Optimization of Fermentation Conditions and Metabolite Profiling of Grape Juice Fermented with Lactic Acid Bacteria for Improved Flavor and Bioactivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Grape Juice Preparation
2.3. Single-Strain Fermentation
2.4. Physicochemical Analyses
2.4.1. Determination of Total Phenolics Content (TPC)
2.4.2. Determination of Total Soluble Solids (TSS), pH, Clarity
2.5. Multi-Strain Fermentation
2.5.1. Optimization of the Proportion of Multi-Strain
2.5.2. Optimization of Multi-Strain Fermentation Conditions
2.6. Metabolite Extraction and UHPLC-QE-MS/MS Analysis of Multi-Strain Fermentation
2.7. VOC Analysis of Multi-Strain Fermentation
2.7.1. Determination of Volatile Compounds
2.7.2. Identification of Key VOCs
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of the Strains’ Function and Optimizing the Fermentation Conditions
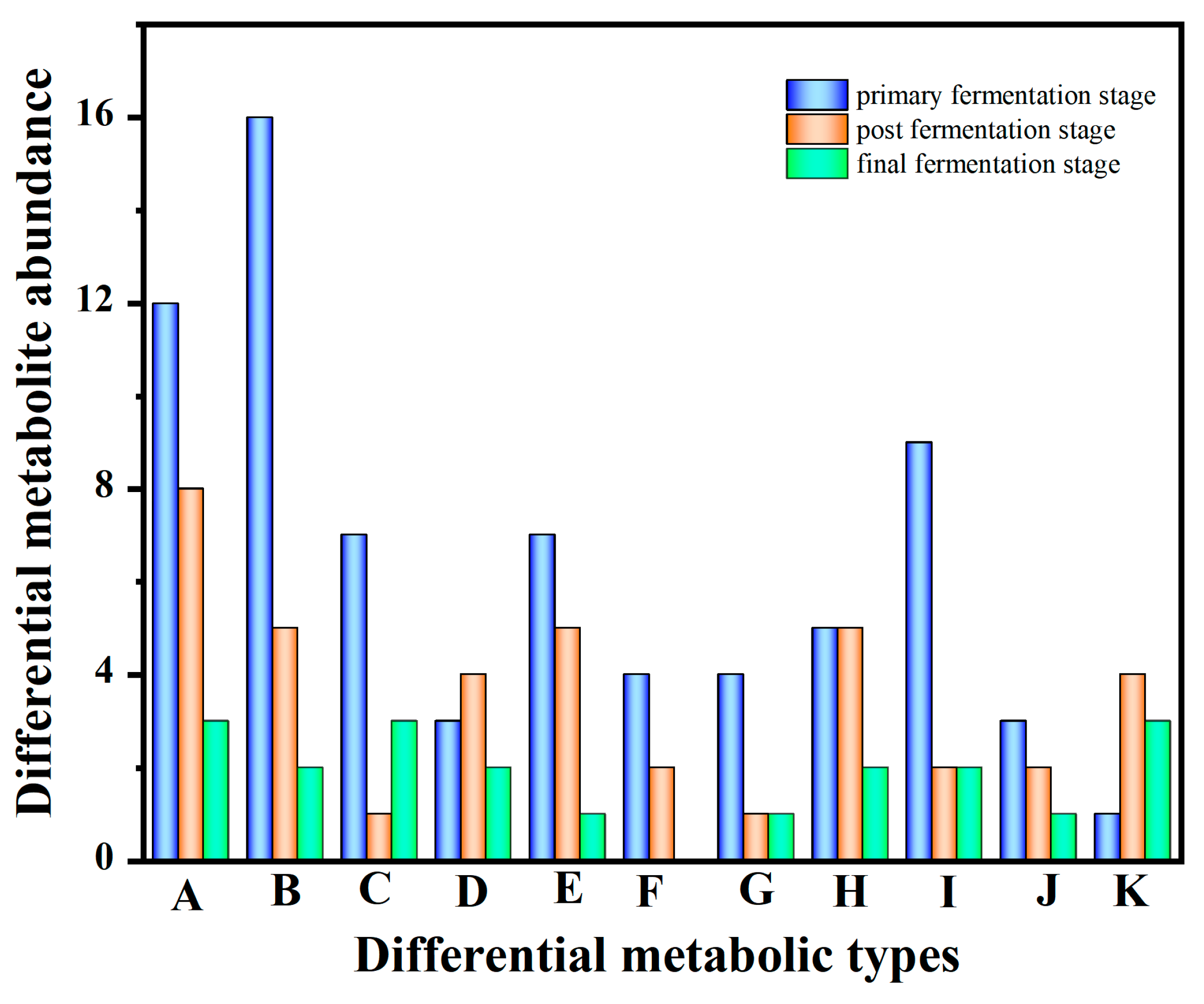
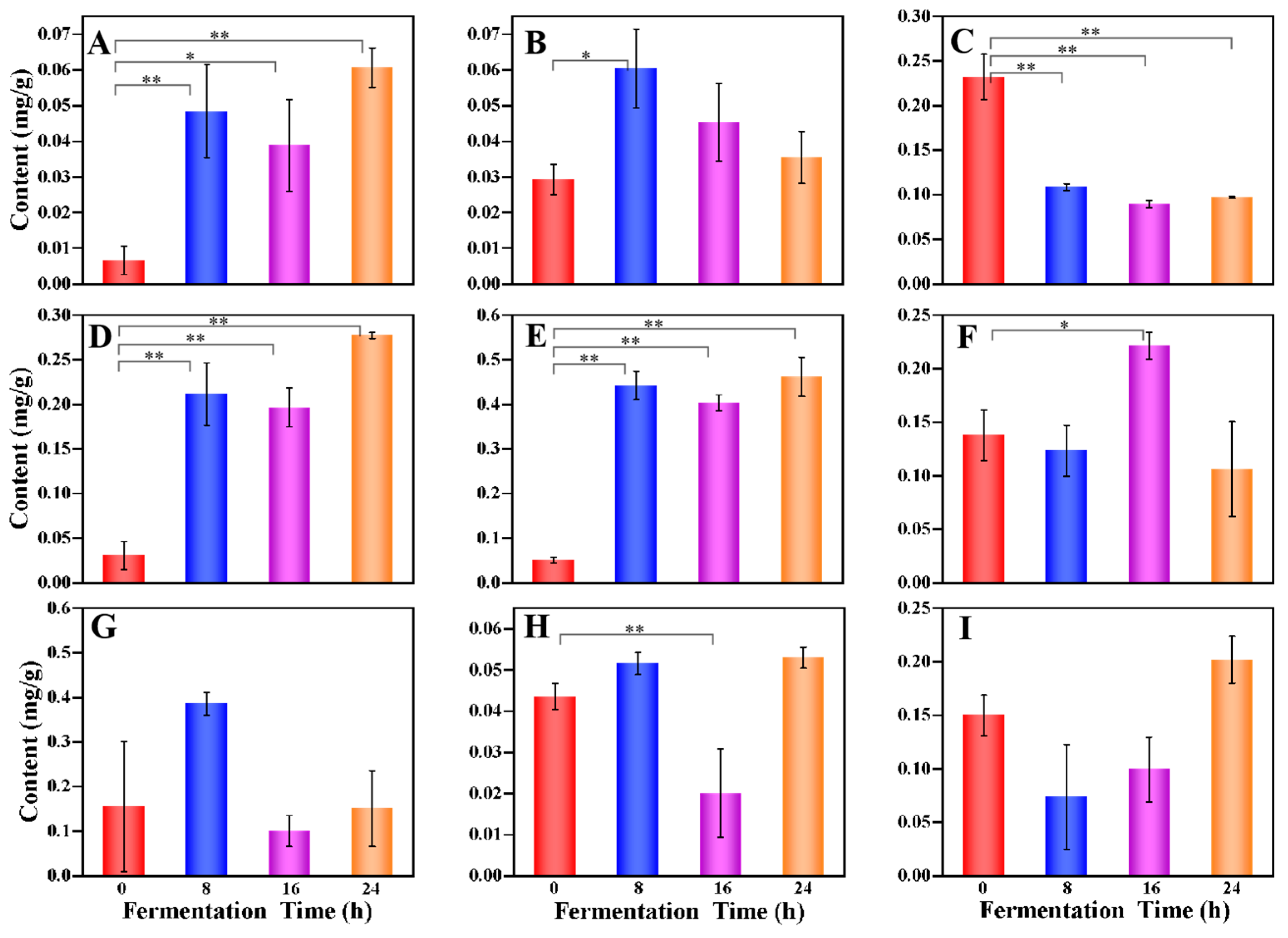
3.2. Profiles of Metabolites during the Multi-Strain Fermentation
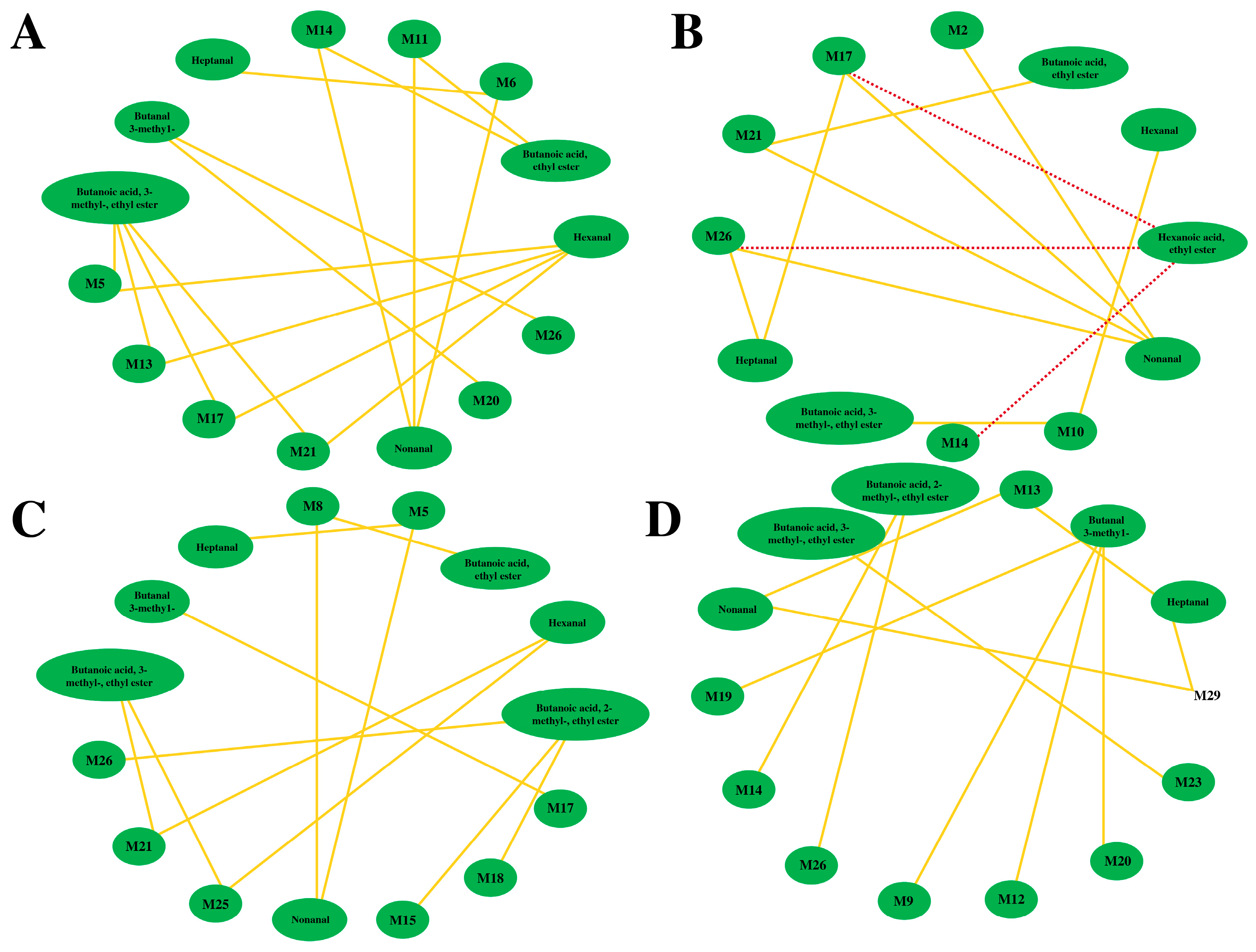
3.3. Analyses of VOC Profiles
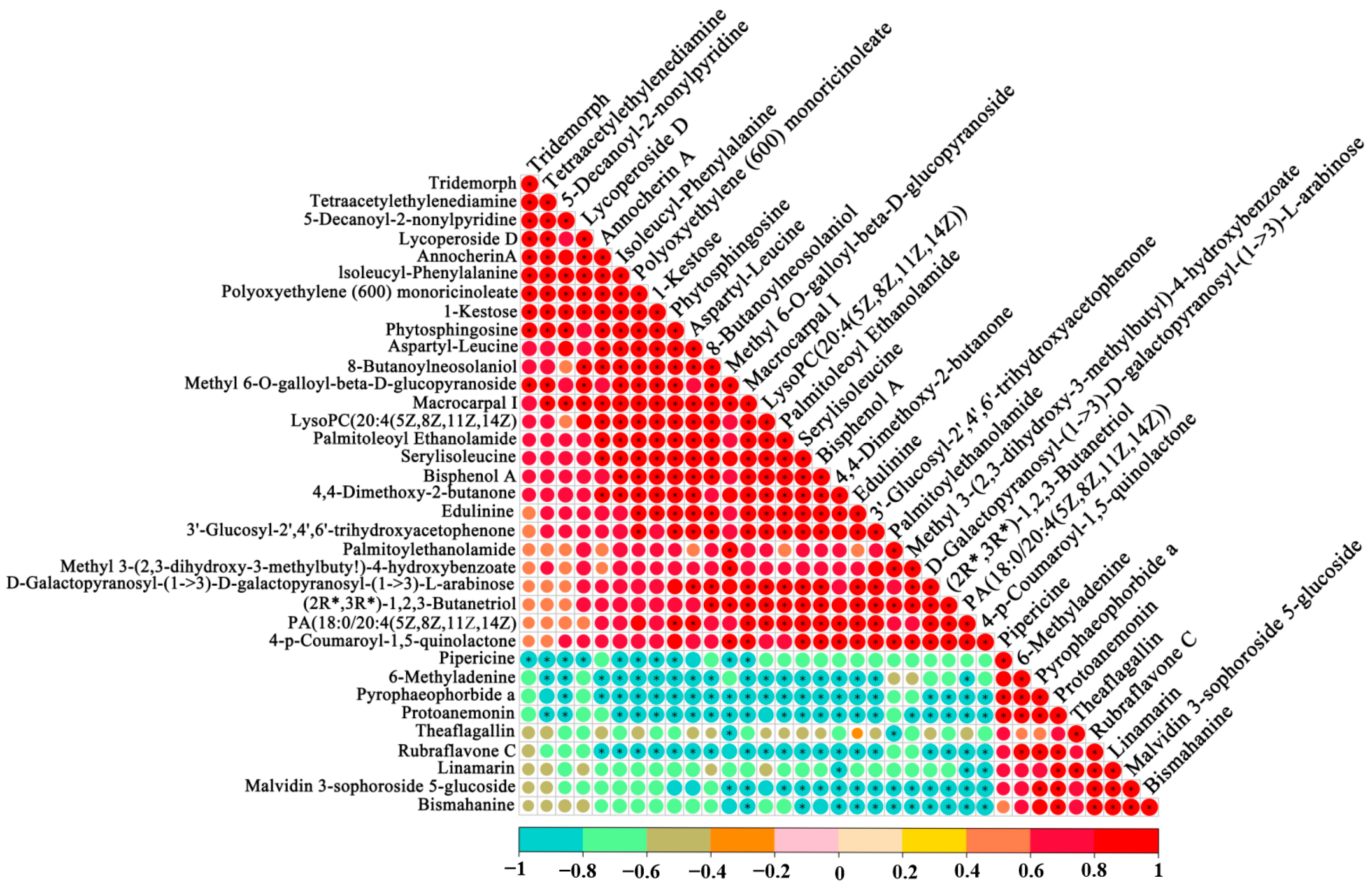
3.4. Association Analysis between the VOCs and Non-Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, Z.Z.; Zhao, Z.Q.; Wu, X.L.; Lin, W.J.; Qin, Y.H.; Chen, H.; Wan, Y.J.; Zhou, C.X.; Bu, T.L.; Chen, H.; et al. A Review on Fruit and Vegetable Fermented Beverage-Benefits of Microbes and Beneficial Effects. Food Rev. Int. 2022, 1–38. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Pobiega, K.; Rybak, K.; Synowiec, A.; Woźniak, L.; Trych, U.; Gniewosz, M.; Witrowa-Rajchert, D. Changes in Physical and Chemical Parameters of Beetroot and Carrot Juices Obtained by Lactic Fermentation. Appl. Sci. 2023, 13, 6113. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, Q.; Zhao, Z.; Shen, N.; Lin, Y.Q.W.; Xiao, Y.; Yuan, M.; Chen, H.; Chen, H.; Bu, T.; et al. Evaluation of fermentation properties, antioxidant capacity in vitro and in vivo, and metabolic profile of a fermented beverage made from apple and cantaloupe. LWT Food Sci. Technol. 2023, 179, 114661. [Google Scholar] [CrossRef]
- Wang, L.Y.; Zhang, H.X.; Lei, H.J. Phenolics Profile, Antioxidant Activity and Flavor Volatiles of Pear Juice: Influence of Lactic Acid Fermentation Using Three Lactobacillus Strains in Monoculture and Binary Mixture. Foods 2022, 11, 11. [Google Scholar] [CrossRef]
- Cele, N.P.; Akinola, S.A.; Manhivi, V.E.; Shoko, T.; Remize, F.; Sivakumar, D. Influence of Lactic Acid Bacterium Strains on Changes in Quality, Functional Compounds and Volatile Compounds of Mango Juice from Different Cultivars during Fermentation. Foods 2022, 11, 682. [Google Scholar] [CrossRef]
- Septembre-Malaterreb, A.; Remize, F.; Pouchereta, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–89. [Google Scholar] [CrossRef]
- Fonseca, H.C.; Melo, D.D.S.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Lactiplantibacillus plantarum CCMA 0743 and Lacticaseibacillus paracasei subsp. paracasei LBC-81 metabolism during the single and mixed fermentation of tropical fruit juices. Braz. J. Microbiol. 2021, 52, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Tkacz, K.; Chmielewska, J.; Turkiewicz, I.P.; Nowicka, P.; Wojdyło, A. Dynamics of changes in organic acids, sugars and phenolic compounds and antioxidant activity of sea buckthorn and sea buckthorn-apple juices during malolactic fermentation. Food Chem. 2020, 32, 127382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.L.; Mu, T.H.; Ma, M.M.; Sun, H.N.; Zhao, G.H. Nutritional composition, antioxidant activity, volatile compounds, and stability properties of sweet potato residues fermented with selected lactic acid bacteria and bifidobacterial. Food Chem. 2022, 374, 131500. [Google Scholar] [CrossRef] [PubMed]
- Kaprasob, R.; Kerdchoechuen, O.; Laohakunjit, N.; Sarkar, D.; Shetty, K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process. Biochem. 2017, 59, 141–149. [Google Scholar] [CrossRef]
- Chen, C.; Lu, Y.Q.; Yu, H.Y.; Chen, Z.Y.; Tian, H.X. Influence of 4 lactic acid bacteria on the flavor profile of fermented apple juice. Food Biosci. 2019, 27, 30–36. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Barba, F.J.; Remize, F.; Garcia, C.; Fessard, A.; Khaneghah, A.M.; Sant, A.S.; Lorenzo, J.M.; Montesano, D.; Meléndez-Martínez, A.J. The impact of fermentation processes on the production, retention and bioavailability of carotenoids: An overview. Trends Food Sci. Technol. 2020, 99, 389–401. [Google Scholar] [CrossRef]
- Ricci, A.; Cirlini, M.; Levante, A.; Asta, C.D.; Galaverna, G.; Lazzi, C. Volatile profile of elderberry juice: Effect of lactic acid fermentation using L. plantarum, L. rhamnosus and L. casei strains. Food Res. Int. 2018, 105, 412–422. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT-Food Sci. Technol. 2020, 131, 109803. [Google Scholar] [CrossRef]
- Xu, X.X.; Bao, Y.J.; Wu, B.B.; Lao, F.; Hu, X.S.; Wu, J.H. Chemical analysis and flavor properties of blended orange, carrot, apple and Chinese jujube juice fermented by selenium-enriched probiotics. Food Chem. 2019, 289, 250–258. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Jayabalan, R. Effect of probiotifification with Lactiplantibacillus plantarum MCC 2974 on quality of Sohiong juice. LWT-Food Sci. Technol. 2019, 108, 55–60. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, S.; Xu, X.X.; Lao, F.I.; Wu, J.H. Volatile and non-volatile profiles in jujube pulp co-fermented with lactic acid bacteria. LWT-Food Sci. Technol. 2022, 154, 112772. [Google Scholar] [CrossRef]
- Chen, R.H.; Chen, W.X.; Chen, H.M.; Zhang, G.F.; Chen, W.J. Comparative Evaluation of the Antioxidant Capacities, Organic Acids, and Volatiles of Papaya Juices Fermented by Lactobacillus acidophilus and Lactobacillus plantarum. Food Qual. 2018, 2018, 9490435. [Google Scholar] [CrossRef] [Green Version]
- Van Gemert, L.J. Odour thresholds. In Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2011; Volume 2. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.Y.; Huang, D.; Pu, D.D.; Zhang, Y.Y.; Chen, H.T.; Sun, B.G.; Ren, F.Z. Multivariate relationships among sensory attributes and volatile components in commercial dry porcini mushrooms (Boletus edulis). Food Res. Int. 2020, 133, 109112. [Google Scholar] [CrossRef]
- Wang, Z.N.; Feng, Y.Z.; Yang, N.N.; Jiang, T.; Xu, H.D.; Lei, H.J. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.P.; Wei, Z.H.; Yin, B.X.; Man, C.X.; Jiang, Y.J. Enhancement of functional characteristics of blueberry juice fermented by Lactobacillus plantarum. LWT Food Sci. Technol. 2021, 139, 110590. [Google Scholar] [CrossRef]
- Mirmohammadi, R.; Zamindar, N.; Razavi, S.H.; Mirmohammadi, M.; Paidari, S. Investigation of the possibility of fermentation of red grape juice and rice flour by Lactobacillus plantarum and Lactobacillus casei. Food Sci. Nutr. 2021, 9, 5370–5378. [Google Scholar] [CrossRef]
- Zhang, J.G.; Huang, X.D.; Cheng, J.H.; Wang, C.Y. Effect of Lactobacillus (L. acidophilus NCIB1899, L. casei CRL 431, L. paracasei LP33) fermentation on free and bound polyphenolic, antioxidant activities in three Chenopodium quinoa cultivars. J. Food Sci. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, H.; Zhu, C. Effect of lactic acid bacteria fermentation on antioxidation and bioactivity of hawthorn pulp. IOP Conf. Ser. Earth Environ. Sci. 2019, 267, 062056. [Google Scholar] [CrossRef] [Green Version]
- Aung, T.; Eun, J. Production and characterization of a novel beverage from laver (Porphyra dentata) through fermentation with kombucha consortium. Food Chem. 2021, 350, 129274. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Chang, L. Effects of lactic acid bacteria fermentation on the phytochemicals content, taste and aroma of blended edible rose and shiitake beverage. Food Chem. 2023, 405, 134722. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Li, T.L.; Qi, J.; Jiang, T.; Xu, H.D.; Lei, H.J. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT Food Sci. Technol. 2020, 122, 109064. [Google Scholar] [CrossRef]
- Benincasa, C.; Muccilli, S.; Amenta, M.; Perri, E.; Romeo, F.V. Phenolic trend and hygienic quality of green table olives fermented with Lactobacillus plantarum starter culture. Food Chem. 2015, 186, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lee, S.Y.; Kim, Y.C.; Choi, I.; Kim, G.B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Viesser, J.A.; Pereira, G.V.D.M.; Neto, D.P.D.C.; Rogez, H.; Neto, A.G.; Azevedo, V.; Brenig, B.; Aburjaile, F.; Soccol, C.R. Co-culturing fructophilic lactic acid bacteria and yeast enhanced sugar metabolism and aroma formation during cocoa beans fermentation. Int. J. Food Microbiol. 2021, 339, 109015. [Google Scholar] [CrossRef]
- Braga, C.M.; Zielinski, A.A.F.; Silva, K.M.D.; Souza, F.K.F.D.; Pietrowski, G.D.A.M.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013, 141, 967–974. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; Angelis, M.D.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 837–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.L.; Jiang, T.; Liu, N.; Wu, C.Y.; Xu, H.D.; Lei, H.J. Biotransformation of phenolic profiles and improvement of antioxidant capacities in jujube juice by select lactic acid bacteria. Food Chem. 2021, 339, 127859. [Google Scholar] [CrossRef] [PubMed]
- Cousin, F.J.; Guellec, R.L.; Schlusselhuber, M.; Dalmasso, M.; Laplace, J.M.; Cretenet, M. Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions. Microorganisms 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| No. | Compounds | a Odor Threshold (mg/L) | b Odor Description | Relative Odor Activity Value (ROAV) |
|---|---|---|---|---|
| V1 | butanoic acid, 2-methyl-, ethyl ester | 0.000063 | Apple | 100.00 |
| V2 | butanoic acid, ethyl ester | 0.003 | Banana, pineapple, strawberry | 15.026 |
| V3 | hexanoic acid, ethyl ester | 0.0022 | Banana, fruit, apple peel | 6.236 |
| V4 | nonanal | 0.008 | Fat, citrus, green | 1.226 |
| V5 | heptanal | 0.0028 | Fat, citrus, rancid | 1.463 |
| V6 | butanal, 3-methyl- | 0.0012 | Ethereal, aldehydic | 1.505 |
| V7 | butanoic acid, 3-methyl-, ethyl ester | 0.00011 | Fruity, sweet, apple | 1.588 |
| V8 | hexanal | 0.073 | Green, tallow, fat | 3.394 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuerban, D.; Lu, J.; Huangfu, Z.; Wang, L.; Qin, Y.; Zhang, M. Optimization of Fermentation Conditions and Metabolite Profiling of Grape Juice Fermented with Lactic Acid Bacteria for Improved Flavor and Bioactivity. Foods 2023, 12, 2407. https://doi.org/10.3390/foods12122407
Kuerban D, Lu J, Huangfu Z, Wang L, Qin Y, Zhang M. Optimization of Fermentation Conditions and Metabolite Profiling of Grape Juice Fermented with Lactic Acid Bacteria for Improved Flavor and Bioactivity. Foods. 2023; 12(12):2407. https://doi.org/10.3390/foods12122407
Chicago/Turabian StyleKuerban, Dilinu, Jing Lu, Zekun Huangfu, Liang Wang, Yanan Qin, and Minwei Zhang. 2023. "Optimization of Fermentation Conditions and Metabolite Profiling of Grape Juice Fermented with Lactic Acid Bacteria for Improved Flavor and Bioactivity" Foods 12, no. 12: 2407. https://doi.org/10.3390/foods12122407





