The Desalting Process for Table Olives and Its Effect on Their Physicochemical Characteristics and Nutrient Mineral Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olives
2.2. Desalting
2.3. Physicochemical Analysis of Brines
2.4. Instrumental Determination of Firmness
2.5. Instrumental Measurement of Colour
2.6. Mineral Analysis in the Pulp
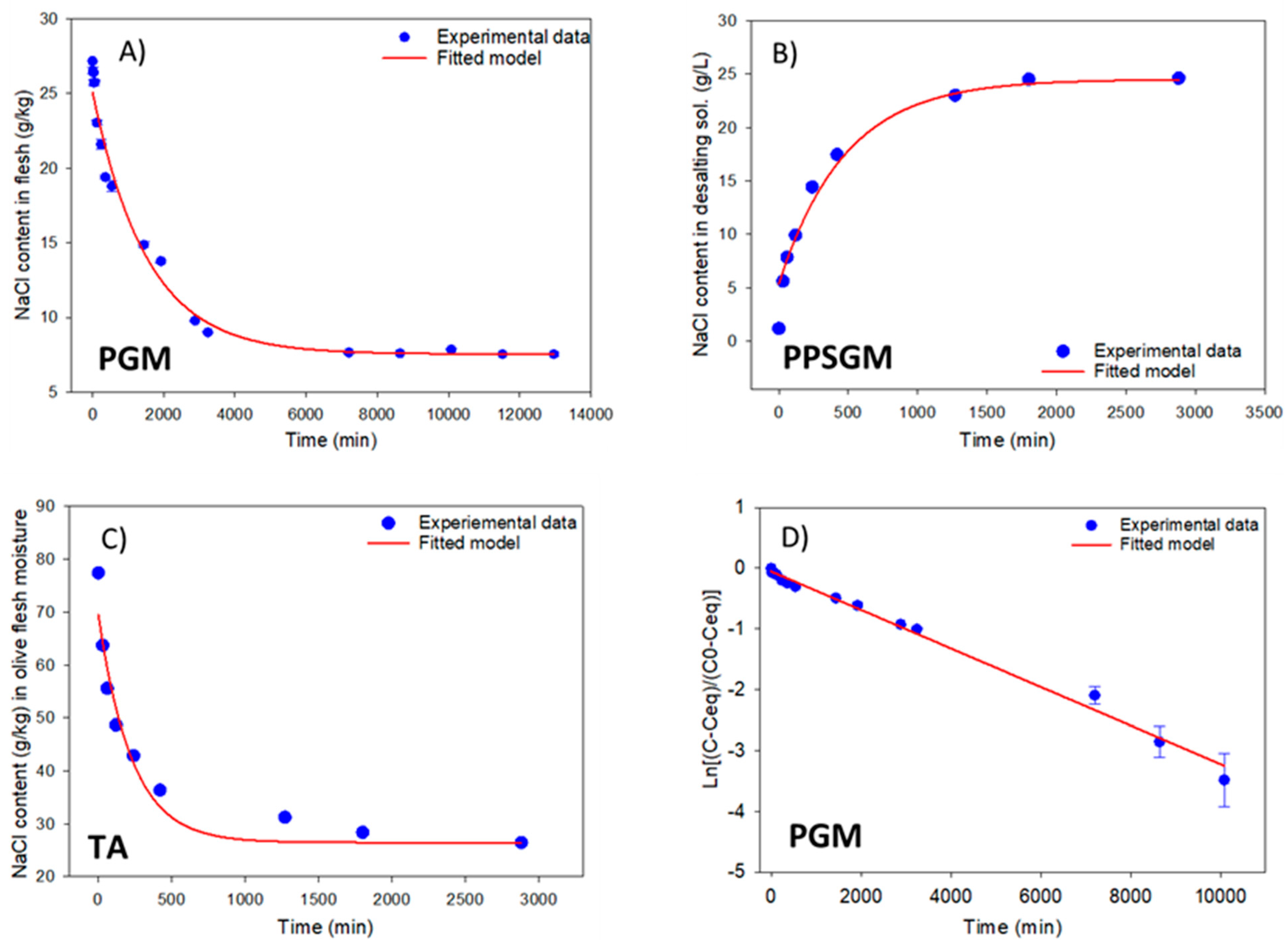
2.7. Kinetics of the Desalting Operation
2.8. Data Analysis
3. Results
3.1. Characterization of the Raw Material
3.2. Estimation of the Approximate Period of Desalting
3.3. Pilot Plant Desalting
3.3.1. Olive Fruit Changes Due to the Desalting Operation
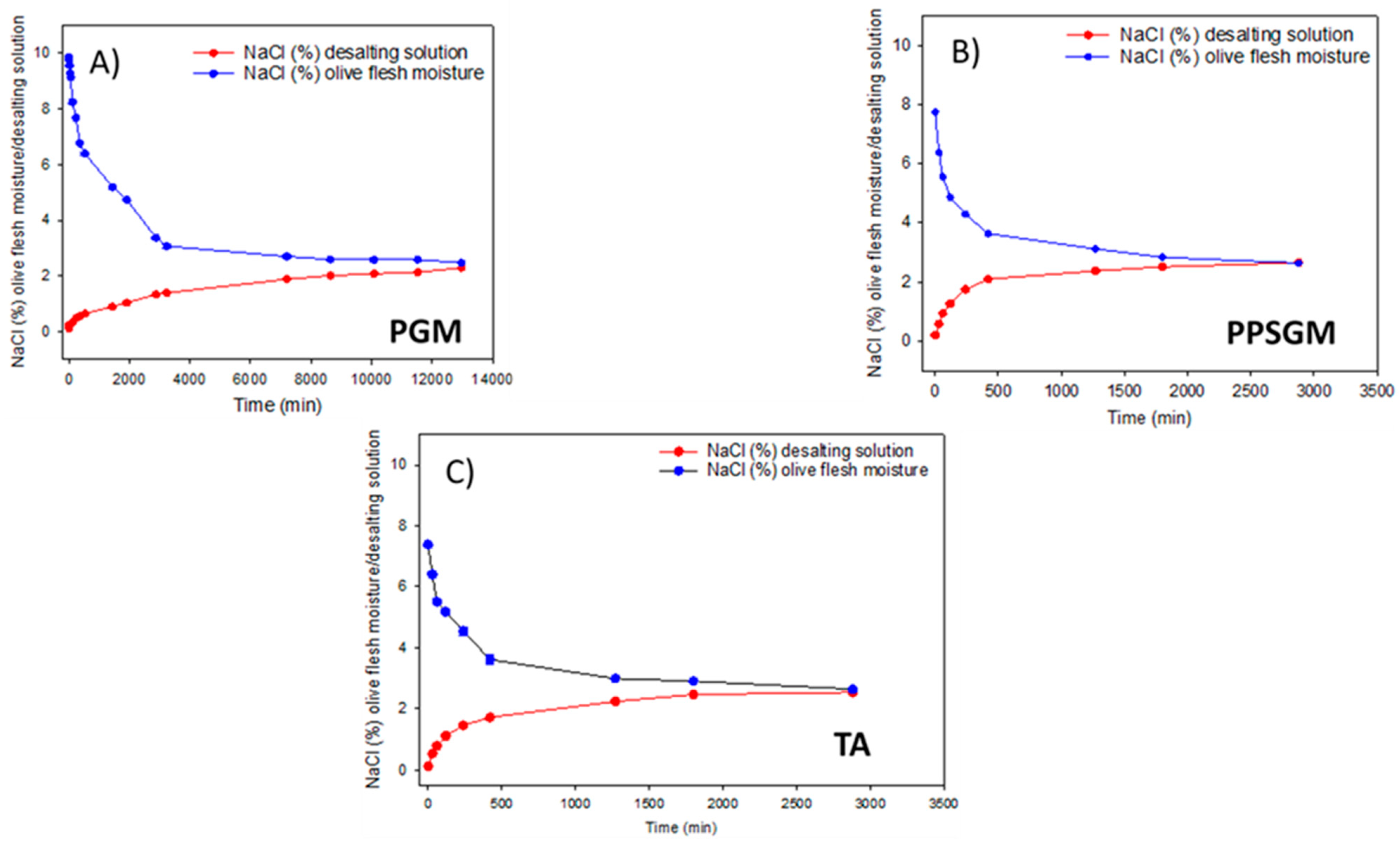
3.3.2. Salt and Mineral Nutrient Diffusion from Flesh into the Desalting Solution
3.3.3. Effect of Desalting on Olive Mineral Nutrient Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Olive Council (IOC). Estadísticas Mundiales Sobre Aceite de Oliva y Aceitunas de Mesa. 2022. Available online: https://www.internationaloliveoil.org/que-hacemos/unidad-de-asuntos-econonicos-y-promocion/?lang=es#figures (accessed on 19 April 2023).
- Garrido-Fernández, A.; Fernández-Díez, M.J.; Adams, R.M. Table Olive Production and Processing; Chapman & Hall: London, UK, 1997. [Google Scholar]
- International Olive Council (IOC). Trade Standards Applying to Table Olives; IOC/OT/NC No. 1/2004; International Olive Council: Madrid, Spain, 2004. [Google Scholar]
- International Olive Council (IOC). Method for the Sensory Analysis of Table Olives; COI/OT/MO No. 1/Rev. 2 November 2011; International Olive Council: Madrid, Spain, 2011; Available online: http://www.internationaloliveoil.org/estaticos/view/70-metodos-de-evaluacion (accessed on 19 April 2023).
- Lee, S.M.; Kitsawad, K.; Sigal, A.; Flynn, D.; Guinard, J.X. Sensory properties and consumer acceptance of imported and domestic sliced black ripe olives. J. Food Sci. 2012, 77, S439–S448. [Google Scholar] [CrossRef] [PubMed]
- López-López, A.; Sánchez-Gómez, A.H.; Montaño, A.; Cortés-Delgado, A.; Garrido-Fernández, A. Sensory profile of Green Spanish-style table olives according to cultivar and origin. Food Res. Int. 2018, 108, 347–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engstrom, A.; Tobelmann, R.C.; Albertson, A.M. Sodium intake trends and food choices. Am. J. Clin. Nutr. 1997, 65, 7045–7075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Survey on Members States’ Implementation of the EU Salt Reduction Framework. 2012. Available online: https://ec.europa.eu/health/sites/health/files/nutrition_physical_activity/docs/salt_report1_en.pdf (accessed on 19 April 2023).
- MordorIntelligence. Spain Sodium Reduction Ingredients Market-Growth, Trends, COVID-19. Impact and Forecast (2023–2028). 2023. Available online: https://www.mordorintelligence.com/industry-reports/spain-sodium-reduction-ingredient-market (accessed on 19 April 2023).
- Agencia Española de Consumo, Seguridad Alimentaria y Nutrición (AECOSAN). Plan to Reduce the Salt Intake within the Estrategia NAOS. 2014. Available online: https://www.aesan.gob.es/AECOSAN/web/nutricion/seccion/estrategia_naos.htm (accessed on 19 April 2023).
- Market Research. Global Reduced Salt Packaged Food Market 2018–2022. 2018. Available online: https://www.marketresearch.com/Infiniti-Research-Limited-v2680/Global-Reduced-Salt-Packaged-Food-11725675/ (accessed on 19 April 2023).
- Kanavouras, A.; Gazouli, M.; Leonidas, L.T.; Petrakis, C. Evaluation of Greek-style black table olives in salt-varying brines. Grasas Aceites 2005, 56, 106–115. [Google Scholar] [CrossRef]
- Tassou, C.C.; Katsaboxakis, C.Z.; Georget, D.M.; Parker, M.L.; Waldron, K.W.; Smith, A.C.; Panagou, E.Z. Effect of calcium chloride on mechanical properties and microbiological characteristics of cv. Conservolea naturally black olives fermented at different sodium chloride levels. J. Sci. Food Agric. 2007, 87, 1123–1131. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Durán-Quintana, M.C.; Garrido-Fernández, A. Fermentation profiles of Manzanilla-Aloreña cracked green table olives in different chloride salt mixtures. Food Microbiol. 2010, 27, 403–412. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Arroyo-López, F.N.; Romero-Gil, V.; Rodríguez-Gómez, F.; García-García, P.; Garrido-Fernández, A. Chloride salt mixtures affect Gordal cv. green Spanish-style table olive fermentation. Food Microbiol. 2011, 28, 1316–1325. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Hondrodimou, O.; Mallouchos, A.; Nychas, G.J.E. A study on the implications of NaCl reduction in the fermentation profile of Conservolea natural black olives. Food Microbiol. 2011, 28, 1301–1307. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Rantsiou, K.; Garrido-Fernández, A.; Cocolin, L.; Arroyo-López, F.N. Salt reduction in vegetable fermentation: Reality or desire? J. Food Sci. 2013, 78, R1095–R1100. [Google Scholar] [CrossRef]
- Montaño, A.; De Castro, A.; Rejano, L.; Brenes, M. 4-Hydroxycyclohexanecarboxilic acid production during the “zapatera” spoilage of Spanish-style green table olives. J. Food Prot. 1996, 59, 657–662. [Google Scholar] [CrossRef]
- Koca, N.; Karadeniz, F.; Burdurlu, H.S. Effect of pH on chlorophyll degradation and colour loss in blanched green peas. Food Chem. 2006, 100, 609–615. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Schijvens, E.P.H.; Biekman, E.S.A. Modelling the change in colour of broccoli and green beans during blanching. Innov. Food Sci. Emerg. Technol. 2001, 2, 303–313. [Google Scholar] [CrossRef]
- Athanasopoulos, N. GBC 932/933 Atomic Absorption Spectrophotometers; Operation Manual: Dandenong, Australia, 1994. [Google Scholar]
- Incropera, F.P.; De Whitt, D.P. Fundamentals of Heat and Mass Transfers, 4th ed.; John Willey and Sons: New York, NY, USA, 1996; p. 886. [Google Scholar]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: New York, NY, USA, 1975; p. 414. [Google Scholar]
- Fasina, O.; Fleming, H.; Thompson, R. Mass transfer and solute diffusion in brined cucumbers. J. Food Sci. 2002, 67, 181–187. [Google Scholar] [CrossRef]
- Azzouz, S.; Guizani, A.; Jomaa, W.; Belghith, A. Moisture diffusivity and drying kinetic equation of convective drying grapes. J. Food Eng. 2002, 55, 323–330. [Google Scholar] [CrossRef]
- Pimiento en Pasta S.A. (PEPSA). Características Pasta de Pimiento con/sin Sorbato. 2018. Available online: http://www.pepsa.es/MarcosQuienesSomos.html (accessed on 19 April 2023).
- Arroyo-López, F.N.; Bautista-Gallego, J.; Garrido-Fernández, A. Aspectos microbiológicos de la aceituna Aloreña de Málaga. In Producción, Elaboración y Valor Nutricional de la Aceituna Aloreña de Málaga; López-López, A., Garrido-Fernández, A., Eds.; Grupo de Desarrollo Rural Valle del Guadalhorce: Pizarra, Spain, 2010. [Google Scholar]
- Hinkley, T.; Pandya, J.; Kinchla, A.J.; Decker, E.A. Determination of quantitative sodium mass transfer coefficient during osmotic processing of potatoes. J. Food Process. Pres. 2015, 40, 963–968. [Google Scholar] [CrossRef]
- Liu, H. A kinetic study of the salt diffusion in potatoes at high temperatures. Int. J. Food Sci. Technol. 1992, 27, 443–455. [Google Scholar] [CrossRef]
- Sarang, S.; Sastry, S.K. Diffusion and equilibrium distribution of salt within the vegetable tissue: Effects of salt concentration and temperature. J. Food Eng. 2007, 82, 377–382. [Google Scholar] [CrossRef]
- Kusnadi, K.; Sastry, S.K. Effect of temperature on salt diffusion into vegetable tissue. Int. J. Food Prop. 2012, 15, 1148–1160. [Google Scholar] [CrossRef]
- López-López, A.; Bautista-Gallego, J.; Moreno-Baquero, J.M.; Garrido-Fernández, A. Fermentation in nutrient salt mixtures affects green Spanish-style Manzanilla table olive characteristics. Food Chem. 2016, 211, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, A.; Guillén, R.; Fernández-Bolaños, J.; Heredia, A. Cell wall composition of olives. J. Food Sci. 1994, 59, 1192–1196. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.; Bautista-Gallego, J.; Romero-Gil, V.; Arroyo-López, F.N.; Garrido-Fernández, A.; García-García, P. Effects of salt mixtures on Spanish green table olive fermentation performance. LWT-Food Sci. Technol. 2012, 46, 56–63. [Google Scholar] [CrossRef]
- Drusas, A.; Vagenas, G.K.; Saravacos, G.D. Diffusion of sodium chloride in green olives. J. Food Eng. 1988, 7, 211–222. [Google Scholar] [CrossRef]
- Maldonado, M.B.; Zuritz, C.A.; Gascón, A.D.; Rey, E. Difusión de sodio en aceitunas verdes durante el tratamiento alcalino. I: Efecto de la concentración de la lejía. Grasas Aceites 2003, 54, 358–364. [Google Scholar] [CrossRef]
- Zuritz, C.A.; Maldonado, M.B.; Gascón, A.D. Difusión de sodio en aceitunas verdes durante el tratamiento alcalino. II: Efecto de la temperatura de la lejía. Grasas Aceites 2003, 54, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Zuritz, C.A.; Maldonado, M.B. A simple method to determine the diffusion of sodium in the epidermis of green olives. J. Food Process Eng. 2004, 27, 328–344. [Google Scholar] [CrossRef]
- Maldonado, M.B.; Zuritz, C.A. Determination of variable diffusion of sodium during debittering of green olives. J. Food Process Eng. 2004, 27, 345–358. [Google Scholar] [CrossRef]
- Maldonado, M.B.; Zuritz, C.A. Difusión de sodio durante el tratamiento alcalino de aceitunas de la variedad Aloreña. Grasas Aceites 2004, 55, 409–414. [Google Scholar] [CrossRef]
- Maldonado, M.B.; Zuritz, C.A.; Assof, M.V. Diffusion of glucose and sodium chloride in green olives during curing as affected by lye treatment. J. Food Eng. 2008, 84, 224–230. [Google Scholar] [CrossRef]
- Maldonado, M.B.; Zuritz, C.A.; Miras, N. Influence of brine concentration on the sugar and sodium chloride diffusion during the processing of the green olive variety Arauco. Grasas Aceites 2008, 59, 267–273. [Google Scholar] [CrossRef]
- Maldonado, M.B.; Zuritz, C.A.; Wuilloud, R.G.; Bageta, C.R.; Terreni, J.; Sánchez, M.J. A simple model of the diffusion phenomena taking place during the debittering process of green table olives. Grasas Aceites 2011, 62, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, M.B.; Perez, R.C. A theoretical model of the diffusion process to spherical and isotropic fruits. Int. J. Innov. Sci. Math. 2014, 2, 161–166. Available online: https://www.ijism.org/administrator/components/com_jresearch/files/publications/IJISM_82_Final.pdf (accessed on 19 April 2023).
- Maldonado, M.B.; Pérez, R.C.; Pérez Iglesias, J.L. The sodium diffusion during the debittering of green table olives. Elliptical coordinates model. Int. J. Agric. Innov. Res. 2014, 2, 610–614. Available online: https://www.ijair.org/administrator/components/com_jresearch/files/publications/IJAIR_469_Final.pdf (accessed on 19 April 2023).
- Chayjan, R.A.; Kaveh, M.; Khayati, S. Modelling drying characteristics of hawthorn fruit under microwave-convective conditions. J. Food Process. Pres. 2015, 39, 239–253. [Google Scholar] [CrossRef]
- Jiménez, A.; Heredia, A.; Guillén, R.; Fernández-Bolaños, J. Correlation between soaking conditions, cation content of cell wall, and olive firmness during “Spanish green olive” processing. J. Agric. Food Chem. 1997, 45, 1653–1658. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers. Off. J. Eur. Union 2011, 304, 18–63. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 19 April 2023).
| Characteristic | PGM | PPSGM | TA |
|---|---|---|---|
| Olive/stuffed product | |||
| Weight (g) | 4.32 (0.11) | 3.15 (0.08) | 4.52 (0.12) |
| Diameter (cm) | 1.76 (0.01) | 1.56 (0.02) | 1.71 (0.02) |
| Height (cm) | 2.19 (0.04) | 1.77 (0.02) | 1.97 (0.03) |
| Volume (cm3) | 4.19 (0.15) | 3.15 (0.05) | 4.83 (0.13) |
| Density (g/mL) | 1.03 (0.02) | 0.99 (0.01) | 0.94 (0.01) |
| Flesh proportion (%) * | 84.58 (0.04) | 84.20 (0.40) | 83.07 (0.57) |
| Pit/stuffing material proportion (%) * | 15.42 (0.40) | 15.77 (0.03) | 16.93 (0.57) |
| Moisture in the flesh/stuffed product | 69.23 (0.28) | 72.96 (0.11) | 61.24 (0.09) |
| Pit/stuffing material | |||
| Weight (g) | 0.67 (0.02) | 0.49 (0.01) | 0.76 (0.02) |
| Volume (cm3) | 0.49 (0.02) | 0.34 (0.02) | 0.46 (0.01) |
| Pit/stuffing material density (g/mL) | 1.37 (0.04) | 1.49 (0.05) | 1.66 (0.03) |
| Pit/stuffing material proportion (%) * | 15.42 (0.40) | 15.77 (0.03) | 16.93 (0.57) |
| Other characteristics | |||
| Stuffing material moisture (%) * | -- | 68.49 (0.14) | -- |
| Olive moisture without stuffing (%) * | -- | 86.90 (0.02) | -- |
| Brine Parameter | PGM | PPSGM | TA |
|---|---|---|---|
| pH | 3.89 (0.03) | 3.90 (0.02) | 4.32 (0.03) |
| Titratable acidity (g/L) | 7.07 (0.17) | 9.20 (0.40) | 3.75 (<0.001) |
| Combined acidity (mEq/L) | 82.5 (1.8) | 101.6 (3.4) | 85.80 (1.56) |
| Lactic acid content (estimated) (g/L) | 14.49 (0.31) | 18.34 (0.09) | NA |
| Lactic acid content (HPLC) (g/L) | 9.86 (0.07) | 17.45 (0.29) | 1.759 (0.018) |
| NaCl (g/L) | 93.7 (0.2) | 73.2 (0.3) | 76.8 (0.06) |
| Parameter | PGM | PPSGM | TA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stored | Desalted | Change | Stored | Desalted | Change | Stored | Desalted | Change | |||
| 5.0% NaCl | 2.5% NaCl | 5.0% NaCl | 2.5% NaCl | 2.5% NaCl | 2.5% NaCl | 2.5% NaCl | 2.5% NaCl | ||||
| Colour index | 27.45 (0.36) | 26.00 (0.12) | 25.67 (1.61) | −5.2% | −6.2% | 25.11 (0.35) | 25.41 (0.38) | 1.2% | 38.94 (1.17) | 31.45 (0.31) | −19.2% |
| L* | 51.20 (0.18) | 49.76 (0.06) | 46.64 (1.44) | −2.8% | −8.8% | 49.62 (0.06) | 50.23 (0.11) | 1.2% | 56.11 (0.47) | 51.31 (0.28) | −8.6% |
| a* | 4.85 (0.13) | 4.77 (0.006) | 4.58 (0.06) | −1.6% | −5.6% | 4.92 (0.07) | 4.56 (0.17) | −7.3% | 5.62 (0.06) | 4.72 (0.07) | −16.0% |
| b* | 35.90 (0.67) | 32.06 (0.06) | 31.91 (0.83) | −10.7% | −11.1% | 32.97 (0.01) | 33.09 (0.30) | 0.4% | 37.87 (0.18) | 33.22 (0.08) | −12.3% |
| Chroma | 36.23 (0.68) | 32.41 (0.06) | 32.23 (0.81) | −10.5% | −11.0% | 33.34 (<0.01) | 33.40 (0.31) | <0.1% | 38.08 (0.16) | 33.55 (0.08) | −11.9% |
| Hue | 82.30 (0.22) | 81.55 (0.17) | 81.84 (0.05) | −0.9% | −0.6% | 81.51 (0.12) | 82.16 (0.21) | 0.8% | 81.51 (0.13) | 81.91 (0.10) | 0.5% |
| −a*/b* | −0.135 (0.002) | −0.148 (0.001) | −0.143 (0.005) | 9.6% | 5.9% | −0.149 (0.002) | −0.138 (0.004) | −7.4% | −0.149 (0.002) | −0.142 (0.002) | −4.7% |
| Firmness(N/g) | 17.98 (0.17) | NA | 13.23 (2.60) | NA | −26.4% | 16.12 (5.10) | 14.22 (4.50) | −12.7% | 33.51 (0.51) | 32.81 (1.81) | −2.1% |
| Moisture (%) | 69.23 (0.28) | 73.41 (0.09) | 76.00 (0.04) | 6.0% | 9.8% | 72.96 (0.50) | 75.72 (0.07) | 3.8% | 61.24 (0.10) | 66.44 (0.13) | 8.5% |
| Lactic acid (g/L) | 10.63 (0.01) | NA | 3.30 (0.01) | NA | −69.0% | 17.95 (0.34) | 6.89 (0.10) | −61.6% | 1.75 (0.013) | 0.73 (0.01) | −58.4% |
| Compound/ Element | y0 | k (min−1) | Fit Parameters | t50 (min) | % ch/h | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-Value | Estimate | SE | p-Value | R2 | SEesti | |||
| Plain green Spanish-style Manzanilla table olives (PGM) | ||||||||||
| Moisture (%) | −4.1 × 10−1 | 6.7 × 10−2 | <0.0001 | −5.0 × 10−4 | 2.2 × 10−5 | <0.0001 | 0.953 | 3.7 × 10−1 | 1386 | 3.0 |
| Lactic acid (g/kg) | −1.6 × 10−1 | 5.6 × 10−2 | 0.0094 | −4.0 × 10−4 | 1.0 × 10−5 | <0.0001 | 0.967 | 2.7 × 10−1 | 1733 | 2.4 |
| NaCl (g/kg) | −1.1 × 10−1 | 2.9 × 10−2 | 0.0005 | −7.0 × 10−4 | 1.1 × 10−5 | <0.0001 | 0.995 | 1.6 × 10−1 | 990 | 3.7 |
| K (g/kg) | −1.3 × 10−1 | 1.0 × 10−1 | 0.2105 ◊ | −7.0 × 10−4 | 1.9 × 10−5 | <0.0001 | 0.949 | 6.2 × 10−1 | 990 | 4.1 |
| Ca (g/kg) | −4.0 × 10−1 | 7.2 × 10−2 | <0.0001 | 3.0 × 10−4 | 1.3 × 10−5 | <0.0001 | 0.920 | 4.1 × 10−1 | 2310 | 1.8 |
| Mg (g/kg) | −9.8 × 10−2 | 5.1 × 10−2 | 0.0595 ◊ | −4.0 × 10−4 | 9.1 × 10−6 | <0.0001 | 0.976 | 2.9 × 10−1 | 1733 | 2.4 |
| P (g/kg) | −1.3 × 10−2 | 4.4 × 10−2 | 0.7702 ◊ | −3.0 × 10−4 | 7.9 × 10−6 | <0.0001 | 0.939 | 2.6 × 10−1 | 2310 | 1.8 |
| Green Spanish-style Manzanilla table olives stuffed with red pepper paste (PPSGM) | ||||||||||
| Moisture (%) | −1.3 × 10−1 | 7.4 × 10−2 | 0.0871 ◊ | −7.3 × 10−3 | 6.0 × 10−4 | <0.0001 | 0.960 | 1.9 × 10−1 | 95 | 43.8 |
| Lactic acid (g/kg) | 3.4 × 10−2 | 6.0 × 10−2 | <0.0001 | −1.3 × 10−3 | 7.5 × 10−5 | <0.0001 | 0.978 | 1.8 × 10−1 | 533 | 7.8 |
| NaCl (g/kg) | −2.3 × 10−1 | 5.5 × 10−2 | 0.0002 | −2.3 × 10−3 | 6.4 × 10−5 | <0.0001 | 0.991 | 2.0 × 10−1 | 301 | 13.8 |
| K (g/kg) | −4.2 × 10−2 | 1.5 × 10−2 | 0.0117 | −3.4 × 10−3 | 7.1 × 10−5 | <0.0001 | 0.997 | 0.4 × 10−1 | 204 | 20.4 |
| Ca (g/kg) | −4.7 × 10−2 | 9.7 × 10−2 | <0.0001 | −2.0 × 10−3 | 1.0 × 10−4 | <0.0001 | 0.963 | 3.7 × 10−1 | 347 | 12.0 |
| Mg (g/kg) | −2.1 × 10−1 | 8.8 × 10−2 | 0.0250 | 2.8 × 10−3 | 1.0 × 10−4 | <0.0001 | 0.983 | 3.4 × 10−1 | 248 | 16.5 |
| P (g/kg) | 4.8 × 10−2 | 6.8 × 10−2 | 0.4897 ◊ | 1.6 × 10−3 | 8.5 × 10−5 | <0.0001 | 0.969 | 2.6 × 10−1 | 433 | 9.59 |
| Traditional Aloreña de Málaga table olives (TA) | ||||||||||
| Moisture (%) | −2.6 × 10−1 | 7.0 × 10−2 | 0.0018 | −4.3 × 10−3 | 3.0 × 10−4 | <0.0001 | 0.953 | 2.1 × 10−1 | 161 | 25.8 |
| Lactic acid (g/kg) | −2.2 × 10−1 | 6.8 × 10−2 | 0.0082 | −3.4 × 10−3 | 3.0 × 10−4 | <0.0001 | 0.956 | 1.7 × 10−1 | 204 | 20.4 |
| NaCl (g/kg) | −2.2 × 10−1 | 4.4 × 10−2 | 0.0002 | −3.7 × 10−3 | 2.0 × 10−4 | <0.0001 | 0.954 | 1.3 × 10−1 | 187 | 22.0 |
| K (g/kg) | −3.6 × 10−1 | 7.0 × 10−2 | <0.0001 | −3.3 × 10−3 | 3.0 × 10−4 | <0.0001 | 0.926 | 2.1 × 10−1 | 210 | 19.8 |
| Ca (g/kg) | −5.1 × 10−2 | 2.9 × 10−2 | 0.1031 ◊ | −3.6 × 10−3 | 2.0 × 10−4 | <0.0001 | 0.974 | 0.7 × 10−1 | 193 | 21.6 |
| Mg (g/kg) | 2.7 × 10−1 | 5.5 × 10−2 | 0.0002 | 3.1 × 10−3 | 3.0 × 10−4 | <0.0001 | 0.945 | 1.6 × 10−1 | 224 | 18.6 |
| P (g/kg) | 3.1 × 10−1 | 5.9 × 10−2 | <0.0001 | 3.2 × 10−3 | 3.0 × 10−4 | <0.0001 | 0.941 | 1.8 × 10−1 | 217 | 19.2 |
| Mineral Nutrient | PGM | PPSGM | TA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content in the Stored Product | Conc. after Desalting | Released Amount (%) | Content in the Stored Product | Conc. after Desalting | Released Amount (%) | Content in the Stored Product | Conc. after Desalting | Released Amount (%) | |||||
| 5.0% NaCl | 2.5% NaCl | 5.0% NaCl | 2.5% NaCl | Stuffed Olives | Olives | Stuffing Material | |||||||
| Na | 27,185 (35) | 15,011 (98) | 7548 (65) | 12,174 (50%) | 19,637 (72%) | 21,198 (36) | 20,069 (126) | 26,541 (126) | 7340 (46) | 13,858 (65%) | 18,590 (223) | 7205 (4) | 11,385 (61%) |
| K | 610 (3) | 334 (9) | 132 (2) | 276 (45%) | 478 (78%) | 659 (8) | 647 (8) | 857 (8) | 232 (1) | 427 (65%) | 2468 (11) | 843 (1) | 1625 (66%) |
| Ca | 761 (1) | 678 (10) | 620 (2) | 90 (12%) | 141 (19%) | 2813 (58) | 2338 (31) | 3226 (31) | 1555 (11) | 1258 (45%) | 1189 (11) | 1035 (1) | 154 (13%) |
| Mg | 140 (1) | 105 (5) | 62 (1) | 35 (25%) | 78.5 (56%) | 194 (3) | 180 (3) | 233 (3) | 77 (1) | 118 (61%) | 126 (1) | 57 (1) | 69 (55%) |
| P | 129 (1) | NA | 89 (1) | NA | 40.1 (31%) | 115 (2) | 120 (2) | 129 (2) | 75 (1) | 40.2 (35%) | 189 (2) | 119 (<1) | 70 (37%) |
| Fe | 29 (1) | 18.4 (0.2) | 16.7 (0.1) | 10.23 (36%) | 11.9 (42%) | 29 (1) | 27 (1) | 23 (1) | 25 (1) | 4.2 (14%) | 5.55 (0.05) | 4.04 (0.03) | 1.46 (27%) |
| Cu | 2.09 (0.02) | 1.55 (0.04) | 1.51 (0.05) | 0.54 (26%) | 0.58 (28%) | 1.30 (0.06) | 1.69 (0.06) | 1.03 (0.06) | 1.08 (0.06) | 0.22 (22%) | 3.31 (0.03) | 2.65 (0.04) | 0.66 (20%) |
| Mn | 1.07 (0.02) | 0.63 (0.02) | 0.40 (0.01) | 0.44 (41.1%) | 0.67 (62.6%) | 0.28 (0.05) | NA | NA | 0.27 (0.02) | 0.10 (36%) | 1.04 (0.06) | 0.52 (0.01) | 0.52 (50%) |
| Zn | 3.95 (0.15) | 2.19 (0.03) | NA | 1.76 (44.5%) | NA | 2.56 (0.10) | 2.58 (0.09) | 2.00 (0.09) | 2.08 (0.11) | 0.48 (19%) | 4.03 (0.02) | 2.57 (0.02) | 1.46 (36%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-López, A.; Moreno-Baquero, J.M.; Garrido-Fernández, A. The Desalting Process for Table Olives and Its Effect on Their Physicochemical Characteristics and Nutrient Mineral Content. Foods 2023, 12, 2307. https://doi.org/10.3390/foods12122307
López-López A, Moreno-Baquero JM, Garrido-Fernández A. The Desalting Process for Table Olives and Its Effect on Their Physicochemical Characteristics and Nutrient Mineral Content. Foods. 2023; 12(12):2307. https://doi.org/10.3390/foods12122307
Chicago/Turabian StyleLópez-López, Antonio, José María Moreno-Baquero, and Antonio Garrido-Fernández. 2023. "The Desalting Process for Table Olives and Its Effect on Their Physicochemical Characteristics and Nutrient Mineral Content" Foods 12, no. 12: 2307. https://doi.org/10.3390/foods12122307
APA StyleLópez-López, A., Moreno-Baquero, J. M., & Garrido-Fernández, A. (2023). The Desalting Process for Table Olives and Its Effect on Their Physicochemical Characteristics and Nutrient Mineral Content. Foods, 12(12), 2307. https://doi.org/10.3390/foods12122307






