Advances in the Metabolic Mechanism and Functional Characteristics of Equol
Abstract
:1. Introduction
2. Physicochemical Properties of Equol
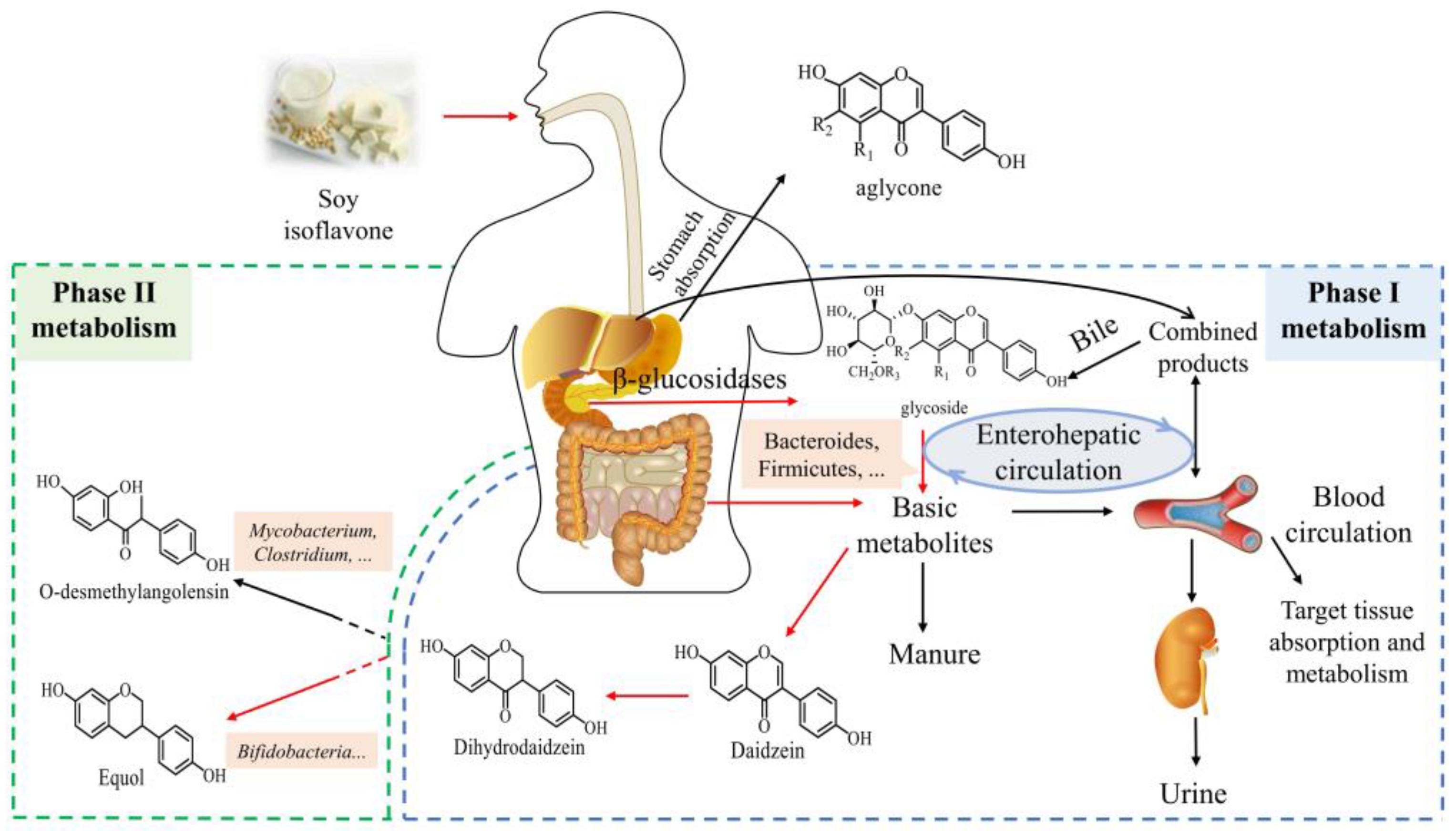
3. Metabolism and Absorption of Equol
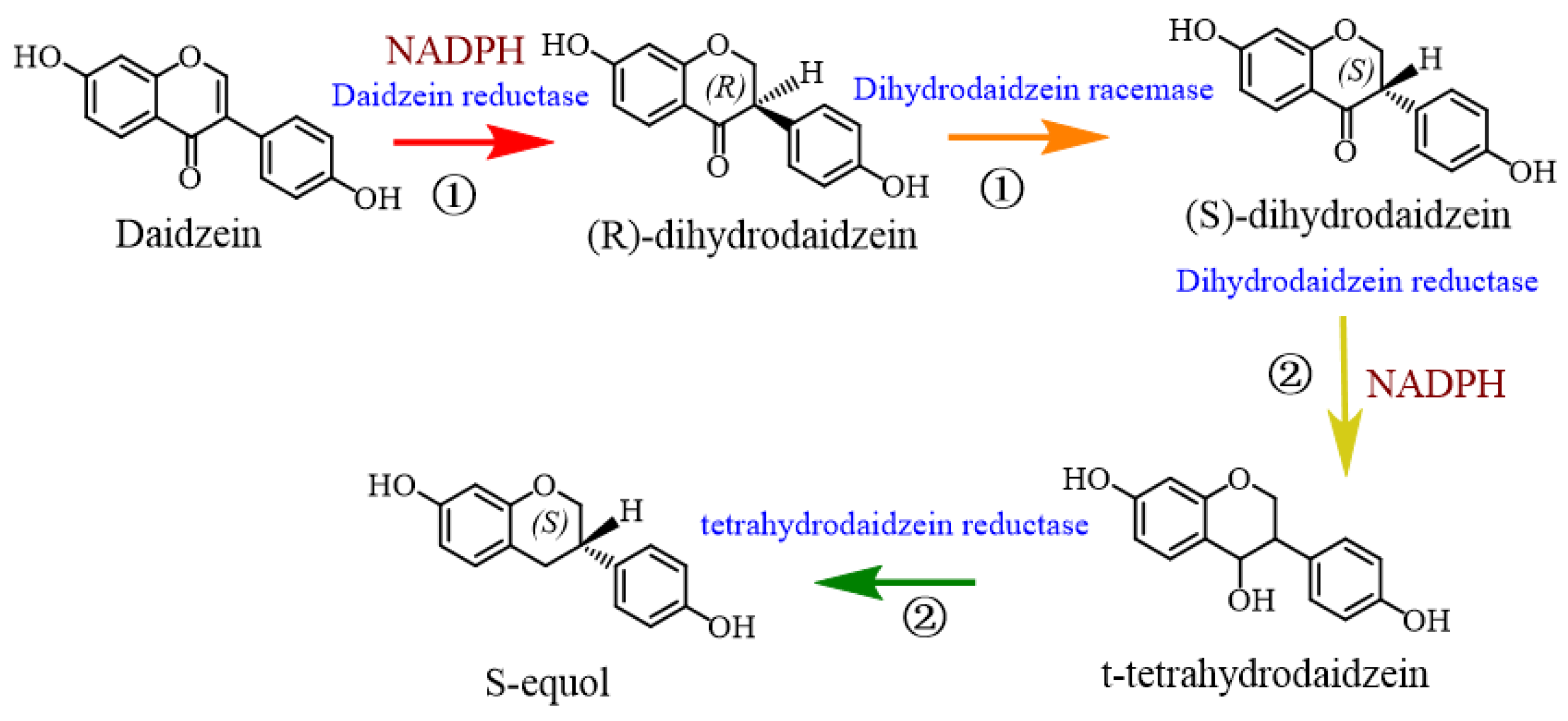
3.1. Metabolism and Regulatory Mechanism of Equol
3.2. Factors Affecting the Absorption of Equol
4. Production Method of Equol
4.1. Chemical Synthesis of Equol
4.2. Microbial Preparation of Equol
4.2.1. Independent Equol-Producing Bacteria
4.2.2. Non-Independent Equol-Producing Bacteria
4.3. Biological Synthesis of Equol
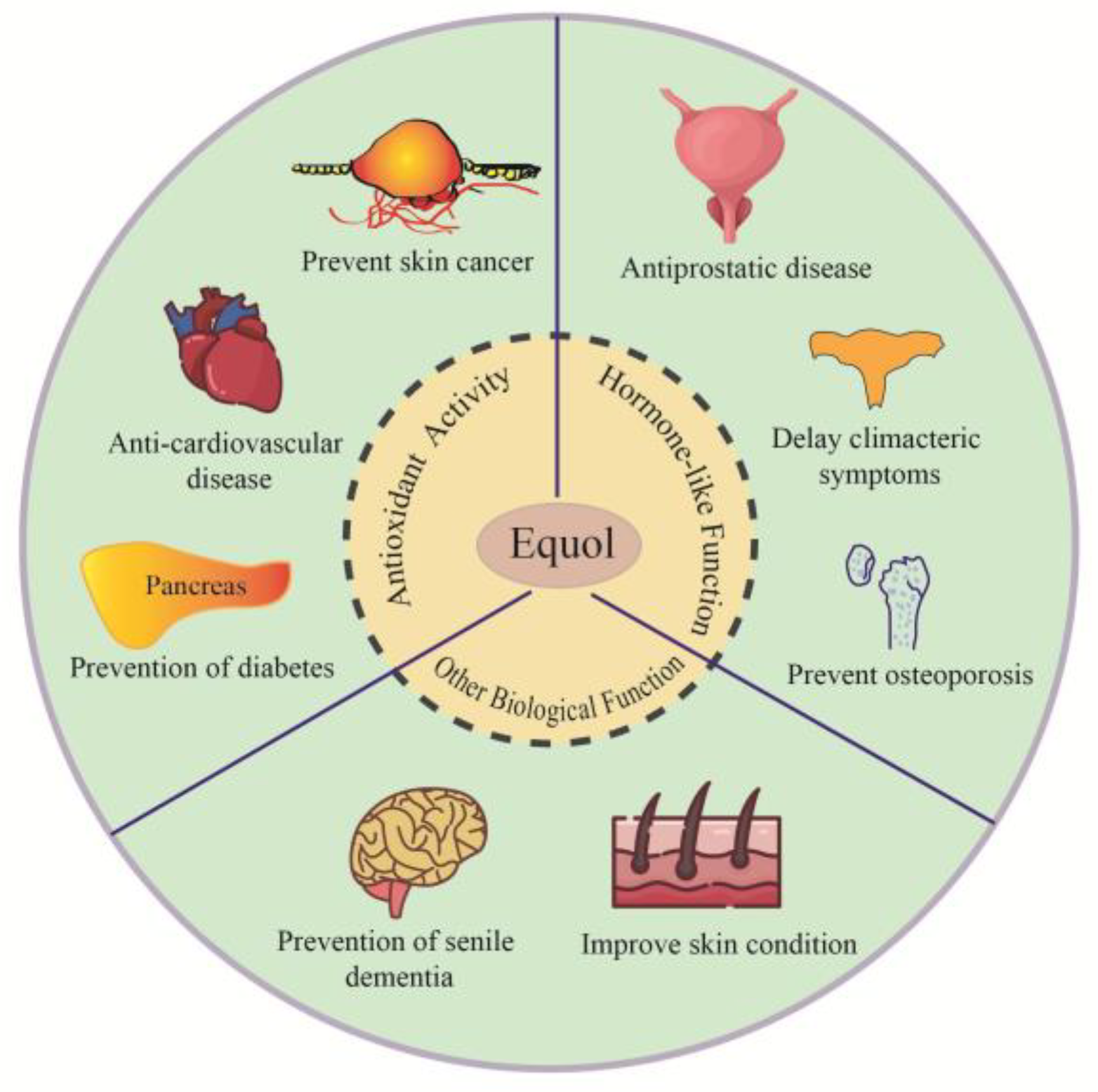
5. Biological Function and Application of Equol
5.1. Hormone-like Effects
5.2. Antioxidant Activity
5.3. Other Biological Functions
6. Detection Method of Equol
7. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jang, C.H.; Oh, J.; Lim, J.S.; Kim, H.J.; Kim, J.-S. Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases. Foods 2021, 10, 636. [Google Scholar] [CrossRef] [PubMed]
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennetts, H.W.; Underwood, E.J.; Shier, F.L. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Br. Vet. J. 1946, 102, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Axelson, M.; Kirk, D.N.; Farrant, R.D.; Cooley, G.; Lawson, A.M.; Setchell, K.D. The identification of the weak oestrogen equol [7-hydroxy-3-(4′-hydroxyphenyl)chroman] in human urine. Biochem. J. 1982, 201, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Zhang, S.; Tong, H.; Shi, S. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytother. Res. 2017, 32, 384–394. [Google Scholar] [CrossRef]
- Tuli, H.S.; Kumar, A.; Sak, K.; Aggarwal, D.; Gupta, D.S.; Kaur, G.; Vashishth, K.; Dhama, K.; Kaur, J.; Saini, A.K.K.; et al. Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone. Pharmaceuticals 2022, 15, 1418. [Google Scholar] [CrossRef]
- Hod, R.; Maniam, S.; Mohd Nor, N.H. A Systematic Review of the Effects of Equol (Soy Metabolite) on Breast Cancer. Molecules 2021, 26, 1105. [Google Scholar] [CrossRef]
- Mayo, B.; Vazquez, L.; Belen Florez, A. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [Green Version]
- de la Bastida, A.R.; Peiroten, A.; Langa, S.; Arques, J.L.; Landete, J.M. Heterologous production of equol by lactic acid bacteria strains in culture medium and food. Int. J. Food Microbiol. 2021, 360, 109328. [Google Scholar] [CrossRef]
- Yoshikata, R.; Myint, K.Z.; Ohta, H.; Ishigaki, Y. Inter-relationship between diet, lifestyle habits, gut microflora, and the equol-producer phenotype: Baseline findings from a placebo-controlled intervention trial. Menopause-J. N. Am. Menopause Soc. 2019, 26, 273–285. [Google Scholar] [CrossRef]
- Soukup, S.T.; Helppi, J.; Müller, D.R.; Zierau, O.; Watzl, B.; Vollmer, G.; Diel, P.; Bub, A.; Kulling, S.E. Phase II metabolism of the soy isoflavones genistein and daidzein in humans, rats and mice: A cross-species and sex comparison. Arch. Toxicol. 2016, 90, 1335–1347. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Ma, Y.; Li, X.; Zhang, J.; Zhao, L. Supplementation with soy isoflavones alleviates depression-like behaviour via reshaping the gut microbiota structure. Food Funct. 2021, 12, 4995–5006. [Google Scholar] [CrossRef]
- Qu, J.D.; Wang, J.Y.; Wang, J.P.; Wang, S. Ldentification of 12 Soybean lsoflavones in Soybean Meal by High Performance Liquid Chromatography-Electrospray lonization-Multi-stageTandem Mass Spectrometry. Mod. Food Sci. Technol. 2013, 29, 863–866, 897. [Google Scholar] [CrossRef]
- Krizova, L.; Krestakova, V.; Dadakova, K.; Kasparovsky, T. Production of Bovine Equol-Enriched Milk: A Review. Animals 2021, 11, 735. [Google Scholar] [CrossRef]
- Sekikawa, A.; Wharton, W.; Butts, B.; Veliky, C.V.; Garfein, J.; Li, J.; Goon, S.; Fort, A.; Li, M.; Hughes, T.M. Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. Int. J. Mol. Sci. 2022, 23, 11921. [Google Scholar] [CrossRef]
- Dufault-Thompson, K.; Hall, B.; Jiang, X. Taxonomic distribution and evolutionary analysis of the equol biosynthesis gene cluster. BMC Genom. 2022, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, B.; Fang, X.; Zhong, Q.; Liao, Z.; Wang, J.; Wu, X.; Ma, Y.; Li, P.; Feng, X.; et al. Soy isoflavone-specific biotransformation product S-equol in the colon: Physiological functions, transformation mechanisms, and metabolic regulatory pathways. Crit. Rev. Food Sci. Nutr. 2022, 1–29. [Google Scholar] [CrossRef]
- Liang, W.O.; Zhao, L.C.; Fang, X.; Wang, L. Progress in the Research of the lnteractions of Soy lsoflavones with Gut Microbiota. Food Sci. 2019, 40, 283–289. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Rüfer, C.E.; Kulling, S.E. Antioxidant Activity of Isoflavones and Their Major Metabolites Using Different in Vitro Assays. J. Agric. Food Chem. 2006, 54, 2926–2931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Spenkelink, B.; Boonpawa, R.; Rietjens, I.M.C.M. Use of Physiologically Based Pharmacokinetic Modeling to Predict Human Gut Microbial Conversion of Daidzein to S-Equol. J. Agric. Food Chem. 2021, 70, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol—A Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [Green Version]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, K.R.; Failla, M.L. Transport and Metabolism of Equol by Caco-2 Human Intestinal Cells. J. Agric. Food Chem. 2009, 57, 8297–8302. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.-Z.; Eun, J.-B. Physicochemical characteristics and isoflavones content during manufacture of short-time fermented soybean product (cheonggukjang). J. Food Sci. Technol. 2020, 57, 2190–2197. [Google Scholar] [CrossRef] [PubMed]
- Langa, S.; Peiroten, A.; Antonio Curiel, J.; Ruiz De la Bastida, A.; Maria Landete, J. Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods 2023, 12, 1293. [Google Scholar] [CrossRef]
- Tamura, A.; Shiomi, T.; Hachiya, S.; Shigematsu, N.; Hara, H. Low activities of intestinal lactase suppress the early phase absorption of soy isoflavones in Japanese adults. Clin. Nutr. 2008, 27, 248–253. [Google Scholar] [CrossRef]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef]
- Hedlund, T.E.; Maroni, P.D.; Ferucci, P.G.; Dayton, R.; Barnes, S.; Jones, K.; Moore, R.; Ogden, L.G.; Wahala, K.; Sackett, H.M.; et al. Long-Term Dietary Habits Affect Soy Isoflavone Metabolism and Accumulation in Prostatic Fluid in Caucasian Men. J. Nutr. 2005, 135, 1400–1406. [Google Scholar] [CrossRef] [Green Version]
- Clavel, T.; Fallani, M.; Lepage, P.; Levenez, F.; Mathey, J.; Rochet, V.; Sérézat, M.; Sutren, M.; Henderson, G.; Bennetau-Pelissero, C.; et al. Isoflavones and Functional Foods Alter the Dominant Intestinal Microbiota in Postmenopausal Women. J. Nutr. 2005, 135, 2786–2792. [Google Scholar] [CrossRef] [Green Version]
- Cortes-Martin, A.; Romo-Vaquero, M.; Garcia-Mantrana, I.; Rodriguez-Varela, A.; Carmen Collado, M.; Carlos Espin, J.; Victoria Selma, M. Urolithin Metabotypes can Anticipate the Different Restoration of the Gut Microbiota and Anthropometric Profiles during the First Year Postpartum. Nutrients 2019, 11, 2079. [Google Scholar] [CrossRef] [Green Version]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.B.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Heemstra, J.M.; Kerrigan, S.A.; Doerge, D.R.; Helferich, W.G.; Boulanger, W.A. Total Synthesis of (S)-Equol. Org. Lett. 2006, 8, 5441–5443. [Google Scholar] [CrossRef]
- Gharpure, S.J.; Sathiyanarayanan, A.M.; Jonnalagadda, P. o-Quinone methide based approach to isoflavans: Application to the total syntheses of equol, 3′-hydroxyequol and vestitol. Tetrahedron Lett. 2008, 49, 2974–2978. [Google Scholar] [CrossRef]
- Gupta, A.; Ray, S. Simple and Efficient Synthesis of (±)-Equol and Related Derivatives. Synthesis 2008, 2008, 3783–3786. [Google Scholar] [CrossRef]
- Li, S.-R.; Chen, P.-Y.; Chen, L.-Y.; Lo, Y.-F.; Tsai, I.-L.; Wang, E.-C. Synthesis of haginin E, equol, daidzein, and formononetin from resorcinol via an isoflavene intermediate. Tetrahedron Lett. 2009, 50, 2121–2123. [Google Scholar] [CrossRef]
- Takashima, Y.; Kaneko, Y.; Kobayashi, Y. Synthetic access to optically active isoflavans by using allylic substitution. Tetrahedron 2010, 66, 197–207. [Google Scholar] [CrossRef]
- Bert, S. Synthesis of Equol. US20120094336A1, 19 April 2012. [Google Scholar]
- Yang, S.; Zhu, S.-F.; Zhang, C.-M.; Song, S.; Yu, Y.-B.; Li, S.; Zhou, Q.-L. Enantioselective iridium-catalyzed hydrogenation of α-arylcinnamic acids and synthesis of (S)-equol-ScienceDirect. Tetrahedron 2012, 68, 5172–5178. [Google Scholar] [CrossRef]
- Li, B.-J. Advances in exploring equol production and application. J. Food Process Preserv. 2019, 43, e14205. [Google Scholar] [CrossRef]
- Brown, N.M.; Galandi, S.L.; Summer, S.S.; Zhao, X.; Heubi, J.E.; King, E.C.; Setchell, K.D. S-(−)equol production is developmentally regulated and related to early diet composition. Nutr. Res. 2014, 34, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-L.; Hur, H.-G.; Lee, J.H.; Kim, K.T.; Kim, S.-I. Enantioselective Synthesis of S-Equol from Dihydrodaidzein by a Newly Isolated Anaerobic Human Intestinal Bacterium. Appl. Environ. Microbiol. 2005, 71, 214–219. [Google Scholar] [CrossRef] [Green Version]
- Rafii, F. The Role of Colonic Bacteria in the Metabolism of the Natural Isoflavone Daidzin to Equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef] [Green Version]
- Song, K.B.; Atkinson, C.; Frankenfeld, C.L.; Jokela, T.; Wähälä, K.; Thomas, W.K.; Lampe, J.W. Prevalence of Daidzein-Metabolizing Phenotypes Differs between Caucasian and Korean American Women and Girls. J. Nutr. 2006, 136, 1347–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2019, 60, 626–659. [Google Scholar] [CrossRef] [PubMed]
- Ahn-Jarvis, J.H.; Sosh, D.; Lombardo, E.; Lesinski, G.B.; Conwell, D.L.; Hart, P.A.; Vodovotz, Y. Short-Term Soy Bread Intervention Leads to a Dose-Response Increase in Urinary Isoflavone Metabolites and Satiety in Chronic Pancreatitis. Foods 2023, 12, 1762. [Google Scholar] [CrossRef] [PubMed]
- Minamida, K.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Production of equol from daidzein by gram-positive rod-shaped bacterium isolated from rat intestine. J. Biosci. Bioeng. 2006, 102, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.-T.; Yao, W.; Zhu, W.-Y. Isolation and identification of equol-producing bacterial strains from cultures of pig faeces. FEMS Microbiol. Lett. 2008, 282, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Tousen, Y.; Abe, F.; Ishida, T.; Uehara, M.; Ishimi, Y. Resistant starch promotes equol production and inhibits tibial bone loss in ovariectomized mice treated with daidzein. Metabolism 2011, 60, 1425–1432. [Google Scholar] [CrossRef]
- Abiru, Y.; Ueno, T.; Uchiyama, S. Isolation and characterization of novel S-equol-producing bacteria from brines of stinky tofu, a traditional fermented soy food in Taiwan. Int. J. Food Sci. Nutr. 2013, 64, 936–943. [Google Scholar] [CrossRef]
- Uchiyama, S.; Ueno, T.; Suzuki, T. Identification of a Newly Isolated Equol-Producing Lactic Acid Bacterium from the Human Feces. J. Intest. Microbiol. 2007, 21, 217–220. [Google Scholar] [CrossRef]
- Yee, S.; Burdock, G.A.; Kurata, Y.; Enomoto, Y.; Narumi, K.; Hamada, S.; Itoh, T.; Shimomura, Y.; Ueno, T. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem. Toxicol. 2008, 46, 2713–2720. [Google Scholar] [CrossRef] [PubMed]
- Antignac, J.-P.; Cariou, R.; Le Bizec, B.; Andre, F. New data regarding phytoestrogens content in bovine milk. Food Chem. 2004, 87, 275–281. [Google Scholar] [CrossRef]
- Lefevre, A.; Daems, F.; Focant, M.; Peeters, J.; Ninane, V.; Larondelle, Y.; Froidmont, E. The effect of commonly used dairy processing techniques and unit operations on the equol content of dairy products. Int. Dairy J. 2019, 93, 30–34. [Google Scholar] [CrossRef]
- Peirotén, Á.; Gaya, P.; J, M.L. Application of recombinant lactic acid bacteria and bifidobacteria able to enrich soy beverage in dihydrodaidzein and dihydrogenistein. Food Res. Int. 2020, 134, 109257. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lim, J.; Kim, I.; Kim, D.; Kang, S.C. Isolation and identification of new bacterial stains producing equol from Pueraria lobata extract fermentation. PLoS ONE 2018, 13, e0192490. [Google Scholar] [CrossRef]
- Langa, S.; de la Bastida, A.R.; Peirot, A.; Curiel, J.A.; Landete, J.M. Development of the first fermented soy beverages enriched in equol and 5-hydroxy-equol. LWT 2022, 168, 113899. [Google Scholar] [CrossRef]
- Lephart, E.D. Review: Anti-Oxidant and Anti-Aging Properties of Equol in Prostate Health (BPH). Open J. Endocr. Metab. Dis. 2014, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lephart, E.D. Determination of S- and/or R-equol in plant-based food products and efficacy of topical or oral 4′,7-isoflavandiol (R/S equol) to improve skin health in adult men, a Placebo-controlled pilot study. J. Funct. Foods 2021, 83, 104563. [Google Scholar] [CrossRef]
- Rosenberg, E.; Delong, E.F.; Lory, S.; Stackebrandt, E.; Thompson, F. The Prokaryotes Actinobacteria, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 201–238. [Google Scholar]
- Hur, H.-G.; Beger, R.D.; Heinze, T.M.; Lay, J.O., Jr.; Freeman, J.P.; Dore, J.; Rafii, F. Isolation of an anaerobic intestinal bacterium capable of cleaving the C-ring of the isoflavonoid daidzein. Arch. Microbiol. 2002, 178, 8–12. [Google Scholar] [CrossRef]
- Yu, F.; Wang, S.Y.; Li, J.; Zhang, Q.; Li, C.D.; Wang, X.L. C-ring cleavage of isoflavone daidzein by a newly-isolated facultative Enterococcus hirae AUH-HM195 from Crossoptilon mantchuricum feces. Acta Microbiol. Sin. 2009, 49, 479–484. [Google Scholar]
- Matthies, A.; Clavel, T.; Gütschow, M.; Engst, W.; Haller, D.; Blaut, M.; Braune, A. Conversion of Daidzein and Genistein by an Anaerobic Bacterium Newly Isolated from the Mouse Intestine. Appl. Environ. Microbiol. 2008, 74, 4847–4852. [Google Scholar] [CrossRef] [Green Version]
- Heng, Y.; Kim, M.J.; Yang, H.J.; Kang, S.; Park, S. Lactobacillus intestinalis efficiently produces equol from daidzein and chungkookjang, short-term fermented soybeans. Arch. Microbiol. 2019, 201, 1009–1017. [Google Scholar] [CrossRef]
- Hur, H.-G.; Lay, J.O., Jr.; Beger, R.D.; Freeman, J.P.; Rafii, F. Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch. Microbiol. 2000, 174, 422–428. [Google Scholar] [CrossRef]
- Wang, X.-L.; Shin, K.-H.; Hur, H.-G.; Kim, S.-I. Enhanced biosynthesis of dihydrodaidzein and dihydrogenistein by a newly isolated bovine rumen anaerobic bacterium. J. Biotechnol. 2005, 115, 261–269. [Google Scholar] [CrossRef]
- Tamura, M.; Tsushida, T.; Shinohara, K. Isolation of an isoflavone-metabolizing, Clostridium-like bacterium, strain TM-40, from human faeces. Anaerobe 2007, 13, 32–35. [Google Scholar] [CrossRef]
- Zhou, B.; Meng, J.Q.; Wang, X.L. lsolation, identification and biotransforming property of an isoflavonereducing bacterium isolated from rabbit intestinal microflora. Microbiol. China 2014, 41, 2301–2309. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Zhao, F.; Wang, C.; Yang, K. Isolation and identification of an isoflavone reducing bacterium from feces from a pregnant horse. PLoS ONE 2019, 14, e0223503. [Google Scholar] [CrossRef]
- Tsangalis, D.; Ashton, J.F.; Mcgill, A.E.J.; Shah, N.P. Enzymic Transformation of Isoflavone Phytoestrogens in Soymilk by β-Glucosidase-Producing Bifidobacteria. J. Food Sci. 2002, 67, 3104–3113. [Google Scholar] [CrossRef]
- Zhang, X. Isolation and Characterizing of Daidzein-Degrading Bacteria from the Intestinal Tract of Pig. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2007. [Google Scholar]
- Maruo, T.; Sakamoto, M.; Ito, C.; Toda, T.; Benno, Y. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 2008, 58, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.-I.; Suzuki, T. Isolation and Characterization of a Novel Equol-Producing Bacterium from Human Feces. Biosci. Biotechnol. Biochem. 2008, 72, 2660–2666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minamida, K.; Ota, K.; Nishimukai, M.; Tanaka, M.; Abe, A.; Sone, T.; Tomita, F.; Hara, H.; Asano, K. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 2008, 58, 1238–1240. [Google Scholar] [CrossRef]
- Wang, X.-L.; Shao, J.-Z.; Wang, S.-Y.; Yu, F.; Zhang, Q.; Li, C.-D. Aci-Netobacter AUH-JLM455 and Its Transformation Method for Preparation of S-Equol. CN101338294B, 7 September 2011. [Google Scholar]
- Tamura, M.; Hori, S.; Nakagawa, H. Lactobacillus collinoides JCM1123(T0: Effects on mouse plasma cholesterol and isoflavonoids in the caecum. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2009, 96, 621–626. [Google Scholar] [CrossRef]
- Matthies, A.; Blaut, M.; Braune, A. Isolation of a Human Intestinal Bacterium Capable of Daidzein and Genistein Conversion. Appl. Environ. Microbiol. 2009, 75, 1740–1744. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Roncaglia, L.; De Lucia, M.; Amaretti, A.; Leonardi, A.; Pagnoni, U.M.; Rossi, M. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl. Microbiol. Biotechnol. 2009, 81, 943–950. [Google Scholar] [CrossRef]
- Jin, J.-S.; Kitahara, M.; Sakamoto, M.; Hattori, M.; Benno, Y. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int. J. Syst. Evol. Microbiol. 2010, 60, 1721–1724. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Miyanaga, N.; Akaza, H. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch. Microbiol. 2010, 192, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Iwami, T.; Hori, S.; Nakagawa, H. Lactobacillus fermentum ATCC9338: Effects on Mouse Intestinal Flora and Plasma Concentration of Isoflavonoids. Food Sci. Technol. Res. 2010, 16, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Elghali, S.; Mustafa, S.; Amid, M.; Abd Manap, M.Y.; Ismail, A.; Abas, F. Bioconversion of daidzein to equol by Bifidobacterium breve 15700 and Bifidobacterium longum BB536. J. Funct. Foods 2012, 4, 736–745. [Google Scholar] [CrossRef]
- Li, Y. Isolation and Characterization of the Eouol-Producing Bacteria from Human Feces. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2012. [Google Scholar]
- Li, H.; Zhou, B.; Wang, X.L. Bioconversion of daidzein by an anaerobic bacterium Eggerthella sp. AUH-Julong365 isolated from human feces. J. Hebei Agric. Univ. 2013, 36, 61–65, 71. [Google Scholar] [CrossRef]
- Tamura, M.; Hori, S.; Nakagawa, H. Intestinal Bacterium TM-30: An S-equol-producing Bacterium Isolated from Human Feces is Involved in Estrogen Metabolism in vitro. Food Sci. Technol. Res. 2014, 20, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.S.; Fang, D.; Zhu, L.Y.; Liu, W.; Wu, J.; Wang, X. Protective effect of an equol-producing Clostridium C1 against Salmonella infection in chicken. Acta Agric. Zhejiangensis 2016, 28, 234–239. [Google Scholar]
- Guo, Y.; Zhao, L.; Fang, X.; Zhong, Q.; Liang, H.; Liang, W.; Wang, L. Isolation and identification of a human intestinal bacterium capable of daidzein conversion. FEMS Microbiol. Lett. 2021, 368, fnab046. [Google Scholar] [CrossRef]
- Schoefer, L.; Mohan, R.; Braune, A.; Birringer, M.; Blaut, M. Anaerobic C-ring cleavage of genistein and daidzein by Eubacterium ramulus. FEMS Microbiol. Lett. 2002, 208, 197–202. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Zhang, C.; Wang, X.L.; Chen, B.H.; Hao, Q.H.; Wang, S.Y. Enhanced biosynthesis of O -desmethylangolensin from daidzein by a novel oxygen-tolerant cock intestinal bacterium in the presence of atmospheric oxygen. J. Appl. Microbiol. 2015, 118, 619–628. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.L.; Li, M.; Wang, X.L.; Hao, Q.H.; Yu, X.M. Isolation, identification and anti-oxidative activity of metabolites from oxygen-tolerant mutant strain Aeroto-AUH-JLC140 capable of biotransforming daidzein. Microbiol. China 2016, 43, 1699–1707. [Google Scholar] [CrossRef]
- Wang, X.-L.; Kim, H.-J.; Kang, S.-I.; Kim, S.-I.; Hur, H.-G. Production of phytoestrogen S-equol from daidzein in mixed culture of two anaerobic bacteria. Arch. Microbiol. 2007, 187, 155–160. [Google Scholar] [CrossRef]
- Fan, H.L.; Liu, X.N.; Liu, D.; Zhao, C.C.; Chen, J.F.; Cheng, Y.Q. The production of equol by mixed culture fermentation of Streptococcus faecium and Enterobacter. Food Sci. Technol. Res. 2011, 36, 2–6. (In Chinese) [Google Scholar] [CrossRef]
- Jin, J.-S.; Nishihata, T.; Kakiuchi, N.; Hattori, M. Biotransformation of C-Glucosylisoflavone Puerarin to Estrogenic (3S)-Equol in Co-culture of Two Human Intestinal Bacteria. Biol. Pharm. Bull. 2008, 31, 1621–1625. [Google Scholar] [CrossRef] [Green Version]
- Soukup, S.T.; Stoll, D.A.; Danylec, N.; Schoepf, A.; Kulling, S.E.; Huch, M. Metabolism of Daidzein and Genistein by Gut Bacteria of the Class Coriobacteriia. Foods 2021, 10, 2741. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H.; Moriyama, K.; Nomoto, K.; Akaza, H. Identification of an Enzyme System for Daidzein-to-Equol Conversion in Slackia sp. Strain NATTS. Appl. Environ. Microbiol. 2012, 78, 1228–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Marsh, E.N.; Kim, S.-U.; Han, J. Conversion of (3S,4R)-Tetrahydrodaidzein to (3S)-Equol by THD Reductase: Proposed Mechanism Involving a Radical Intermediate. Biochemistry 2010, 49, 5582–5587. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-G.; Kim, J.; Kim, E.-J.; Jung, E.; Pandey, B.P.; Kim, B.-G. P212A Mutant of Dihydrodaidzein Reductase Enhances (S)-Equol Production and Enantioselectivity in a Recombinant Escherichia coli Whole-Cell Reaction System. Appl. Environ. Microbiol. 2016, 82, 1992–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, Y.; Takahashi, M.; Miyazawa, N.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Identification of a Novel Dihydrodaidzein Racemase Essential for Biosynthesis of Equol from Daidzein in Lactococcus sp. Strain 20-92. Appl. Environ. Microbiol. 2012, 78, 4902–4907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Gao, S.; Zhang, W.; Zhang, T.; Li, N.; Zhou, J. High Titer of (S)-Equol Synthesis from Daidzein in Escherichia coli. ACS Synth. Biol. 2022, 11, 4043–4053. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Yasuda, S.; Takahashi, M.; Hayashi, T.; Miyazawa, N.; Sato, I.; Abiru, Y.; Uchiyama, S.; Hishigaki, H. Cloning and Expression of a Novel NADP(H)-Dependent Daidzein Reductase, an Enzyme Involved in the Metabolism of Daidzein, from Equol-Producing Lactococcus Strain 20-92. Appl. Environ. Microbiol. 2010, 76, 5892–5901. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, L.; Belen Florez, A.; Rodriguez, J.; Mayo, B. Heterologous expression of equol biosynthesis genes from Adlercreutzia equolifaciens. FEMS Microbiol. Lett. 2021, 368, fnab082. [Google Scholar] [CrossRef]
- Gao, Y.N.; Hao, Q.H.; Zhang, H.L.; Zhou, B.; Yu, X.M.; Wang, X.L. Reduction of soy isoflavones by use of Escherichia coli whole-cell biocatalyst expressing isoflavone reductase under aerobic conditions. Lett. Appl. Microbiol. 2016, 63, 111–116. [Google Scholar] [CrossRef]
- Lee, P.-G.; Lee, S.-H.; Kim, J.; Kim, E.-J.; Choi, K.-Y.; Kim, B.-G. Polymeric solvent engineering for gram/liter scale production of a water-insoluble isoflavone derivative, (S)-equol. Appl. Microbiol. Biotechnol. 2018, 102, 6915–6921. [Google Scholar] [CrossRef]
- Li, H.; Mao, S.; Chen, H.; Zhu, L.; Liu, W.; Wang, X.; Yin, Y. To Construct an Engineered (S)-Equol Resistant E. coli for in Vitro (S)-Equol Production. Front. Microbiol. 2018, 9, 1182. [Google Scholar] [CrossRef] [Green Version]
- Yoshikata, R.; Myint, K.Z.Y.; Ohta, H. Effects of Equol Supplement on Bone and Cardiovascular Parameters in Middle-Aged Japanese Women: A Prospective Observational Study. J. Altern. Complement. Med. 2018, 24, 701–708. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.; Cianci, S.; Cariola, M.; Fava, V.; Rapisarda, A.M.C.; Cianci, A. Effects of nutraceuticals on quality of life and sexual function of perimenopausal women. J. Endocrinol. Investig. 2017, 40, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Clerici, C. Equol: Pharmacokinetics and Biological Actions. J. Nutr. 2010, 140, 1363S–1368S. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fujii, S.; Inoue, H.; Takahashi, N.; Ishimi, Y.; Uehara, M. (S)-Equol Is More Effective than (R)-Equol in Inhibiting Osteoclast Formation and Enhancing Osteoclast Apoptosis, and Reduces Estrogen Deficiency–Induced Bone Loss in Mice. J. Nutr. 2022, 152, 1831–1842. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.F.; Huang, H.Y. Study on the effectiveness of soybean powder urine metabolites estrol, dihydrodaidzein, o-demethylated Angolan red sandalwood and perimenopausal symptoms. J. Pract. Med. 2022, 38, 1667–1670. [Google Scholar]
- Lu, Z.; Zhou, R.; Kong, Y.; Wang, J.; Xia, W.; Guo, J.; Liu, J.; Sun, H.; Liu, K.; Yang, J.; et al. S-equol, a Secondary Metabolite of Natural Anticancer Isoflavone Daidzein, Inhibits Prostate Cancer Growth In Vitro and In Vivo, though Activating the Akt/FOXO3a Pathway. Curr. Cancer Drug Targets 2016, 16, 455–465. [Google Scholar] [CrossRef]
- Stewart, K.L.; Lephart, E.D. Overview of BPH: Symptom Relief with Dietary Polyphenols, Vitamins and Phytochemicals by Nutraceutical Supplements with Implications to the Prostate Microbiome. Int. J. Mol. Sci. 2023, 24, 5486. [Google Scholar] [CrossRef]
- Trinchieri, A. Soy in benign prostate hyperplasia and prostate cancer: A literature review. Longhua Chin. Med. 2021, 5, 9. [Google Scholar] [CrossRef]
- Fatima, A.; Khan, M.S.; Ahmad, W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020, 26, 5837–5843. [Google Scholar] [CrossRef]
- Nishimura, Y.; Mabuchi, K.; Takano, A.; Hara, Y.; Negishi, H.; Morimoto, K.; Ueno, T.; Uchiyama, S.; Takamata, A. S-equol Exerts Estradiol-Like Anorectic Action with Minimal Stimulation of Estrogen Receptor-α in Ovariectomized Rats. Front. Endocrinol. 2017, 8, 281. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zuo, Q.; Hai, Y.; Sun, X.J. Lactulose: An indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med. Hypotheses 2011, 76, 325–327. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, Y.; Yao, Y.; Li, J.; Wang, W.; Wu, X. Equol Induces Mitochondria-Dependent Apoptosis in Human Gastric Cancer Cells via the Sustained Activation of ERK1/2 Pathway. Mol. Cells 2016, 39, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.; Wang, J.; Morazzoni, P.; Hodis, H.N.; Sevanian, A. The phytoestrogen equol increases nitric oxide availability by inhibiting superoxide production: An antioxidant mechanism for cell-mediated LDL modification. Free. Radic. Biol. Med. 2003, 34, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Han, M.H.; Han, S.-B.; Lee, K.; Park, S.-K.; Kim, H.M. Equol inhibits nitric oxide production and inducible nitric oxide synthase gene expression through down-regulating the activation of Akt. Int. Immunopharmacol. 2007, 7, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J. Evaluation of Equol Function on Anti- or Prooxidant Status in vivo. J. Food Sci. 2009, 74, H65–H71. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.J.; Ni, Y.D.; Lu, L.Z.; Grossmann, R.; Zhao, R.Q. The effect of equol injection in ovo on posthatch growth, meat quality and antioxidation in broilers. Animal 2011, 5, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Magnet, U.; Urbanek, C.; Gaisberger, D.; Tomeva, E.; Dum, E.; Pointner, A.; Haslberger, A. Topical equol preparation improves structural and molecular skin parameters. Int. J. Cosmet. Sci. 2017, 39, 535–542. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Aresta, A.; Di Grumo, F.; Zambonin, C. Determination of Major Isoflavones in Soy Drinks by Solid-Phase Micro Extraction Coupled to Liquid Chromatography. Food Anal. Methods 2015, 9, 925–933. [Google Scholar] [CrossRef]
- Mazur, W.; Fotsis, T.; Wahala, K.; Ojala, S.; Salakka, A.; Adlercreutz, H. Isotope Dilution Gas Chromatographic–Mass Spectrometric Method for the Determination of Isoflavonoids, Coumestrol, and Lignans in Food Samples. Anal. Biochem. 1996, 233, 169–180. [Google Scholar] [CrossRef]
- Bustamante-Rangel, M.; Milagros Delgado-Zamarreno, M.; Perez-Martin, L.; Rodriguez-Gonzalo, E.; Dominguez-Alvarez, J. Analysis of Isoflavones in Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef] [Green Version]
- Clarke, D.B.; Lloyd, A.S.; Botting, N.P.; Oldfield, M.F.; Needs, P.W.; Wiseman, H. Measurement of intact sulfate and glucuronide phytoestrogen conjugates in human urine using isotope dilution liquid chromatography-tandem mass spectrometry with [13C3]isoflavone internal standards. Anal. Biochem. 2002, 309, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Plomley, J.B.; Jackson, R.L.; Schwen, R.J.; Greiwe, J.S. Development of chiral liquid chromatography–tandem mass spectrometry isotope dilution methods for the determination of unconjugated and total S-equol in human plasma and urine. J. Pharm. Biomed. Anal. 2011, 55, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Yerramsetty, V.; Roe, M.; Cohen, J.; Hegeman, A.; Ismail, B. Development of a Simple, Fast, and Accurate Method for the Direct Quantification of Selective Estrogen Receptor Modulators Using Stable Isotope Dilution Mass Spectrometry. J. Agric. Food Chem. 2013, 61, 7028–7037. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Cole, S.J. Method of Defining Equol-Producer Status and Its Frequency among Vegetarians. J. Nutr. 2006, 136, 2188–2193. [Google Scholar] [CrossRef] [Green Version]
- Bensaada, S.; Raymond, I.; Pellegrin, I.; Viallard, J.-F.; Bennetau-Pelissero, C. Validation of ELISAs for Isoflavones and Enterolactone for Phytoestrogen Intake Assessment in the French Population. Nutrients 2023, 15, 967. [Google Scholar] [CrossRef] [PubMed]
| Key Steps | Precursor | Number of Steps | Overall Yield | References |
|---|---|---|---|---|
| Reduction reaction | Daidzein | 1 | 61% b | [32] |
| Evans alkylation | Benzyl chlorides | 6 | 9.8% | [33] |
| Diels–Alder reaction | o-Quinone methides and aryl-substituted enol ethers | 3 a | 30.75% b | [34] |
| Friedel–Crafts acylation reaction | M-methoxyphenol and p-hydroxyphenylacetic acid | 4 | 22.3% b | [35] |
| Wittig reaction | Resorcinol | 7 | 31.0% b | [36] |
| Allylic substitution | Ethyl L-(-)-lactate | 11 | 31.6% | [37] |
| Reduced in a enanti-oselective manner | Daidzein | 4 | 44.46% | [38] |
| Asymmetric hydrogenation | α-arylcinnamic acids | 6 | 48.4% | [39] |
| Substrate | Product | Bacterial Strains | Origins | Conversion Efficiency/Time | Classifications | References |
|---|---|---|---|---|---|---|
| Group I | ||||||
| Daidzein | DHD | |||||
| HGH6 | Human | 9.3%/7 d | Clostridium | [65] | ||
| Niu-O16 | Bovine | 100%/40 h | Lactobacillus | [66] | ||
| TM-40 | Human | 61.1%/24 h | Coprobacillus | [67] | ||
| AUH-JLR41 | Rabbit | NA | Slackia equolifaciens | [68] | ||
| HXBM408 | Pregnant horse | NA | Pediococcus acidilactici | [69] | ||
| Group II | ||||||
| Daidzein | Equol | |||||
| - | Human | NA | Bifidobacterium | [70] | ||
| Pure culture | Bifidobacterium animalis | |||||
| EP | Human | NA | Veillonella | [28] | ||
| EPI1 | Enterococcus faecium | |||||
| EPI3 | Finegoldia magna | |||||
| EPI2 | Lactobacillus mucosae | |||||
| AHU1763 | Rat | NA | Asaccharobacter celatus | [47] | ||
| zx-5, zx-7 | Suzhong sows | NA | Clostridium bifermentans | [71] | ||
| 20-92 | Human | 89.4%/1 h | Lactococcus garvieae | [51] | ||
| FJC-B9 | Human | NA | Adlercreutzia equolifaciens | [72] | ||
| YY7918 | Human | 100%/72 h | Eggerthella | [73] | ||
| do03 | Rat | 17%/96 h | Asaccharobacter celatus | [74] | ||
| LH-52 | Rat | NA | Proteus mirabilis | [63] | ||
| MT1B8 | Mouse | 100%/18 h | Enterorhabdus mucosicola | |||
| AUH-JLM455 | Mouse | NA | Acinetobacter sp. (Patent) | [75] | ||
| D1/D2 | Pig | 1.75%/48 h | Eubacterium | [48] | ||
| JCM1123(T) | Mouse | NA | Lactobacillus collinoides | [76] | ||
| HE8 | Human | 61.9%/14 h | Slackia isoflavoniconvertens | [77] | ||
| 22 strains | NA | Bifidobacterium | [78] | |||
| DZE | 85.6%/120 h | Slackia equolifaciens | [79] | |||
| NATTS | ≥90%/8 h | Slackia | [80] | |||
| ATCC9338 | Mouse | NA | Lactobacillus fermentum | [81] | ||
| ATCC15700 | Human | 78.5%/96 h | Bifidobacterium breve | [82] | ||
| BB536 | 77.2%/96 h | Bifidobacterium longum | ||||
| HY-1 | Human | NA | Enterococcus faecium | [83] | ||
| HY-2 | Slackia isoflavoniconvert ens | |||||
| AUH-Julong365 | Human | NA | Eggerthella | [84] | ||
| SNR | Stinky tofu | 12~90%/24 h | Coriobacteriaceae | [50] | ||
| TM-30 | Human | 52%/72 h | Coriobacteriaceae | [85] | ||
| C1 | Chicken | NA | Clostridium | [86] | ||
| CS1 | Human | NA | Pediococcus pentosaceus | [56] | ||
| CS2(JS1) | Lactobacillus paracasei | |||||
| CS3 | Lactobacillus sakei/graminis | |||||
| JCM 7548 | Rat | 29.5%/48 h | Lactobacillus intestinalis | [64] | ||
| Y11 | Human | 56%/120 h | Slackia equiolifaciens | [87] | ||
| DHD | Equol | |||||
| SNU Julong 732 | Human | >80%/96 h | Eggerthella | [42] | ||
| FJC-A10/FJC-A161 | Human | NA | Adlercreutzia equolifaciens | [72] | ||
| Group III | ||||||
| Daidzein | O-DMA | |||||
| HGH 136 | Human | NA | Clostridium | [61] | ||
| wK1 | Human | NA | Eubacterium ramulus | [88] | ||
| AUH-HM195 | Brown pheasant | NA | Enterococcus hirae | [62] | ||
| AUH-JLC108 | Chicken | 80%/24 h | Clostridium | [89] | ||
| AUH-JLC140 | Chicken | NA | Clostridium | [90] | ||
| Detection Methods | Principle | Advantages | Disadvantages |
|---|---|---|---|
| HPLC | Separation is carried out by taking advantage of the difference in the distribution of analytes with mobile and stationary phases. | High sensitivity, high flow rate, high separation efficiency, suitable for macromolecules, thermally unstable substances | Long analysis time, low resolution, and short column lifetime |
| MS | Analyze by measuring the mass-to-charge ratio of the tested sample ions. | Good specificity, high sensitivity and fast analysis | High cost, the complex preparation and labor-intensive sample preparation |
| UV-Vis | Molecules and ions in a substance are used to absorb light in its wavelength range. | Easy operation, high accuracy, fast detection speed, low measurement cost | Poor characterization and limitations of qualitative analysis |
| ELISA | Assay using the color displayed after the analyte reacts with the enzyme. | Simple operation, good reproducibility and high sensitivity | Sometimes nonspecific reactions occur |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, Y.; Lv, J.; Pang, X.; Zhang, S.; Zhang, G.; Liu, L.; Wang, Y.; Li, C. Advances in the Metabolic Mechanism and Functional Characteristics of Equol. Foods 2023, 12, 2334. https://doi.org/10.3390/foods12122334
Gong Y, Lv J, Pang X, Zhang S, Zhang G, Liu L, Wang Y, Li C. Advances in the Metabolic Mechanism and Functional Characteristics of Equol. Foods. 2023; 12(12):2334. https://doi.org/10.3390/foods12122334
Chicago/Turabian StyleGong, Yining, Jiaping Lv, Xiaoyang Pang, Shuwen Zhang, Guofang Zhang, Libo Liu, Yunna Wang, and Chun Li. 2023. "Advances in the Metabolic Mechanism and Functional Characteristics of Equol" Foods 12, no. 12: 2334. https://doi.org/10.3390/foods12122334
APA StyleGong, Y., Lv, J., Pang, X., Zhang, S., Zhang, G., Liu, L., Wang, Y., & Li, C. (2023). Advances in the Metabolic Mechanism and Functional Characteristics of Equol. Foods, 12(12), 2334. https://doi.org/10.3390/foods12122334