Pungency Perception and the Interaction with Basic Taste Sensations: An Overview
Abstract
:1. Introduction
2. Common Pungent Ingredients
2.1. Capsaicinoids
2.2. Allicin
2.3. Allyl Isothiocyanate (AITC)
2.4. Piperine
2.5. Gingerols and Derivatives
2.6. Sanshools
2.7. Other Pungent Substances
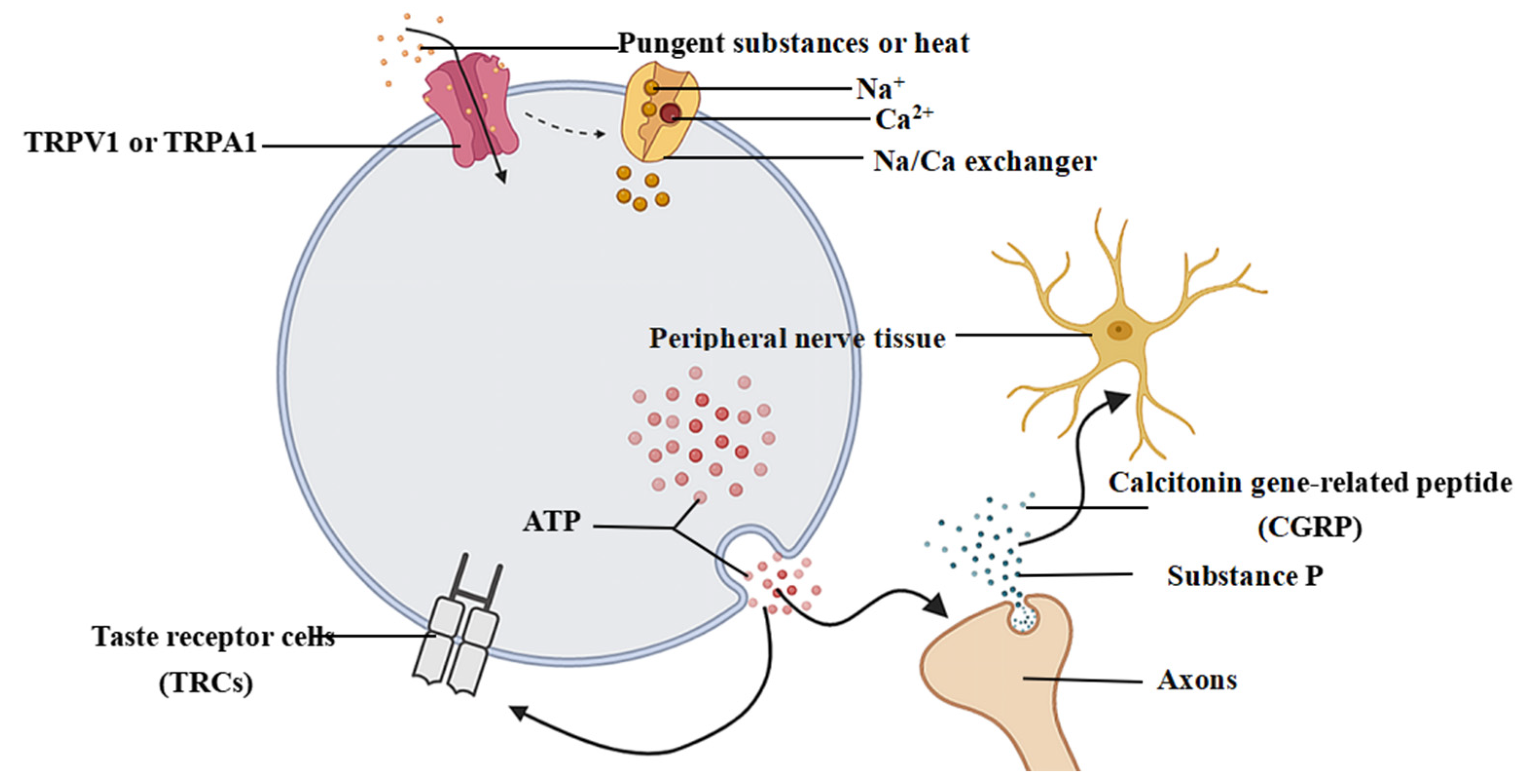
3. Sensory Basis and Transmission of Pungent Sensation
3.1. Perceptual Mechanisms Associated with Pungent Sensory Information
3.2. Transmission and Generation of Pungent Sensation
4. The Interaction between Pungent Sensation and Taste Sensation
4.1. The Interaction between Pungent Sensation and Taste Sensation in the Saliva
4.2. Interaction between Pungent Sensation and Various Tastes
4.2.1. Interaction between Pungent Sensation and Salty Sensation
4.2.2. Interaction between Pungent Sensation and Umami, Sweet, and Bitter Sensation
4.2.3. Interaction between Pungent Sensation and Sour Sensation
4.3. Interaction between Pungent Sensation and Taste Sensation in the Peripheral and Central Nerves
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Govindarajan, V.S.; Sathyanarayana, M.N. Capsicum—Production, technology, chemistry, and quality. Part V. Impact on physiology, pharmacology, nutrition, and metabolism; structure, pungency, pain, and desensitization sequences. Crit. Rev. Food Sci. Nutr. 1991, 29, 435–474. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawless, H.; Rozin, P.; Shenker, J. Effects of oral capsaicin on gustatory, olfactory and irritant sensations and flavor identification in humans who regularly or rarely consume chili pepper. Chem. Senses 1985, 10, 579–589. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Zhong, K.; Shi, B.; Wang, H.; Wang, S. Pungency of Chinese pepper: Its perception and preference. Sci. Talks 2022, 2, 100009. [Google Scholar] [CrossRef]
- Green, B.G. Chemesthesis: Pungency as a component of flavor. Trends Food Sci. Technol. 1996, 7, 415–420. [Google Scholar] [CrossRef]
- Spencer, M.; Dalton, P. The third dimension of flavor: A chemesthetic approach to healthier eating (a review). J. Sens. Stud. 2020, 35, e12551. [Google Scholar] [CrossRef]
- Aroke, E.N.; Powell-Roach, K.L.; Jaime-Lara, R.B.; Tesfaye, M.; Roy, A.; Jackson, P.; Joseph, P.V. Taste the Pain: The Role of TRP Channels in Pain and Taste Perception. Int. J. Mol. Sci. 2020, 21, 5929. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, X.; Jiang, S.; Zhang, Y.; Zhang, L.; Liu, Y. Multi-dimensional pungency and sensory profiles of powder and oil of seven chili peppers based on descriptive analysis and Scoville heat units. Food Chem. 2023, 411, 135488. [Google Scholar] [CrossRef]
- Justyna, W.; Robert, F.; Tomasz, G.; Agnieszka, Z. High-performance liquid chromatography with fluorescence detection for the determination of capsaicin and dihydrocapsaicin in fat-burning dietary supplements. Anal. Lett. 2021, 54, 2097–2112. [Google Scholar] [CrossRef]
- Araceli, P.; Luis, A.; Luz, E.V. Analysis of capsaicin and dihydrocapsaicin in hot peppers by ultrasound assisted extraction followed by gas chromatography-mass spectrometry. Instrum. Sci. Technol. 2012, 40, 429–440. [Google Scholar] [CrossRef]
- Tian, K.; Wang, W.; Yao, Y.; Nie, X.; Lu, A.; Wu, Y.; Han, C. Rapid identification of gutter oil by detecting the capsaicin using surface enhanced Raman spectroscopy. J. Raman Spectrosc. 2018, 49, 472–481. [Google Scholar] [CrossRef]
- Sarma, M.; del Valle, M. Improved sensing of capsaicin with TiO2 nanoparticles modified epoxy graphite electrode. Electroanalysis. 2020, 32, 230. [Google Scholar] [CrossRef]
- Ivet, J.; Clara, P.; Núria, S.; Manel, D.; José, M. Carbon based electrodes for the voltammetric determination of capsaicin in spicy samples. Microchem. J. 2023, 191, 108757. [Google Scholar] [CrossRef]
- Braud, A.; Boucher, Y. Intra-oral trigeminal-mediated sensations influencing taste perception: A systematic review. J. Oral Rehabil. 2020, 47, 258–269. [Google Scholar] [CrossRef]
- Prasad, N.S.; Raghavendra, R.; Lokesh, B.R.; Naidu, K.A. Spice phenolics inhibit human PMNL 5-lipoxygenase. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 521–528. [Google Scholar] [CrossRef]
- Tanrıkulu-Küçük, S.; Başaran-Küçükgergin, C.; Seyithanoğlu, M.; Doğru-Abbasoğlu, S.; Koçak, H.; Beyhan-Özdaş, Ş.; Öner-İyidoğan, Y. Effect of dietary curcumin and capsaicin on testicular and hepatic oxidant-antioxidant status in rats fed a high-fat diet. Appl. Physiol. Nutr. Metab. 2019, 4, 774–782. [Google Scholar] [CrossRef]
- Montanari, T.; Boschi, F.; Colitti, M. Comparison of the Effects of Browning-Inducing Capsaicin on Two Murine Adipocyte Models. Front. Physiol. 2019, 10, 1380. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Q.; Guo, W.; Tang, X.; Cui, S.; Zhang, F.; Liu, X.; Zhao, J.; Zhang, H.; Mao, B.; Chen, W. Capsaicin—The spicy ingredient of chili peppers: A review of the gastrointestinal effects and mechanisms. Trends Food Sci. Technol. 2021, 116, 755–765. [Google Scholar] [CrossRef]
- Stevens, R.M.; Ervin, J.; Nezzer, J.; Nieves, Y.; Guedes, K.; Burges, R.; Hanson, P.D.; Campbell, J.N. Randomized, double-Blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthritis Rheumatol. 2019, 71, 1524–1533. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.; Wang, W.; Huang, K.; Huang, J.; Chu, X.; Wang, F.; Pang, L.; Wang, Y.; Sun, X. Novel capsaicin releasing system targeted protects ischemic brain from cardiac arrest. J. Drug. Deliv. Sci. Technol. 2022, 70, 103229. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, P.; Xia, F.; Tang, H.; Chen, J.; Zhang, J.; Liu, D.; Zhu, Y.; Liu, Y.; Gu, L.; et al. Capsaicin ameliorates inflammation in a TRPV1-independent mechanism by inhibiting PKM2-LDHA-mediated Warburg effect in sepsis. Cell Chem. Biol. 2022, 29, 1248–1259.e1246. [Google Scholar] [CrossRef] [PubMed]
- Thongin, S.; Den-Udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laolob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Guo, Y.; Zhang, S. Protective effect and molecular mechanism of dihydrocapsaicin independent of induced mild hypothermia on brain injury after the resuscitation in cardiac arrest patients. Chin. J. Crit. Care Med. 2022, 42, 850–857. [Google Scholar]
- Tiwari, A.; Mahadik, K.R.; Gabhe, S.Y. Piperine: A comprehensive review of methods of isolation, purification, and biological properties. Med. Drug Discov. 2020, 7, 100027. [Google Scholar] [CrossRef]
- Arora, P.; Athari, S.S.; Nainwal, L.M. Piperine attenuates production of inflammatory biomarkers, oxidative stress and neutrophils in lungs of cigarette smoke-exposed experimental mice. Food Biosci. 2022, 49, 101909. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, J.; Jin, W.; Yang, J.; Yu, G.; Shi, H.; Shi, K. Piperine alleviates acute pancreatitis: A possible role for FAM134B and CCPG1 dependent ER-phagy. Phytomedicine 2022, 105, 154361. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Huang, J.; Himabindu, K.; Tewari, D.; Horbańczuk, J.O.; Xu, S.; Chen, Z.; Atanasov, A.G. Cardiovascular protective effect of black pepper (Piper nigrum L.) and its major bioactive constituent piperine. Trends Food Sci. Technol. 2021, 117, 34–45. [Google Scholar] [CrossRef]
- He, Q.; Xu, J.; Gu, J.; Tong, X.; Wan, Z.; Gu, Y.; Fang, C.; Qin, L. Piperine is capable of improving pancreatic β-cell apoptosis in high fat diet and streptozotocin induced diabetic mice. J. Funct. Foods 2022, 88, 104890. [Google Scholar] [CrossRef]
- Carp, O.E.; Moraru, A.; Pinteala, M.; Arvinte, A. Electrochemical behaviour of piperine. Comparison with control antioxidants. Food Chem. 2021, 339, 128110. [Google Scholar] [CrossRef]
- He, J.; Le, Q.; Wei, Y.; Yang, L.; Cai, B.; Liu, Y.; Hong, B. Effect of piperine on the mitigation of obesity associated with gut microbiota alteration. Curr. Res. Food Sci. 2022, 5, 1422–1432. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, L.; Wang, W.; Che, H.; Zhang, Y. Piperine attenuates hepatic steatosis and insulin resistance in high-fat diet-induced obesity in Sprague-Dawley rats. Nutr. Res. 2022, 108, 9–21. [Google Scholar] [CrossRef]
- Chang, P.Y.; Tsai, F.J.; Bau, D.T.; Hsu, Y.M.; Yang, J.S.; Tu, M.G.; Chiang, S.L. Potential effects of allyl isothiocyanate on inhibiting cellular proliferation and inducing apoptotic pathway in human cisplatin-resistant oral cancer cells. J. Formos. Med. Assoc. 2021, 120, 515–523. [Google Scholar] [CrossRef]
- Park, S.; Barton, M.; Pendleton, P. Controlled release of allyl isothiocyanate for bacteria growth management. Food Control 2012, 23, 478–484. [Google Scholar] [CrossRef]
- Yang, B.; Li, L.; Geng, H.; Zhang, C.; Wang, G.; Yang, S.; Gao, S.; Zhao, Y.; Xing, F. Inhibitory effect of allyl and benzyl isothiocyanates on ochratoxin a producing fungi in grape and maize. Food Microbiol. 2021, 100, 103865. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y. Allicin promotes autophagy and ferroptosis in esophageal squamous cell carcinoma by activating AMPK/mTOR signaling. Heliyon 2022, 8, e11005. [Google Scholar] [CrossRef]
- Li, X.; Ni, J.; Tang, Y.; Wang, X.; Tang, H.; Li, H.; Zhang, S.; Shen, X. Allicin inhibits mouse colorectal tumorigenesis through suppressing the activation of STAT3 signaling pathway. Nat. Prod. Res. 2019, 33, 2722–2725. [Google Scholar] [CrossRef] [PubMed]
- Reiter, J.; Hubbers, A.M.; Albrecht, F.; Leichert, L.I.O.; Slusarenko, A.J. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria. Int. J. Med. Microbiol. 2020, 310, 151359. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, S.; Liang, S.; Duan, C.; Xu, Z.; Zhao, L.; Wen, F.; Li, Q.; Li, Y.; Zhang, J. Hypertensive vascular and cardiac remodeling protection by allicin in spontaneous hypertension rats via CaMK Ⅱ/NF-kappaB pathway. Biomed. Pharmacother. 2022, 155, 113802. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, A.; Kumari, A.; Singh, M.; Kumar, S.; Kumar, S.; Dabla, A.; Chaturvedi, S.; Yadav, V.; Chattopadhyay, D.; Prakash Dwivedi, V. [6]-Gingerol exhibits potent anti-mycobacterial and immunomodulatory activity against tuberculosis. Int. Immunopharmacol. 2020, 87, 106809. [Google Scholar] [CrossRef]
- Dutta, A.; Hsiao, S.; Hung, C.; Chang, C.; Lin, Y.; Lin, C.; Chen, T.; Huang, C. Effect of [6]-gingerol on viral neuraminidase and hemagglutinin-specific T cell immunity in severe influenza. Phytomed. Plus 2023, 3, 100387. [Google Scholar] [CrossRef]
- Zahoor, A.; Yang, C.; Yang, Y.; Guo, Y.; Zhang, T.; Jiang, K.; Guo, S.; Deng, G. 6-Gingerol exerts anti-inflammatory effects and protective properties on LTA-induced mastitis. Phytomedicine 2020, 76, 153248. [Google Scholar] [CrossRef]
- Mi, H.; Guo, X.; Li, J. Effect of 6-gingerol as natural antioxidant on the lipid oxidation in red drum fillets during refrigerated storage. LWT 2016, 74, 70–76. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L. Inhibitory effect 6-gingerol on adipogenesis through activation of the Wnt/beta-catenin signaling pathway in 3T3-L1 adipocytes. Toxicol. Vitr. 2015, 30 Pt B, 394–401. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Sun, L.; Pei, K.; Chen, L.; Zhang, S.; Hu, M. Protective effects of hydroxy-α-sanshool from the pericarp of Zanthoxylum bungeanum Maxim. On D-galactose/AlCl3-induced Alzheimer’s disease-like mice via Nrf2/HO-1 signaling pathways. Eur. J. Pharmacol. 2022, 914, 174691. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Chen, X.; Yuan, S.; Xu, T.; Zhao, W.; Li, M.; Wang, F.; Hoffman, R.M.; Jia, L. Evodiamine inhibits ESCC by inducing M-phase cell-cycle arrest via CUL4A/p53/p21 axis and activating noxa-dependent intrinsic and DR4-dependent extrinsic apoptosis. Phytomedicine 2023, 108, 154493. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Pickersgill, B. Peppers and chillies. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 4460–4467. [Google Scholar]
- Miron, T.; Rabinkov, A.; Mirelman, D.; Wilchek, M.; Weiner, L. The mode of action of allicin: Its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim. Biophys. Acta Biomembr. 2000, 1463, 20–30. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.K.; Maulik, S.K. Effect of garlic on cardiovascular disorders: A review. Nutr. J. 2002, 1, 4. [Google Scholar] [CrossRef]
- Khanum, F.; Anilakumar, K.R.; Viswanathan, K.R. Anticarcinogenic properties of garlic: A review. Crit. Rev. Food Sci. Nutr. 2004, 44, 479–488. [Google Scholar] [CrossRef]
- Salazar, H.; Llorente, I.; Jara-Oseguera, A.; Garcia-Villegas, R.; Munari, M.; Gordon, S.E.; Islas, L.D.; Rosenbaum, T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 2008, 11, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Jancso, N.; Jancso-Gabor, A.; Szolcsanyi, J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. 1967, 31, 138–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daxenbichler, M.E.; Spencer, G.F.; Carlson, D.G.; Rose, G.B.; Brinker, A.M.; Powell, R.G. Glucosinolate composition of seeds from 297 species of wild plants. Phytochemistry 1991, 30, 2623–2638. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Xue, C.; He, Z.; Wang, Z.; Qin, F.; Chen, J.; Zeng, M. The inhibitory effects of yellow mustard (Brassica juncea) and its characteristic pungent ingredient allyl isothiocyanate (AITC) on PhIP formation: Focused on the inhibitory pathways of AITC. Food Chem. 2022, 373 Pt A, 131398. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Boschin, G.; Resta, D. Alkaloids Derived from Lysine: Quinolizidine (a Focus on Lupin Alkaloids). In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 381–403. [Google Scholar]
- Szallasi, A. Piperine: Researchers discover new flavor in an ancient spice. Trends Pharmacol. Sci. 2005, 26, 437–439. [Google Scholar] [CrossRef]
- Morera, E.; De Petrocellis, L.; Morera, L.; Moriello, A.S.; Nalli, M.; Di Marzo, V.; Ortar, G. Synthesis and biological evaluation of [6]-gingerol analogues as transient receptor potential channel TRPV1 and TRPA1 modulators. Bioorg. Med. Chem. 2012, 22, 1674–1677. [Google Scholar] [CrossRef]
- Menon, V.; Elgharib, M.; El-Awady, R.; Saleh, E. Ginger: From serving table to salient therapy. Food Biosci. 2021, 41, 100934. [Google Scholar] [CrossRef]
- Luo, J.; Ke, J.; Hou, X.; Li, S.; Luo, Q.; Wu, H.; Shen, G.; Zhang, Z. Composition, structure and flavor mechanism of numbing substances in Chinese prickly ash in the genus Zanthoxylum: A review. Food Chem. 2022, 373 Pt B, 131454. [Google Scholar] [CrossRef]
- Li, Y.; Hao, D.; Jiang, X. Advances in pharmacological research of sanshool. Chin. Pharmacol. Bull. 2019, 35, 172–175. [Google Scholar]
- Ivane, N.M.A.; Haruna, S.A.; Zekrumah, M.; Roméo Elysé, F.K.; Hassan, M.O.; Hashim, S.B.H.; Tahir, H.E.; Zhang, D. Composition, mechanisms of tingling paresthesia, and health benefits of Sichuan pepper: A review of recent progress. Trends Food Sci. Technol. 2022, 126, 1–12. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Narula, A.; Liu, L.; Ahn, K. Molecular targets and anticancer potential of evodiamine. Phytochem. Lett. 2022, 52, 92–103. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Ugawa, S.; Ueda, T.; Murakami, S.; Shimada, S. Vanilloid receptor subtype-1 (VR1) is specifically localized to taste papillae. Mol. Brain Res. 2002, 107, 17–22. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [Green Version]
- Nilius, B.; Appendino, G. Spices: The savory and beneficial science of pungency. Rev. Physiol. Biochem. Pharmacol. 2013, 164, 1–76. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Yin, Y.; Vu, S.; Yang, F.; Yarov-Yarovoy, V.; Tian, Y.; Zheng, J. A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem. Biophys. Res. Commun. 2019, 516, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Molecular mechanism of TRP channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riera, C.E.; Menozzi-Smarrito, C.; Affolter, M.; Michlig, S.; Munari, C.; Robert, F.; Vogel, H.; Simon, S.A.; le Coutre, J. Compounds from Sichuan and Melegueta peppers activate, covalently and non-covalently, TRPA1 and TRPV1 channels. Br. J. Pharmacol. 2009, 157, 1398–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roper, S.D. TRPs in taste and chemesthesis. Handb. Exp. Pharmacol. 2014, 223, 827–871. [Google Scholar] [CrossRef] [Green Version]
- Choy, M.; El Fassi, S.; Treur, J. An adaptive network model for pain and pleasure through spicy food and its desensitization. Cogn. Syst. Res. 2021, 66, 211–220. [Google Scholar] [CrossRef]
- Tizzano, M.; Gulbransen, B.D.; Vandenbeuch, A.; Clapp, T.R.; Herman, J.P.; Sibhatu, H.M.; Churchill, M.E.; Silver, W.L.; Kinnamon, S.C.; Finger, T.E. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc. Natl. Acad. Sci. USA 2010, 107, 3210–3215. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Angeles, M.; Morales-Lazaro, S.L.; Juarez-Gonzalez, E.; Rosenbaum, T. TRPV1: Structure, endogenous agonists, and mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Akopian, A.N. Regulation of nociceptive transmission at the periphery via TRPA1-TRPV1 interactions. Curr. Pharm. Biotechnol. 2011, 12, 89–94. [Google Scholar] [CrossRef]
- Vandewauw, I.; Owsianik, G.; Voets, T. Systematic and quantitative mRNA expression analysis of TRP channel genes at the single trigeminal and dorsal root ganglion level in mouse. BMC Neurosci. 2013, 14, 21. [Google Scholar] [CrossRef] [Green Version]
- Paulsen, C.E.; Armache, J.P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 ion channel suggests regulatory mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The pungency of garlic: Activation of TRPA1 and TRPV1 in response to allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef] [Green Version]
- Iwata, H.; Kanda, N.; Araki, M.; Sagae, Y.; Masuda, K.; Okuno, Y. Discovery of natural TRPA1 activators through pharmacophore-based virtual screening and a biological assay. Bioorg. Med. Chem. 2021, 31, 127639. [Google Scholar] [CrossRef]
- Rhyu, M.R.; Kim, Y.; Lyall, V. Interactions between chemesthesis and taste: Role of TRPA1 and TRPV1. Int. J. Mol. Sci. 2021, 22, 3360. [Google Scholar] [CrossRef]
- Eib, S.; Schneider, D.J.; Hensel, O.; Seuß-Baum, I. Evaluation of trigeminal pungency perception of allyl isothiocyanate—A time intensity (TI) study. Food Qual. Prefer. 2021, 87, 104039. [Google Scholar] [CrossRef]
- Iwasaki, Y.; Morita, A.; Iwasawa, T.; Kobata, K.; Sekiwa, Y.; Morimitsu, Y.; Kubota, K.; Watanabe, T. A nonpungent component of steamed ginger—[10]-shogaol—Increases adrenaline secretion via the activation of TRPV1. Nutr. Neurosci. 2013, 9, 169–178. [Google Scholar] [CrossRef]
- Delmas, P.; Coste, B. SnapShot: Orofacial sensation. Cell 2020, 183, 284. [Google Scholar] [CrossRef]
- Klein, A.H. The orotrigeminal system. Handb. Clin. Neurol. 2019, 164, 205–216. [Google Scholar] [CrossRef]
- Leijon, S.C.M.; Neves, A.F.; Breza, J.M.; Simon, S.A.; Chaudhari, N.; Roper, S.D. Oral thermosensing by murine trigeminal neurons: Modulation by capsaicin, menthol and mustard oil. J. Physiol. 2019, 597, 2045–2061. [Google Scholar] [CrossRef] [Green Version]
- Yarmolinsky, D.A.; Peng, Y.; Pogorzala, L.A.; Rutlin, M.; Hoon, M.A.; Zuker, C.S. Coding and plasticity in the mammalian thermosensory system. Neuron 2016, 92, 1079–1092. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, G.; Allison, T.; Spencer, D.D. Localization of the face area of human sensorimotor cortex by intracranial recording of somatosensory evoked potentials. J. Neurosurg. 1993, 79, 874–884. [Google Scholar] [CrossRef]
- Saito, H.; Katagiri, A.; Okada, S.; Mikuzuki, L.; Kubo, A.; Suzuki, T.; Ohara, K.; Lee, J.; Gionhaku, N.; Iinuma, T.; et al. Ascending projections of nociceptive neurons from trigeminal subnucleus caudalis: A population approach. Exp. Neurol. 2017, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Carstens, E.; Kuenzler, N.; Handwerker, H.O. Activation of neurons in rat trigeminal subnucleus caudalis by different irritant chemicals applied to oral or ocular mucosa. J. Neurophysiol. 1998, 80, 465–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, A.; Carstens, M.I.; Carstens, E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J. Neurophysiol. 2011, 106, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Aicher, S.A.; Hermes, S.M.; Hegarty, D.M. Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience 2013, 232, 182–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, M.C.; Bowen, A.; Schier, L.A.; Tupone, D.; Uddin, O.; Heinricher, M.M. Parabrachial complex: A hub for pain and aversion. J. Neurosci. 2019, 39, 8225–8230. [Google Scholar] [CrossRef] [Green Version]
- Chiang, M.C.; Nguyen, E.K.; Canto-Bustos, M.; Papale, A.E.; Oswald, A.M.; Ross, S.E. Divergent neural pathways emanating from the lateral parabrachial nucleus mediate distinct components of the pain response. Neuron 2020, 106, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.M.; Duus, E.M.; Friend, J. Experience of weight loss and its burden in patients with non-small cell lung cancer: Results of an online survey. J. Clin. Oncol. 2016, 34 (Suppl. S26), 71. [Google Scholar] [CrossRef]
- Campos, C.A.; Bowen, A.J.; Roman, C.W.; Palmiter, R.D. Encoding of danger by parabrachial CGRP neurons. Nature 2018, 555, 617–622. [Google Scholar] [CrossRef]
- Chen, J.Y.; Campos, C.A.; Jarvie, B.C.; Palmiter, R.D. Parabrachial CGRP neurons establish and sustain aversive taste memories. Neuron 2018, 100, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Han, J.S.; Adwanikar, H.; Li, Z.; Ji, G.; Neugebauer, V. Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol. Pain 2010, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Morrison, S.F.; Nakamura, K. Central mechanisms for thermoregulation. Annu. Rev. Physiol. 2019, 81, 285–308. [Google Scholar] [CrossRef]
- Brown, F.; Mackie, A.; He, Q.; Branch, A.; Sarkar, A. Protein-saliva interactions: A systematic review. Food Funct. 2021, 12, 3324–3351. [Google Scholar] [CrossRef]
- Yang, N.; Galves, C.; Racioni Goncalves, A.C.; Chen, J.; Fisk, I. Impact of capsaicin on aroma release: In vitro and in vivo analysis. Food Res. Int. 2020, 133, 109197. [Google Scholar] [CrossRef]
- Kono, Y.; Kubota, A.; Taira, M.; Katsuyama, N.; Sugimoto, K. Effects of oral stimulation with capsaicin on salivary secretion and neural activities in the autonomic system and the brain. J. Dent. Sci. 2018, 13, 116–123. [Google Scholar] [CrossRef]
- Galaniha, L.T.; Nolden, A.A. The role of saliva in taste dysfunction among cancer patients: Mechanisms and potential treatment. Oral Oncol. 2022, 133, 106030. [Google Scholar] [CrossRef]
- Gardner, A.; So, P.W.; Carpenter, G. Endogenous salivary citrate is associated with enhanced rheological properties following oral capsaicin stimulation. Exp. Physiol. 2020, 105, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Heinzerling, C.I.; Stieger, M.; Bult, J.H.; Smit, G. Individually Modified Saliva Delivery Changes the Perceived Intensity of Saltiness and Sourness. Chemosens. Percept. 2011, 4, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Munoz-Gonzalez, C.; Feron, G.; Canon, F. Main effects of human saliva on flavour perception and the potential contribution to food consumption. Proc. Nutr. Soc. 2018, 77, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Stolle, T.; Grondinger, F.; Dunkel, A.; Meng, C.; Medard, G.; Kuster, B.; Hofmann, T. Salivary Proteome Patterns Affecting Human Salt Taste Sensitivity. J. Agric. Food Chem. 2017, 65, 9275–9286. [Google Scholar] [CrossRef]
- Ferry, A.-L.S.; Mitchell, J.R.; Hort, J.; Hill, S.E.; Taylor, A.J.; Lagarrigue, S.; Vallès-Pàmies, B. In-Mouth Amylase Activity Can Reduce Perception of Saltiness in Starch-Thickened Foods. J. Agric. Food Chem. 2006, 54, 8869–8873. [Google Scholar] [CrossRef]
- Matsuo, R. Role of Saliva in the Maintenance of Taste Sensitivity. Crit. Rev. Oral Biol. Med. 2000, 11, 216–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padiglia, A.; Zonza, A.; Atzori, E.; Chillotti, C.; Calo, C.; Tepper, B.J.; Barbarossa, I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010, 92, 539–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scinska-Bienkowska, A.; Wrobel, E.; Turzynska, D.; Bidzinski, A.; Jezewska, E.; Sienkiewicz-Jarosz, H.; Golembiowska, K.; Kostowski, W.; Kukwa, A.; Plaznik, A.; et al. Glutamate concentration in whole saliva and taste responses to monosodium glutamate in humans. Nutr. Neurosci. 2006, 9, 25–31. [Google Scholar] [CrossRef]
- Lawless, H.T.; Gillette, M. Sensory Responses to Oral Chemical Heat; American Chemical Society: Washington, DC, USA, 1985; Volume VIII. [Google Scholar]
- Le, B.; Yu, B.; Amin, M.S.; Liu, R.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends Food Sci. Technol. 2022, 129, 657–666. [Google Scholar] [CrossRef]
- Feldman, G.M.; Mogyorosi, A.; Heck, G.L.; Desimone, J.A.; Lyall, V. Salt-evoked lingual surface potential in humans. J. Neurophysiol. 2003, 90, 2060–2064. [Google Scholar] [CrossRef]
- Lyall, V.; Alam, R.I.; Phan, T.-H.T.; Russell, O.F.; Malik, S.A.; Heck, G.L.; DeSimone, J.A. Modulation of rat chorda tympani NaCl responses and intracellular Na+ activity in polarized taste receptor cells by pH. J. Gen. Physiol. 2002, 120, 793–815. [Google Scholar] [CrossRef] [Green Version]
- Rhyu, M.R.; Lyall, V. Interaction of taste-active nutrients with taste receptors. Curr. Opin. Physiol. 2021, 20, 64–69. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Benham, C.D.; Randall, A.; Davis, J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002, 23, 183–191. [Google Scholar] [CrossRef]
- Kasahara, Y.; Narukawa, M.; Ishimaru, Y.; Kanda, S.; Umatani, C.; Takayama, Y.; Tominaga, M.; Oka, Y.; Kondo, K.; Kondo, T.; et al. TMC4 is a novel chloride channel involved in high-concentration salt taste sensation. J. Physiol. Sci. 2021, 71, 23. [Google Scholar] [CrossRef]
- Huang, A.Y.; Wu, S.Y. Calcitonin gene-related peptide reduces taste-evoked ATP secretion from mouse taste buds. J. Neurosci. 2015, 35, 12714–12724. [Google Scholar] [CrossRef] [Green Version]
- Rhyu, M.R.; Song, A.Y.; Kim, E.Y.; Son, H.J.; Kim, Y.; Mummalaneni, S.; Qian, J.; Grider, J.R.; Lyall, V. Kokumi taste active peptides modulate salt and umami taste. Nutrients 2020, 12, 1198. [Google Scholar] [CrossRef] [Green Version]
- Graudal, N.; Jurgens, G.; Baslund, B.; Alderman, M.H. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: A meta-analysis. Am. J. Hypertens. 2014, 27, 1129–1137. [Google Scholar] [CrossRef] [Green Version]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.; de Araujo, I.E.; Gutierrez, R.; Nicolelis, M.A.L. The neural mechanisms of gustation: A distributed processing code. Nat. Rev. Neurosci. 2006, 7, 890–901. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Jin, R.; Lang, H.; Yu, H.; Sun, F.; He, C.; Ma, T.; Li, Y.; Zhou, X.; et al. Enjoyment of spicy flavor enhances central salty-taste perception and reduces salt intake and blood pressure. Hypertension 2017, 70, 1291–1299. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, D.; Zhang, J.; Hao, Z.; Zhao, X.; Sun, B.; Zhang, Y. Identification of novel umami peptides in chicken breast soup through a sensory-guided approach and molecular docking to the T1R1/T1R3 taste receptor. J. Agric. Food Chem. 2023, 71, 7803–7811. [Google Scholar] [CrossRef]
- Liang, L.; Zhou, C.; Zhang, J.; Huang, Y.; Zhao, J.; Sun, B.; Zhang, Y. Characteristics of umami peptides identified from porcine bone soup and molecular docking to the taste receptor T1R1/T1R3. Food Chem. 2022, 387, 132870. [Google Scholar] [CrossRef]
- Liang, L.; Duan, W.; Zhang, J.; Huang, Y.; Zhang, Y.; Sun, B. Characterization and molecular docking study of taste peptides from chicken soup by sensory analysis combined with nano-lc-q-tof-ms/ms. Food Chem. 2022, 383, 132455. [Google Scholar] [CrossRef]
- Taruno, A.; Nomura, K.; Kusakizako, T.; Ma, Z.; Nureki, O.; Foskett, J.K. Taste transduction and channel synapses in taste buds. Pflügers Arch. 2021, 473, 3–13. [Google Scholar] [CrossRef]
- Smutzer, G.; Devassy, R.K. Integrating TRPV1 Receptor Function with Capsaicin Psychophysics. Adv. Pharmacol. Sci. 2016, 2016, 1512457. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Escobedo, L.; Ornelas-Paz, J.J.; Olivas, G.I.; Guerrero-Beltran, J.A.; Jimenez-Castro, J.; Sepulveda, D.R. Determination of absolute threshold and just noticeable difference in the sensory perception of pungency. J. Food Sci. 2012, 77, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.T.; O’Mahony, M.; Carstens, E. Taste suppression following lingual capsaicin pre-treatment in humans. Chem. Senses 2002, 27, 353–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taruno, A.; Vingtdeux, V.; Ohmoto, M.; Ma, Z.; Dvoryanchikov, G.; Li, A.; Adrien, L.; Zhao, H.; Leung, S.; Abernethy, M.; et al. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013, 495, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef] [Green Version]
- Liman, E.R. Thermal gating of TRP ion channels: Food for thought? Sci. STKE 2006, 2006, 12. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.A.; Pereira, E.; Roper, S.D. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS ONE 2011, 6, 25471. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.A.; Dando, R.; Roper, S.D. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J. Neurosci. 2009, 29, 13909–13918. [Google Scholar] [CrossRef] [Green Version]
- Kapaun, C.L.; Dando, R. Deconvoluting physical and chemical heat: Temperature and spiciness influence flavor differently. Physiol. Behav. 2017, 170, 54–61. [Google Scholar] [CrossRef]
- Kuhn, C.; Bufe, B.; Winnig, M.; Hofmann, T.; Frank, O.; Behrens, M.; Lewtschenko, T.; Slack, J.P.; Ward, C.D.; Meyerhof, W. Bitter taste receptors for saccharin and acesulfame K. J. Neurosci. 2004, 24, 10260–10265. [Google Scholar] [CrossRef] [Green Version]
- Riera, C.E.; Vogel, H.; Simon, S.A.; le Coutre, J. Artificial sweeteners and salts producing a metallic taste sensation activate TRPV1 receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, 626–634. [Google Scholar] [CrossRef] [Green Version]
- Lindemann, B. Receptors and transduction in taste. Nature 2001, 413, 219–225. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Inada, H.; Kubota, M.; Zhuang, H.; Matsunami, H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 12569–12574. [Google Scholar] [CrossRef] [Green Version]
- Gilbertson, T.; Avenet, P.; Kinnamon, S.; Roper, S. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: Enhancement by vasopressin and cAMP. Neuron 1993, 10, 931–942. [Google Scholar] [CrossRef]
- Stevens, D.R.; Seifert, R.; Bufe, B.; Müller, F.; Kremmer, E.; Gauss, R.; Meyerhof, W.; Kaupp, U.B.; Lindemann, B. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature 2001, 413, 631–635. [Google Scholar] [CrossRef]
- Araki, M.; Kanda, N.; Iwata, H.; Sagae, Y.; Masuda, K.; Okuno, Y. Identification of a new class of non-electrophilic TRPA1 agonists by a structure-based virtual screening approach. Bioorg. Med. Chem. Lett. 2020, 30, 127142. [Google Scholar] [CrossRef]
- Lyall, V.; Alam, R.I.; Phan, D.Q.; Ereso, G.L.; Phan, T.-H.T.; Malik, S.A.; Montrose, M.H.; Chu, S.; Heck, G.L.; Feldman, G.M.; et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am. J. Physiol. Cell Physiol. 2001, 281, C1005–C1013. [Google Scholar] [CrossRef] [Green Version]
- Richter, T.A.; Caicedo, A.; Roper, S.D. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J. Physiol. 2003, 547, 475–483. [Google Scholar] [CrossRef]
- Hayakawa, T.; Kuwahara, S.; Maeda, S.; Tanaka, K.; Seki, M. Calcitonin gene-related peptide immunoreactive neurons innervating the soft palate, the root of tongue, and the pharynx in the superior glossopharyngeal ganglion of the rat. J. Chem. Neuroanat. 2010, 39, 221–227. [Google Scholar] [CrossRef]
- Green, B.G. Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem. Senses 2012, 37, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.A.; Grant, J.; Roper, S. Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS ONE 2012, 7, 30662. [Google Scholar] [CrossRef] [Green Version]
- Vandenbeuch, A.; Larson, E.D.; Anderson, C.B.; Smith, S.A.; Ford, A.P.; Finger, T.E.; Kinnamon, S.C. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J. Physiol. 2015, 593, 1113–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyall, V.; Heck, G.L.; Vinnikova, A.K.; Ghosh, S.; Phan, T.H.; Alam, R.I.; Russell, O.F.; Malik, S.A.; Bigbee, J.W.; DeSimone, J.A. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J. Physiol. 2004, 558, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, H.; Zhang, W.; Ding, C.; O’Keeffe, S.; Ye, M.; Zuker, C.S. Sour sensing from the tongue to the brain. Cell 2019, 179, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Felizardo, R.; Boucher, Y.; Braud, A.; Carstens, E.; Dauvergne, C.; Zerari-Mailly, F. Trigeminal projections on gustatory neurons of the nucleus of the solitary tract: A double-label strategy using electrical stimulation of the chorda tympani and tracer injection in the lingual nerve. Brain Res. 2009, 1288, 60–68. [Google Scholar] [CrossRef]
- Zerari-Mailly, F.; Buisseret, P.; Buisseret-Delmas, C.; Nosjean, A. Trigemino-solitarii-facial pathway in rats. J. Comp. Neurol. 2005, 487, 176–189. [Google Scholar] [CrossRef]
- Smith, D.V.; Li, C.S.; Cho, Y.K. Forebrain modulation of brainstem gustatory processing. Chem. Senses 2005, 30 (Suppl. S1), 176–177. [Google Scholar] [CrossRef]
- Li, J.; Lemon, C.H. Mouse parabrachial neurons signal a relationship between bitter taste and nociceptive stimuli. J. Neurosci. 2018, 39, 1631–1648. [Google Scholar] [CrossRef] [Green Version]
- Palmiter, R.D. The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 2018, 41, 280–293. [Google Scholar] [CrossRef]
- Wang, X.; Geng, L.; Qin, J.; Yao, S. The potential relationship between spicy taste and risk seeking. Judgm. Decis. Mak. 2017, 11, 547–553. [Google Scholar] [CrossRef]

| Natural Pungent Ingredients | Main Ingredients | Molecular Formula | Chemical Formula | CAS# | Threshold Pungency (105 SHU) | Natural Sources |
|---|---|---|---|---|---|---|
| Capsaicinoids | Capsaicin | C18H27NO3 |  | 404-86-4 | 160 | Capsicum annuum L. (e.g., chili peppers) |
| Dihydrocapsaicin | C18H29NO3 |  | 19408-84-5 | 160 | ||
| Nordihydrocapsaicin | C17H27NO3 |  | 28789-35-7 | 91 | ||
| Homocapsaicin | C19H29NO3 | 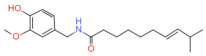 | 58493-48-4 | 86 | ||
| Homodihydrocapsaicin | C19H31NO3 | 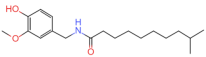 | 20279-06-5 | 86 | ||
| Piperine | Piperine | C17H19NO3 | 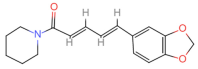 | 94-62-2 | 1.0 | Piperaceae Giseke. |
| Allyl isothiocyanate | Allyl isothiocyanate | C4H5NS |  | 57-06-7 | - | Brassicaceae Burnett. (e.g., mustard, wasabi, kohlrabi, radish) |
| Allicin | Allicin | C6H10OS2 |  | 539-86-6 | - | Liliaceae Juss. (e.g., garlic, onion) |
| Natural Pungent Ingredients | Physiological Effects | Experimental Phenomenon | References |
|---|---|---|---|
| Capsaicin | Antioxidant | In vitro: reduced lipoxygenase activity and lipid peroxidation significantly. Animal experiment: The oxidative stress levels in the liver and testis of SD rats were significantly decreased, and the contents of GSH-Px and GSH were significantly increased. | [16,17] |
| Anti-obesity | Reduced neutral fat content, fat accumulation, lipid droplet size, and surface area; Improved the release of glucagon and the absorption of glucose in the gastrointestinal tract; Improved postprandial hyperglycemia and hyperinsulinemia and fasting lipid metabolic disorders in women with GDM, reduced the incidence of LGA newborns. | [18,19] | |
| Analgesic | Relieved knee osteoarthritis pain, fibromyalgia, and postherpetic neuralgia. | [20] | |
| Anti-cardiovascular and cerebrovascular diseases | Significant neuroprotective effect on hypoxic neuron model in vitro and cardiac arrest model in vivo. | [21] | |
| Anti-inflammatory | Alleviated the inflammation response and the Warburg effect in a TRPV1-independent manner by targeting PKM2-LDHA and COX-2 in sepsis. | [22] | |
| Dihydrocapsaicin | Anti-cardiovascular and cerebrovascular diseases | Protective mechanisms of brain injury after cardiac arrest and resuscitation; Markedly abrogated TNFα-induced expression of the adhesion molecules VCAM-1 and ICAM-1, IL-6 production, and activation of NFκB, Reduced inflammatory damage in human vascular endothelial cell cultures. | [23,24] |
| Piperine | Anticancer | Inhibited the epithelial-mesenchymal transition (EMT) activated by TGF-β and prevented the invasion and metastasis of HepG2 cells in hepatocellular carcinoma. | [25] |
| Anti-inflammatory | Enhanced FAM134B and CCPG1-dependent ER phagocytosis to reduce ER stress, thereby alleviating pancreatitis injury; Repressed CS-induced infiltration of inflammatory cells and thereby exaggerated production of pro-inflammatory mediators and oxidative stress. | [26,27] | |
| Anti-cardiovascular and cerebrovascular diseases | Improved myocardial ischemia, cardiac injury, and cardiac fibrosis, inhibited vascular smooth muscle cell proliferation, and prevented arterial stenosis. | [28] | |
| Immunoregulation | Regulated PI3K/AkT-mediated anti-apoptosis signal transduction and improves pancreatic β-cell dysfunction. | [29] | |
| Antioxidant | Easy to react with high oxidation free radicals, scavenged DPPH, TEMPO, hydrogen peroxide, and reduced Fe3+. | [30] | |
| Anti-obesity | Reversed HFD-induced liver lipid accumulation and insulin resistance via the inactivation of adiponectin-AMPK and PI3K-Akt signaling; Regulated energy homeostasis and inflammation and alleviates obesity associated with GM regulation. | [31,32] | |
| Allyl isothiocyanate | Anticancer | Inhibited Akt/mTOR proliferation signaling and promoted mitochondria-dependent apoptotic pathway through AITC-enhanced activities of caspase-3 and caspase-9 in CAR cells | [33] |
| Antibacterial | Prevented A. niger, A. carbonarius and A. ochraceus from infecting grapes and maize and controlled Ochratoxin A contamination; More effective in controlling yeast and Gram-negative bacteria than Gram-positive bacteria. | [34,35] | |
| Allicin | Anticancer | Inhibited the proliferation and promoted apoptosis of various colorectal cancer cells. | [36,37] |
| Antibacterial | Inhibited DNA gyrase activity in bacteria and has natural antibacterial properties. | [38] | |
| Anti-cardiovascular and cerebrovascular diseases | Decreased serum levels of IL-1β, IL-6, and TNF-α, improved calcium homeostasis in cardiomyocytes, and downregulated calcium transport related CaMK II and inflammation related NF-κB and NLRP3, inhibited the activation of CaMK II/NF-κB pathway and protected hypertensive vascular and cardiac remodeling in spontaneously hypertensive rats. | [39] | |
| Gingerols | Immunoregulation | Inhibited viral neuraminidase activity and boosted hemagglutinin-specific CD4 T cell response to the infection; Increased expression of pro-inflammatory cytokines and enhanced Th1/Th17 responses. | [40,41] |
| Anti-inflammatory | Attenuated NF-κB/MAPK signaling pathways, formation of ECM, production of inflammatory cytokines, and injury to mammary gland cells both in vivo and in vitro. | [42] | |
| Antioxidant | Had a high scavenging capacity of DPPH and ATBS radicals, retarded lipid oxidation, and hydrolysis. | [43] | |
| Anti-obesity | Inhibited adipogenic differentiation and lipid accumulation and activated the Wnt/β-catenin signaling pathway during adipogenic differentiation. | [44] | |
| Sanshools | Antioxidant | Ameliorated spontaneous locomotion deficit of mice induced by D-galactose (D-gal) and AlCl3 treatment, reduced malondialdehyde production, and increased the activity of antioxidative enzymes, showing an inhibitory effect on oxidative stress. | [45] |
| Evodiamine | Anticancer | Induced M-phase cell-cycle arrest by inactivation of CUL4A E3 ligase, and suppressed the growth of esophageal squamous cell carcinoma both in vitro and in vivo. | [46] |
| Cinnamaldehyde | Antibacterial | Inhibited the growth of an array of microorganisms such as bacteria, molds, and yeasts, inhibited toxin production by micro-organisms. | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Liang, L.; Zhang, Y. Pungency Perception and the Interaction with Basic Taste Sensations: An Overview. Foods 2023, 12, 2317. https://doi.org/10.3390/foods12122317
He W, Liang L, Zhang Y. Pungency Perception and the Interaction with Basic Taste Sensations: An Overview. Foods. 2023; 12(12):2317. https://doi.org/10.3390/foods12122317
Chicago/Turabian StyleHe, Wei, Li Liang, and Yuyu Zhang. 2023. "Pungency Perception and the Interaction with Basic Taste Sensations: An Overview" Foods 12, no. 12: 2317. https://doi.org/10.3390/foods12122317
APA StyleHe, W., Liang, L., & Zhang, Y. (2023). Pungency Perception and the Interaction with Basic Taste Sensations: An Overview. Foods, 12(12), 2317. https://doi.org/10.3390/foods12122317





