Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects
Abstract
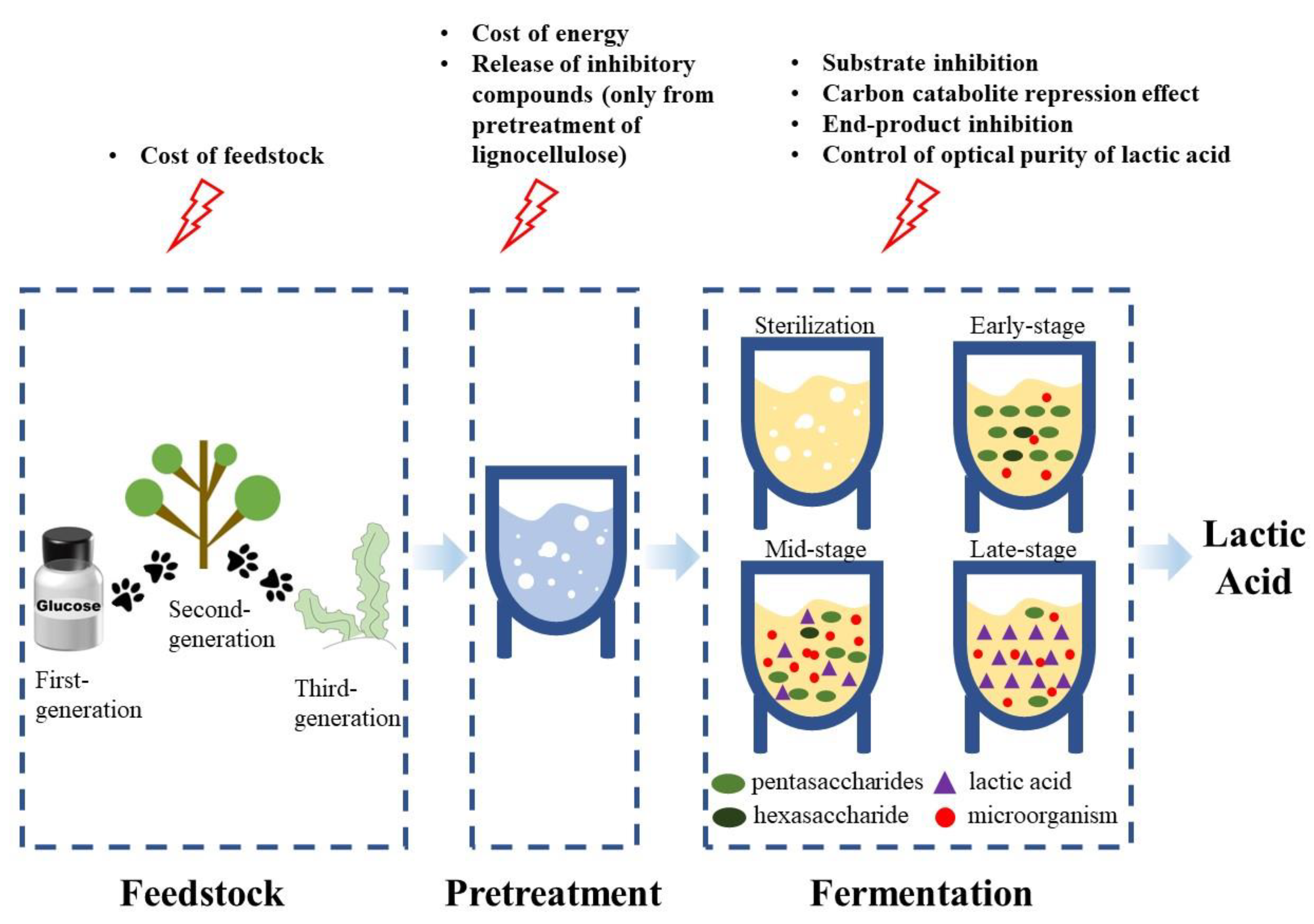
:1. Introduction
2. Ways to Reduce the Costs of LA Fermentation
2.1. Inexpensive Feedstock Application for Fermentation
2.2. Change the Production Process to Save Energy Consumption
3. Problems in the Fermentation Process and Their Solutions
3.1. Select Appropriate Strains and Fermentation Processes to Accommodate High Concentrations of Substrates
3.2. Methods to Control the Carbon Catabolite Repression Effect
3.3. Release of Inhibitory Compounds during Feedstock Pretreatment and the Detoxification Methods
3.4. Suitable Strains and Fermentation Processes for Solving Product Inhibition Problems
3.5. Methods to Control of Optical Purity of Lactic Acid
4. Selecting Suitable Fermentation Processes for LA Production
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nwamba, M.C.; Sun, F.; Mukasekuru, M.R.; Song, G.; Harindintwali, J.D.; Boyi, S.A.; Sun, H. Trends and hassles in the microbial production of lactic acid from lignocellulosic biomass. Environ. Technol. Innov. 2021, 21, 101337. [Google Scholar] [CrossRef]
- Djukic-Vukovic, A.; Mladenovic, D.; Ivanovic, J.; Pejin, J.; Mojovic, L. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew. Sustain. Energy Rev. 2019, 108, 238–252. [Google Scholar] [CrossRef]
- Rombouts, J.L.; Kranendonk, E.; Regueira, A.; Weissbrodt, D.G.; Kleerebezem, R.; Loosdrecht, M. Selecting for lactic acid producing and utilising bacteria in anaerobic enrichment cultures. Biotechnol. Bioeng. 2020, 117, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Acid, M.L. Microbial Lactic Acid, Its Polymer Poly(lactic acid), and Their Industrial Applications. Microbiol. Monogr. 2010, 14, 323–346. [Google Scholar]
- Feng, L.Y.; Yuan, F.Y.; Liu, F.; Wang, T.T.; Chen, Y.G. Research Progress on Lactic Acid Production from Food Waste by Fermentation. J. Tongji Univ. Nat. Sci. 2021, 49, 1688–1700. [Google Scholar]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Szliszka, E.; Czuba, Z.P.; Domino, M.; Mazur, B.; Zydowicz, G.; Krol, W. Ethanolic extract of propolis (EEP) enhances the apoptosis- inducing potential of TRAIL in cancer cells. Molecules 2009, 14, 738–754. [Google Scholar] [CrossRef]
- Guo, W.; He, R.; Ma, L.; Jia, W.; Li, D.; Chen, S. Construction of a constitutively expressed homo-fermentative pathway in Lactobacillus brevis. Appl. Microbiol. Biotechnol. 2014, 98, 6641–6650. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Grabar, T.B.; Zhou, S.; Shanmugam, K.T.; Yomano, L.P.; Ingram, L.O.N. Methylglyoxal Bypass Identified as Source of Chiral Contamination in l(+) and d(−)-lactate Fermentations by Recombinant Escherichia coli. Biotechnol. Lett. 2006, 28, 1527–1535. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Yuan, S.F.; Wang, C.A.; Huang, Y.J.; Guo, G.L.; Hwang, W.S. Production of optically pure L-lactic acid from lignocellulosic hydrolysate by using a newly isolated and D-lactate dehydrogenase gene-deficient Lactobacillus paracasei strain. Bioresour. Technol. 2015, 198, 651–657. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Sonomoto, K. Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J. Biotechnol. 2016, 236, 176–192. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Schneider, R.; Vaz Rossell, C.E.; Maciel Filho, R.; Venus, J. Polymer grade l-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour. Technol. Rep. 2019, 6, 26–31. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, P.; Tao, F. l-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour. Technol. Rep. 2019, 6, 131–137. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Z.; Zheng, Y.; Zhou, J.; Xiu, Z. Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochem. 2019, 81, 132–138. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Klupsaite, D. Application of acid tolerant Pedioccocus strains for increasing the sustainability of lactic acid production from cheese whey. LWT Food Sci. Technol. 2016, 72, 399–406. [Google Scholar] [CrossRef]
- Pleissner, D.; Lin, C.S.K. Valorisation of food waste in biotechnological processes. Sustain. Chem. Process 2013, 1, 21. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, A.; Bastidas-Oyanedel, J.R.; Schmidt, J.E. Effect of total solid content and pretreatment on the production of lactic acid from mixed culture dark fermentation of food waste. Waste Manag. 2018, 77, 516–521. [Google Scholar] [CrossRef]
- Song, L.; Liu, S.; Liu, R.; Yang, D.; Dai, X. Direct lactic acid production from household food waste by lactic acid bacteria. Sci. Total Environ. 2022, 840, 156479. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Huang, M.-Y.; Kao, T.-Y.; Lu, W.-J.; Lin, H.-J.; Pan, C.-L. Production of Lactic Acid from Seaweed Hydrolysates via Lactic Acid Bacteria Fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Sudhakar, M.P.; Dharani, G. Evaluation of seaweed for the production of lactic acid by fermentation using Lactobacillus plantarum. Bioresour. Technol. Rep. 2022, 17, 100890. [Google Scholar] [CrossRef]
- Nagarajan, D.; Nandini, A.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Lactic Acid Production from Renewable Feedstocks Using Poly(vinyl alcohol)-Immobilized Lactobacillus plantarum 23. Ind. Eng. Chem. Res. 2020, 59, 17156–17164. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljevic, M.; Pribic, M.; Kocic-Tanackov, S.; Mladenovic, D.; Djukic-Vukovic, A.; Mojovic, L. Possibility of L-(+)-lactic acid fermentation using malting, brewing, and oil production by-products. Waste Manag. 2018, 79, 153–163. [Google Scholar] [CrossRef]
- Karnaouri, A.; Asimakopoulou, G.; Kalogiannis, K.G.; Lappas, A.; Topakas, E. Efficient d-lactic acid production by Lactobacillus delbrueckii subsp. bulgaricus through conversion of organosolv pretreated lignocellulosic biomass. Biomass Bioenergy 2020, 140, 105672. [Google Scholar] [CrossRef]
- Santos-Corona, A.M.; Lázaro-Mixteco, P.E.; Vargas-Tah, A.A.; Castro-Montoya, A.J. Lactic acid production from food waste using the lactogenic Escherichia coli strain JU15: Optimization of reducing sugar recovery. J. Chem. Technol. Biotechnol. 2021, 97, 668–675. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Devaraj, K.; Devaraj, T.; Subramanian, S. Enhancement of lactic acid production from food waste through simultaneous saccharification and fermentation using selective microbial strains. Biomass Convers. Biorefin. 2020, 12, 5947–5958. [Google Scholar] [CrossRef]
- Bustamante, D.; Tortajada, M.; Ramón, D.; Rojas, A. Production of D-Lactic Acid by the Fermentation of Orange Peel Waste Hydrolysate by Lactic Acid Bacteria. Fermentation 2019, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Koo, J.-R.; Min Park, H.; Kyung Kim, S.; Shik Yun, H. Lactic Acid Fermentation from Coffee Ground Waste Hydrolysate by Lactobacillus Rhamnosus. J. Renew. Mater. 2019, 7, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Wischral, D.; Arias, J.M.; Modesto, L.F.; de Franca Passos, D.; Pereira, N., Jr. Lactic acid production from sugarcane bagasse hydrolysates by Lactobacillus pentosus: Integrating xylose and glucose fermentation. Biotechnol. Prog. 2019, 35, e2718. [Google Scholar] [CrossRef] [Green Version]
- Alexandri, M.; Blanco-Catala, J.; Schneider, R.; Turon, X.; Venus, J. High L(+)-lactic acid productivity in continuous fermentations using bakery waste and lucerne green juice as renewable substrates. Bioresour. Technol. 2020, 316, 123949. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Z.; Xu, Q.; Qian, Z.; Liu, J.; Ouyang, J. Valorization of dairy waste for enhanced D-lactic acid production at low cost. Process Biochem. 2018, 71, 18–22. [Google Scholar] [CrossRef]
- Nagarajan, D.; Oktarina, N.; Chen, P.T.; Chen, C.Y.; Lee, D.J.; Chang, J.S. Fermentative lactic acid production from seaweed hydrolysate using Lactobacillus sp. And Weissella sp. Bioresour. Technol. 2022, 344, 126166. [Google Scholar] [CrossRef]
- Chai, C.Y.; Tan, I.S.; Foo, H.C.Y.; Lam, M.K.; Tong, K.T.X.; Lee, K.T. Sustainable and green pretreatment strategy of Eucheuma denticulatum residues for third-generation l-lactic acid production. Bioresour. Technol. 2021, 330, 124930. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.S.; Hwang, H.J.; Park, M.S.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Production of l-lactic acid from a green microalga, Hydrodictyon reticulum, by Lactobacillus paracasei LA104 isolated from the traditional Korean food, makgeolli. Bioresour. Technol. 2012, 110, 552–559. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Enterococcus faecium QU 50: A novel thermophilic lactic acid bacterium for high-yield l-lactic acid production from xylose. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cai, Y.; Liu, L.; Zhan, R.; Zhao, S.; Liang, Y.; Peng, N.; Zhu, J.; Li, H.; Xiao, N.; et al. Enhanced lactic acid production by Bacillus coagulans through simultaneous saccharification, biodetoxification, and fermentation. Biofuels Bioprod. Bioref. 2020, 14, 533–543. [Google Scholar] [CrossRef]
- Zhao, K.; Qiao, Q.; Chu, D.; Gu, H.; Dao, T.H.; Zhang, J.; Bao, J. Simultaneous saccharification and high titer lactic acid fermentation of corn stover using a newly isolated lactic acid bacterium Pediococcus acidilactici DQ2. Bioresour. Technol. 2013, 135, 481–489. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Y.; Liu, H.; Wei, C.; Qi, W.; Xiu, Z. Simultaneous liquefaction, saccharification, and fermentation of L-lactic acid using aging paddy rice with hull by an isolated thermotolerant Enterococcus faecalis DUT1805. Bioprocess Biosyst. Eng. 2020, 43, 1717–1724. [Google Scholar] [CrossRef]
- Shahri, S.Z.; Vahabzadeh, F.; Mogharei, A. Lactic acid production by loofah-immobilized Rhizopus oryzae through one-step fermentation process using starch substrate. Bioprocess Biosyst. Eng. 2020, 43, 333–345. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Chu, J.; Mohsin, A.; Zhuang, Y. Exploring cellular fatty acid composition and intracellular metabolites of osmotic-tolerant mutant Lactobacillus paracasei NCBIO-M2 for highly efficient lactic acid production with high initial glucose concentration. J. Biotechnol. 2018, 286, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, L.; Wu, J.C. Efficient production of L-lactic acid by newly isolated thermophilic Bacillus coagulans WCP10-4 with high glucose tolerance. Appl. Microbiol. Biotechnol. 2013, 97, 4309–4314. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Chen, C.-Y.; Ariyadasa, T.U.; Lee, D.-J.; Chang, J.-S. Macroalgal biomass as a potential resource for lactic acid fermentation. Chemosphere 2022, 309, 136694. [Google Scholar] [CrossRef] [PubMed]
- Sivagurunathan, P.; Raj, T.; Chauhan, P.S.; Kumari, P.; Satlewal, A.; Gupta, R.P.; Kumar, R. High-titer lactic acid production from pilot-scale pretreated non-detoxified rice straw hydrolysate at high-solid loading. Biochem. Eng. J. 2022, 187, 108668. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Wei, C.; Qi, W.; Xiu, Z. An aptly industrialized bioprocess for lactic acid production from corn stover using thermotolerant microbial consortia. Bioprocess Biosyst. Eng. 2021, 44, 2445–2454. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tan, J.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Non-carbon loss long-term continuous lactic acid production from mixed sugars using thermophilic Enterococcus faecium QU 50. Biotechnol. Bioeng. 2020, 117, 1673–1683. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Alrefaey, H.M.A.; Elsakhawy, T. Efficient Co-Utilization of Biomass-Derived Mixed Sugars for Lactic Acid Production by Bacillus coagulans Azu-10. Fermentation 2021, 7, 28. [Google Scholar] [CrossRef]
- Moreno, A.D.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for Lignocellulosic Biomass Deconstruction Within a Biorefinery Perspective. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels; Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 379–399. [Google Scholar]
- Yi, X.; Zhang, P.; Sun, J.; Tu, Y.; Gao, Q.; Zhang, J.; Bao, J. Engineering wild-type robust Pediococcus acidilactici strain for high titer L- and D-lactic acid production from corn stover feedstock. J. Biotechnol. 2016, 217, 112–121. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Saddler, J.N. Extent of Enzyme Inhibition by Phenolics Derived from Pretreated Biomass Is Significantly Influenced by the Size and Carbonyl Group Content of the Phenolics. ACS Sustain. Chem. Eng. 2018, 6, 3823–3829. [Google Scholar] [CrossRef]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [Green Version]
- Roque, L.R.; Morgado, G.P.; Nascimento, V.M.; Ienczak, J.L.; Rabelo, S.C. Liquid-liquid extraction: A promising alternative for inhibitors removing of pentoses fermentation. Fuel 2019, 242, 775–787. [Google Scholar] [CrossRef]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet Explosion: A Universal and Efficient Pretreatment Process for Lignocellulosic Biorefineries. BioEnergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Ouyang, S.; Zou, L.; Qiao, H.; Shi, J.; Zheng, Z.; Ouyang, J. One-pot process for lactic acid production from wheat straw by an adapted Bacillus coagulans and identification of genes related to hydrolysate-tolerance. Bioresour. Technol. 2020, 315, 123855. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; Gonzalez-Fernandez, C.; Ballesteros, M.; Tomas-Pejo, E. Lactobacillus pentosus CECT 4023 T co-utilizes glucose and xylose to produce lactic acid from wheat straw hydrolysate: Anaerobiosis as a key factor. Biotechnol. Prog. 2019, 35, e2739. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Bae, J.H.; Ko, H.J.; Lee, S.H.; Sohn, J.H. Low-pH production of D-lactic acid using newly isolated acid tolerant yeast Pichia kudriavzevii NG7: PARK et al. Biotechnol. Bioeng. 2018, 115, 2232–2242. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of lactic acid: The latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [Google Scholar] [CrossRef]
- Jang, B.K.; Ju, Y.; Jeong, D.; Jung, S.K.; Kim, C.K.; Chung, Y.S.; Kim, S.R. l-Lactic Acid Production Using Engineered Saccharomyces cerevisiae with Improved Organic Acid Tolerance. J. Fungi 2021, 7, 928. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, M.; Jia, J.; Bao, J. Increasing Acid Tolerance of an Engineered Lactic Acid Bacterium Pediococcus acidilactici for L-Lactic Acid Production. Fermentation 2022, 8, 96. [Google Scholar] [CrossRef]
- Lee, J.J.; Crook, N.; Sun, J.; Alper, H.S. Improvement of lactic acid production in Saccharomyces cerevisiae by a deletion of ssb1. J. Ind. Microbiol. Biotechnol. 2016, 43, 87–96. [Google Scholar] [CrossRef]
- Baek, S.H.; Kwon, E.Y.; Yong, H.K.; Hahn, J.S. Metabolic engineering and adaptive evolution for efficient production of D-lactic acid in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2016, 100, 2737–2748. [Google Scholar] [CrossRef]
- Baek, S.H.; Kwon, E.Y.; Bae, S.J.; Cho, B.R.; Kim, S.Y.; Hahn, J.S. Improvement of d-Lactic Acid Production in Saccharomyces cerevisiae Under Acidic Conditions by Evolutionary and Rational Metabolic Engineering. Biotechnol. J. 2017, 12, 1700015. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Traverso, J.J.; Murali, N.; Srinivas, K. Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity. Biochem. Eng. J. 2016, 109, 162–169. [Google Scholar] [CrossRef]
- Pimtong, V.; Ounaeb, S.; Thitiprasert, S.; Tolieng, V.; Sooksai, S.; Boonsombat, R.; Tanasupawat, S.; Assabumrungrat, S.; Thongchul, N. Enhanced effectiveness of Rhizopus oryzae by immobilization in a static bed fermentor for L-lactic acid production. Process Biochem. 2017, 52, 44–52. [Google Scholar] [CrossRef]
- Shi, Z.; Wei, P.; Zhu, X.; Cai, J.; Huang, L.; Xu, Z. Efficient production of l-lactic acid from hydrolysate of Jerusalem artichoke with immobilized cells of Lactococcus lactis in fibrous bed bioreactors. Enzym. Microb. Technol. 2012, 51, 263–268. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Qi, B.; Luo, J.; Shen, F.; Su, Y.; Khan, R.; Wan, Y. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour. Technol. 2014, 163, 160–166. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y. Dynamic membrane-assisted fermentation of food wastes for enhancing lactic acid production. Bioresour. Technol. 2017, 234, 40–47. [Google Scholar] [CrossRef]
- Viana, R.; Yebra, M.J.; Galán, J.; Monedero, V.; Pérez-Martínez, G. Pleiotropic effects of lactate dehydrogenase inactivation in Lactobacillus casei. Res. Microbiol. 2005, 156, 641–649. [Google Scholar] [CrossRef]
- Jian, Q.; Li, X.; Chen, Y.; Liu, Y.; Pan, Y. Production of high optical purity l-lactic acid from waste activated sludge by supplementing carbohydrate: Effect of temperature and pretreatment time. Environ. Technol. 2016, 37, 2457–2466. [Google Scholar] [CrossRef]
- Gu, S.A.; Jun, C.; Joo, J.C.; Kim, S.; Lee, S.H.; Kim, Y.H. Higher thermostability of l-lactate dehydrogenases is a key factor in decreasing the optical purity of d-lactic acid produced from Lactobacillus coryniformis. Enzym. Microb. Technol. 2014, 58–59, 29–35. [Google Scholar] [CrossRef]

| Feedstock | Strain (s) | Strategy | Lactic Acid | References | ||
|---|---|---|---|---|---|---|
| Concentrations (g·L) | Yield (g·g−1) | Productivity (g·(L·h) −1) | ||||
| Brewer’s spent grain, malt rootlets | L. rhamnosus ATCC 7469 | Batch fermentation | 25.73 | - | 0.95 | [24] |
| Fed-batch fermentation | 58.01 | - | 1.19 | |||
| Beechwood | Lactobacillus delbrueckii subsp. bulgaricus (ATCC® 11842) | Simultaneous saccharification and fermentation (batch) | 62.00 | 0.69 | 0.86 | [25] |
| Food waste | E.coli JU15 | Batch fermentation | 22.66 | 0.99 | 0.73 | [26] |
| Food waste | Lacobacillus manihotivorans DSM 13343 | Batch fermentation | 18.69 | 0.73 | - | [27] |
| L. plantarum DSM 20174 | 17.03 | 0.69 | - | |||
| Orange peel waste | Lactobacillus delbrueckii ssp. bulgaricus CECT 5037 | Batch fermentation | 39.00 | - | - | [28] |
| Coffee ground waste | L. rhamnosus ATCC 10863 | Batch fermentation | 24.95 | - | 0.59 | [29] |
| Sugarcane bagasse | Lactobacillus pentosus ATCC 8041 | Batch fermentation | 65.00 | 0.93 | 1.01 | [30] |
| Sugarcane molasses | microbial consortium CEE-DL15 | Batch fermentation | 112.34 | 0.81 | 4.94 | [16] |
| Bakery waste hydrolysates and lucerne green juice | B. coagulans | Batch fermentation | 62.20 | 0.57 | 2.59 | [31] |
| Continuous fermentation | 55.00 | 0.48 | 11.28 | |||
| Cheese whey powder | Lactobacillus bulgaricus CGMCC 1.6970 | Batch fermentation | 70.70 | - | 1.47 | [32] |
| Continuous fermentation | 113.18 | - | 2.36 | |||
| Sargassum cristaefolium | L. plantarum 23 | Batch fermentation | 26.00 | 0.81 | 7.53 | [33] |
| Ulva sp. | L. rhamnosus | Batch fermentation | 30.93 | 0.85 | 7.53 | [33] |
| Gracilaria sp. | Weissella paramesenteroides 24 | Batch fermentation | 29.00 | 0.94 | 3.58 | [33] |
| Eucheuma denticulatum | Bacillus coagulans ATCC 7050 | Prehydrolysis simultaneous saccharification and fermentation (batch) | 14.00 | - | - | [34] |
| Hydrodictyon reticulum | Lactobacillus paracasei LA104 | Simultaneous saccharification and cofermentation | 37.11 | 0.46 | 1.03 | [35] |
| Fermentation Process | Feedstock | Pretreatment | Applicable Scope |
|---|---|---|---|
| Fed-batch fermentation | First-generation feedstocks | No need |
|
| Second-generation feedstocks | Solids (e.g., food waste, corn stover): mill (except powder)/dissolve/mix (with other base feedstocks)/detoxification (lignocellulose as substrate)/sterilization/saccharification (or SSF) Liquid (e.g., whey, molasses): mix (with other base materials)/sterilization/saccharification (or SSF) | ||
| Third-generation feedstocks | Mill/dissolve/mix (with other base materials)/detoxification/sterilization/saccharification (or SSF) | ||
| Continuous fermentation | First-generation feedstocks | Same as above |
|
| Second-generation feedstocks | |||
| Third-generation feedstocks | |||
| Cell immobilization fermentation | First-generation feedstocks | Same as above |
|
| Second-generation feedstocks | Same as above (pretreatment of feedstocks (e.g., lignocellulose) that may produce inhibitory substances is not recommended) | ||
| Third-generation feedstocks | Same as above | ||
| Cell-recycled batch fermentation | First-generation feedstocks | Same as above |
|
| Second-generation feedstocks | Same as above (pretreatment of feedstocks (e.g., lignocellulose) that may produce inhibitory substances is not recommended) | ||
| Third-generation feedstocks | Same as above |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wang, Y.; Shang, N.; Li, P. Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects. Foods 2023, 12, 2311. https://doi.org/10.3390/foods12122311
Huang Y, Wang Y, Shang N, Li P. Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects. Foods. 2023; 12(12):2311. https://doi.org/10.3390/foods12122311
Chicago/Turabian StyleHuang, Yueying, Yu Wang, Nan Shang, and Pinglan Li. 2023. "Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects" Foods 12, no. 12: 2311. https://doi.org/10.3390/foods12122311
APA StyleHuang, Y., Wang, Y., Shang, N., & Li, P. (2023). Microbial Fermentation Processes of Lactic Acid: Challenges, Solutions, and Future Prospects. Foods, 12(12), 2311. https://doi.org/10.3390/foods12122311





