Byproducts of Sesame Oil Extraction: Composition, Function, and Comprehensive Utilization
Abstract
:1. Introduction
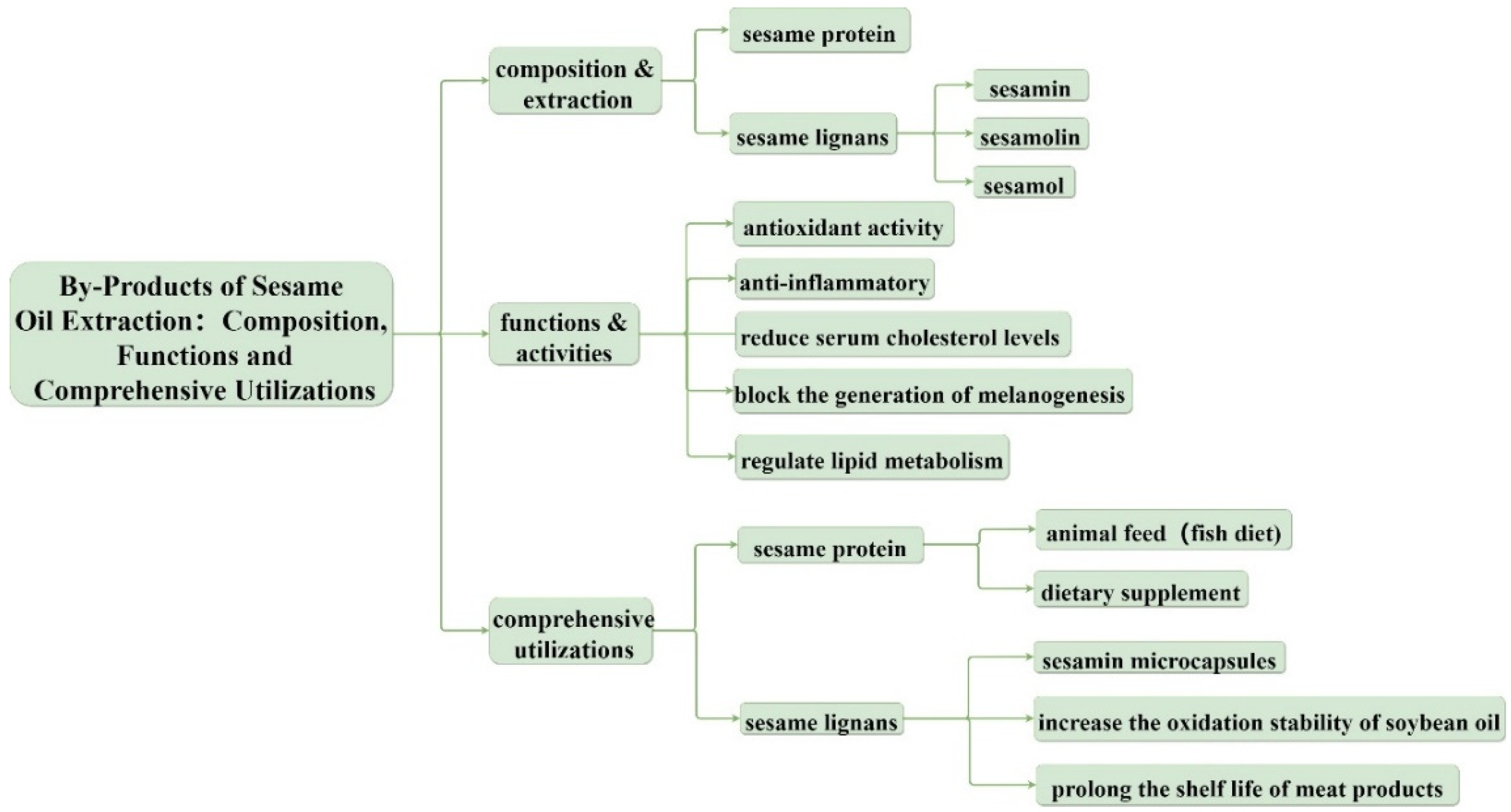
2. Composition and Extraction of Bioactive Substances in Sesame Meal
2.1. Sesame Protein
Extraction of Sesame Protein
2.2. Sesame Lignans
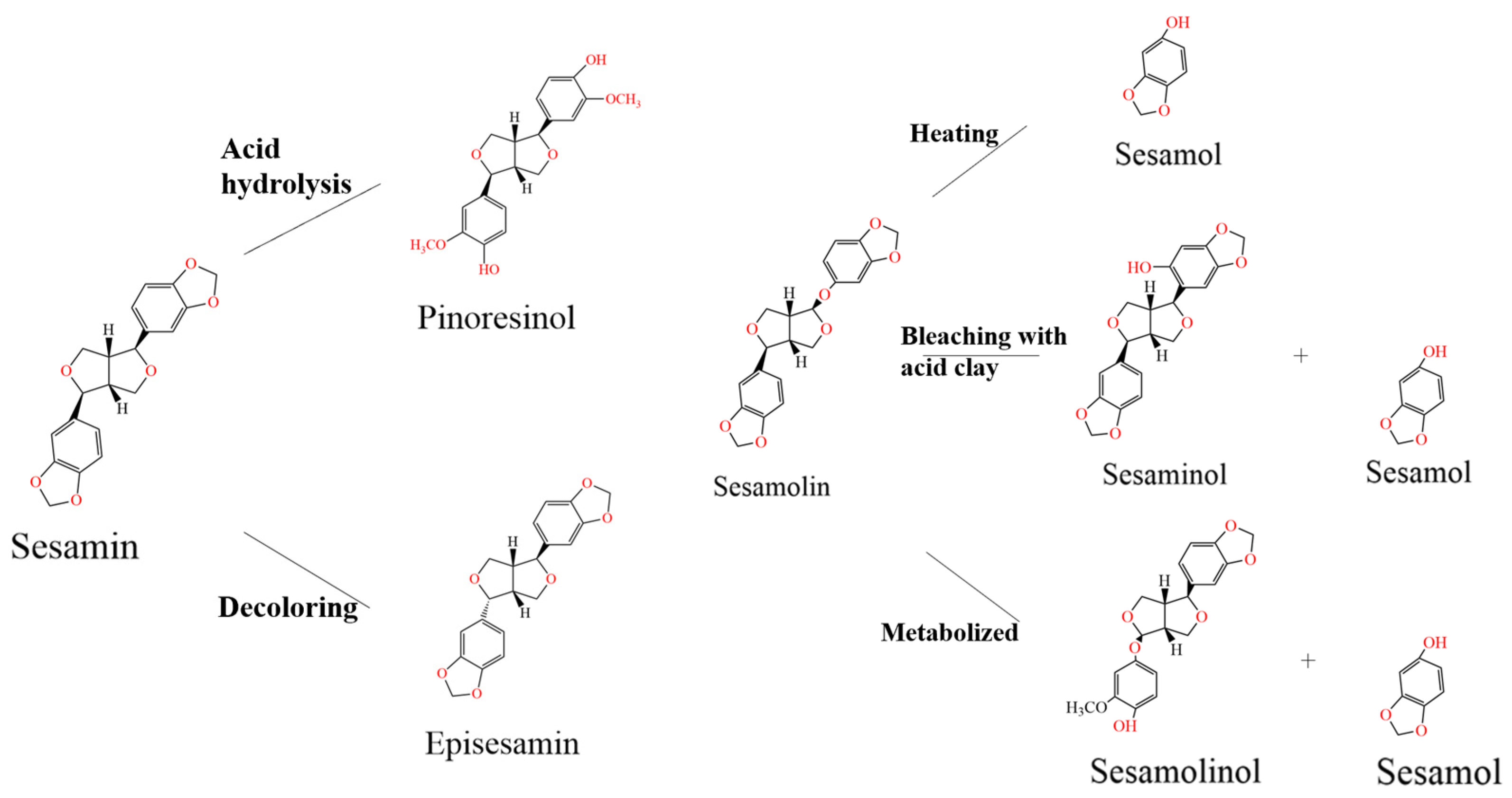
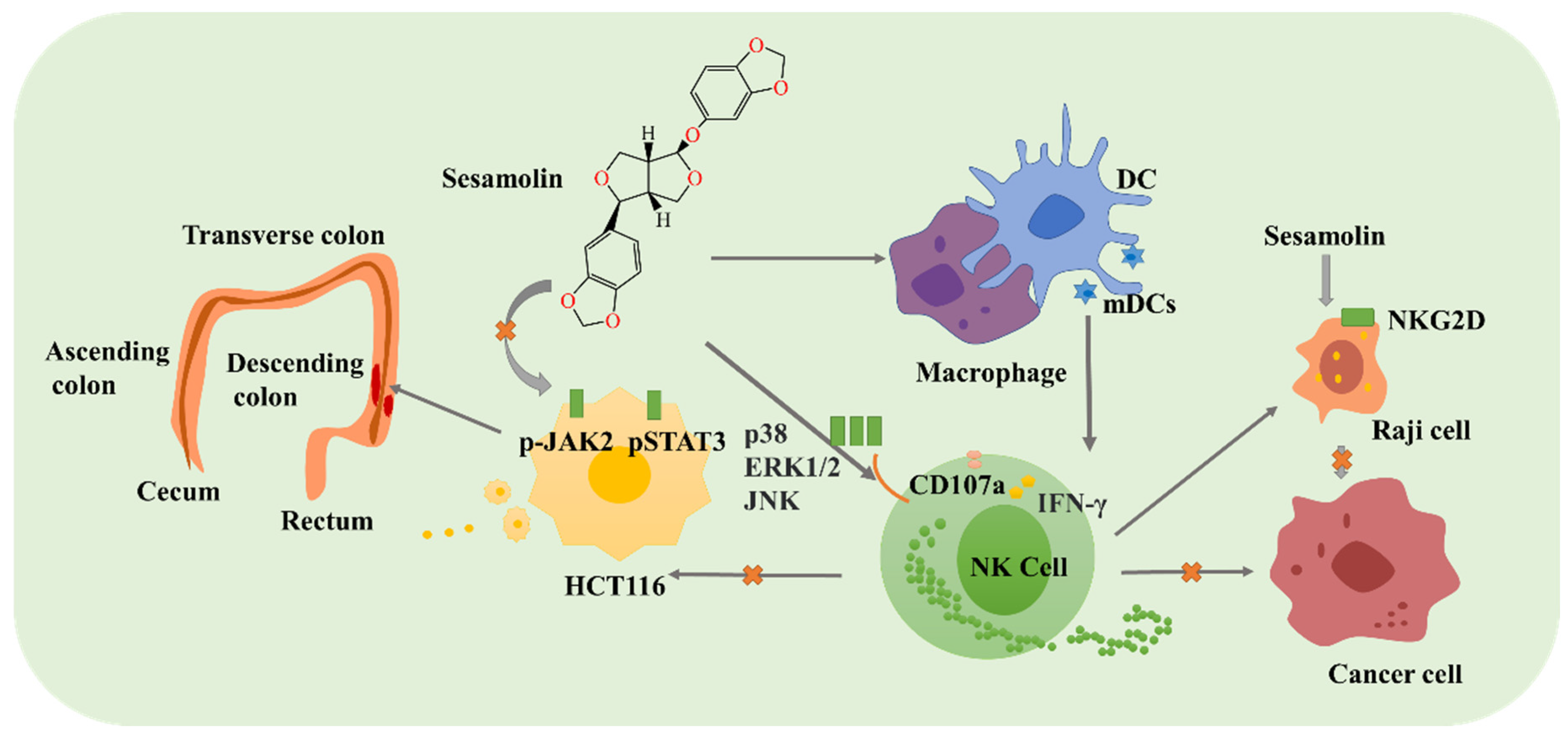
2.2.1. Sesamin, Sesamolin, and Sesamol
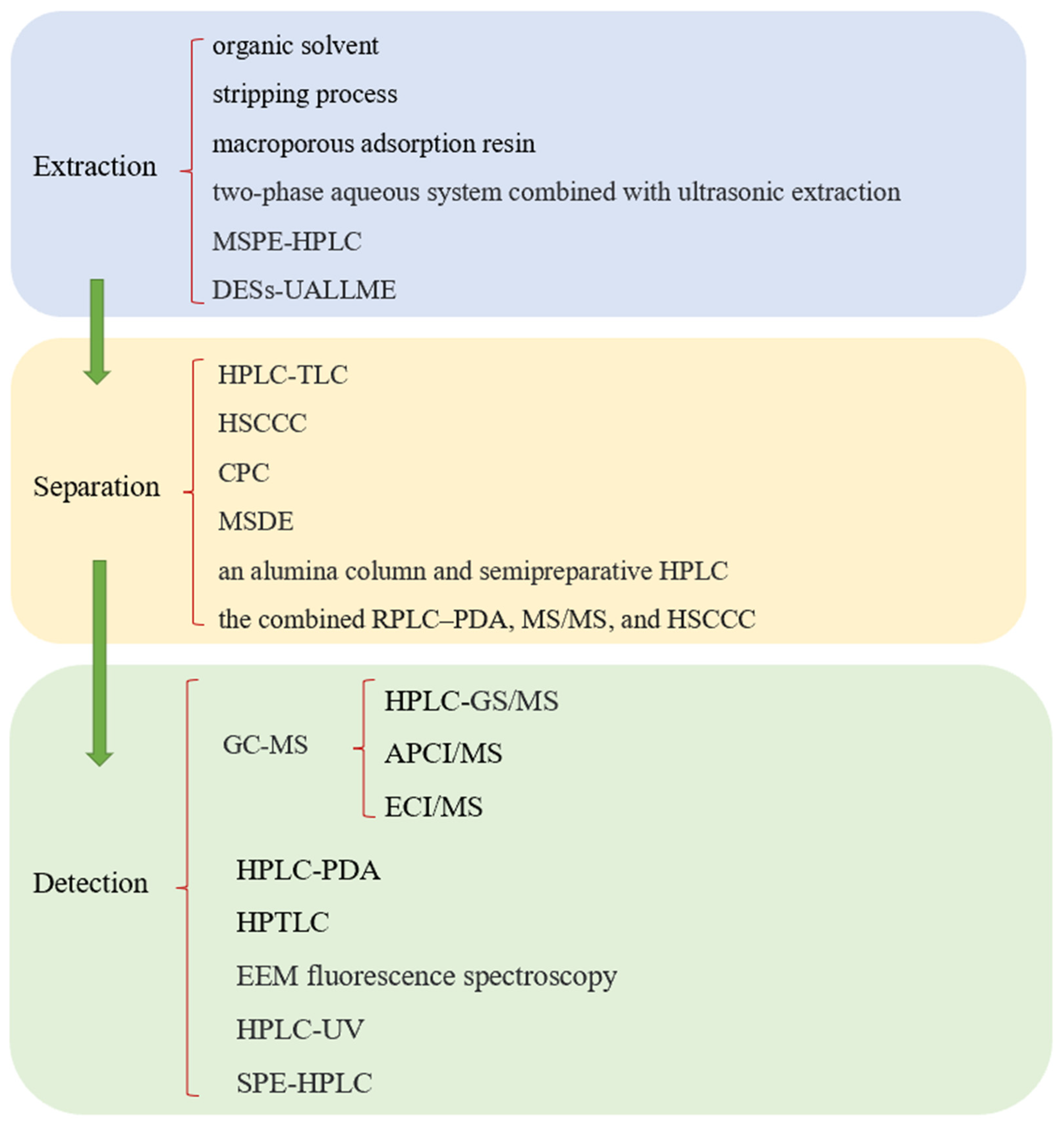
2.2.2. Extraction, Separation, and Detection of Sesame Lignans
3. Functions and Activities of Sesame Protein and Sesame Lignans
3.1. Sesame Proteins
3.1.1. Functional Properties of Sesame Proteins
3.1.2. Biological Activities of Sesame Protein/Peptide
3.2. Sesame Lignans
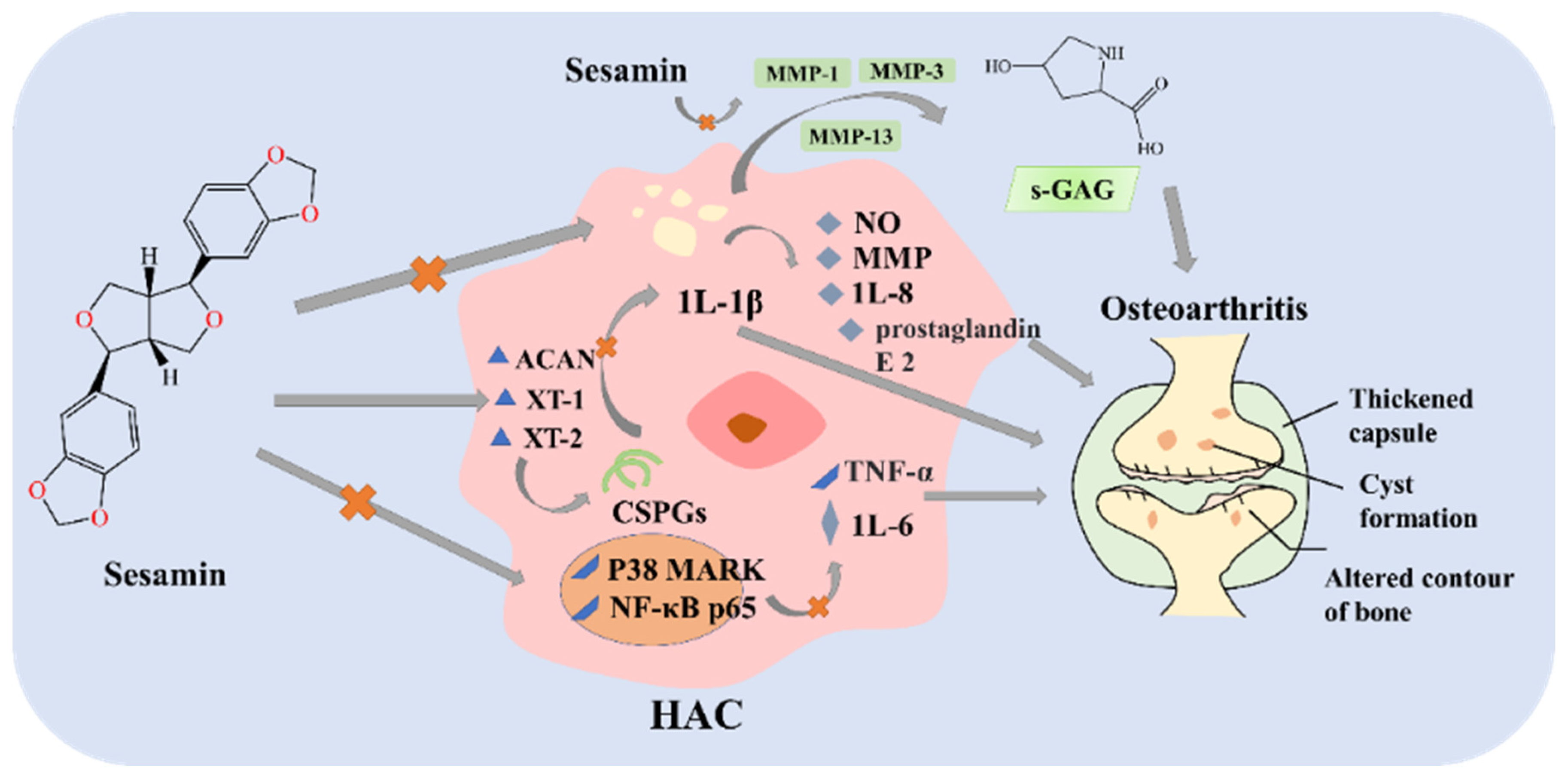
3.2.1. Biological Activities of Sesamin
3.2.2. Biological Activities of Sesamolin
3.2.3. Biological Activities of Sesamol
4. Comprehensive Utilizations of Sesame Protein and Sesame Lignans
4.1. Utilization of Sesame Protein
4.2. Sesame Lignans
5. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, L.K.; Liu, R.J.; Jin, Q.Z.; Wang, X.G. The Contents of Lignans in Sesame Seeds and Commercial Sesame Oils of China. J. Am. Oil Chem. Soc. 2017, 94, 1035–1044. [Google Scholar] [CrossRef]
- Budowski, P.; Markley, K.S. The Chemical And Physiological Properties Of Sesame Oil. Chem. Rev. 1951, 48, 125–151. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, Z.; Shi, L.; Li, Y. Bioactive beta-Carbolines Harman and Norharman in Sesame Seed Oils in China. Molecules 2022, 27, 402. [Google Scholar] [CrossRef]
- Chatterjee, R.; Dey, T.K.; Ghosh, M.; Dhar, P. Enzymatic modification of sesame seed protein, sourced from waste resource for nutraceutical application. Food Bioprod. Process. 2015, 94, 70–81. [Google Scholar] [CrossRef]
- Sharma, L.; Singh, C.; Sharma, H.K. Assessment of functionality of sesame meal and sesame protein isolate from Indian cultivar. J. Food Meas. Charact. 2016, 10, 520–526. [Google Scholar] [CrossRef]
- Aahin, S.; Elhussein, E.A.A. Assessment of sesame (Sesamum indicum L.) cake as a source of high-added value substances: From waste to health. Phytochem. Rev. 2018, 17, 691–700. [Google Scholar]
- Achouri, A.; Nail, V.; Boye, J.I. Sesame protein isolate: Fractionation, secondary structure and functional properties. Food Res. Int. 2012, 46, 360–369. [Google Scholar] [CrossRef]
- Onsaard, E. Sesame proteins. Int. Food Res. J. 2012, 19, 1287–1295. [Google Scholar]
- Li, J.; Xu, H.; Liu, J.J.; Li, B.; Gao, S.J. Optimization of extraction process of sesame protein using response surface methodology. China Brew. 2016, 35, 140–144. [Google Scholar]
- Gandhi, A.P.; Srivastava, J.J.A.F.J. Studies on the Production of Protein Isolates From Defatted Sesame Seed (Sesamum indicum) Flour and Their Nutritional Profile. ASEAN Food J. 2007, 14, 175–180. [Google Scholar]
- Yuan, D.Z.; Zhang, G.Z.; Huang, J.N. Study on preparation of sesame protein from subcritical sesame cake. J. Henan Univ. Technol. Nat. Sci. Ed. 2014, 35, 49–55. [Google Scholar]
- Lu, X.; Gao, J.H.; Zhang, G.H.; Song, G.H.; Sun, Q.; Huang, J.N. Preparation of sesame protein by combination of ultrasonic extraction and membrane separation. Food Sci. Technol. 2017, 42, 242–248. [Google Scholar]
- Zhu, X.; Dai, Q.; Jia, D.; Li, P.; Xia, N.; Hu, C.; Hu, L. Study on the ultrafiltration concentration of sesame protein extracting solution and its functional properties. Sci. Technol. Food Ind. 2015, 36, 244–249. [Google Scholar]
- Aondona, M.M.; Ikya, J.K.; Ukeyima, M.T.; Gborigo, T.-W.j.a.; Aluko, R.E.; Girgih, A.T. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. J. Food Biochem. 2021, 45, e13587. [Google Scholar] [CrossRef] [PubMed]
- Li, R.Y.; Zhang, J.N.; Huang, J.N.; Lu, X.; Zhang, W.X.; Yang, F. The preparation and structure property of sesame 11S protein. J. Henan Univ. Technol. Nat. Sci. Ed. 2017, 38, 6–11. [Google Scholar]
- Sun, L.-H.; Yu, F.; Wang, Y.-Y.; Lv, S.-W.; He, L.-Y. Effects of ultrasound extraction on the physicochemical and emulsifying properties of rice bran protein. Int. J. Food Eng. 2021, 17, 327–335. [Google Scholar] [CrossRef]
- Gorguc, A.; Bircan, C.; Yilmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Lin, H.; Deng, S.; Pang, J.; Chen, D.; Ma, P. Optimization of composite enzymatic hydrolysis technology for preparation of ferrous chelating polypeptide from hairtail protein. J. Chin. Inst. Food Sci. Technol. 2011, 11, 87–94. [Google Scholar]
- Guo, Y.Y.; Dou, L.X.; Hao, M.Y.; Dong, T.T.; Wang, C.; Sui, X. Effect of enzymatic efficiency of sesame protein by different process conditions. J. Food Saf. Qual. 2015, 6, 2109–2114. [Google Scholar]
- Pi, S.Y.; Wang, C.G.; Du, X.; Cai, J. Optimization of enzymatic extraction process and component analysis of protein from sesame meal. China Brew. 2020, 39, 202–207. [Google Scholar]
- Latif, S.; Anwar, F. Aqueous enzymatic sesame oil and protein extraction. Food Chem. 2011, 125, 679–684. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar] [CrossRef]
- Rosalina, R.; Weerapreeyakul, N. An Insight into Sesamolin: Physicochemical Properties, Pharmacological Activities, and Future Research Prospects. Molecules 2021, 26, 5849. [Google Scholar] [CrossRef] [PubMed]
- Majdalawieh, A.F.; Mansour, Z.R. Sesamol, a major lignan in sesame seeds (Sesamum indicum): Anti-cancer properties and mechanisms of action. Eur. J. Pharmacol. 2019, 855, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-C.; Feng, D.-W.; Zheng, G.-S. Extraction of sesamin from sesame oil using macroporous resin. J. Food Eng. 2010, 100, 289–293. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Chen, X.; Liu, Y.; Di, D. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem. 2010, 123, 1027–1034. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Ding, X.; Li, P.; Dai, X.; Chen, X.; Zhou, H.; Bai, Y.; Ding, J. Magnetic solid-phase extraction based on graphene oxide for the determination of lignans in sesame oil. Food Chem. 2017, 217, 320–325. [Google Scholar] [CrossRef]
- Dar, A.A.; Verma, N.K.; Arumugam, N. An updated method for isolation, purification and characterization of clinically important antioxidant lignans—Sesamin and sesamolin, from sesame oil. Ind. Crops Prod. 2015, 64, 201–208. [Google Scholar] [CrossRef]
- Buranachokpaisan, K.; Muangrat, R.; Chalermchat, Y. Preservation. Supercritical CO2 extraction of residual oil from pressed sesame seed cake: Optimization and its physicochemical properties. J. Food Process. Perservation 2021, 45, e15722. [Google Scholar]
- Schwertner, H.A.; Rios, D.C. Analysis of Sesamin, Asarinin, and Sesamolin by HPLC with Photodiode and Fluorescent Detection and by GC/MS: Application to Sesame Oil and Serum Samples. J. Am. Oil Chem. Soc. 2012, 89, 1943–1950. [Google Scholar] [CrossRef]
- Michailidis, D.; Angelis, A.; Aligiannis, N.; Mitakou, S.; Skaltsounis, L. Recovery of Sesamin, Sesamolin, and Minor Lignans From Sesame Oil Using Solid Support-Free Liquid-Liquid Extraction and Chromatography Techniques and Evaluation of Their Enzymatic Inhibition Properties. Front. Pharmacol. 2019, 10, 723. [Google Scholar] [CrossRef]
- Mikropoulou, E.V.; Petrakis, E.A.; Argyropoulou, A.; Mitakou, S.; Halabalaki, M.; Skaltsounis, L.A. Quantification of bioactive lignans in sesame seeds using HPTLC densitometry: Comparative evaluation by HPLC-PDA. Food Chem. 2019, 288, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Xia, Z.; Wu, Y.; Li, Y.; Gong, Z. Simultaneous and rapid determination of sesamin and sesamolin in sesame oils using excitation-emission matrix fluorescence coupled with self-weighted alternating trilinear decomposition. J. Sci. Food Agric. 2020, 100, 4418–4424. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizaki, Y.; Sugimoto, N.; Takeuchi, H.; Takeuchi, H.; Nakagawa, K.; Akiyama, H.; Sato, K.; Inoue, K. Determination and purification of sesamin and sesamolin in sesame seed oil unsaponified matter using reversed-phase liquid chromatography coupled with photodiode array and tandem mass spectrometry and high-speed countercurrent chromatography. J. Sep. Sci. 2016, 39, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Z.; Gu, H.Y.; Yang, F.; Zhang, L.; Yang, L. Improved method to obtain essential oil, asarinin and sesamin from Asarum heterotropoides var. mandshuricum using microwave-assisted steam distillation followed by solvent extraction and antifungal activity of essential oil against Fusarium spp. Ind. Crops Prod. 2021, 162, 113295. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, R. Determination of sesamol in sesame oil by anion exchange solid phase extraction coupled with HPLC. Anal. Methods 2014, 6, 6432–6436. [Google Scholar] [CrossRef]
- Aslisen, B.; Kocak, C.C.; Kocak, S. Electrochemical Determination of Sesamol in Foods by Square Wave Voltammetry at a Boron-Doped Diamond Electrode. Anal. Lett. 2020, 53, 343–354. [Google Scholar] [CrossRef]
- Zhou, S.; Zou, H.; Huang, G.; Chen, G. Preparations and antioxidant activities of sesamol and it’s derivatives. Bioorg. Med. Chem. Lett. 2021, 31, 127716. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Qin, Y.; Yu, J. A simple and green ultrasonic-assisted liquidliquid microextraction technique based on deep eutectic solvents for the HPLC analysis of sesamol in sesame oils. Anal. Methods 2017, 9, 4184–4189. [Google Scholar] [CrossRef]
- David, R.B.; Jose, M.F.; Luis, A.G.Z.; Maria Franco, J.; Alberto Garcia-Zapateiro, L. Rheological properties of oil-in-water emulsions prepared with oil and protein isolates from sesame (Sesamum indicum). Food Sci. Technol. 2016, 36, 64–69. [Google Scholar]
- Eladawy, T.A. Effect of sesame seed protein supplementation on the nutritional, physical, chemical and sensory properties of wheat flour bread. Food Chemistry. 1997, 59, 7–14. [Google Scholar] [CrossRef]
- Alicia, C.M.; Hugo, J.I.; Luc, D.; Rosalba, P.H.; Guillermo, G.A.; Eleazar, M.E.S. Emulsifying and foaming capacity and emulsion and foam stability of sesame protein concentrates. Food Res. Int. 2011, 44, 684–692. [Google Scholar]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Herceg, Z.; Herceg, I.L. Effect of ultrasound treatment on solubility and foaming properties of whey protein suspensions. J. Food Eng. 2008, 86, 281–287. [Google Scholar] [CrossRef]
- Badifu, G.I.O.; Akpagher, E.M. Effects of debittering methods on the proximate composition, organoleptic and functional properties of sesame (Sesamum indicum L) seed flour. Plant Foods Hum. Nutrition. 1996, 49, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Li, X.; Chang, X.; Gu, R.; Duan, X.; Liu, F.; Wang, Y. Impact of germination on structural, functional properties and in vitro protein digestibility of sesame (Sesamum indicum L.) protein. Lwt-Food Sci. Technol. 2022, 154, 112651. [Google Scholar] [CrossRef]
- Kajihausa, O.E.; Fasasi, R.A.; Atolagbe, Y.M. Effect of different soaking time and boiling on the proximate composition and functional properties of sprouted sesame seed flour. Niger. Food J. 2014, 32, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Amita, M.; Seema, B.; Saroj, D. Traditional processing treatments as a promising approach to enhance the functional properties of rapeseed (Brassica campestris var. toria) and sesame seed (Sesamum indicum) meals. J. Agric. Food Chem. 1999, 47, 3093–3098. [Google Scholar]
- Davis, C.K.; Vemuganti, R. Antioxidant therapies in traumatic brain injury. Neurochem. Int. 2022, 152. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Elena Diaz-Rubio, M.; Saura-Calixto, F. Contribution of Macromolecular Antioxidants to Dietary Antioxidant Capacity: A Study in the Spanish Mediterranean Diet. Plant Foods Hum. Nutr. 2015, 70, 365–370. [Google Scholar] [CrossRef]
- Esfandi, R.; Walters, M.E.; Tsopmo, A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon 2019, 5, e01538. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, W.D.; Lee, S.K.; Lee, Y.B.; Park, C.H.; Ryu, H.W.; Lee, J.H. Comparative assessment of compositional components, antioxidant effects, and lignan extractions from Korean white and black sesame (Sesamum indicum L.) seeds for different crop years. J. Funct. Foods 2014, 7, 495–505. [Google Scholar] [CrossRef]
- Mousavi Nasab, A.; Sadeghi Mahoonak, A.; Mohammad, G.; Mehran, A.; Nasim, M. The optimization of hydrolyzed protein production with high anti-oxidation ability from sesame meal by response surface methodology. J. Res. Innov. Food Sci. Technol. 2019, 8, 45–52. [Google Scholar]
- Laohakunjit, N.; Kerdchoechuen, O.; Kaprasob, R.; Matta, F.B. Volatile Flavor, Antioxidant Activity And Physicochemical Properties Of Enzymatic Defatted Sesame Hydrolysate. J. Food Process. Preserv. 2017, 41, e13075. [Google Scholar] [CrossRef]
- Lu, X.; Hu, D.; Jia, C.; Gao, J.; Sun, Q.; Huang, J. Antioxidant analysis of peptides produced by in vitro simulated digestion of sesame protein. China Oils Fats 2020, 45, 63–66, 81. [Google Scholar]
- Qian, S.; Ge, Y.; Zhao, S.; Wei, M.; Yang, C.; Xue, Z. Preparation of sesame peptides from sesame meal by ultrasound pretreatment. China Oils Fats 2016, 41, 105–108. [Google Scholar]
- Jia, C.; Meng, X.; You, J.; Sun, Q.; Lu, X.; Gao, J.; Huang, J. Preparation, amino acid composition and structure analysis of low bitterness sesame ACE inhibitory peptides. Food Ferment. Ind. 2021, 47, 172–178. [Google Scholar]
- Liu, W.; Cheng, G.; Liu, H.; Kong, Y. Purification and Identification of a Novel Angiotensin I-Converting Enzyme Inhibitory Peptide from Sesame Meal. Int. J. Pept. Res. Ther. 2015, 21, 433–442. [Google Scholar] [CrossRef]
- Han, R.; Alvarez, A.J.H.; Maycock, J.; Murray, B.S.; Boesch, C. Comparison of alcalase- and pepsin-treated oilseed protein hydrolysates—Experimental validation of predicted antioxidant, antihypertensive and antidiabetic properties. Curr. Res. Food Sci. 2021, 4, 141–149. [Google Scholar] [CrossRef]
- Maria-Neto, S.; Honorato, R.V.; Costa, F.T.; Almeida, R.G.; Amaro, D.S.; Oliveira, J.T.A.; Vasconcelos, I.M.; Franco, O.L. Bactericidal Activity Identified in 2S Albumin from Sesame Seeds and In silico Studies of Structure-Function Relations. Protein J. 2011, 30, 340–350. [Google Scholar] [CrossRef]
- Duan, X.; Leng, Y.; Chen, F.; Zhang, M.; Li, Z. Evaluation of oilseed proteins as precursors of antimicrobial peptides using bioinformatics method. Amino Acids 2023, 55, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.T.; Neto, S.M.; Bloch, C., Jr.; Franco, O.L. Susceptibility of human pathogenic bacteria to antimicrobial peptides from sesame kernels. Curr. Microbiol. 2007, 55, 162–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, Y.Y.; Meng, J.; Wang, Y.Y.; Zhang, S.B. Preparation of antimicrobial peptides from sesame by enzymatic hydrolysis and study on antimicrobial activity. Food Sci. Technol. 2020, 45, 8–14. [Google Scholar]
- Anand, T.D.; Pothiraj, C.; Gopinath, R.M.; Kayalvizhi, B. Effect of oil-pulling on dental caries causing bacteria. Afr. J. Microbiol. Res. 2008, 2, 63–66. [Google Scholar]
- Sallam, K.I.; Abd-Elghany, S.M.; Imre, K.; Morar, A.; Herman, V.; Hussein, M.A.; Mahros, M.A. Ensuring safety and improving keeping quality of meatballs by addition of sesame oil and sesamol as natural antimicrobial and antioxidant agents. Food Microbiol. 2021, 99, 103834. [Google Scholar] [CrossRef]
- Srisuthtayanont, W.; Pruksakorn, D.; Kongtawelert, P.; Pothacharoen, P. Effects of sesamin on chondroitin sulfate proteoglycan synthesis induced by interleukin-1beta in human chondrocytes. BMC Complement. Altern. Med. 2017, 17, 286. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yan, G.; Qin, X.; Li, L. Sesamin attenuates mast cell-mediated inflammatory cytokines release through suppressing p38 and NF-kappaB activation. Immunol. J. 2014, 30, 1073–1076. [Google Scholar]
- Helli, B.; Shahi, M.M.; Mowla, K.; Jalali, M.T.; Haghighian, H.K. A randomized, triple-blind, placebo-controlled clinical trial, evaluating the sesamin supplement effects on proteolytic enzymes, inflammatory markers, and clinical indices in women with rheumatoid arthritis. Phytother. Res. 2019, 33, 2421–2428. [Google Scholar] [CrossRef]
- Phitak, T.; Pothacharoen, P.; Settakorn, J.; Poompimol, W.; Caterson, B.; Kongtawelert, P. Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry 2012, 80, 77–88. [Google Scholar] [CrossRef]
- Fan, D.; Yang, Z.; Liu, F.-Y.; Jin, Y.-G.; Zhang, N.; Ni, J.; Yuan, Y.; Liao, H.-H.; Wu, Q.-Q.; Xu, M.; et al. Sesamin Protects Against Cardiac Remodeling Via Sirt3/ROS Pathway. Cell. Physiol. Biochem. 2017, 44, 2212–2227. [Google Scholar] [CrossRef]
- Wanachewin, O.; Pothacharoen, P.; Kongtawelert, P.; Phitak, T. Inhibitory effects of sesamin on human osteoclastogenesis. Arch. Pharmacal Res. 2017, 40, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Yamaori, S.; Kamijo, S.; Aikawa, K.; Ohmori, S. In Vitro Inhibitory Effects of Sesamin on CYP4F2 Activity. Biol. Pharm. Bull. 2020, 43, 688–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, T.; Iwase, H.; Amano, S.; Sunahara, S.; Tachihara, A.; Yagi, M.; Watanabe, T. Physiological effects of gamma-linolenic acid and sesamin on hepatic fatty acid synthesis and oxidation. J. Nutr. Biochem. 2017, 41, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Anilakumar, K.R.; Pal, A.; Khanum, F.; Bawa, A.S. Nutritional, Medicinal and Industrial Uses of Sesame (Sesamum indicum L.) Seeds—An Overview. Agric. Conspec. Sci. 2010, 75, 159–168. [Google Scholar]
- Ide, T. Combined effect of sesamin and soybean phospholipid on hepatic fatty acid metabolism in rats. J. Clin. Biochem. Nutr. 2014, 54, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Rogi, T.; Tomimori, N.; Ono, Y.; Kiso, Y. The Mechanism Underlying the Synergetic Hypocholesterolemic Effect of Sesamin and alpha-Tocopherol in Rats Fed a High-Cholesterol Diet. J. Pharmacol. Sci. 2011, 115, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Zhu, H.; Zhang, C.; Wang, X.; Ma, K.Y.; Wang, L.; Zhao, Y.; Chen, Z.-Y. Dietary beta-sitosterol is more potent in reducing plasma cholesterol than sesamin in hypercholesterolemia hamsters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600349. [Google Scholar] [CrossRef]
- Ye, S.; Wang, W.; Chen, X.; Deng, Y. Sesamin promotes angiogenesis and accelerates wound healing in rats via alleviates TBHP-induced apoptosis in human umbilical vein endothelial cells. Biosci. Biotechnol. Biochem. 2020, 84, 887–897. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liu, K.-J.; Wu, Y.-C.; Lin, S.-J.; Chang, C.-C.; Huang, T.-S. Sesamin Inhibits Macrophage-Induced Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9 Expression and Proangiogenic Activity in Breast Cancer Cells. Inflammation 2011, 34, 209–221. [Google Scholar] [CrossRef]
- Hsieh, C.-C.; Kuo, C.-H.; Kuo, H.-F.; Chen, Y.-S.; Wang, S.-L.; Chao, D.; Lee, M.-S.; Hung, C.-H. Sesamin suppresses macrophage-derived chemokine expression in human monocytes via epigenetic regulation. Food Funct. 2014, 5, 2494–2500. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, M.; Zhao, Y.; Hui, Y.; Pan, J.; Yu, H.; Yan, S.; Dai, X.; Liu, X.; Liu, Z. Supplementation of Sesamin Alleviates Stress-Induced Behavioral and Psychological Disorders via Reshaping the Gut Microbiota Structure. J. Agric. Food Chem. 2019, 67, 12441–12451. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, L.; Huang, J.; Song, G.; Ai, Z. Research on Antioxidative Activity of Sesamolin in Soybean Oil at High Temperature. J. Chin. Cereals Oils Assoc. 2016, 31, 112–116, 22. [Google Scholar]
- Li, X.; Wang, X.; Wang, N.; Ma, Y. Acidic Catalysis and Antioxidant Activity of Sesamolin. Food Sci. 2018, 39, 59–64. [Google Scholar]
- Wang, M.; Zhang, L.; Huang, J.; Song, G.; Ai, Z. Antioxidative activity and mechanism of sesamolin in soybean oil at high temperature. Sci. Technol. Food Ind. 2015, 36, 76–80. [Google Scholar]
- Lee, J.K. Sesamolin promotes cytolysis and migration activity of natural killer cells via dendritic cells. Arch. Pharmacal Res. 2020, 43, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.K. Sesamolin enhances NK cell lysis activity by increasing the expression of NKG2D ligands on Burkitt’s lymphoma cells. Int. Immunopharmacol. 2015, 28, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, X.-P.; Zhang, W. Sesamolin exerts anti-proliferative and apoptotic effect on human colorectal cancer cells via inhibition of JAK2/STAT3 signaling pathway. Cell. Mol. Biol. 2019, 65, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Kang, M.-G.; Park, D. Inhibitory Effect of Sesamolin on Melanogenesis in B16F10 Cells Determined by In Vitro and Molecular Docking Analyses. Curr. Pharm. Biotechnol. 2020, 21, 169–178. [Google Scholar] [CrossRef]
- Kitipaspallop, W.; Sillapaprayoon, S.; Phuwapraisirisan, P.; Kim, W.-K.; Chanchao, C.; Pimtong, W. Developmental effects of sesamolin on zebrafish (Danio rerio) embryos. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2022, 256, 109319. [Google Scholar] [CrossRef]
- Keowkase, R.; Shoomarom, N.; Bunargin, W.; Sitthithaworn, W.; Weerapreeyakul, N. Sesamin and sesamolin reduce amyloid-beta toxicity in a transgenic Caenorhabditis elegans. Biomed. Pharmacother. 2018, 107, 656–664. [Google Scholar] [CrossRef]
- Meysam, N. Antioxidant Activity of Sesamol Derivatives and Their Drug Delivery via C60 Nanocage: A Theoretical Study. Chin. J. Struct. Chem. 2019, 38, 195–200. [Google Scholar]
- Srisayam, M.; Weerapreeyakul, N.; Barusrux, S.; Kanokmedhakul, K. Antioxidant, antimelanogenic, and skin-protective effect of sesamol. J. Cosmet. Sci. 2014, 65, 69–79. [Google Scholar] [PubMed]
- You, Y.-J.; Wu, P.-Y.; Liu, Y.-J.; Hou, C.-W.; Wu, C.-S.; Wen, K.-C.; Lin, C.-Y.; Chiang, H.-M. Sesamol Inhibited Ultraviolet Radiation-Induced Hyperpigmentation and Damage in C57BL/6 Mouse Skin. Antioxidants 2019, 8, 207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Chang, S.-H.; Yang, D.K.; Song, N.-J.; Yun, U.J.; Park, K.W. Sesamol Increases Ucp1 Expression in White Adipose Tissues and Stimulates Energy Expenditure in High-Fat Diet-Fed Obese Mice. Nutrients 2020, 12, 1459. [Google Scholar] [CrossRef]
- Yuan, T.; Chu, C.; Shi, R.; Cui, T.; Zhang, X.; Zhao, Y.; Shi, X.; Hui, Y.; Pan, J.; Qian, R.; et al. ApoE-Dependent Protective Effects of Sesamol on High-Fat Diet-Induced Behavioral Disorders: Regulation of the Microbiome-Gut-Brain Axis. J. Agric. Food Chem. 2019, 67, 6190–6201. [Google Scholar] [CrossRef]
- Liu, X.B.; Liu, X.N.; Yuan, T.; Liu, Z.G. Neuroprotective effects of sesamol, potential mechanisms of action. J. Chin. Inst. Food Sci. Technol. 2017, 17, 1–7. [Google Scholar]
- Liou, C.-J.; Chen, Y.-L.; Yu, M.-C.; Yeh, K.-W.; Shen, S.-C.; Huang, W.-C. Sesamol Alleviates Airway Hyperresponsiveness and Oxidative Stress in Asthmatic Mice. Antioxidants 2020, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Kaya, H.; Pekel, G.; Yorukoglu, A.; Hiraali, M.C.; Sahin, B. Is Sesamol Effective in Corneal Neovascularization? Eye Contact Lens-Sci. Clin. Pract. 2018, 44, S414–S419. [Google Scholar] [CrossRef]
- Yashaswini, P.S.; Rao, A.G.A.; Singh, S.A. Inhibition of lipoxygenase by sesamol corroborates its potential anti-inflammatory activity. Int. J. Biol. Macromol. 2017, 94, 781–787. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, B.; Li, X.; Zhang, L.; Liu, X.; Shi, R.; Kou, R.; Liu, Z.; Liu, X. Sesamol Supplementation Attenuates DSS-Induced Colitis via Mediating Gut Barrier Integrity, Inflammatory Responses, and Reshaping Gut Microbiome. J. Agric. Food Chem. 2020, 68, 10697–10708. [Google Scholar] [CrossRef]
- Prakash, K.; Naik, S.N.; Vadivel, D.; Hariprasad, P.; Gandhi, D.; Saravanadevi, S. Utilization of defatted sesame cake in enhancing the nutritional and functional characteristics of biscuits. J. Food Process. Preserv. 2018, 42, e13751. [Google Scholar] [CrossRef]
- Lou, L.; Zhang, S. Tween -assisted simultaneous aqueous extraction of sesame oil and sesame protein. China Oils Fats 2018, 43, 5–8, 21. [Google Scholar]
- Olude, O.; George, F.; Alegbeleye, W. Utilization of autoclaved and fermented sesame (Sesamum indicum L.) seed meal in diets for Til-aqua natural male tilapia. Anim. Nutr. 2016, 2, 339–344. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.; Ray, A.K. Effect of fermentation on the nutritive value of sesame seed meal in the diets for rohu, Labeo rohita (Hamilton), fingerlings. Aquac. Nutr. 1999, 5, 229–236. [Google Scholar] [CrossRef]
- Sereewatthanawut, I.; Prapintip, S.; Watchiraruji, K.; Goto, M.; Sasaki, M.; Shotipruk, A. Extraction of protein and amino acids from deoiled rice bran by subcritical water hydrolysis. Bioresour. Technol. 2008, 99, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Choommongkol, V.; Ruangsuriya, J.; Suttiarporn, P.; Punyodom, W.; Thapsukhon, B. Polyester-releasing sesamin by electrospinning technique for the application of bone tissue engineering. Des. Monomers Polym. 2022, 25, 231–244. [Google Scholar] [CrossRef]
- Ren, X.; Zeng, S.; Xie, X.; Zhu, K.; Chen, X. Study on the Metabolism of Sesamin to Ethanol and the Preparation of Sesamin Microcapsules. In Proceedings of the International Conference on Advances in Civil Engineering, Energy Resources and Environment Engineering (ACCESE), Jilin Jianzhu University, College Civil Engineering, Changchun, China, 28–30 June 2019. [Google Scholar]
- Higashino, H.; Minami, K.; Kataoka, M.; Tomimori, N.; Rogi, T.; Shibata, H.; Yamashita, S. Control of oral absorption of nutritional supplement using lipid-based formulations (LBFs): Application to the poorly water-soluble ingredient. J. Drug Deliv. Sci. Technol. 2020, 57, 101675. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, X.-D.; Liu, H.-M.; Ma, Y.-X. Preparation and Characterization of Solid Acid Catalysts for the Conversion of Sesamin into Asarinin in Sesame Oil. Foods 2022, 11, 1225. [Google Scholar] [CrossRef]
- Rana, J.; Diwakar, G.; Evenocheck, H.; Schneider, L.M.; Shenoy, D.; Rebhun, J.; Roloff, S.; Scholten, J.; Rothouse, J.N. Compositions including sesamin, methods of making and using the same in skin anti-aging and skin lightening applications. U.S. Patent 10383806, 2019. Access Business Group International Llc. [Google Scholar]
- Sun, Y.; Ren, J.; Zhu, S.; Zhang, Z.; Guo, Z.; An, J.; Yin, B.; Ma, Y. The Effects of Sesamin Supplementation on Obesity, Blood Pressure, and Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 842152. [Google Scholar] [CrossRef]
- Vasanthi, E.A.P.; Rajavel, D.S. Effect of sesamin on termites, Odontotermes wallonensis (Wasmann) in groundnut. J. Entomol. Res. 2016, 40, 17–20. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Song, G.; Wang, M.; Sun, Q.; Huang, J. Effect of Sesamolin on the Quality of Soybean Oil during the Frying Process. Food Sci. 2018, 39, 78–83. [Google Scholar]
- Huang, J.; Song, G.; Zhang, L.; Sun, Q.; Lu, X. A novel conversion of sesamolin to sesaminol by acidic cation exchange resin. Eur. J. Lipid Sci. Technol. 2012, 114, 842–848. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, W.; Zhang, F.; Li, J.; Yang, H.; Bi, Y. Effects of free fatty acids and peroxide on thermal loss of sesamol and formation of its transformation products in soybean oil. Lwt-Food Sci. Technol. 2022, 159, 113236. [Google Scholar] [CrossRef]
- Han, P.; Zhong, Y.; An, N.; Lu, S.; Wang, Q.; Dong, J. Preparation, characterization, and molecular modeling of sesamol/beta-cyclodextrin derivatives inclusion complexes. J. Mol. Liq. 2021, 339, 116790. [Google Scholar] [CrossRef]
- Hwang, H.-S.; Winkler-Moser, J.K.; Vermillion, K.; Liu, S.X. Enhancing Antioxidant Activity of Sesamol at Frying Temperature by Addition of Additives through Reducing Volatility. J. Food Sci. 2014, 79, C2164–C2173. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Jo, S.; Kim, M.-J.; Park, S.; Lee, S.; Lee, J.; Lee, J. Addition of Sesamol Increases the Oxidative Stability of Beeswax Organogels and Beef Tallow Matrix Under UV Light Irradiation and Thermal Oxidation. J. Food Sci. 2019, 84, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, J.; Wang, S.; Cheng, W.; Ma, L.; Liu, G.; Han, D.; Niu, L. Sesamol can inhibit the formation of glycidyl ester in deep frying palm oil. J. Food Process. Preserv. 2022, 46, e16236. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qiao, Q.; Zhao, T.; Zhang, W.; Ren, B.; Liu, Q.; Liu, X. Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system. Food Funct. 2017, 8, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.H.; Bi, Y.L.; Wang, X.D.; Yang, G.L. Research status of the processing technology and application of sesame in China: A review. China Oils Fats 2010, 35, 4–8. Available online: http://www.fao.org/faostat/en/#data/QC/ (accessed on 9 August 2021).
| Source of Peptide | Detection Technology | Preparation | Activity | References |
|---|---|---|---|---|
| Defatted sesame meal | Bromelain flavourzyme | Hydroxyl and DPPH radical-scavenging activity | [54] | |
| Sesame protein | Ultrafiltration and preparative HPLC | Dual-enzyme system comprised alcalase and trypsin | Nano liquid chromatography electrospray ionization–tandem mass spectrometry (Nano-LC-ESI-MS/MS)-CoMFA; DPPH and ABTS radical-scavenging activity; IC50 | [51] |
| Sesame seed meal | Alkalase enzyme | DPPH free radical scavenging activity | [53] | |
| Defatted sesame meal | Membrane ultrafiltration | Consecutive additions of pepsin and pancreatin | Radical scavenging and metal ion chelation | [14] |
| Sesame bran | Standard alkaline method | Viscozyme L, alcalase, ultrasound and ultrasound-assisted enzymatic extractions | DPPH and ABTS radical-scavenging activity | [17] |
| Defatted sesame meal | Pepsin, trypsin, chymotrypsin | PPH, ABTS and FRAP free radical | [55] | |
| Sesame 11S protein | Alcalase and tripsin are mixed in an enzyme activity ratio of 1:1 | DPPH and ABTS free radical scavenging rate | [15] | |
| Sesame seed meal | Ultrasound-assisted technology | Broken; centrifugal | DPPH free radical and hydroxyl free radical | [56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Y.; Zhou, Q.; Zhao, M.; Hou, T. Byproducts of Sesame Oil Extraction: Composition, Function, and Comprehensive Utilization. Foods 2023, 12, 2383. https://doi.org/10.3390/foods12122383
Wan Y, Zhou Q, Zhao M, Hou T. Byproducts of Sesame Oil Extraction: Composition, Function, and Comprehensive Utilization. Foods. 2023; 12(12):2383. https://doi.org/10.3390/foods12122383
Chicago/Turabian StyleWan, Yuan, Qiaoyun Zhou, Mengge Zhao, and Tao Hou. 2023. "Byproducts of Sesame Oil Extraction: Composition, Function, and Comprehensive Utilization" Foods 12, no. 12: 2383. https://doi.org/10.3390/foods12122383
APA StyleWan, Y., Zhou, Q., Zhao, M., & Hou, T. (2023). Byproducts of Sesame Oil Extraction: Composition, Function, and Comprehensive Utilization. Foods, 12(12), 2383. https://doi.org/10.3390/foods12122383





