Quantitative Detection of Viable but Nonculturable Vibrio parahaemolyticus in Frozen Bivalve Molluscs
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Detection and Enumeration of V. parahaemolyticus via Standard Culture Methods
2.3. PMA Assay and Genomic DNA Extraction
2.4. Real-Time qPCR Analysis
2.5. Bacterial Strain and Growth Conditions
2.6. Preparation of Heat-Killed Cell Suspension
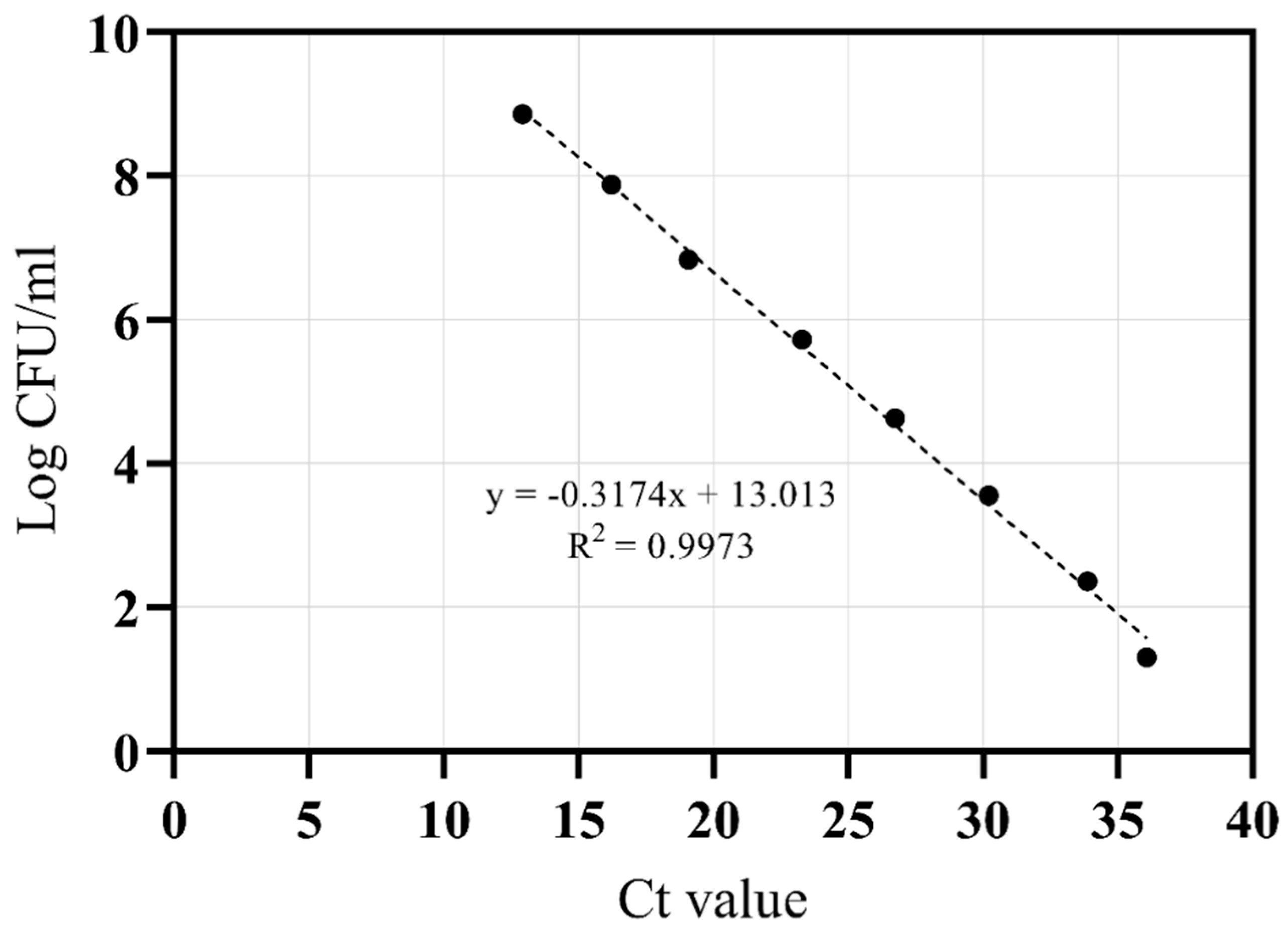
2.7. Standard Curve
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ansaruzzaman, M.; Lucas, M.; Deen, J.L.; Bhuiyan, N.A.; Wang, X.Y.; Safa, A.; Sultana, M.; Chowdhury, A.; Balakrish Nair, G.; Sack, D.A.; et al. Pandemic Serovars (O3:K6 and O4:K68) of Vibrio parahaemolyticus Associated with Diarrhea in Mozambique: Spread of the Pandemic into the African Continent. J. Clin. Microbiol. 2005, 43, 2559–2562. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Liu, C. Vibrio parahaemolyticus: A Concern of Seafood Safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Balter, S.; Hanson, H.; Kornstein, L.; Lee, L.; Reddy, V.; Sahl, S. Vibrio parahaemolyticus Infections Associated with Consumption of Raw Shellfish—Three States, 2006. Morb. Mortal. Wkly. Rep. 2006, 55, 854–857. [Google Scholar]
- Sferlazzo, G.; Meloni, D.; Lamon, S.; Marceddu, M.; Mureddu, A.; Consolati, S.G.; Pisanu, M.; Virgilio, S. Evaluation of Short Purification Cycles in Naturally Contaminated Mediterranean Mussels (Mytilus galloprovincialis) Harvested in Sardinia (Italy). Food Microbiol. 2018, 74, 86–91. [Google Scholar] [CrossRef]
- Murphree, R.L.; Tamplin, M.L. Uptake and Retention of Vibrio Cholerae O1 in the Eastern Oyster, Crassostrea Virginica. Appl. Environ. Microbiol. 1991, 61, 3656–3660. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Bae, Y.M.; Lee, S.Y. Effects of Varying Concentrations of Sodium Chloride and Acidic Conditions on the Behavior of Vibrio parahaemolyticus and Vibrio Vulnificus Cold-Starved in Artificial Sea Water Microcosms. Food Sci. Biotechnol. 2017, 26, 829–839. [Google Scholar] [CrossRef]
- Jiang, Y.; Chu, Y.; Xie, G.; Li, F.; Wang, L.; Huang, J.; Zhai, Y.; Yao, L. Antimicrobial Resistance, Virulence and Genetic Relationship of Vibrio parahaemolyticus in Seafood from Coasts of Bohai Sea and Yellow Sea, China. Int. J. Food Microbiol. 2019, 290, 116–124. [Google Scholar] [CrossRef]
- Daniels, N.A.; Mackinnon, L.; Bishop, R.; Altekruse, S.; Ray, B.; Hammond, R.M.; Thompson, S.; Wilson, S.; Bean, N.H.; Griffin, P.M.; et al. Vibrio parahaemolyticus Infections in the United States, 1973–1998. J. Infect. Dis. 2000, 181, 1661–1666. [Google Scholar] [CrossRef]
- Feldhusen, F. The role of seafood in bacterialfoodborne diseases. Microbes Infect. 2000, 2, 1651–1660. [Google Scholar] [CrossRef]
- Lozano-León, A.; Torres, J.; Osorio, C.R.; Martínez-Urtaza, J. Identification of Tdh-Positive Vibrio parahaemolyticus from an Outbreak Associated with Raw Oyster Consumption in Spain. FEMS Microbiol. Lett. 2003, 226, 281–284. [Google Scholar] [CrossRef]
- Martinez-Urtaza, J.; Lozano-Leon, A.; DePaola, A.; Ishibashi, M.; Shimada, K.; Nishibuchi, M.; Liebana, E. Characterization of Pathogenic Vibrio parahaemolyticus Isolates from Clinical Sources in Spain and Comparison with Asian and North American Pandemic Isolates. J. Clin. Microbiol. 2004, 42, 4672–4678. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urtaza, J.; Powell, A.; Jansa, J.; Rey, J.L.C.; Montero, O.P.; Campello, M.G.; López, M.J.Z.; Pousa, A.; Valles, M.J.F.; Trinanes, J.; et al. Epidemiological Investigation of a Foodborne Outbreak in Spain Associated with U.S. West Coast Genotypes of Vibrio parahaemolyticus. Springerplus 2016, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Stockley, L.; Rangdale, R.; Martinez-Urtaza, J. Environmental Occurrence and Clinical Impact of Vibrio vulnificus and Vibrio parahaemolyticus: A European Perspective. Environ. Microbiol. Rep. 2010, 2, 7–18. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- European Commission (EC). Commission Regulation (EC) No. 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission (EC): Brussels, Belgium, 2005. [Google Scholar]
- Savichtcheva, O.; Okabe, S. Alternative Indicators of Fecal Pollution: Relations with Pathogens and Conventional Indicators, Current Methodologies for Direct Pathogen Monitoring and Future Application Perspectives. Water Res. 2006, 40, 2463–2476. [Google Scholar] [CrossRef]
- Suffredini, E.; Mioni, R.; Mazzette, R.; Bordin, P.; Serratore, P.; Fois, F.; Piano, A.; Cozzi, L.; Croci, L. Detection and Quantification of Vibrio parahaemolyticus in Shellfish from Italian Production Areas. Int. J. Food. Microbiol. 2014, 184, 14–20. [Google Scholar] [CrossRef]
- Bonnin-Jusserand, M.; Copin, S.; Bris, C.L.; Brauge, T.; Gay, M.; Brisabois, A.; Grard, T.; Midelet-Bourdin, G. Vibrio Species Involved in Seafood-Borne Outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of Microbiological versus Recent Molecular Detection Methods in Seafood Products. Crit. Rev. Food Sci. Nutr. 2019, 59, 597–610. [Google Scholar] [CrossRef]
- Boutin, B.K.; Reyes, A.L.; Peeler, J.T.; Twedt, R.M. Effect of Temperature and Suspending Vehicle on Survival of Vibrio parahaemolyticus and Vibrio vulnificus. J. Food Prot. 1985, 48, 875–878. [Google Scholar] [CrossRef]
- Bryan, P.J.; Steffan, R.J.; Depaola, A.; Foster, J.W.; Bej, A.K. Adaptive Response to Cold Temperatures in Vibrio vulnificus. Curr. Microbiol. 1999, 38, 168–175. [Google Scholar] [CrossRef]
- Howard, C.; Johnson, J.L. Sensitivity of Vibrio parahaemolyticus to Cold in Oysters, Fish Fillets and Crabmeat. J. Food Sci. 1973, 38, 437–441. [Google Scholar] [CrossRef]
- Shen, X.; Cai, Y.; Liu, C.; Liu, W.; Hui, Y.; Su, Y.C. Effect of Temperature on Uptake and Survival of Vibrio parahaemolyticus in Oysters (Crassostrea plicatula). Int. J. Food Microbiol. 2009, 136, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Telli, A.E.; Doğruer, Y. Discrimination of Viable and Dead Vibrio parahaemolyticus Subjected to Low Temperatures Using Propidium Monoazide—Quantitative Loop Mediated Isothermal Amplification (PMA-QLAMP) and PMA-QPCR. Microb. Pathog. 2019, 132, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Marek, P.; Daigle, S.; Hoagland, T.; Venkitanarayanan’, K.S. Effect of Chilling and Freezing on Survival of Vibrio parahaemolyticus on Fish Fillets. J. Food Saf. 2002, 22, 209–217. [Google Scholar] [CrossRef]
- Wong, H.-C.; Chen, L.-L.; Yu, C.-M. Survival of psychrotrophic Vibrio mimicus, Vibrio fluvialis and Vibrio parahaemolyticus in culture broth at low temperatures. J. Food Prot. 1994, 57, 607–610. [Google Scholar] [CrossRef]
- Caburlotto, G.; Bianchi, F.; Gennari, M.; Ghidini, V.; Socal, G.; Aubry, F.B.; Bastianini, M.; Tafi, M.C.; Lleo, M.M. Integrated Evaluation of Environmental Parameters Influencing Vibrio Occurrence in the Coastal Northern Adriatic Sea (Italy) Facing the Venetian Lagoon. Microb. Ecol. 2012, 63, 20–31. [Google Scholar] [CrossRef]
- Tang, J.Y.H.; Wan-Rosli, W.F.; Abdul-Razak, N.H.; Yeo, C.C.; Bakar, C.A.; Son, R. Incidence and antibiogram of Vibrio parahaemolyticus in processed and frozen bivalve mollusks in Kuala Terengganu, Malaysia. Int. Food Res. J. 2014, 21, 1349. [Google Scholar]
- Panebianco, A.; Ruolo, A.; Giarratana, F.; Ziino, G. Occurrence of halophilic vibrions in frozen bivalve molluscs from retail outlets. LXV Annu. Meet. Ital. Soc. Vet. Sci. 2011, 93, 341–343. [Google Scholar]
- Xu, X.; Wu, Q.; Zhang, J.; Cheng, J.; Zhang, S.; Wu, K. Prevalence, Pathogenicity, and Serotypes of Vibrio parahaemolyticus in Shrimp from Chinese Retail Markets. Food Control 2014, 46, 81–85. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Jiao, X.A.; Zhou, X.H.; Cao, G.X.; Fang, W.M.; Gu, R.X. Isolation and Molecular Characterization of Vibrio parahaemolyticus from Fresh, Low-Temperature Preserved, Dried, and Salted Seafood Products in Two Coastal Areas of Eastern China. Int. J. Food Microbiol. 2008, 125, 279–285. [Google Scholar] [CrossRef]
- Singleton, F.L.; Attwell, R.; Jangi, S.; Colwell, R.R. Effects of Temperature and Salinity on Vibrio Cholerae Growth. Appl. Environ. Microbiol. 1982, 44, 1047–1058. [Google Scholar] [CrossRef]
- Huq, A.; West’, P.A.; Small, E.B.; Imdadul Huq, M.; Colwell’, R.R. Influence of Water Temperature, Salinity, and PH on Survival and Growth of Toxigenic Vibrio Cholerae Serovar 01 Associated with Live Copepods in Laboratory Microcosms. Appl. Environ. Microbiol. 1984, 48, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Tamplin, M.L.; Colwell, R.R. Effects of Microcosm Salinity and Organic Substrate Concentration on Production of Vibrio cholerae Enterotoxin. Appl. Environ. Microbiol. 1986, 52, 297–301. [Google Scholar] [CrossRef]
- Amako, K.; Shimodori, S.; Imoto, T.; Miake, S.; Umeda’, A. Effects of Chitin and Its Soluble Derivatives on Survival of Vibrio cholerae 01 at Low Temperature. Appl. Environ. Microbiol. 1987, 53, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Karunasagar, I.; Venugopal, M.N.; Karunasagar, I.; Segar, K. Role of Chitin in the Survival of Vibrio parahaemolyticus at Different Temperatures. Can. J. Microbiol. 1986, 32, 889–891. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Su, Y.-C. Effects of flash freezing, followed by frozen storage, on reducing Vibrio parahaemolyticus in Pacific raw oysters (Crassostrea gigas). J. Food Prot. 2009, 72, 174–177. [Google Scholar] [CrossRef]
- Yoon, J.H.; Moon, S.K.; Choi, C.; Ryu, B.Y.; Lee, S.Y. Detection of Viable but Nonculturable Vibrio parahaemolyticus Induced by Prolonged Cold-Starvation Using Propidium Monoazide Real-Time Polymerase Chain Reaction. Lett. Appl. Microbiol. 2019, 68, 537–545. [Google Scholar] [CrossRef]
- Wagley, S.; Morcrette, H.; Kovacs-Simon, A.; Yang, Z.R.; Power, A.; Tennant, R.K.; Love, J.; Murray, N.; Titball, R.W.; Butler, C.S. Bacterial Dormancy: A Subpopulation of Viable but Non-Culturable Cells Demonstrates Better Fitness for Revival. PLoS Pathog. 2021, 17, e1009194. [Google Scholar] [CrossRef]
- Oliver, J.D. The Viable but Nonculturable State in Bacteria. J. Microbiol. 2005, 43, 93–100. [Google Scholar] [PubMed]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.W.; Ding, T. Current Perspectives on Viable but Non-Culturable State in Foodborne Pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef]
- Dong, K.; Pan, H.; Yang, D.; Rao, L.; Zhao, L.; Wang, Y.; Liao, X. Induction, Detection, Formation, and Resuscitation of Viable but Non-Culturable State Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 149–183. [Google Scholar] [CrossRef]
- Cole, K.M.; Supan, J.; Ramirez, A.; Johnson, C.N. Suspension of Oysters Reduces the Populations of Vibrio parahaemolyticus and Vibrio vulnificus. Lett. Appl. Microbiol. 2015, 61, 209–213. [Google Scholar] [CrossRef]
- Ferro, S.; Amorico, T.; Deo, P. Role of Food Sanitising Treatments in Inducing the ‘Viable but Nonculturable’ State of Microorganisms. Food Control 2018, 91, 321–329. [Google Scholar] [CrossRef]
- Schottroff, F.; Fröhling, A.; Zunabovic-Pichler, M.; Krottenthaler, A.; Schlüter, O.; Jäger, H. Sublethal Injury and Viable but Non-Culturable (VBNC) State in Microorganisms during Preservation of Food and Biological Materials by Non-Thermal Processes. Front. Microbiol. 2018, 9, 2773. [Google Scholar] [CrossRef] [PubMed]
- Shamloei, S.; Nabavi-Rad, A.; Nazem, H.; Yadegar, A. Current Perspectives on Viable but Non-Culturable Bacteria in Food Safety and Public Health. Avicenna J. Clin. Microbiol. Infect. 2022, 9, 31–40. [Google Scholar] [CrossRef]
- Ayrapetyan, M.; Oliver, J.D. The Viable but Non-Culturable State and Its Relevance in Food Safety. Curr. Opin. Food Sci. 2016, 8, 127–133. [Google Scholar] [CrossRef]
- Gao, R.; Liao, X.; Zhao, X.; Liu, D.; Ding, T. The Diagnostic Tools for Viable but Nonculturable Pathogens in the Food Industry: Current Status and Future Prospects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2146–2175. [Google Scholar] [CrossRef] [PubMed]
- İzgördü, Ö.K.; Darcan, C.; Kariptaş, E. Overview of VBNC, a Survival Strategy for Microorganisms. 3 Biotech 2022, 12, 307. [Google Scholar] [CrossRef] [PubMed]
- Vattakaven, T.; Bond, P.; Bradley, G.; Munn, C.B. Differential Effects of Temperature and Starvation on Induction of the Viable-but-Nonculturable State in the Coral Pathogens Vibrio shiloi and Vibrio tasmaniensis. Appl. Environ. Microbiol. 2006, 72, 6508–6513. [Google Scholar] [CrossRef]
- Oliver, J.D. Recent Findings on the Viable but Nonculturable State in Pathogenic Bacteria. FEMS Microbiol. Rev. 2010, 34, 415–425. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The Importance of the Viable but Non-Culturable State in Human Bacterial Pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Yoon, J.H.; Bae, Y.M.; Jo, S.; Moon, S.K.; Oh, S.W.; Lee, S.Y. Optimization of Resuscitation-Promoting Broths for the Revival of Vibrio parahaemolyticus from a Viable but Nonculturable State. Food Sci. Biotechnol. 2021, 30, 159–169. [Google Scholar] [CrossRef]
- Baffone, W.; Citterio, B.; Vittoria, E.; Casaroli, A.; Campana, R.; Falzano, L.; Donelli, G. Retention of Virulence in Viable but Non-Culturable Halophilic Vibrio Spp. Int. J. Food Microbiol. 2003, 89, 31–39. [Google Scholar] [CrossRef]
- Zhang, X.H.; Ahmad, W.; Zhu, X.Y.; Chen, J.; Austin, B. Viable but Nonculturable Bacteria and Their Resuscitation: Implications for Cultivating Uncultured Marine Microorganisms. Mar. Life Sci. Technol. 2021, 3, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ren, Q. Wake Up! Resuscitation of Viable but Nonculturable Bacteria: Mechanism and Potential Application. Foods 2023, 12, 82. [Google Scholar] [CrossRef]
- Wu, B.; Liang, W.; Kan, B. Enumeration of Viable Non-Culturable Vibrio cholerae Using Propidium Monoazide Combined with Quantitative PCR. J. Microbiol. Methods 2015, 115, 147–152. [Google Scholar] [CrossRef]
- Wong, H.C.; Wang, P. Induction of Viable but Nonculturable State in Vibrio parahaemolyticus and Its Susceptibility to Environmental Stresses. J. Appl. Microbiol. 2004, 96, 359–366. [Google Scholar] [CrossRef]
- Nocker, A.; Cheung, C.Y.; Camper, A.K. Comparison of Propidium Monoazide with Ethidium Monoazide for Differentiation of Live vs. Dead Bacteria by Selective Removal of DNA from Dead Cells. J. Microbiol. Methods 2006, 67, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Sheet, O.H.; Grabowski, N.T.; Klein, G.; Abdulmawjood, A. Development and Validation of a Loop Mediated Isothermal Amplification (LAMP) Assay for the Detection of Staphylococcus aureus in Bovine Mastitis Milk Samples. Mol. Cell Probes 2016, 30, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zhang, Z.; Sun, X.; Pan, Y.; Zhao, Y. Development of a Quantitative Real-Time PCR Assay for Viable salmonella Spp. without Enrichment. Food Control 2015, 57, 185–189. [Google Scholar] [CrossRef]
- Yoon, J.H.; Wei, S.; Oh, D.H. A Highly Selective Enrichment Broth Combined with Real-Time PCR for Detection of Staphylococcus aureus in Food Samples. LWT 2018, 94, 103–110. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Zhang, J.; Chen, X.; Shi, L.; Fang, X.; Xie, H.; Chang, Y.; Wang, L. Detection of Viable but Nonculturable Vibrio parahaemolyticus in Shrimp Samples Using Improved Real-Time PCR and Real-Time LAMP Methods. Food Control 2019, 103, 145–152. [Google Scholar] [CrossRef]
- Ling, N.; Shen, J.; Guo, J.; Zeng, D.; Ren, J.; Sun, L.; Jiang, Y.; Xue, F.; Dai, J.; Li, B. Rapid and Accurate Detection of Viable Vibrio parahaemolyticus by Sodium Deoxycholate-Propidium Monoazide-QPCR in Shrimp. Food Control 2020, 109, 106883. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Q.; Wang, J.; Lei, S. Enumeration of Vibrio parahaemolyticus in VBNC State by PMA-Combined Real-Time Quantitative PCR Coupled with Confirmation of Respiratory Activity. Food Control 2018, 91, 85–91. [Google Scholar] [CrossRef]
- Zhong, Q.; Tian, J.; Wang, B.; Wang, L. PMA Based Real-Time Fluorescent LAMP for Detection of Vibrio parahaemolyticus in Viable but Nonculturable State. Food Control 2016, 63, 230–238. [Google Scholar] [CrossRef]
- Zhu, R.G.; Li, T.P.; Jia, Y.F.; Song, L.F. Quantitative Study of Viable Vibrio parahaemolyticus Cells in Raw Seafood Using Propidium Monoazide in Combination with Quantitative PCR. J. Microbiol. Methods 2012, 90, 262–266. [Google Scholar] [CrossRef]
- Zhao, L.; Lv, X.; Cao, X.; Zhang, J.; Gu, X.; Zeng, H.; Wang, L. Improved Quantitative Detection of VBNC Vibrio parahaemolyticus Using Immunomagnetic Separation and PMAxx-QPCR. Food Control 2020, 110, 106962. [Google Scholar] [CrossRef]
- Fittipaldi, M.; Nocker, A.; Codony, F. Progress in Understanding Preferential Detection of Live Cells Using Viability Dyes in Combination with DNA Amplification. J. Microbiol. Methods 2012, 91, 276–289. [Google Scholar] [CrossRef]
- Kobayashi, H.; Oethinger, M.; Tuohy, M.J.; Hall, G.S.; Bauer, T.W. Improving Clinical Significance of PCR: Use of Propidium Monoazide to Distinguish Viable from Dead Staphylococcus aureus and Staphylococcus epidermidis. J. Orthop. Res. 2009, 27, 1243–1247. [Google Scholar] [CrossRef]
- Yang, X.; Badoni, M.; Gill, C.O. Use of Propidium Monoazide and Quantitative PCR for Differentiation of Viable Escherichia Coli from E. Coli Killed by Mild or Pasteurizing Heat Treatments. Food Microbiol. 2011, 28, 1478–1482. [Google Scholar] [CrossRef]
- Lee, J.L.; Levin, R.E. A Comparative Study of the Ability of EMA and PMA to Distinguish Viable from Heat Killed Mixed Bacterial Flora from Fish Fillets. J. Microbiol. Methods 2009, 76, 93–96. [Google Scholar] [CrossRef]
- Dinu, L.D.; Bach, S. Detection of Viable but Non-Culturable Escherichia coli O157: H7 from Vegetable Samples Using Quantitative PCR with Propidium Monoazide and Immunological Assays. Food Control 2013, 31, 268–273. [Google Scholar] [CrossRef]
- Xiao, X.; Tian, C.; Yu, Y.; Wu, H. Detection of Viable but Nonculturable Escherichia coli O157:H7 Using Propidium Monoazide Treatments and QPCR. Can. J. Microbiol. 2013, 59, 157–163. [Google Scholar] [CrossRef]
- Lv, X.; Wang, L.; Zhang, J.; Zeng, H.; Chen, X.; Shi, L.; Cui, H.; He, X.; Zhao, L. Rapid and Sensitive Detection of VBNC Escherichia coli O157: H7 in Beef by PMAxx and Real-Time LAMP. Food Control 2020, 115, 107292. [Google Scholar] [CrossRef]
- Zhang, H.-N.; Hou, P.-B.; Chen, Y.-Z.; Ma, Y.; Li, X.-P.; Lv, H.; Wang, M.; Tan, H.-L.; Bi, Z.-W. Prevalence of Foodborne Pathogens in Cooked Meat and Seafood from 2010 to 2013 in Shandong Province, China. Iran. J. Public Health 2016, 45, 1577. [Google Scholar]
- El-Aziz, N.K.A.; Tartor, Y.H.; El-Aziz Gharib, A.A.; Ammar, A.M. Propidium Monoazide Quantitative Real-Time Polymerase Chain Reaction for Enumeration of Some Viable but Nonculturable Foodborne Bacteria in Meat and Meat Products. Foodborne Pathog. Dis. 2018, 15, 226–234. [Google Scholar] [CrossRef]
- Rowan, N.J. Viable but Non-Culturable Forms of Food and Waterborne Bacteria: Quo Vadis? Trends Food Sci. Technol. 2004, 15, 462–467. [Google Scholar] [CrossRef]
- Fakruddin, M.; Mannan, K.S.B.; Andrews, S. Viable but Nonculturable Bacteria: Food Safety and Public Health Perspective. ISRN Microbiol. 2013, 2013, 703813. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 21872-1: 2017; Microbiology of the Food Chian-Horizontal Method for the Determination of Vibrio spp. Part 1: Detection of Potentially Enteropathogenic Vibrio parahaemolyticus, Vibrio cholerae and Vibrio vulnificus. International Organization for Standardization: Geneva, Switzerland, 2017.
- Bej, A.K.; Patterson, D.P.; Brasher, C.W.; Vickery, M.C.L.; Jones, D.D.; Kaysner, C.A. Detection of Total and Hemoly-sin-Producing Vibrio parahaemolyticus in Shellfish Using Multiplex PCR Amplification of Tl, Tdh and Trh. J. Microbiol. Methods 1999, 36, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, R. Quantification on the LightCycler. In Rapid Cycle Real-Time PCR; Springer: Berlin/Heidelberg, Germany, 2001; pp. 21–34. [Google Scholar]
- Caraguel, C.G.B.; Stryhn, H.; Gagné, N.; Dohoo, I.R.; Hammell, K.L. Selection of a Cutoff Value for Real-Time Polymerase Chain Reaction Results to Fit a Diagnostic Purpose: Analytical and Epidemiologic Approaches. J. Vet. Diagn. Investig. 2011, 23, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Okuda, J.U.N.; Matsumoto, C.; Takahashi, N.; Hashimoto, S.; Nishibuchi, M. Identification of Vibrio parahaemolyticus Strains at the Species Level by PCR Targeted to the ToxR Gene. J. Clin. Microbiol. 1999, 37, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Tada, J.; Ohashi, T.; Nishimura, N.; Shirasaki, Y.; Ozaki, H.; Fukushima, S.; Takano, J.; Nishibuchi, M.; Takeda, Y. Detection of the Thermostable Direct Hemolysin Gene (Tdh) and the Thermostable Direct Hemolysin-Related Hemolysin Gene (Trh) of Vibrio parahaemolyticus by Polymerase Chain Reaction. Mol. Cell Probes 1992, 6, 477–487. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Pan, S.-F.; Chen, C.-H. Sequence of a Cloned PR72H Fragment and Its Use for Detection of Vibrio parahaemolyticus in Shellfish with the PCR. Appl. Environ. Microbiol 1995, 61, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, K.; Dohmoto, N.; Harayama, S. Cloning and Nucleotide Sequence of the GyrB Gene of Vibrio parahaemolyticus and Its Application in Detection of This Pathogen in Shrimp. Appl. Environ. Microbiol. 1998, 64, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Oshima, K.; Kurokawa, K.; Yokoyama, K.; Uda, T.; Tagomori, K.; Iijima, Y.; Najima, M.; Nakano, M.; Yamashita, A.; et al. Genome Sequence of Vibrio parahaemolyticus: A Pathogenic Mechanism Distinct from That of V Cholerae. Lancet 2003, 361, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Lüdeke, C.H.M.; Bowers, J.C.; Garrett, N.; Fischer, M.; Parsons, M.B.; Bopp, C.A.; DePaola, A. Biochemical, Serological, and Virulence Characterization of Clinical and Oyster Vibrio parahaemolyticus Isolates. J. Clin. Microbiol. 2012, 50, 2343–2352. [Google Scholar] [CrossRef]
- Nocker, A.; Camper, A.K. Novel Approaches toward Preferential Detection of Viable Cells Using Nucleic Acid Amplification Techniques. FEMS Microbiol. Lett. 2009, 291, 137–142. [Google Scholar] [CrossRef]
- Lv, R.; Wang, K.; Feng, J.; Heeney, D.D.; Liu, D.; Lu, X. Detection and Quantification of Viable but Non-Culturable Campylobacter Jejuni. Front. Microbiol. 2020, 10, 2920. [Google Scholar] [CrossRef]
- Wang, R.; Zhong, Y.; Gu, X.; Yuan, J.; Saeed, A.F.; Wang, S. The Pathogenesis, Detection, and Prevention of Vibrio parahaemolyticus. Front. Microbiol. 2015, 6, 144. [Google Scholar] [CrossRef]
- Chahorm, K.; Prakitchaiwattana, C. Application of Reverse Transcriptase-PCR-DGGE as a Rapid Method for Routine Determination of Vibrio Spp. in Foods. Int. J. Food Microbiol. 2018, 264, 46–52. [Google Scholar] [CrossRef]
- Shimodori, S.; Moriya, T.; Kohashi, O.; Faming, D.; Amako, K. Extraction from Prawn Shells of Substances Cryoprotective for Vibrio Cholerae. Appl. Environ. Microbiol. 1989, 55, 2726–2728. [Google Scholar] [CrossRef]
- Bang, W.; Drake, M.A. Resistance of cold-and starvation-stressed Vibrio vulnificus to heat and freeze-thaw exposure. J. Food Prot. 2002, 65, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Johnston, M.D. An Investigation into the Changed Physiological State of Vibrio Bacteria as a Survival Mechanism in Response to Cold Temperatures and Studies on Their Sensitivity to Heating and Freezing. J. Appl. Microbiol. 2002, 92, 1066–1077. [Google Scholar] [CrossRef]
- Yu, H.; Fang, J.; Ma, B.; Li, J.; Zhang, M. One Real-Time Fluorescent Loop-Mediated Isothermal Amplification Combined with Propidium Monoazide for Detection of Viable Vibrio parahaemolyticus in Seafood. Am. J. Biochem. Biotechnol. 2019, 15, 91–100. [Google Scholar] [CrossRef]
- Niu, B.; Hong, B.; Zhang, Z.; Mu, L.; Malakar, P.K.; Liu, H.; Pan, Y.; Zhao, Y. A Novel QPCR Method for Simultaneous Detection and Quantification of Viable Pathogenic and Non-Pathogenic Vibrio parahaemolyticus (Tlh+, Tdh+, and UreR+). Front. Microbiol. 2018, 9, 1747. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Khezri, M.; Ollivier, J.; Le Guyader, F.S.; Rodríguez-Díaz, J.; Aznar, R.; Sánchez, G. Optimization of PMAxx Pretreatment to Distinguish between Human Norovirus with Intact and Altered Capsids in Shellfish and Sewage Samples. Int. J. Food Microbiol. 2018, 266, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bouju-Albert, A.; Saltaji, S.; Dousset, X.; Prévost, H.; Jaffrès, E. Quantification of Viable Brochothrix Thermosphacta in Cold-Smoked Salmon Using PMA/PMAxx-QPCR. Front. Microbiol. 2021, 12, 654178. [Google Scholar] [CrossRef]
- Lamon, S.; Bastardo, A.; Meloni, D.; Consolati, S.G.; Fois, F.; Porcheddu, G.; Agus, V.; Pes, M.; Cambula, M.G.; Mureddu, A.; et al. Clonal Relationship among Vibrio parahaemolyticus Isolated from Mediterranean Mussels (Mytilus galloprovincialis) and Grooved Carpet Shells (Ruditapes decussatus) Harvested in Sardinia (Italy). Food Microbiol. 2019, 84, 103258. [Google Scholar] [CrossRef]
- Bacian, C.; Verdugo, C.; García, K.; Perez-Larruscain, J.; de Blas, I.; Cachicas, V.; Lopez-Joven, C. Longitudinal Study of Total and Pathogenic Vibrio parahaemolyticus (Tdh+ and/or Trh+) in Two Natural Extraction Areas of Mytilus Chilensis in Southern Chile. Front. Microbiol. 2021, 12, 621737. [Google Scholar] [CrossRef]
- Lamon, S.; Consolati, S.G.; Fois, F.; Cambula, M.G.; Pes, M.; Porcheddu, G.; Agus, V.; Esposito, G.; Mureddu, A.; Meloni, D. Occurrence, Seasonal Distribution, and Molecular Characterization of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in Shellfish (Mytilus galloprovincialis and Ruditapes decussatus) Collected in Sardinia (Italy). J. Food Prot. 2019, 82, 1851–1856. [Google Scholar] [CrossRef]
- Lopatek, M.; Wieczorek, K.; Osek, J. Prevalence and Antimicrobial Resistance of Vibrio parahaemolyticus Isolated from Raw Shellfish in Poland. J. Food Prot. 2015, 78, 1029–1033. [Google Scholar] [CrossRef]
- Roque, A.; Lopez-Joven, C.; Lacuesta, B.; Elandaloussi, L.; Wagley, S.; Furones, M.D.; Ruiz-Zarzuela, I.; De Blas, I.; Rangdale, R.; Gomez-Gil, B. Detection and Identification of Tdh- And Trh-Positive Vibrio parahaemolyticus Strains from Four Species of Cultured Bivalve Molluscs on the Spanish Mediterranean Coast. Appl. Environ. Microbiol. 2009, 75, 7574–7577. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Bowers, J.C.; Griffitt, K.J.; Molina, V.; Clostio, R.W.; Pei, S.; Laws, E.; Paranjpye, R.N.; Strom, M.S.; Chen, A.; et al. Ecology of Vibrio parahaemolyticus and Vibrio vulnificus in the Coastal and Estuarine Waters of Louisiana, Maryland, Mississippi, and Washington (United States). Appl. Environ. Microbiol. 2012, 78, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Froelich, B.; Oliver, J. Increases in the Amounts of Vibrio Spp. in Oysters upon Addition of Exogenous Bacteria. Appl. Environ. Microbiol. 2013, 79, 5208–5213. [Google Scholar] [CrossRef]
- Randa, M.A.; Polz, M.F.; Lim, E. Effects of Temperature and Salinity on Vibrio vulnificus Population Dynamics as Assessed by Quantitative PCR. Appl. Environ. Microbiol. 2004, 70, 5469–5476. [Google Scholar] [CrossRef] [PubMed]
- Motes, M.L.; Depaola, A.; Cook, D.W.; Veazey, J.E.; Hunsucker, J.C.; Garthright, W.E.; Blodgett, R.J.; Chirtel, S.J. Influence of water temperature and salinity on Vibrio vulnificus in Northern Gulf and Atlantic Coast oysters (Crassostrea virginica). Appl. Environ. Microbiol. 1998, 64, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.W.; Oliver, J.D. Temperature Effects on the Viable but Non-culturable State of Vibrio vulnificus. FEMS Microbiol. Lett. 1992, 101, 33–39. [Google Scholar] [CrossRef]
- Makino, S.-I.; Kii, T.; Asakura, H.; Shirahata, T.; Ikeda, T.; Takeshi, K.; Itoh, K. Does Enterohemorrhagic Escherichia coli O157:H7 Enter the Viable but Nonculturable State in Salted Salmon Roe? Appl. Environ. Microbiol. 2000, 66, 5536–5539. [Google Scholar] [CrossRef]
- Asakura, H.; Panutdaporn, N.; Kawamoto, K.; Igimi, S.; Yamamoto, S.; Makino, S.I. Proteomic characterization of enter-ohemorrhagic Escherichia coli O157: H7 in the oxidation-induced viable but non-culturable state. Microbiol. Immunol. 2007, 51, 875–881. [Google Scholar] [CrossRef]
- Aurass, P.; Prager, R.; Flieger, A. EHEC/EAEC O104:H4 Strain Linked with the 2011 German Outbreak of Haemolytic Uremic Syndrome Enters into the Viable but Non-Culturable State in Response to Various Stresses and Resuscitates upon Stress Relief. Environ. Microbiol. 2011, 13, 3139–3148. [Google Scholar] [CrossRef]
- Nicolò, M.S.; Guglielmino, S.P.P. Viable but nonculturable bacteria in food. In Public Health–Methodology, Environmental and Systems Issues; Maddock, J., Ed.; InTech: Rjeka, Croatia, 2012; pp. 189–216. [Google Scholar] [CrossRef]
- Panebianco, F.; Nava, V.; Giarratana, F.; Gervasi, T.; Cicero, N. Assessment of Heavy- and Semi-Metals Contamination in Edible Seaweed and Dried Fish Sold in Ethnic Food Stores on the Italian Market. J. Food Compos. Anal. 2021, 104, 104150. [Google Scholar] [CrossRef]
- Panebianco, F.; Giusti, A.; Giarratana, F.; Armani, A. Ethnic Seafood Products Sold on the Italian Market: Labelling Assessment and Biological, Chemical and Physical Risk Characterization. Food Control 2019, 105, 198–208. [Google Scholar] [CrossRef]
| Sample ID | Species | Type | FAO Fishing Area | Sampling Date |
|---|---|---|---|---|
| 1 | Paphia undulata | shelled clams | 71 | 10 February 2020 |
| 2 | Mytilus chilensis | shelled mussels | 87 | 10 February 2020 |
| 3 | Paphia undulata | shelled clams | 71 | 10 February 2020 |
| 4 | Mytilus chilensis | half shell mussels | 87 | 10 February 2020 |
| 5 | Mytilus chilensis | shelled mussels | 87 | 10 February 2020 |
| 6 | Chamelea gallina | shelled clams | 71 | 10 February 2020 |
| 7 | Mytilus chilensis | shelled mussels | 87 | 10 February 2020 |
| 8 | Paphia undulata | shelled clams | 71 | 10 February 2020 |
| 9 | Mytilus chilensis | shelled mussels | 87 | 16 March 2020 |
| 10 | Mytilus chilensis | shelled mussels | 87 | 16 March 2020 |
| 11 | Paphia undulata | shelled clams | 71 | 16 March 2020 |
| 12 | Mytilus chilensis | shelled mussels | 87 | 16 March 2020 |
| 13 | Paphia undulata | whole shell clams | 71 | 16 March 2020 |
| 14 | Chamelea gallina | shelled clams | 37 | 16 March 2020 |
| 15 | Mytilus chilensis | shelled mussels | 87 | 16 March 2020 |
| 16 | Chamelea gallina | shelled clams | 71 | 16 March 2020 |
| 17 | Chamelea gallina | shelled clams | 71 | 16 March 2020 |
| 18 | Mytilus chilensis | shelled mussels | 87 | 20 April 2020 |
| 19 | Chamelea gallina | shelled clams | 71 | 20 April 2020 |
| 20 | Paphia textile | shelled clams | 61 | 20 April 2020 |
| 21 | Chamelea gallina | shelled clams | 71 | 20 April 2020 |
| 22 | Paphia textile | shelled clams | 61 | 20 April 2020 |
| 23 | Chamelea gallina | shelled clams | 71 | 20 April 2020 |
| 24 | Mytilus chilensis | shelled mussels | 87 | 20 April 2020 |
| 25 | Chamelea gallina | shelled clams | 71 | 20 April 2020 |
| 26 | Mytilus chilensis | shelled mussels | 87 | 20 April 2020 |
| 27 | Mytilus chilensis | shelled mussels | 87 | 22 June 2020 |
| 28 | Mytilus chilensis | shelled mussels | 87 | 22 June 2020 |
| 29 | Meretix lyrata | whole shell clams | 61 | 22 June 2020 |
| 30 | Paphia undulata | shelled clams | 71 | 22 June 2020 |
| 31 | Mytilus chilensis | whole shell mussels | 87 | 22 June 2020 |
| 32 | Mytilus chilensis | shelled mussels | 87 | 22 June 2020 |
| 33 | Paphia undulata | shelled clams | 71 | 22 June 2020 |
| 34 | Mytilus chilensis | shelled mussels | 87 | 22 June 2020 |
| 35 | Mytilus chilensis | shelled mussels | 87 | 27 July 2020 |
| 36 | Paphia undulata | shelled clams | 71 | 27 July 2020 |
| 37 | Paphia textile | whole shell clams | 61 | 27 July 2020 |
| 38 | Mytilus chilensis | shelled mussels | 87 | 27 July 2020 |
| 39 | Paphia undulata | shelled clams | 71 | 27 July 2020 |
| 40 | Mytilus chilensis | shelled mussels | 87 | 27 July 2020 |
| 41 | Paphia textile | shelled clams | 37 | 27 July 2020 |
| 42 | Paphia textile | shelled clams | 61 | 27 July 2020 |
| 43 | Paphia textile | shelled clams | 61 | 27 July 2020 |
| 44 | Mytilus chilensis | shelled mussels | 87 | 7 September 2020 |
| 45 | Paphia undulata | shelled clams | 71 | 7 September 2020 |
| 46 | Mytilus chilensis | shelled mussels | 87 | 7 September 2020 |
| 47 | Chamelea gallina | shelled clams | 71 | 7 September 2020 |
| 48 | Mytilus chilensis | shelled mussels | 87 | 7 September 2020 |
| 49 | Mytilus chilensis | shelled mussels | 87 | 7 September 2020 |
| 50 | Meretrix meretrix | whole shell clams | 61 | 7 September 2020 |
| 51 | Paphia undulata | shelled clams | 71 | 7 September 2020 |
| 52 | Paphia undulata | shelled clams | 71 | 13 October 2020 |
| 53 | Mytilus chilensis | whole shell mussels | 87 | 13 October 2020 |
| 54 | Meretrix lyrata | whole shell clams | 61 | 13 October 2020 |
| 55 | Paphia undulata | shelled clams | 71 | 13 October 2020 |
| 56 | Mytilus chilensis | shelled mussels | 87 | 13 October 2020 |
| 57 | Paphia textile | shelled clams | 61 | 13 October 2020 |
| 58 | Mytilus chilensis | shelled mussels | 87 | 13 October 2020 |
| 59 | Meretrix lyrata | shelled clams | 61 | 13 October 2020 |
| 60 | Mytilus chilensis | shelled mussels | 87 | 13 October 2020 |
| 61 | Mytilus chilensis | shelled mussels | 87 | 14 December 2020 |
| 62 | Chamelea gallina | shelled clams | 71 | 14 December 2020 |
| 63 | Chamelea gallina | shelled clams | 71 | 14 December 2020 |
| 64 | Mytilus chilensis | shelled mussels | 87 | 14 December 2020 |
| 65 | Mytilus chilensis | shelled mussels | 87 | 14 December 2020 |
| 66 | Chamelea gallina | shelled clams | 71 | 14 December 2020 |
| 67 | Chamelea gallina | shelled clams | 71 | 14 December 2020 |
| 68 | Paphia textile | shelled clams | 61 | 14 December 2020 |
| 69 | Meretrix lyrata | whole shell clams | 61 | 11 January 2021 |
| 70 | Mytilus chilensis | shelled mussels | 87 | 11 January 2021 |
| 71 | Mytilus chilensis | shelled mussels | 87 | 11 January 2021 |
| 72 | Mytilus chilensis | shelled mussels | 87 | 11 January 2021 |
| 73 | Paphia textile | shelled clams | 61 | 11 January 2021 |
| 74 | Mytilus chilensis | shelled mussels | 87 | 11 January 2021 |
| 75 | Mytilus chilensis | shelled mussels | 87 | 11 January 2021 |
| 76 | Paphia textile | shelled clams | 61 | 11 January 2021 |
| 77 | Meretrix meretrix | whole shell clams | 61 | 11 January 2021 |
| No. | Species | Strain | Source | qPCR Result |
|---|---|---|---|---|
| 1 | V. parahaemolyticus | ATCC 17802 | Shirasu food poisoning, Japan | + |
| 2 | V. parahaemolyticus | ATCC 33847 | human clinical isolate | + |
| 3 | V. parahaemolyticus | CCUG 43363 | unknown | + |
| 4 | V. parahaemolyticus | MELAB 772 | mussels | + |
| 5 | V. parahaemolyticus | MELAB 777 | mussels | + |
| 6 | V. parahaemolyticus | MELAB 778 | mussels | + |
| 7 | V. parahaemolyticus | MELAB 547 | fish | + |
| 8 | V. alginolyticus | ATCC 17749 | fish | − |
| 9 | V. vulnificus | ATCC 27562 | blood | − |
| 10 | V. cholerae | CCUG 37531 | unknown | − |
| 11 | V. mimicus | CCUG 13624 | human clinical isolate | − |
| 12 | A. hydrophila | ATCC 7966T | tin of milk with fishy odor | − |
| 13 | A. molluscorum | CECT 5864 | wedge shells | − |
| 14 | A. sobria | CECT 4245T | fish | − |
| 15 | E. coli | ATCC 8739 | feces | − |
| 16 | L. monocytogenes | ATCC 13932 | human clinical isolate | − |
| 17 | P. aeruginosa | ATCC 15442 | water | − |
| Sample ID | Type | Species | FAO Fishing Area | Sampling Period | Plate Count | CT Values | Log CFU/g Predicted | ||
|---|---|---|---|---|---|---|---|---|---|
| qPCR (Dead + VBNC) | PMA-qPCR (VBNC) | qPRC (Dead + VBNC) | PMA-qPCR (VBNC) | ||||||
| 6 | shelled clams | Chamelea gallina | 71 | February 2020 | UD | 29.05 a | UD | 3.79 a | UD |
| 8 | shelled clams | Paphia undulata | 71 | March 2020 | UD | 31.18 b | 34.54 a | 3.12 b | 2.05 a |
| 11 | shelled clams | Paphia undulata | 71 | March 2020 | UD | 29.26 c | 35.1 b | 3.73 c | 1.87 b |
| 16 | shelled clams | Chamelea gallina | 71 | March 2020 | UD | 32.08 d | 35.73 c | 2.83 d | 1.67 c |
| 19 | shelled clams | Chamelea gallina | 71 | April 2020 | UD | 33.37 e | 34.74 d | 2.42 e | 1.99 d |
| 20 | shelled clams | Paphia textile | 61 | April 2020 | UD | 30.21 f | 33.78 e | 3.42 f | 2.29 e |
| 23 | shelled clams | Chamelea gallina | 71 | April 2020 | UD | 35.21 g | UD | 1.84 g | UD |
| 33 | shelled clams | Paphia undulata | 71 | June 2020 | UD | 29.74 h | 35.58 f | 3.57 h | 1.72 f |
| 36 | shelled clams | Paphia undulata | 71 | July 2020 | UD | 32.86 i | UD | 2.58 i | UD |
| 45 | shelled clams | Paphia undulata | 71 | September 2020 | UD | 27.32 l | 34.62 g | 4.34 l | 2.03 a |
| 51 | shelled clams | Paphia undulata | 71 | September 2020 | UD | 35.67 m | UD | 1.69 m | UD |
| 62 | shelled clams | Chamelea gallina | 71 | December 2020 | UD | 35.59 n | UD | 1.72 n | UD |
| 63 | shelled clams | Chamelea gallina | 71 | December 2020 | UD | 31.19 b | 35.51 h | 3.11 b | 1.74 f |
| 66 | shelled clams | Chamelea gallina | 71 | December 2020 | UD | 33.65 o | 35.48 i | 2.33 o | 1.75 fg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Salvo, E.; Panebianco, F.; Panebianco, A.; Ziino, G. Quantitative Detection of Viable but Nonculturable Vibrio parahaemolyticus in Frozen Bivalve Molluscs. Foods 2023, 12, 2373. https://doi.org/10.3390/foods12122373
Di Salvo E, Panebianco F, Panebianco A, Ziino G. Quantitative Detection of Viable but Nonculturable Vibrio parahaemolyticus in Frozen Bivalve Molluscs. Foods. 2023; 12(12):2373. https://doi.org/10.3390/foods12122373
Chicago/Turabian StyleDi Salvo, Eleonora, Felice Panebianco, Antonio Panebianco, and Graziella Ziino. 2023. "Quantitative Detection of Viable but Nonculturable Vibrio parahaemolyticus in Frozen Bivalve Molluscs" Foods 12, no. 12: 2373. https://doi.org/10.3390/foods12122373
APA StyleDi Salvo, E., Panebianco, F., Panebianco, A., & Ziino, G. (2023). Quantitative Detection of Viable but Nonculturable Vibrio parahaemolyticus in Frozen Bivalve Molluscs. Foods, 12(12), 2373. https://doi.org/10.3390/foods12122373






