruit seed (GSE)—TiO2 NPs |
| TS (MPa): 60.6 ± 1.1, 57.9 ± 1.3, 63.4 ± 1.5, 61.5 ± 1.7, 58.3 ± 1.9, 55.2 ± 1.6 Gel, (respectively for Gel/GSE, Gel/GSE/0.5%TiO2, Gel/GSE/1%TiO2, Gel/GSE/2%TiO2, Gel/GSE/5%TiO2) EB (%)10.6 ± 0.8, 12.7 ± 1.2, 9.6 ± 1.7, 10.4 ± 2.0, 12.5 ± 1.1, 13.3 ± 1.9 (respectively, for Gel/GSE, Gel/GSE/0.5%TiO2, Gel/GSE/1%TiO2, Gel/GSE/2%TiO2, Gel/GSE/5%TiO2) | Initial weight loss at 80–120 °C, subsequent degradation varied between 200 and 300 °C, and third step of weight loss around 320 °C | Potential active food packaging material | [44] |
| Gelatin—PLA |
| TS (MPa): 24.90 ± 5.59, 31.21 ± 2.88 (respectively, for Gelatin-PLA and Epigallocatechin gallate, laminated with PLA and emulsified with gelatin) EB (%): 8.27 ± 3.26, 11.83 ± 3.05 (respectively, for Gelatin-PLA and Epigallo-catechin gallate, laminated with PLA and emulsified with gelatin) | - | Control lipid oxidation and increased shelf-life of fried salmon skins up to 30 days. Suitable active packaging material for high-lipid-content foods. | [43] |
| Gelatin-chitosan-3-phenylacetic acid |
| The first weight loss occurred around 75–150 °C A major loss occurred at 200–300 °C | Potential active food packaging material | [164] | |
| Gelatin-agar bilayer—vine leaves |
| TS (MPa): 68.15 ± 1.20, 62.50 ± 1.10 (respectively, for Gelatin-agar bilayer and Gelatin-agar bilayer—5 mg/mL vine leaves) EB (%): 21.20 ± 1.91, 25.20 ± 1.10 (respectively, for Gelatin-agar bilayer and Gelatin-agar bilayer—5 mg/mL vine leaves) | Tg (°C): 65.15, 65.24 (respectively, for Gelatin-agar bilayer and Gelatin-agar bilayer—5 mg/mL vine leaves) | Potential active food packaging material | [23] |
| Gelatin-oxidized chitin nanocrystals (Ch)—black rice bran anthocyanins (BACNs) |
| TS: 9.44 ± 0.29, 2.53 ± 0.12 (respectively, for BACNs-Ch0 and BACNs-Ch100) EB (%): 115.33 ± 3.06, 141.67 ± 3.06 (respectively, for BACNs-Ch0 and BACNs-Ch100) | - | Monitor the freshness of shrimp and hairtail by visible color changes. Potential intelligent packaging material for freshness monitoring of high protein foods | [165] |
| Gelatin-carrageenan—carbon dots |
| TS (MPa): 52.8 ± 6.3, 81.2 ± 5.3 (respectively, for gelatin-carrageenan and gelatin-carrageenan-10% carbon dots) EB (%): 3.9 ± 1.1, 6.4 ± 0.6 (respectively, for gelatin-carrageenan and gelatin-carrageenan—10% carbon dots) | The first weight loss at 55−110 °C The second thermal degradation from 125 °C to 280 °C The third thermal degradation from 285 °C to 350 °C | Potential active food packaging material | [171] |
| Gelatin-carrageenan-shikonin—propolis |
| TS(MPa): 43.9 ± 2.3, 41.7 ± 3.0 (respectively, for Gelatin-Carrageenan and Gelatin-carrageenan-shikonin—propolis) EB (%): 3.2 ± 0.2, 3.6 ± 0.1 (respectively, for Gelatin-Carrageenan and Gelatin-carrageenan-shikonin—proplis) | Three step degradation between 290 and 350 °C | Potential intelligent food packaging material | [172] |
| Gelatin—tea polyphenol/ε-poly (L-lysine) |
| - | - | Potential active food packaging material | [173] |
| Keratins | |||||
| Keratin—citric acid |
| TS(MPa): 1.49 ± 0.80 EB (%):138 ± 21 | First stage of weight loss 60 °C for pure keratin and 80 °C for keratin—citric acid The second stage at 224 °C for pure keratin and 195 °C for keratin—citric acid | Increased shelf-life of carrot. Active packaging material suitable for food preservation | [58] |
| Keratin—glycerol |
| TS(MPa): 9.59, 0.0409 (respectively, for 15 wt% glycerol-sugar palm starch film, 10 wt% keratin bioplastic film) | - | Potential active food packaging material | [168] |
| Feather keratin—dialdehyde carboxymethyl cellulose |
| TS(MPa): 17.6 ± 3.0, 30.8 ± 4.6 (respectively, for keratin, keratin—dialdehyde carboxymethyl cellulose) EB (%): 4.0 ± 0.9, 0.7 ± 0.4 (respectively, for keratin, keratin—dialdehyde carboxymethyl cellulose) | - | Potential edible food packaging material | [59] |
| Keratin—starch |
| TS(MPa): 8.3 ± 0.2, 13.8 ± 0.2 (respectively, for starch-keratin 20:0, starch-keratin 20:5) EB (%): 19.7 ± 0.1, 33.3 ± 0 (respectively, for starch-keratin 20:0, starch-keratin 20:5) | - | Potential active food packaging material | [174] |
| Keratin–gelatin–glycerin–curcumin |
| TS(MPa): 12.45, 13.73 (respectively, for 7% keratin–10% gelatin–1% curcumin, 7% keratin–10% gelatin–2% glycerin–1% curcumin) | The initial degradation weight loss occurs between 25 °C and 130 °C The second degradation step is observed in the temperature range of 130–400 °C The third step of degradation occurs between 400 and 800 °C | Potential active food packaging material | [175] |
| Whey proteins | |||||
| Whey protein–furcellaran–yerba mate–white tea extracts |
| TS(MPa): 1.36 ± 0.32, 1.31 ± 0.20 (respectively, for whey protein–furcellaran, whey protein-–furcellaran–yerba mate–white tea extracts) EB (%): 25.99 ± 3.32, 25.13 ± 2.79 (respectively, for whey protein–furcellaran, whey protein–furcellaran–yerba mate–white tea extracts) | Peak temperature (Tm) (°C) (1st transition endothermic): 218.2 ± 1.1, 219.4 ± 2.3 (respectively, for whey protein–furcellaran, whey protein–furcellaran–yerba mate–white tea extracts) | Potential edible film for cheese packaging with decreased microbial growth and water content | [52] |
| Whey protein-corn oil—TiO2 NPs |
| TS(MPa): 8.62 ± 0.59, 16.24 ± 0.29 (respectively, for 2.5% whey protein–corn oil–0% TiO2 NPs, 2.5% whey protein–corn oil–0.5% TiO2 NPs) EB (%): 30 ± 8, 67 ± 7 (respectively, for 2.5% whey protein–corn oil–0% TiO2 NPs, 2.5% whey protein–corn oil–0.5% TiO2 NPs) | The first stage of weight loss 50 to 110 °C The second stage of weight loss 120–220 °C The third stage of weight loss 250–340 °C Finally stage of weight loss 350 to 500 °C | Potential active food packaging material for cheese packaging | [50] |
| Whey protein–chitosan nanofiber–nano-formulated cinnamon oil |
| TS(MPa): 4.09 ± 0.38, 3.41 ± 0.47 (respectively, for whey protein, whey protein–chitosan nanofiber–nano-formulated cinnamon oil) EB (%): 77.21 ± 0.49, 35.57 ± 5.85 (respectively, for whey protein, whey protein–chitosan nanofiber–nano-formulated cinnamon oil) | Potential active food packaging material | [163] | |
| Whey protein isolate-coated multilayer film |
| TS(MPa): 45.80 ± 1.53, 33.57 ± 0.93 (respectively, for polyethylene terephthalate–whey protein isolate, low-density polyethylene–linear low-density polyethylene, polyethylene terephthalate–whey protein isolate, low-density polyethylene–linear low-density polyethylene–aluminum oxide) EB (%): 84.10 ± 14.67, 60.56 ± 4.94 (respectively, for polyethylene terephthalate–whey protein isolate, low-density polyethylene–linear low-density polyethylene, polyethylene terephthalate–whey protein isolate, low-density polyethylene–linear low-density polyethylene–aluminum oxide) | Preservation of physicochemical and sensory properties of frozen marinated meatloaf up to 6 months Potential frozen food packaging material | [53] | |
| Whey protein—nanoemulsions of orange peel (Citrus sinensis) essential oil |
| TS(MPa): 2.64 ± 0.62, 1.76 ± 0.44 (respectively, for whey protein, whey protein—5% of nanoemulsions of Citrus sinensis) EB (%): 11.40 ± 1.68, 18.65 ± 1.78 (respectively, for whey protein, whey protein—5% of nanoemulsions of Citrus sinensis) | - | Suitable active food packaging material for the preservation of food against oxidation and microbial spoilage | [51] |
| Whey protein isolate–polyvinyl alcohol–nano-silica |
| TS(MPa): 7.13, 10.2 (respectively, for whey protein isolate–polyvinyl alcohol, whey protein isolate–polyvinyl alcohol–4% nano silica) | Tg (°C): 19, 26 (respectively, for whey protein isolate–polyvinyl alcohol, whey protein isolate–polyvinyl alcohol–4% nano silica) | Potential active food packaging material | [176] |
| Zein | |||||
| Zein–potato starch–chitosan NPs incorporated with curcumin |
| TS(MPa): 7.9 ± 0.8, 13.1 ± 2.3 (respectively, for zein–potato starch, zein–potato starch–chitosan NPs incorporated with curcumin) EB (%): 19.1 ± 1.6, 50.3 ± 4.1 (respectively, for zein–potato starch, zein–potato starch–chitosan NPs incorporated with curcumin) | - | Delayed physicochemical changes in Schizothorax prenati fillets and prolonged shelf-life up to 15 days. Potential bioactive packaging material for Schizothorax prenati fillets | [166] |
| Zein–chitosan–cinnamodendron dinisii schwanke essential oil |
| - | The endothermic peaks at −7.8 °C and −6.2 °C (respectively, for zein, zein–chitosan–Cinnamodendron dinisii schwanke essential oil) | Stabilizing deterioration reactions and preserving the color of ground beef | [55] |
| Zein-sodium alginate-TiO2 NPs-betanin |
| TS(MPa): 2.01 ± 0.26, 12.62 ± 1.24 (respectively, for zein–sodium alginate, zein–sodium alginate-TiO2 NPs–betanin) EB (%): 10.74 ± 2.11, 40.49 ± 3.72 (respectively, for zein–sodium alginate, zein–sodium alginate-TiO2 NPs–betanin) | - | Potential active food packaging material | [56] |
| Zein–pomegranate peel extract–chitosan NPs |
| TS(MPa): 12.22 ± 1.2, 28 ± 1.06 (respectively, for zein, zein–pomegranate peel extract–chitosan NPs) EB (%): 2.6 ± 0.22, 4.1 ± 0.21 (respectively, for zein, zein–pomegranate peel extract–chitosan NPs) | The initial stage happened between 100 and 150 °C. The second stage of weight loss occurred at 200–250 °C | Restricted microbial growth in pork sample. Potential antimicrobial active food packaging material | [167] |
| Zein-TiO2 nanofibers |
| - | Zein nanofibers (0% TiO2) presented a one-step weight loss which peaked at approximately 240–390 °C | Potential active food packaging material | [54] |
| Zein–catechin-loaded β-cyclodextrin metal |
| TS(MPa): 2.53 ± 0.18, 19.24 ± 0.61 (respectively, for zein, zein–8% catechin-loaded β-cyclodextrin metal) EB (%): 1.65 ± 0.04, 4.51 ± 0.14 (respectively, for zein, zein–8% catechin-loaded β-cyclodextrin metal) | - | Potential active food packaging material | [177] |
| Collagen | |||||
| Collagen–chitosan–lemon essential oil |
| TS (MPa): 30.97 ± 5.26, 20.73 ± 3.88 (respectively, for collagen, collagen–chitosan–lemon essential oil 40%) EB (%): 65.41 ± 10.28, 84.57 ± 11.12 (respectively, for collagen, collagen–chitosan–lemon essential oil 40%) | - | Delay deterioration of pork at 4 °C for 21 days by preventing lipid oxidation and microbial proliferation | [60] |
| Collagen–alginate-SiO2 |
| - | - | Potential active food packaging material | [169] |
6.3.2. Poly (Butylene Adipate Terephthalate) (PBAT)
6.3.3. Polycaprolactone (PCL)
6.3.4. Polybutylene Succinate (PBS)
6.3.5. Polyhydroxyalkanoate (PHAs)
| Packaging Material | Characteristics of Food Packaging System | Mechanical Properties | Thermal Properties | Application | Reference |
|---|---|---|---|---|---|
| Poly lactic acid (PLA) | |||||
| PLA-cellulose nanocrystals–green tea extract |
| TS(MPa): 39.8 ± 5.8, 36.3 ± 3.5 (respectively, for PLA, PLA-2% cellulose nanocrystals–green tea extract) EB (%): 2.7 ± 0.4, 2.3 ± 0.1 (respectively, for PLA, PLA-2% cellulose nanocrystals–green tea extract) | Tg (°C): 63.2, 59.6 (respectively, for PLA, PLA-2% cellulose nanocrystals–green tea extract) | Extended the shelf-life of salami slices exhibiting an oxidation reduction | [65] |
| PLA—alginate microbeads containing silver NPs |
| TS(MPa): 15.5 ± 1.5, 14.0 ± 1.1 (respectively, for PLA, PLA composite) EB (%): 477 ± 26, 77 ± 23 (respectively, for PLA, PLA composite) | - | Potential active food packaging material | [94] |
| PLA—carbon NPs |
| - | Tg (°C): 280, 215 (respectively, for PLA, PLA—0.09% carbon nanotubes) | Potential active food packaging material | [183] |
| PLA—thymol–kesum–curry |
| - | Initial decomposition temperature (°C): 352.9, 342.7 (respectively, for PLA, PLA—thymol–kesum–curry) | Increased shelf-life of chicken up to 15 days. Active packaging material suitable for meats, fruits, and vegetables products | [98] |
| PLA—lignin micro particles |
| - | Tg (°C): 62.1 ± 0.3, 64.8 ± 0.4 (respectively, for PLA, PLA—ethylene−vinyl acetate−glycidyl methacrylate) | Potential active food packaging material | [184] |
| PLA—ZnO NPs |
| - | Tg (°C): The pure PLA film has shown Tg around 60 °C and Tm around 156 °C | Potential active food packaging material | [185] |
| PLA—fenugreek essential oil-curcumin |
| TS(MPa): 30.27 ± 1.0, 36.79 ± 0.88 (respectively, for PLA, PLA—fenugreek essential oil–curcumin) EB (%): 16.68 ± 1.68, 53.08 ± 5.12 (respectively, for PLA, PLA—fenugreek essential oil–curcumin) | Tg (°C): 58.67, 63.02 (respectively, for PLA, PLA—fenugreek essential oil–curcumin) | Potential active food packaging material | [186] |
| PLA—PBAT-tannic acid–gallic acid |
| TS(MPa): 4.80 ± 0.06, 8.63 ± 0.3, 7.01 ± 0.95 (respectively, for PLA, PLA-PBAT-10% tannic acid, PLA, PLA-PBAT—10% gallic acid) EB (%): 21.94 ± 11.42, 23.52 ± 9.18, 22.09 ± 18.64 (respectively, for PLA, PLA-PBAT—10% tannic acid, PLA, PLA-PBAT—10% gallic acid) | The first weight loss at around 30 to 70 °C | Potential active food packaging material | [187] |
| Poly(butylene adipate terephthalate) (PBAT) | |||||
| PBAT-PLA—ferulic acid |
| TS(MPa): 5.42 ± 0.03, 10.78 ± 0.83 (respectively, for PBAT-PLA, PBAT-PLA—ferulic acid) EB (%): 21.93 ± 17.42, 22.13 ± 21.34 (respectively, for PBAT-PLA, PBAT-PLA—ferulic acid) | The first weight loss stage was around 60 to 80 °C | Potential active food packaging material | [64] |
| PBAT-lignin—melanin NPs |
| One-step degradation process with an initial decomposition temperature of 369 °C major weight loss occurring around 400 °C | Potential active food packaging material where high UV resistance is required | [35] | |
| PBAT-PLA—nano-polyhedral oligomeric silsesquioxane |
| - | - | Potential active food packaging material | [9] |
| PBAT—glycerol–zeolite–citric acid–cassava starch |
| TS(MPa): 2.44 ± 0.23, 2.44 ± 0.24 (respectively, for control films, zeolites) EB (%): 74.84 ± 23.74, 97.74 ± 19.99 (respectively, for control films, zeolites) | Preserved the color and vitamin C content broccoli florets for 7 days. Senescence indicator of labels were able to detect CO2 in packages | [188] | |
| PBAT-PLA—carvacrol |
| TS(MPa): 26.8 ± 3.9, 16.4 ± 1.4 (respectively, for PBAT 70-PLA 30, PBAT 70-PLA 30—5% carvacrol) EB (%): 267.3 ± 37.3 (respectively, for PBAT 70-PLA 30, PBAT 70-PLA 30—5% carvacrol) | Weight loss at degradation temperatures of 100, 310 and 350 °C | Potential active food packaging material | [189] |
| PBAT—zinc oxide–graphene oxide |
| TS(MPa): 7.65 ± 0.55, 27.43 ± 0.83 (respectively, for PBAT, PBAT—zinc oxide–graphene oxide) EB (%): 121.96 ± 6.35, 304.38 ± 14.84 (respectively, for PBAT, PBAT—zinc oxide–graphene oxide) | Final thermal decomposition at about 650 °C | Potential active food packaging material | [190] |
| PBAT—SiO2 NP-grape seed essential oil |
| TS(MPa): 35, 43 (respectively, for PBAT, PBAT-GEO-SiO2NP (87:10:3)) EB (%): 590, 595 (respectively, for PBAT, PBAT-GEO-SiO2NP (87:10:3)) | Initial weight loss at temperatures of 70–90 °C. Second thermal decomposition at 320–411 °C | Potential active food packaging material | [179] |
| Poly caprolactone (PCL) | |||||
| PCL-PLA—thymol–carvacrol |
| TS(MPa): 29.6 ± 1.47, 6.42 ± 0.6783 (respectively, for PCL–PLA, PCL-PLA—thymol–carvacrolzinc oxide–graphene oxide) EB (%): 603.4 ± 48.7, 10.68 ± 2.30 (respectively, for PCL –PLA, PCL-PLA-thymol–carvacrolzinc oxide–graphene oxide) | - | Potential active food packaging material | [180] |
| PCL-α-tocopherol |
| - | - | Potential active food packaging material | [191] |
| PCL-poly(propylene carbonate) |
| TS(MPa): 9.6 ± 1.0, 19.9 ± 0.9 (respectively, for PCL-40, PCL-60) EB (%): 371 ± 43.9, 465 ± 36.9 (respectively, for PCL-40, PCL-60) | - | Potential active food packaging material | [192] |
| Poly (butylene succinate adipate) (PBSA) | |||||
| PBSA-PLA |
| TS(MPa): 48.61 ± 1.22, 36.68 ± 1.74 (respectively, for 90 wt% PLA +10 wt% PBSA, 82.8 wt% PLA +9.2 wt% PBSA +8 wt% Thymol) EB (%): 55.70 ± 3.56, 353.80 ± 24.80b (respectively, for 90 wt% PLA +10 wt% PBSA, 82.8 wt% PLA +9.2 wt% PBSA +8 wt% Thymol) | Endothermic peak of melting at 149 °C | Extended the shelf-life of salmon slices by 3–4 days during cold storage. Active packaging material suitable for fishery products | [67] |
| PBSA-poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| TS(MPa): 2153, 1297 (respectively, for PHBV/PBSA 100/0, PHBV/PBSA 70/30) EB (%): 0.98 ± 0.1, 134.8 ± 48 (respectively, for PHBV/PBSA 100/0, PHBV/PBSA 70/30) | Tm (°C): 88 ± 3, 86 ± 2, (respectively, for PHBV/PBSA 0/100, PHBV/PBSA 70/30) Tg (°C): −45.9 ± 1.6, −48.6 ± 2.3 (respectively, for PHBV/PBSA 0/100, PHBV/PBSA 70/30) | Potential active food packaging material | [193] |
| Polyhydroxyalkanoate (PHAs) | |||||
| PHB-graphene nanoplatelets |
| TS (MPa): 4.5, 12.2 (respectively, for PHB, PHB-1.3 wt% graphene nanoplatelets) | Tmax: 279.4 °C, 284.1 °C (respectively, for PHB, PHB-1.3 wt% graphene nanoplatelets) | Active packaging material suitable for moisture and oxygen-sensitive food items (potato chips and milk product) | [190] |
| PHB-polycaprolactone-organo-clays (Cloisite® 30 B and 10A) |
| TS(MPa): 6.29 ± 1.42, 7.06 ± 1.96 (respectively, for PHB-PLA, PHB-PLA-Cloisite® 30 B) EB (%): 3.03 ± 1.71, 0.72 ± 0.19 (respectively, for PHB-PLA, PHB-PLA-Cloisite® 30 B) | Increased shelf-life of sliced ham. Active packaging material suitable for processed meat packaging | [69] | |
| PHBV-PHB-eugenol |
| TS(MPa): 1491 ± 207, 1446 ± 190 (respectively, for active multilayer with cellulose nanocrystal, active multilayer with cellulose nanocrystal) EB (%): 59.1 ± 56, 51.6 ± 45 (respectively, for active multilayer with cellulose nanocrystal, active multilayer with cellulose nanocrystal) | - | Potential multilayer antimicrobial active food packaging material | [70] |
| PHBV-PHB–cellulose nanofibrils-lignocellulose nanofibrils |
| TS(MPa): 4504.2 ± 105, 2991.4 ± 184 (respectively, for cellulose nanofibrils, lignocellulose nanofibrils) EB (%): 18.1 ± 2.2, 13.7 ± 0.5 (respectively, for cellulose nanofibrils, lignocellulose nanofibrils) | - | Potential active food packaging material | [194] |
| PHBV-thermoplastic starch |
| - | - | Build on current knowledge on multilayered TPS-PHBV film for food packaging applications | [195] |
| PHA-cellulose nanocrystals |
| TS(MPa): 24.5 ± 0.6, 39.0 ± 1.9 (respectively, for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) containing 8 mol.%, 2 mol.% EB (%): 2.6 ± 0.2, 1.4 ± 0.1 (respectively, for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) containing 8 mol.%, 2 mol.% | - | Potential active food packaging material | [182] |
7. SWOT Analysis of Biodegradable Polymers in the Food Packaging Industry
7.1. Strengths
7.2. Weaknesses
7.3. Opportunities
7.4. Threats
8. Future Trends and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Horst, C.; Pagno, C.H.; Flores, S.H.; Costa, T.M.H. Hybrid starch/silica films with improved mechanical properties. J. Sol-Gel Sci. Technol. 2020, 95, 52–65. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of Nanomaterials in Food Packaging and Its Advanced Future Prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Xue, Z.; Xia, Y.; Qin, Y.; Zhang, G.; Liu, H.; Li, K. Effect of SiO2 nanoparticle on the physical and chemical properties of eco-friendly agar/sodium alginate nanocomposite film. Int. J. Biol. Macromol. 2019, 125, 1289–1298. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean. Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biol. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Silva, R.B.S.; Carvalho, C.W.P.; Rossi, A.L.; Teixeira, J.A.; Freitas-Silva, O.; Cabral, L.M.C. Cellulose nanocrystals from grape pomace and their use for the development of starch-based nanocomposite films. Int. J. Biol. Macromol. 2020, 159, 1048–1061. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Active bilayer films based on cassava starch incorporating ZnO nanorods and PVA electrospun mats containing rosemary extract. Food Hydrocoll. 2020, 108, 106054. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Hou, H. Effects of high starch content on the physicochemical properties of starch/PBAT nanocomposite films prepared by extrusion blowing. Carbohydr. Polym. 2020, 239, 116231. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and characterization of chitosan-titanium dioxide nanocomposite film as ethylene scavenging and antimicrobial active food packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Paiva, C.A.; Vilvert, J.C.; Menezes, F.L.G.; Leite, R.H.d.L.; Santos, F.K.G.; Medeiros, J.F.; Aroucha, E.M.M. Extended shelf life of melons using chitosan and graphene oxide-based biodegradable bags. J. Food Process. Preserv. 2020, 44, e14871. [Google Scholar] [CrossRef]
- de Lima Barizão, C.; Crepaldi, M.I.; Junior, O.D.O.S.; de Oliveira, A.C.; Martins, A.F.; Garcia, P.S.; Bonafé, E.G. Biodegradable films based on commercial κ-carrageenan and cassava starch to achieve low production costs. Int. J. Biol. Macromol. 2020, 165, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Liu, Y.-K.; Chiu, F.-C. Fabrication of Cellulose Nanocrystal/Silver/Alginate Bionanocomposite Films with Enhanced Mechanical and Barrier Properties for Food Packaging Application. Nanomaterials 2019, 9, 1523. [Google Scholar] [CrossRef] [PubMed]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Nouri, A.; Tavakkoli Yaraki, M.; Lajevardi, A.; Rahimi, T.; Tanzifi, M.; Ghorbanpour, M. An investigation of the role of fabrication process in the physicochemical properties of κ-carrageenan-based films incorporated with Zataria multiflora extract and nanoclay. Food Packag. Shelf Life 2020, 23, 100435. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial cellulose as a biodegradable food packaging material: A review. Food Hydrocoll. 2021, 113, 106530. [Google Scholar] [CrossRef]
- Noshirvani, N.; Ghanbarzadeh, B.; Rezaei Mokarram, R.; Hashemi, M. Novel active packaging based on carboxymethyl cellulose-chitosan-ZnO NPs nanocomposite for increasing the shelf life of bread. Food Packag. Shelf Life 2017, 11, 106–114. [Google Scholar] [CrossRef]
- El-Hefnawy, M.E. Biodegradable Films from Phytosynthesized TiO2 Nanoparticles and Nanofungal Chitosan as Probable Nanofertilizers. Int. J. Polym. Sci. 2020, 2020, 6727132. [Google Scholar] [CrossRef]
- Salama, H.E.; Aziz, M.S.A. Optimized carboxymethyl cellulose and guanidinylated chitosan enriched with titanium oxide nanoparticles of improved UV-barrier properties for the active packaging of green bell pepper. Int. J. Biol. Macromol. 2020, 165, 1187–1197. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Agar-based antioxidant composite films incorporated with melanin nanoparticles. Food Hydrocoll. 2019, 94, 391–398. [Google Scholar] [CrossRef]
- Fekete, E.; Bella, E.; Csiszar, E.; Moczo, J. Improving physical properties and retrogradation of thermoplastic starch by incorporating agar. Int. J. Biol. Macromol. 2019, 136, 1026–1033. [Google Scholar] [CrossRef]
- Jridi, M.; Abdelhedi, O.; Zouari, N.; Fakhfakh, N.; Nasri, M. Development and characterization of grey triggerfish gelatin/agar bilayer and blend films containing vine leaves bioactive compounds. Food Hydrocoll. 2019, 89, 370–378. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V.; Viscusi, G.; Vittoria, V. Physical and Barrier Properties of Chemically Modified Pectin with Polycaprolactone through an Environmentally Friendly Process. Colloid Polym. Sci. 2021, 299, 429–437. [Google Scholar] [CrossRef]
- Mellinas, A.C.; Jimenez, A.; Garrigos, M.C. Pectin-Based Films with Cocoa Bean Shell Waste Extract and ZnO/Zn-NPs with Enhanced Oxygen Barrier, Ultraviolet Screen and Photocatalytic Properties. Foods 2020, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, T.I.A.; Biernacki, K.; Castro, M.C.R.; Gonçalves, M.P.; Souza, H.K.S. A new approach to develop biodegradable films based on thermoplastic pectin. Food Hydrocoll. 2019, 97, 105175. [Google Scholar] [CrossRef]
- Norcino, L.B.; Mendes, J.F.; Natarelli, C.V.L.; Manrich, A.; Oliveira, J.E.; Mattoso, L.H.C. Pectin films loaded with copaiba oil nanoemulsions for potential use as bio-based active packaging. Food Hydrocoll. 2020, 106, 105862. [Google Scholar] [CrossRef]
- Cheikh, D.; Martín-Sampedro, R.; Majdoub, H.; Darder, M. Alginate Bionanocomposite Films Containing Sepiolite Modified with Polyphenols from Myrtle Berries Extract. Int. J. Biol. Macromol. 2020, 165, 2079–2088. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Duffy, B.; Pathania, S.; Jaiswal, A.K.; Jaiswal, S. An Active Biodegradable Layer-by-Layer Film Based on Chitosan-Alginate-TiO2 for the Enhanced Shelf Life of Tomatoes. Food Packag. Shelf Life 2022, 34, 100971. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Ruan, C.; Zhang, Y.; Wang, J.; Sun, Y.; Gao, X.; Xiong, G.; Liang, J. Preparation and Antioxidant Activity of Sodium Alginate and Carboxymethyl Cellulose Edible Films with Epigallocatechin Gallate. Int. J. Biol. Macromol. 2019, 134, 1038–1044. [Google Scholar] [CrossRef]
- Pavli, F.; Argyri, A.A.; Skandamis, P.; Nychas, G.J.; Tassou, C.; Chorianopoulos, N. Antimicrobial Activity of Oregano Essential Oil Incorporated in Sodium Alginate Edible Films: Control of Listeria monocytogenes and Spoilage in Ham Slices Treated with High Pressure Processing. Materials 2019, 12, 3726. [Google Scholar] [CrossRef] [PubMed]
- Raschip, I.E.; Fifere, N.; Varganici, C.D.; Dinu, M.V. Development of Antioxidant and Antimicrobial Xanthan-Based Cryogels with Tuned Porous Morphology and Controlled Swelling Features. Int. J. Biol. Macromol. 2020, 156, 608–620. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Ismail, F.R.M.; Manoharan, R.K.; Kim, S.S.; Lee, J. Blends of Gellan Gum/Xanthan Gum/Zinc Oxide Based Nanocomposites for Packaging Application: Rheological and Antimicrobial Properties. Int. J. Biol. Macromol. 2020, 148, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.J. Biodegradable UV-Blocking Films through Core-Shell Lignin-Melanin Nanoparticles in Poly(Butylene Adipate-Co-Terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Zadeh, E.M.; O’Keefe, S.F.; Kim, Y.T. Utilization of Lignin in Biopolymeric Packaging Films. ACS Omega 2018, 3, 7388–7398. [Google Scholar] [CrossRef]
- Dou, J.; Vuorinen, T.; Koivula, H.; Forsman, N.; Sipponen, M.; Hietala, S. Self-Standing Lignin-Containing Willow Bark Nanocellulose Films for Oxygen Blocking and UV Shielding. ACS Appl. Nano Mater. 2021, 4, 2921–2929. [Google Scholar] [CrossRef]
- Esakkimuthu, E.S.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional lignin-poly (lactic acid) biocomposites for packaging applications. Bioeng. Biotechnol. 2022, 10, 1025076. [Google Scholar] [CrossRef]
- Yeasmin, S.; Yeum, J.H.; Yang, S.B. Fabrication and characterization of pullulan-based nanocomposites reinforced with montmorillonite and tempo cellulose nanofibril. Carbohydr. Polym. 2020, 240, 116307. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, S.M.; Rhim, J.W. Pectin/pullulan blend films for food packaging: Effect of blending ratio. Food Chem. 2021, 347, 129022. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.; Ashokkumar, V. Preparation and characterization of curdlan/polyvinyl alcohol/ thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Fu, J.; Bian, L.; Chang, T.; Zhang, C. Preparation of a novel curdlan/bacterial cellulose/cinnamon essential oil blending film for food packaging application. Int. J. Biol. Macromol. 2022, 212, 211–219. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Guerrero, P.; Caba, K.D.L.; Benjakul, S.; Prodpran, T. Fish gelatin films laminated with emulsified gelatin film or poly(lactic) acid film: Properties and their use as bags for storage of fried salmon skin. Food Hydrocoll. 2021, 111, 106199. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.W.; Bagheri, R. Gelatin-based functional films integrated with grapefruit seed extract and TiO2 for active food packaging applications. Food Hydrocoll. 2021, 112, 106314. [Google Scholar] [CrossRef]
- Pattarasiriroj, K.; Kaewprachu, P.; Rawdkuen, S. Properties of rice flour-gelatine-nanoclay film with catechin-lysozyme and its use for pork belly wrapping. Food Hydrocoll. 2020, 107, 105951. [Google Scholar] [CrossRef]
- Zubair, M.; Ullah, A. Recent advances in protein derived bionanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 406–434. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Tafaghodi, M. Environment-friendly green composites based on soluble soybean polysaccharide: A review. Int. J. Biol. Macromol. 2019, 122, 216–223. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F.; Birjand, S.M.A.; Bazeli, J. Characterization of a green nanocomposite prepared from soluble soy bean polysaccharide/Cloisite 30B and evaluation of its toxicity. Int. J. Biol. Macromol. 2018, 120, 109–118. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F.; Mohammad Aboutorabzade, S.; Fathi, M. pH-sensitive soluble soybean polysaccharide/SiO2 incorporated with curcumin for intelligent packaging applications. Food Sci. Nutr. 2021, 9, 2169–2179. [Google Scholar] [CrossRef]
- Montes-de-Oca-Ávalos, J.M.; Altamura, D.; Herrera, M.L.; Huck-Iriart, C.; Scattarella, F.; Siliqi, D.; Giannini, C.; Candal, R.J. Physical and structural properties of whey protein concentrate—Corn oil—TiO2 nanocomposite films for edible food-packaging. Food Packag. Shelf Life 2020, 26, 100590. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghadertaj, A.; Mehryar, L. Whey protein isolate-based films incorporated with nanoemulsions of orange peel (Citrus sinensis) essential oil: Preparation and characterization. Food Process. Preserv. 2021, 45, e15196. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Jamróz, E.; Kawecka, A.; Juszczak, L.; Krzyściak, P. Active edible furcellaran/whey protein films with yerba mate and white tea extracts: Preparation, characterization and its application to fresh soft rennet-curd cheese. Int. J. Biol. Macromol. 2020, 155, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Song, H.G.; Choi, I.; Choi, Y.J.; Yoon, C.S.; Han, J. High gas barrier properties of whey protein isolate-coated multi-layer film at pilot plant facility and its application to frozen marinated meatloaf packaging. Food Packag. Shelf Life 2020, 26, 100599. [Google Scholar] [CrossRef]
- Böhmer-Maas, B.W.; Fonseca, L.M.; Otero, D.M.; da Rosa Zavareze, E.; Zambiazi, R.C. Photocatalytic zein-TiO2 nanofibers as ethylene absorbers for storage of cherry tomatoes. Food Packag. Shelf Life 2020, 24, 100508. [Google Scholar] [CrossRef]
- Xavier, L.O.; Sganzerla, W.G.; Rosa, G.B.; da Rosa, C.G.; Agostinetto, L.; Veeck, A.P.D.L.; Bretanha, L.C.; Micke, G.A.; Dalla Costa, M.; Bertodi, F.C.; et al. Chitosan packaging functionalized with Cinnamodendron dinisii essential oil loaded zein: A proposal for meat conservation. Int. J. Biol. Macromol. 2021, 169, 183–193. [Google Scholar] [CrossRef]
- Amjadi, S.; Almasi, H.; Ghorbani, M.; Ramazani, S. Preparation and characterization of TiO2NPs and betanin loaded zein/sodium alginate nanofibers. Food Packag. Shelf Life 2020, 24, 100504. [Google Scholar] [CrossRef]
- Dong, S.; Li, X.-y.; Guo, P.; Chen, Y.; Li, H.-j. Preparation and characterization of PCL-grafted zein film via atmospheric-pressure cold plasma pretreatment. Plasma Process. Polym. 2021, 18, 2000242. [Google Scholar] [CrossRef]
- Ramirez, D.O.S.; Carletto, R.A.; Tonetti, C.; Giachet, F.T.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf Life 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.W.; He, L.; Liu, Y.W. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Mangaraj, S.; Thakur, R.R.; Yadav, A. Development and characterization of PLA and Cassava starch-based novel biodegradable film used for food packaging application. J. Food Process. Preserv. 2022, 46, e16314. [Google Scholar] [CrossRef]
- Kostic, D.; Vukasinovic-Sekulic, M.; Armentano, I.; Torre, L.; Obradovic, B. Multifunctional ternary composite films based on PLA and Ag/alginate microbeads: Physical characterization and silver release kinetics. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1159–1168. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Waterhouse, G.I.N.; Gong, R.; Xie, J.; Zhang, K.; Xu, J. Optimizing interfacial adhesion in PBAT/PLA nanocomposite for biodegradable packaging films. Food Chem. 2021, 334, 127487. [Google Scholar] [CrossRef]
- Sharma, S.; Jaiswal, A.K.; Duffy, B.; Jaiswal, S. Ferulic acid incorporated active films based on poly(lactide)/poly(butylene adipate-co-terephthalate) blend for food packaging. Food Packag. Shelf Life 2020, 24, 100491. [Google Scholar] [CrossRef]
- Vilarinho, F.; Stanzione, M.; Buonocore, G.G.; Barbosa-Pereira, L.; Sendón, R.; Vaz, M.F.; Sanches Silva, A. Green tea extract and nanocellulose embedded into polylactic acid film: Properties and efficiency on retarding the lipid oxidation of a model fatty food. Food Packag. Shelf Life 2021, 27, 100609. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2018, 3, 77–96. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2019, 154, 866–877. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Pinto Collares, M.; Meireles Oliveira, J.R.; Prentice-Hernández, C.; Guimarães Martins, V. Improvement of fish protein films properties for food packaging through glow discharge plasma application. Food Hydrocoll. 2019, 87, 970–976. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Alabi, O.A.; Ologbonjaye, K.I.; Awosolu, O.; Alalade, O.E. Public and Environmental Health Effects of Plastic Wastes Disposal: A Review. J. Toxicol. Risk Assess. 2019, 5, 021. [Google Scholar] [CrossRef]
- Commission Regulation. 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the C; 2015. Available online: https://www.legislation.gov.uk/eur/2015/2283/contents (accessed on 6 April 2023).
- Association of Plastic Manufacturers (Organization). Plastics—The Facts 2020; PlasticEurope: Brussels, Belgium, 2020; p. 16. [Google Scholar]
- European Commission. Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the Implementation of the Circular Economy Action Plan; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martinez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- European Bioplastics. Bioplastics Market Data. Available online: https://www.european-bioplastics.org/market/ (accessed on 6 April 2023).
- Ahmed, J.; Arfat, Y.A.; Bher, A.; Mulla, M.; Jacob, H.; Auras, R. Active Chicken Meat Packaging Based on Polylactide Films and Bimetallic Ag–Cu Nanoparticles and Essential Oil. Food Sci. 2018, 83, 1299–1310. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 16. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Li, S.; Ma, Y.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; Li, S.; Liu, Y. Cassava starch/carboxymethylcellulose edible films embedded with lactic acid bacteria to extend the shelf life of banana. Carbohydr. Polym. 2020, 248, 116805. [Google Scholar] [CrossRef]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- Caroline da Silva Rocha, A.; Rodrigues Menezes, L.; Silva, E.O.d.; Pedrosa, M.C.G. Synergistic effect of carbon nanoparticles on the mechanical and thermal properties of poly(lactic acid) as promising systems for packaging. J. Compos. Mater. 2020, 54, 4133–4144. [Google Scholar] [CrossRef]
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M.D. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Lee, H.; Rukmanikrishnan, B.; Lee, J. Rheological, morphological, mechanical, and water-barrier properties of agar/gellan gum/montmorillonite clay composite films. Int. J. Biol. Macromol. 2019, 141, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Polman, E.M.N.; Gruter, G.M.; Parsons, J.R.; Tietema, A. Comparison of the aerobic biodegradation of biopolymers and the corresponding bioplastics: A review. Sci. Total Environ. 2021, 753, 141953. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, W.A.; Hussain, A.; Lin, C.; Nguyen, M.K. Biodegradation of Different Types of Bioplastics through Composting—A Recent Trend in Green Recycling. Catalysts 2023, 13, 294. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. [Google Scholar]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and Oxygen Barrier Properties of Cellulose Nanomaterial-Based Films. ACS Sustain. Chem. Eng. 2018, 6, 49–70. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W.; Jaiswal, L. Bioactive agar-based functional composite film incorporated with copper sulfide nanoparticles. Food Hydrocoll. 2019, 93, 156–166. [Google Scholar] [CrossRef]
- Rukmanikrishnan, B.; Ramalingam, S.; Rajasekharan, S.K.; Lee, J.; Lee, J. Binary and ternary sustainable composites of gellan gum, hydroxyethyl cellulose and lignin for food packaging applications: Biocompatibility, antioxidant activity, UV and water barrier properties. Int. J. Biol. Macromol. 2020, 153, 55–62. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Mathew, J.; Radhakrishnan, E.K. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Cao, W.; Yan, J.; Liu, C.; Zhang, J.; Wang, H.; Gao, X.; Yan, H.; Niu, B.; Li, W. Preparation and characterization of catechol-grafted chitosan/gelatin/modified chitosan-AgNP blend films. Carbohydr. Polym. 2020, 247, 116643. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Fouad, H.; Jawaid, M. Development of active agents filled polylactic acid films for food packaging application. Int. J. Biol. Macromol. 2020, 163, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, W.R.; Zhang, Y.C.; Han, X.D.; Chen, C.; Chen, A. In situ generated silica reinforced polyvinyl alcohol/liquefied chitin biodegradable films for food packaging. Carbohydr. Polym. 2020, 238, 116182. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, X.; He, J.; Wang, R.; Jin, Z. Effects of electron beam irradiation on the properties of waxy maize starch and its films. Int. J. Biol. Macromol. 2020, 151, 239–246. [Google Scholar] [CrossRef]
- Zheng, Q.; Tian, Y.; Ye, F.; Zhou, Y.; Zhao, G. Fabrication and application of starch-based aerogel: Technical strategies. Trends Food Sci. Technol. 2020, 99, 608–620. [Google Scholar] [CrossRef]
- Chi, K.; Wang, H.; Catchmark, J.M. Sustainable starch-based barrier coatings for packaging applications. Food Hydrocoll. 2020, 103, 105696. [Google Scholar] [CrossRef]
- Chaireh, S.; Ngasatool, P.; Kaewtatip, K. Novel composite foam made from starch and water hyacinth with beeswax coating for food packaging applications. Int. J. Biol. Macromol. 2020, 165, 1382–1391. [Google Scholar] [CrossRef]
- Zhang, K.; Su, T.; Cheng, F.; Lin, Y.; Zhou, M.; Zhu, P.; Li, R.; Wu, D. Effect of sodium citrate/polyethylene glycol on plasticization and retrogradation of maize starch. Int. J. Biol. Macromol. 2020, 154, 1471–1477. [Google Scholar] [CrossRef]
- Tibolla, H.; Czaikoski, A.; Pelissari, F.M.; Menegalli, F.C.; Cunha, R.L. Starch-based nanocomposites with cellulose nanofibers obtained from chemical and mechanical treatments. Int. J. Biol. Macromol. 2020, 161, 132–146. [Google Scholar] [CrossRef]
- Othman, S.H.; Wane, B.M.; Nordin, N.; Noor Hasnan, N.Z.; ATalib, R.; Karyadi, J.N.W. Physical, Mechanical, and Water Vapor Barrier Properties of Starch/Cellulose Nanofiber/Thymol Bionanocomposite Films. Polymers 2021, 13, 4060. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Cui, Y.; Yan, X.; Zhang, R.; Wang, J.; Wang, X. Effect of dual-modified cassava starches on intelligent packaging films containing red cabbage extracts. Food Hydrocoll. 2022, 124, 107225. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; He, L.; Liu, Y. Improving nisin production by encapsulated Lactococcus lactis with starch/carboxymethyl cellulose edible films. Carbohydr. Polym. 2021, 251, 117062. [Google Scholar] [CrossRef] [PubMed]
- Merz, B.; Capello, C.; Leandro, G.C.; Moritz, D.E.; Monteiro, A.R.; Valencia, G.A. A novel colorimetric indicator film based on chitosan, polyvinyl alcohol and anthocyanins from jambolan (Syzygium cumini) fruit for monitoring shrimp freshness. Int. J. Biol. Macromol. 2020, 153, 625–632. [Google Scholar] [CrossRef]
- Indumathi, M.P.; Sarojini, K.S.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biol. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Process. Preserv. 2020, 44, e14536. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Vootla, S.K.; Mudigoudra, B.S. Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohydr. Polym. 2022, 277, 118866. [Google Scholar] [CrossRef]
- Dong, W.; Su, J.; Chen, Y.; Xu, D.; Cheng, L.; Mao, L.; Gao, Y.; Yuan, F. Characterization and antioxidant properties of chitosan film incorporated with modified silica nanoparticles as an active food packaging. Food Chem. 2022, 373, 131414. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.N.; Cao, Y.Y.; Zhang, Y.L.; Zhe, T.T.; Guo, Z.R.; Sun, X.Y.; Wang, Q.Z.; Wang, L. Copper sulfide nanoparticle-carrageenan films for packaging application. Food Hydrocoll. 2020, 109, 106094. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Li, C.; Qin, Y.; Xiao, L.; Liu, J. Comparison of the structural, physical and functional properties of κ-carrageenan films incorporated with pomegranate flesh and peel extracts. Int. J. Biol. Macromol. 2020, 147, 1076–1088. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Chiu, F.C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Sani, M.; Tavassoli, M.; Salim, S.A.; Azizi-lalabadi, M.; McClements, D.J. Development of green halochromic smart and active packaging materials: TiO2 nanoparticle- and anthocyanin-loaded gelatin/κ-carrageenan films. Food Hydrocoll. 2022, 124, 107324. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Valenzuela, L.M.; Giordano, A.; Cabrera-Barjas, G.; Martin-Belloso, O. κ-carrageenan edible films for beef: Honey and bee pollen phenolic compounds improve their antioxidant capacity. Food Hydrocoll. 2022, 124, 107250. [Google Scholar] [CrossRef]
- da Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Tavares, K.M.; Campos, A.D.; Luchesi, B.R.; Resende, A.A.; Oliveira, J.E.D.; Marconcini, J.M. Effect of carboxymethyl cellulose concentration on mechanical and water vapor barrier properties of corn starch films. Carbohydr. Polym. 2020, 246, 116521. [Google Scholar] [CrossRef]
- Saedi, S.; Shokri, M.; Kim, J.T.; Shin, G.H. Semi-transparent regenerated cellulose/ZnONP nanocomposite film as a potential antimicrobial food packaging material. J. Food Eng. 2021, 307, 110665. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Ahmed, A.A.Q.; Awad, M.F.; Ul-Islam, M.; Subhan, F.; Ullah, M.W.; Yang, G. Development and Characterization of Yeast-Incorporated Antimicrobial Cellulose Biofilms for Edible Food Packaging Application. Polymer. 2021, 13, 2310. [Google Scholar] [CrossRef]
- Zhang, R.; Zhai, X.; Wang, W.; Hou, H. Preparation and evaluation of agar/maltodextrin-beeswax emulsion films with various hydrophilic-lipophilic balance emulsifiers. Food Chem. 2022, 384, 132541. [Google Scholar] [CrossRef]
- Hong, S.-I.; Cho, Y.; Rhim, J.-W. Effect of Agar/AgNP Composite Film Packaging on Refrigerated Beef Loin Quality. Membranes 2021, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Hashim, S.B.H.; Elrasheid Tahir, H.; Liu, L.; Zhang, J.; Zhai, X.; Ali Mahdi, A.; Nureldin Awad, F.; Hassan, M.M.; Xiaobo, Z.; Jiyong, S. Intelligent colorimetric pH sensoring packaging films based on sugarcane wax/agar integrated with butterfly pea flower extract for optical tracking of shrimp freshness. Food Chem. 2022, 373, 131514. [Google Scholar] [CrossRef] [PubMed]
- Dash, K.K.; Ali, N.A.; Das, D.; Mohanta, D. Thorough evaluation of sweet potato starch and lemon-waste pectin based-edible films with nano-titania inclusions for food packaging applications. Int. J. Biol. Macromol. 2019, 139, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Song, K.B. Antioxidant properties of watermelon (Citrullus lanatus) rind pectin films containing kiwifruit (Actinidia chinensis) peel extract and their application as chicken thigh packaging. Food Packag. Shelf Life 2021, 28, 100636. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of pectin/agar-based functional films integrated with zinc sulfide nano petals for active packaging applications. Colloids Surf. B Biointerfaces 2021, 207, 111999. [Google Scholar] [CrossRef]
- Singh, P.; Baisthakur, P.; Yemul, O.S. Synthesis, characterization and application of crosslinked alginate as green packaging material. Heliyon 2020, 6, e03026. [Google Scholar] [CrossRef]
- Vizzini, P.; Beltrame, E.; Zanet, V.; Vidic, J.; Manzano, M. Development and Evaluation of qPCR Detection Method and Zn-MgO/Alginate Active Packaging for Controlling Listeria monocytogenes Contamination in Cold-Smoked Salmon. Foods 2020, 9, 1353. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Hu, X.W.; Wang, M.H. Physical and bioactivities of biopolymeric films incorporated with cellulose, sodium alginate and copper oxide nanoparticles for food packaging application. Int. J. Biol. Macromol. 2020, 153, 207–214. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E. Developing multifunctional edible coatings based on alginate for active food packaging. Int. J. Biol. Macromol. 2021, 190, 837–844. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Kim, H.-J.; Rhim, J.-W. Effect of sulfur nanoparticles on properties of alginate-based films for active food packaging applications. Food Hydrocoll. 2021, 110, 106155. [Google Scholar] [CrossRef]
- Mohsin, A.; Zaman, W.Q.; Guo, M.; Ahmed, W.; Khan, I.M.; Niazi, S.; Rehman, A.; Hang, H.; Zhuang, Y. Xanthan-Curdlan nexus for synthesizing edible food packaging films. Int. J. Biol. Macromol. 2020, 162, 43–49. [Google Scholar] [CrossRef]
- Wei, Y.C.; Cheng, C.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Active gellan gum/purple sweet potato composite films capable of monitoring pH variations. Food Hydrocoll. 2017, 69, 491–502. [Google Scholar] [CrossRef]
- Goudar, N.; Vanjeri, V.N.; Dixit, S.; Hiremani, V.; Sataraddi, S.; Gasti, T.; Vootla, S.K.; Masti, S.P.; Chougale, R.B. Evaluation of multifunctional properties of gallic acid crosslinked Poly (vinyl alcohol)/Tragacanth Gum blend films for food packaging applications. Int. J. Biol. Macromol. 2020, 158, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Vilela, C.; Almeida, A.; Marrucho, I.M.; Freire, C.S.R. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Han, K.; Liu, Y.; Liu, Y.; Huang, X.; Sheng, L. Characterization and film-forming mechanism of egg white/pullulan blend film. Food Chem. 2020, 315, 126201. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, R.; Mayakrishnan, V.; Kesavan, R.; Shanmugam, K.; Veeramani, S.; Ilangovan, R. Mechanical, Barrier, Adhesion and Antibacterial Properties of Pullulan/Graphene Bio Nanocomposite Coating on Spray Coated Nanocellulose Film for Food Packaging Applications. J. Polym. Environ. 2022, 30, 1749–1757. [Google Scholar] [CrossRef]
- Khan, M.J.; Ramiah, S.K.; Selamat, J.; Shameli, K.; Sazili, A.Q.; Mookiah, S. Utilisation of pullulan active packaging incorporated with curcumin and pullulan mediated silver nanoparticles to maintain the quality and shelf life of broiler meat. Ital. J. Anim. Sci. 2022, 21, 244–262. [Google Scholar] [CrossRef]
- Chen, F.; Chi, C. Development of pullulan/carboxylated cellulose nanocrystal/tea polyphenol bionanocomposite films for active food packaging. Int. J. Biol. Macromol. 2021, 186, 405–413. [Google Scholar] [CrossRef]
- Duan, M.; Yu, S.; Sun, J.; Jiang, H.; Zhao, J.; Tong, C.; Hu, Y.; Pang, J.; Wu, C. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int. J. Biol. Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Gniewosz, M.; Pobiega, K.; Kraśniewska, K.; Synowiec, A.; Chaberek, M.; Galus, S. Characterization and Antifungal Activity of Pullulan Edible Films Enriched with Propolis Extract for Active Packaging. Foods 2022, 11, 2319. [Google Scholar] [CrossRef]
- Ceballos, R.L.; Ochoa-Yepes, O.; Goyanes, S.; Bernal, C.; Fama, L. Effect of yerba mate extract on the performance of starch films obtained by extrusion and compression molding as active and smart packaging. Carbohydr. Polym. 2020, 244, 116495. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Cen, C.N.; Chen, J.; Fu, L.L. MgO/carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohydr. Polym. 2020, 236, 116078. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhu, J.; Guan, G.; Wu, H. Preparation of chitosan-sodium alginate films through layer-by-layer assembly and ferulic acid crosslinking: Film properties, characterization, and formation mechanism. Int. J. Biol. Macromol. 2019, 122, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Rusman, R.; Khaldun, I.; Ardana, L.; Mudatsir, M.; Fansuri, H. Active edible sugar palm starch-chitosan films carrying extra virgin olive oil: Barrier, thermo-mechanical, antioxidant, and antimicrobial properties. Int. J. Biol. Macromol. 2020, 163, 766–775. [Google Scholar] [CrossRef]
- Younis, H.G.R.; Zhao, G. Physicochemical properties of the edible films from the blends of high methoxyl apple pectin and chitosan. Int. J. Biol. Macromol. 2019, 131, 1057–1066. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; de Oliveira Rios, A.; Alves, V.D.; Bandarra, N.; Moldão-Martins, M.; Flôres, S.H. Active food packaging prepared with chitosan and olive pomace. Food Hydrocoll. 2018, 74, 139–150. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent pH-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and characterization of chitosan-based antimicrobial active food packaging film incorporated with apple peel polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef]
- Wang, X.; Yong, H.; Gao, L.; Li, L.; Jin, M.; Liu, J. Preparation and characterization of antioxidant and pH-sensitive films based on chitosan and black soybean seed coat extract. Food Hydrocoll. 2019, 89, 56–66. [Google Scholar] [CrossRef]
- Riaz, A.; Lagnika, C.; Luo, H.; Dai, Z.; Nie, M.; Hashim, M.M.; Liu, C.; Song, J.; Li, D. Chitosan-based biodegradable active food packaging film containing Chinese chive (Allium tuberosum) root extract for food application. Int. J. Biol. Macromol. 2020, 150, 595–604. [Google Scholar] [CrossRef]
- do Vale, D.A.; Vieira, C.B.; Vidal, M.F.; Claudino, R.L.; Andrade, F.K.; Sousa, J.R.; Souza Filho, M.d.S.M.; da Silva, A.L.C.; de Souza, B.W.S. Chitosan-Based Edible Films Produced from Crab-Uçá (Ucides cordatus) Waste: Physicochemical, Mechanical and Antimicrobial Properties. J. Polym. Environ. 2021, 29, 694–706. [Google Scholar] [CrossRef]
- Lin, D.R.; Yang, Y.M.; Wang, J.; Yan, W.J.; Wu, Z.J.; Chen, H.; Zhang, Q.; Wu, D.T.; Qin, W.; Tu, Z.C. Preparation and characterization of TiO2-Ag loaded fish gelatin-chitosan antibacterial composite film for food packaging. Int. J. Biol. Macromol. 2020, 154, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Panariello, L.; Coltelli, M.-B.; Buchignani, M.; Lazzeri, A. Chitosan and nano-structured chitin for biobased anti-microbial treatments onto cellulose based materials. Eur. Polym. 2019, 113, 328–339. [Google Scholar] [CrossRef]
- Sun, G.; Chi, W.; Zhang, C.; Xu, S.; Li, J.; Wang, L. Developing a green film with pH-sensitivity and antioxidant activity based on κ-carrageenan and hydroxypropyl methylcellulose incorporating Prunus maackii juice. Food Hydrocoll. 2019, 94, 345–353. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Ren, Z.; Xie, S.; Wu, Y.; Chen, W.; Ma, F.; Liu, X. Characterization and antibacterial properties of biodegradable films based on CMC, mucilage from Dioscorea opposita Thunb. and Ag nanoparticles. Int. J. Biol. Macromol. 2020, 163, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Sá, N.M.S.M.; Mattos, A.L.A.; Silva, L.M.A.; Brito, E.S.; Rosa, M.F.; Azeredo, H.M.C. From cashew byproducts to biodegradable active materials: Bacterial cellulose-lignin-cellulose nanocrystal nanocomposite films. Int. J. Biol. Macromol. 2020, 161, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, W.; Zhang, H.; Dai, Y.; Dong, H.; Kong, L.; Hou, H. Effects of preparation conditions on the properties of agar/maltodextrin-beeswax pseudo-bilayer films. Carbohydr. Polym. 2020, 236, 116029. [Google Scholar] [CrossRef]
- Kontominas, M.G. Use of Alginates as Food Packaging Materials. Foods 2020, 9, 1440. [Google Scholar] [CrossRef]
- Mohammadi, M.; Mirabzadeh, S.; Shahvalizadeh, R.; Hamishehkar, H. Development of novel active packaging films based on whey protein isolate incorporated with chitosan nanofiber and nano-formulated cinnamon oil. Int. J. Biol. Macromol. 2020, 149, 11–20. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/chitosan/3-phenylacetic acid food-packaging nanofiber antibacterial films by electrospinning. Int. J. Biol. Macromol. 2021, 169, 161–170. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent gelatin/oxidized chitin nanocrystals nanocomposite films containing black rice bran anthocyanins for fish freshness monitorings. Int. J. Biol. Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for Schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Surendhiran, D.; Li, C.Z.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Oluba, O.M.; Obi, C.F.; Akpor, O.B.; Ojeaburu, S.I.; Ogunrotimi, F.D.; Adediran, A.A.; Oki, M. Fabrication and characterization of keratin starch biocomposite film from chicken feather waste and ginger starch. Sci. Rep. 2021, 11, 8768. [Google Scholar] [CrossRef]
- Marangoni Júnior, L.; Silva, R.G.d.; Vieira, R.P.; Alves, R.M.V. Water vapor sorption and permeability of sustainable alginate/collagen/SiO2 composite films. LWT 2021, 152, 112261. [Google Scholar] [CrossRef]
- Figueroa-Lopez, K.J.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Escuin, J.M.; Bourbon, A.I.; Cabedo, L.; Nevo, Y.; Cerqueira, M.A.; Lagaron, J.M. Development of active barrier multilayer films based on electrospun antimicrobial hot-tack food waste derived poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and cellulose nanocrystal interlayers. Nanomaterials 2020, 10, 2356. [Google Scholar] [CrossRef]
- Roy, S.; Ezati, P.; Rhim, J.-W. Gelatin/Carrageenan-Based Functional Films with Carbon Dots from Enoki Mushroom for Active Food Packaging Applications. ACS Appl. Polym. Mater. 2021, 3, 6437–6445. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of Gelatin/Carrageenan-Based Color-Indicator Film Integrated with Shikonin and Propolis for Smart Food Packaging Applications. ACS Appl. Bio Mater. 2021, 4, 770–779. [Google Scholar] [CrossRef]
- Lan, X.; Luo, T.; Zhong, Z.; Huang, D.; Liang, C.; Liu, Y.; Wang, H.; Tang, Y. Green cross-linking of gelatin/tea polyphenol/ε-poly (L-lysine) electrospun nanofibrous membrane for edible and bioactive food packaging. Food Packag. Shelf Life 2022, 34, 100970. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and Characterization of Edible Dialdehyde Carboxymethyl Cellulose Crosslinked Feather Keratin Films for Food Packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, S.; Yang, H.; Liao, Z.; Gong, Z.; Zhao, W.; Li, Y.; Gu, J.; Wei, Z.; Yang, J. Keratin-Based Composite Bioactive Films and Their Preservative Effects on Cherry Tomato. Molecules 2022, 27, 6331. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.R.B.; de Andrade, P.S.; Guimarães Junior, M.; Dias, M.V.; Alcântara, L.A.P. Novel Whey Protein Isolate/Polyvinyl Biocomposite for Packaging: Improvement of Mechanical and Water Barrier Properties by Incorporation of Nano-silica. J. Polym. Environ. 2021, 29, 2397–2408. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, F.; Wang, F.; Zhang, H.; Kang, M. Development and characterization of zein-based active packaging films containing catechin loaded β-cyclodextrin metal-organic frameworks. Food Packag. Shelf Life 2022, 31, 100810. [Google Scholar] [CrossRef]
- Andrade-Del Olmo, J.; Pérez-Álvarez, L.; Hernáez, E.; Ruiz-Rubio, L.; Vilas-Vilela, J.L. Antibacterial multilayer of chitosan and (2-carboxyethyl)- β-cyclodextrin onto polylactic acid (PLLA). Food Hydrocoll. 2019, 88, 228–236. [Google Scholar] [CrossRef]
- Arumugam, S.; Kandasamy, J.; Thiyaku, T.; Saxena, P. Effect of Low Concentration of SiO2 Nanoparticles on Grape Seed Essential Oil/PBAT Composite Films for Sustainable Food Packaging Application. Sustainability 2022, 14, 8073. [Google Scholar] [CrossRef]
- Lukic, I.; Vulic, J.; Ivanovic, J. Antioxidant activity of PLA/PCL films loaded with thymol and/or carvacrol using scCO2 for active food packaging. Food Packag. Shelf Life 2020, 26, 100578. [Google Scholar] [CrossRef]
- Diken, M.E.; Koçer Kizilduman, B.; Doğan, S.; Doğan, M. Antibacterial and antioxidant phenolic compounds loaded PCL biocomposites for active food packaging application. J. Appl. Polym. Sci. 2022, 139, e52423. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Hilliou, L.; Escuin, J.M.; Cabedo, L.; Nevo, Y.; Prieto, C.; Lagaron, J.M. High-Oxygen-Barrier Multilayer Films Based on Polyhydroxyalkanoates and Cellulose Nanocrystals. Nanomaterials 2021, 11, 1443. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Niu, N.; Chen, Z.; Li, S.; Liu, S.-X.; Li, J. Fluorescent Poly(vinyl alcohol) Films Containing Chlorogenic Acid Carbon Nanodots for Food Monitoring. ACS Appl. Nano Mater. 2020, 3, 7611–7620. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Akshaykranth, A.; Jayarambabu, N.; Venkatappa Rao, T.; Rakesh Kumar, R.; Srinivasa Rao, L. Antibacterial activity study of ZnO incorporated biodegradable poly (lactic acid) films for food packaging applications. Polym. Bull. 2023, 80, 1369–1384. [Google Scholar] [CrossRef]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Perera, K.Y.; Pradhan, D.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Active Packaging Film Based on Poly Lactide-Poly (Butylene Adipate-Co-Terephthalate) Blends Incorporated with Tannic Acid and Gallic Acid for the Prolonged Shelf Life of Cherry Tomato. Coatings 2022, 12, 1902. [Google Scholar] [CrossRef]
- Marzano-Barreda, L.A.; Yamashita, F.; Bilck, A.P. Effect of biodegradable active packaging with zeolites on fresh broccoli florets. J. Food Sci. Technol. 2021, 58, 197–204. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly(butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Charoensri, K.; Rodwihok, C.; Ko, S.H.; Wongratanaphisan, D.; Park, H.J. Enhanced antimicrobial and physical properties of poly (butylene adipate-co-terephthalate)/zinc oxide/reduced graphene oxide ternary nanocomposite films. Mater. Today Commun. 2021, 28, 102586. [Google Scholar] [CrossRef]
- Dumitriu, R.P.; Stoleru, E.; Mitchell, G.R.; Vasile, C.; Brebu, M. Bioactive Electrospun Fibers of Poly(ε-Caprolactone) Incorporating α-Tocopherol for Food Packaging Applications. Molecules 2021, 26, 5498. [Google Scholar] [CrossRef]
- Cheng, P.-f.; Liang, M.; Yun, X.-y.; Dong, T. Biodegradable blend films of poly(ε-caprolactone)/poly(propylene carbonate) for shelf life extension of whole white button mushrooms. J. Food Sci. Technol. 2022, 59, 144–156. [Google Scholar] [CrossRef]
- Rech, C.R.; Brabes, K.C.S.; Silva, B.E.B.; Martines, M.A.U.; Silveira, T.F.S.; Alberton, J.; Amadeu, C.A.A.; Caon, T.; Arruda, E.J.; Martelli, S.M. Antimicrobial and Physical–Mechanical Properties of Polyhydroxybutyrate Edible Films Containing Essential Oil Mixtures. J. Polym. Environ. 2021, 29, 1202–1211. [Google Scholar] [CrossRef]
- Le Delliou, B.; Vitrac, O.; Castro, M.; Bruzaud, S.; Domenek, S. Characterization of a new bio-based and biodegradable blends of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(butylene-co-succinate-co-adipate). J. Appl. Polym. Sci. 2022, 139, 52124. [Google Scholar] [CrossRef]
- La Fuente Arias, C.I.; González-Martínez, C.; Chiralt, A. Lamination of starch/polyesters by thermocompression for food packaging purposes. Sustain. Food Technol. 2023, 1, 296–305. [Google Scholar] [CrossRef]

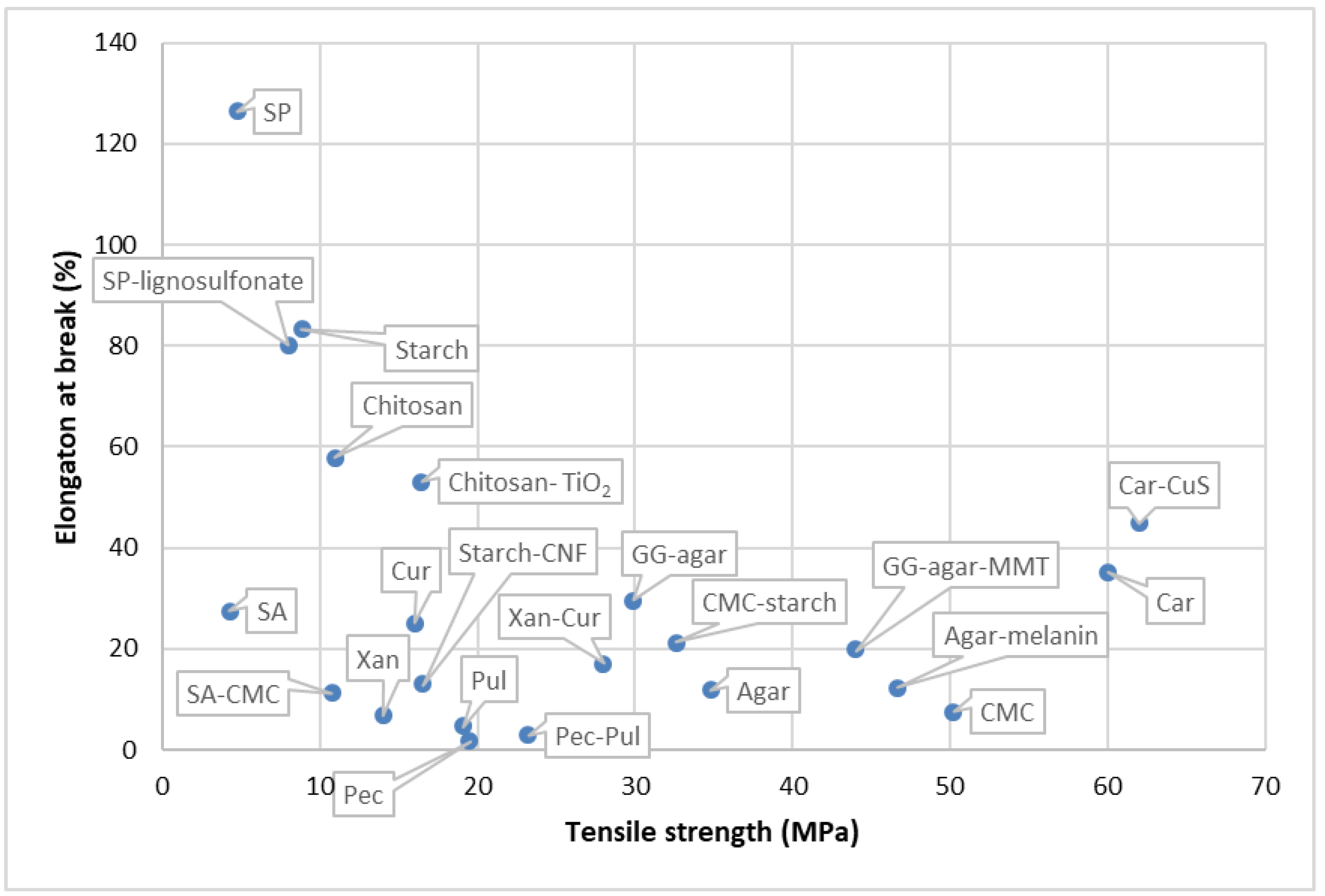
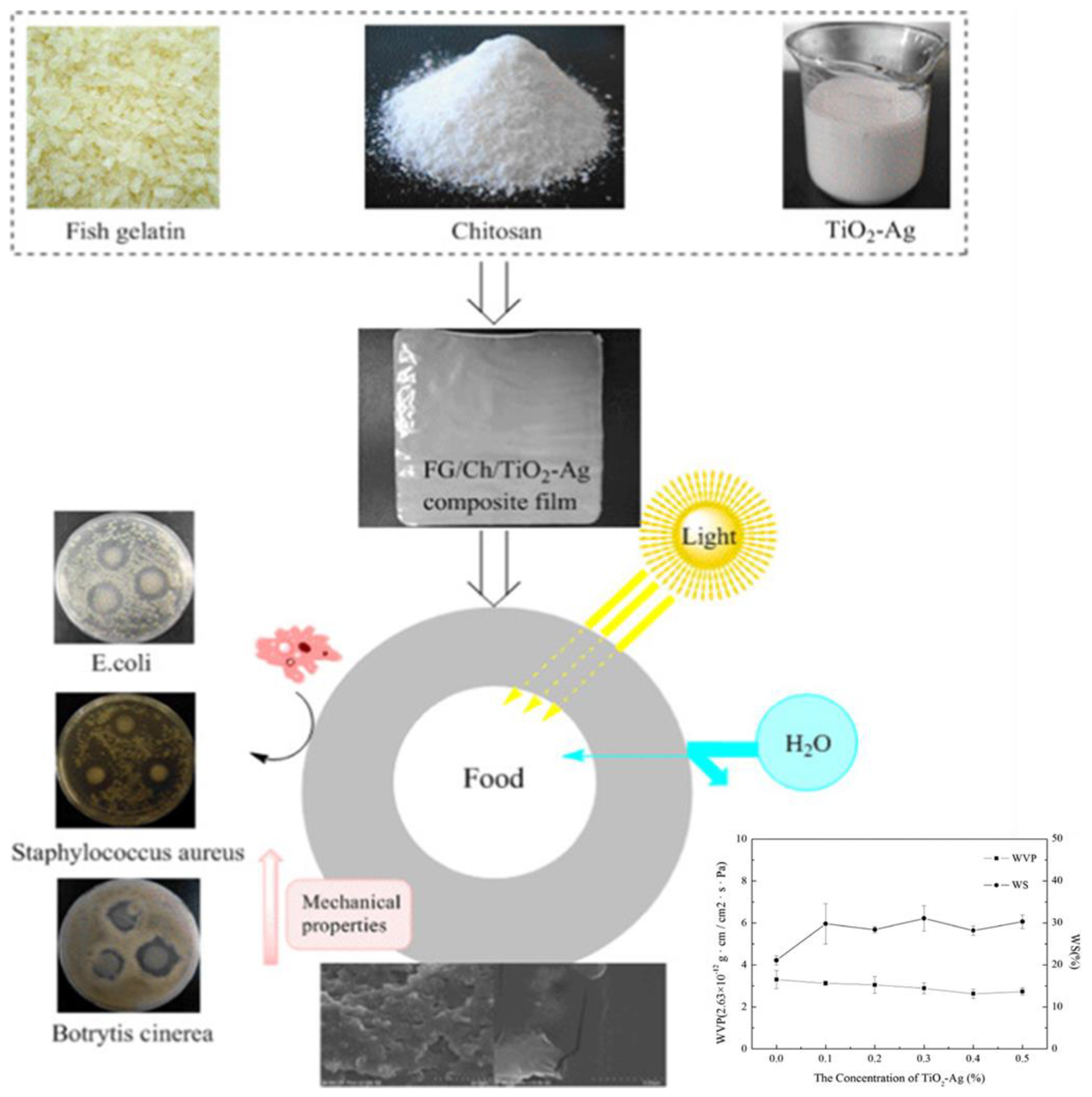
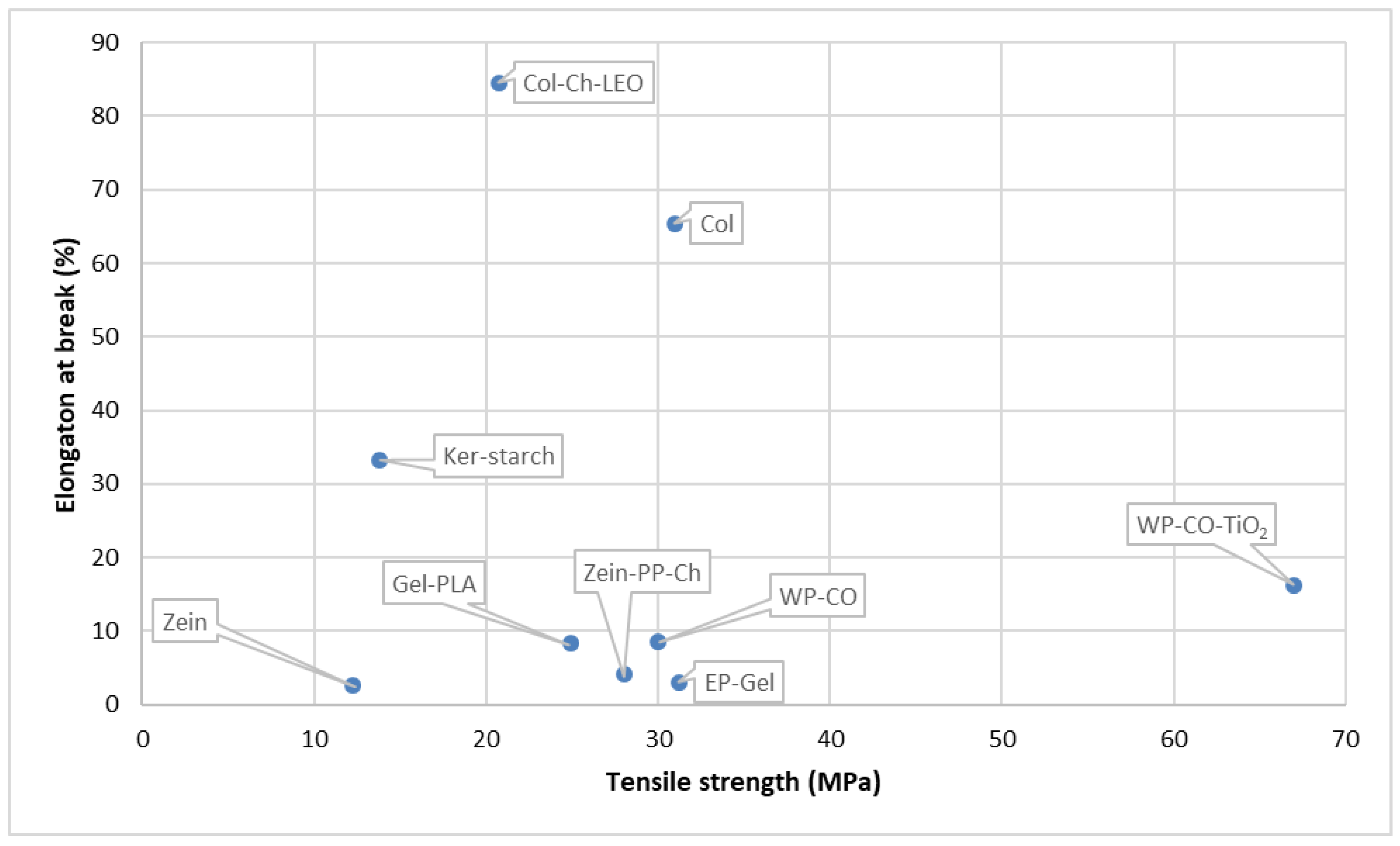

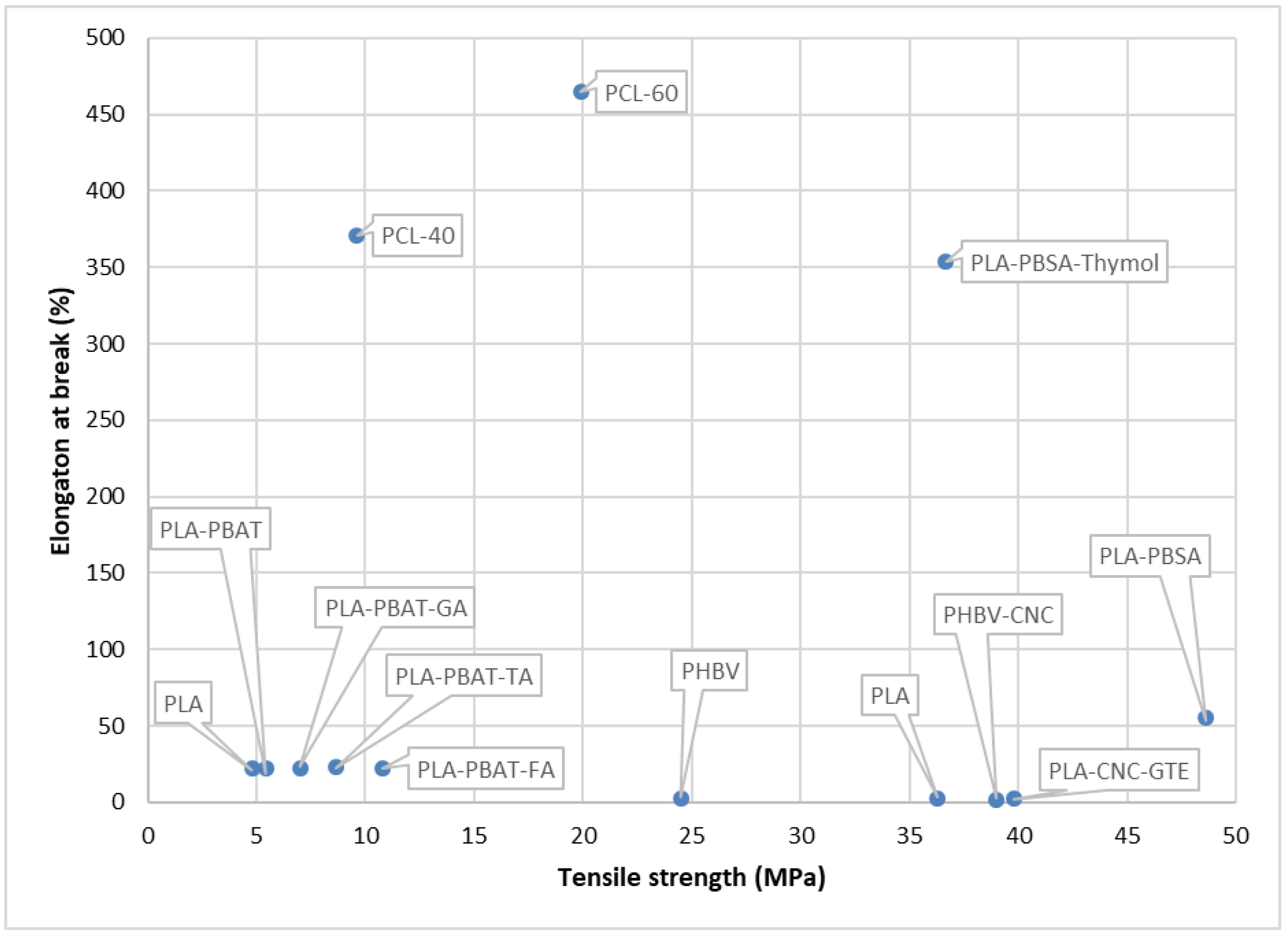

| Biopolymer | Positive Characteristics | Negative Characteristics | References |
|---|---|---|---|
| Starch-based biopolymers | |||
| Starch |
|
| [1,6,7,8,9] |
| Chitosan |
|
| [10,11,12] |
| Carrageenan |
|
| [13,14,15,16,17] |
| Cellulose |
|
| [18,19,20,21] |
| Agar |
|
| [3,4,22,23,24] |
| Pectins |
|
| [25,26,27,28] |
| Alginate |
|
| [29,30,31,32,33] |
| Gums |
|
| [34,35,36] |
| Lignin |
|
| [4,34,37,38] |
| Pullulan |
|
| [20,39,40] |
| Curdlan |
|
| [41,42] |
| Protein-based biopolymers | |||
| Gelatin |
|
| [24,43,44,45] |
| Soy protein |
|
| [46,47,48,49] |
| whey proteins |
|
| [50,51,52,53] |
| zein |
|
| [54,55,56,57] |
| Keratin |
|
| [58,59] |
| Collagen |
|
| [60] |
| Aliphatic polyester-based biopolymers | |||
| Poly lactic acid (PLA) |
|
| [61,62,63,64,65] |
| poly(butylene adipate terephthalate) (PBAT) |
|
| [9,63,66] |
| Poly caprolactone (PCL) |
|
| [57] |
| polybutylene succinate (PBS) |
|
| [67] |
| Polyhydroxyalkanoate (PHAs) |
|
| [68,69,70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perera, K.Y.; Jaiswal, A.K.; Jaiswal, S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods 2023, 12, 2422. https://doi.org/10.3390/foods12122422
Perera KY, Jaiswal AK, Jaiswal S. Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods. 2023; 12(12):2422. https://doi.org/10.3390/foods12122422
Chicago/Turabian StylePerera, Kalpani Y., Amit K. Jaiswal, and Swarna Jaiswal. 2023. "Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications" Foods 12, no. 12: 2422. https://doi.org/10.3390/foods12122422
APA StylePerera, K. Y., Jaiswal, A. K., & Jaiswal, S. (2023). Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications. Foods, 12(12), 2422. https://doi.org/10.3390/foods12122422