Physicochemical, Antioxidant, Sensory, and Starch Digestibility Properties of Steamed Bread Fortified with Tamarillo Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Steamed Bread Making
2.3. Determination of Proximate Composition, Total Dietary Fiber, and Water Holding Capacity
2.4. Determination of Physical Characteristics
2.5. Determination of Bioactive Components
2.6. Determination of Antioxidant Properties
2.7. Determination of Starch Hydrolysis and Predicted Glycemic Index (pGI)
2.8. Sensory Evaluation
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Qualities of Tamarillo Powder and Wheat Flour
3.2. Physical Characteristics of the Steamed Bread
3.3. Proximate Composition, Total Dietary Fiber, and Water Activity of the Steamed Bread
3.4. Bioactive Components and Antioxidant Activity of the Steamed Bread Extract
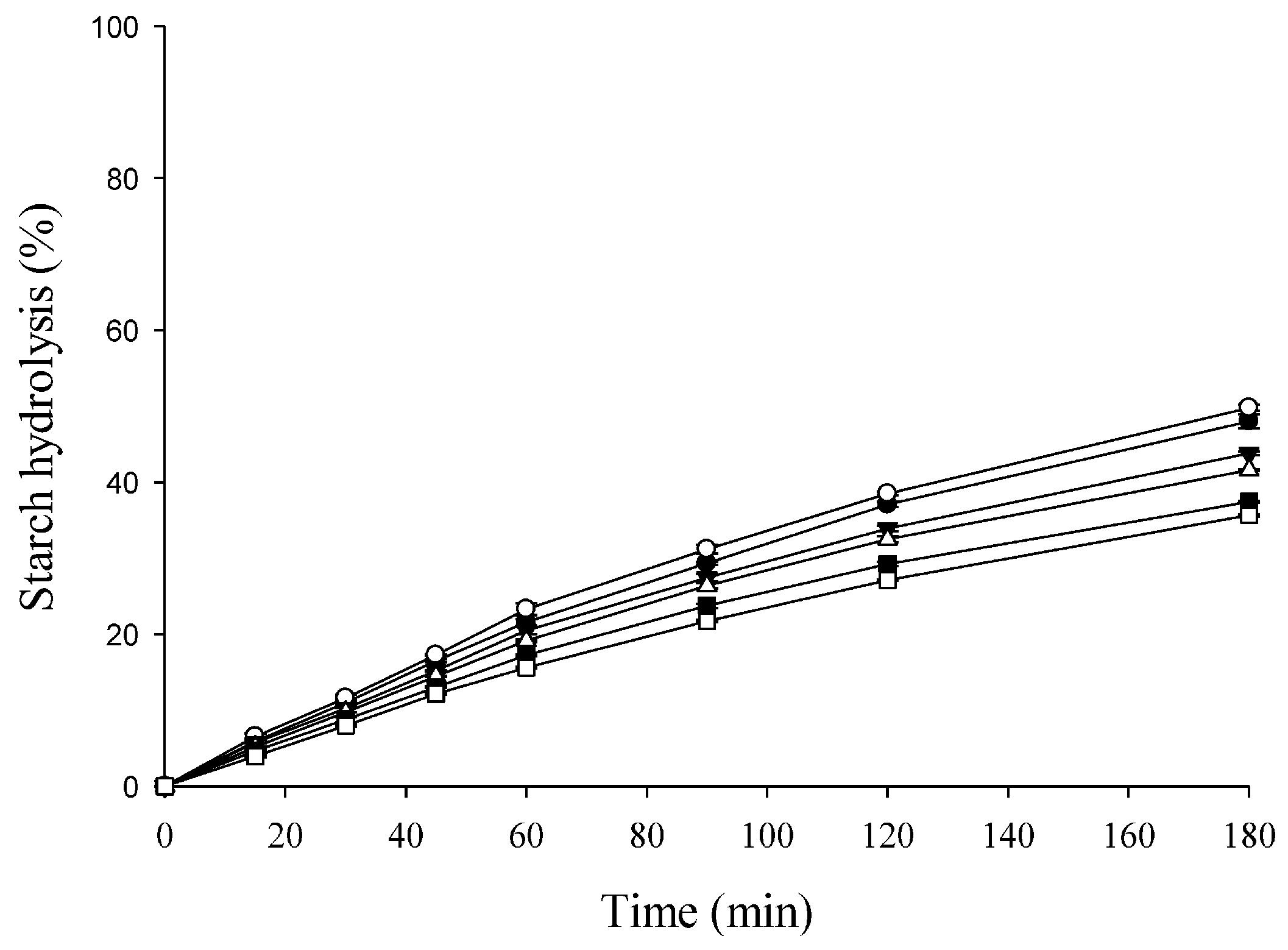
3.5. Starch Hydrolysis and pGI of the SB
3.6. Sensory Evaluation
3.7. Effect of Added Water Amount on the Physical and Sensory Properties of T20
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, F. Influence of ingredients and chemical components on the quality of Chinese steamed bread. Food Chem. 2014, 163, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.S.; Yu, M.; Xia, C.; Meng, N.; Wang, B.T.; Mo, J.X.; Che, S.P. Analysis on glycemic indexes of the staple foods in Tianjin City. J. Tianjin Med. Univ. 2003, 9, 152–155. [Google Scholar]
- Lan, S.Q.; Meng, Y.N.; Li, X.P.; Zhang, Y.L.; Song, G.Y.; Ma, H.J. Effect of consumption of micronutrient enriched wheat steamed bread on postprandial plasma glucose in healthy and type 2 diabetic subjects. Nutr. J. 2013, 12, 64. [Google Scholar]
- Yang, Y.X.; Wang, H.W.; Cui, H.M.; Wang, Y.; Yu, L.D.; Xiang, S.X.; Zhou, S.Y. Glycemic index of cereal and tubers produced in China. World J. Gastroenterol. 2006, 12, 3430–3433. [Google Scholar] [CrossRef]
- Ma, X.Y.; Liu, J.P.; Song, Z.Y. Glycemic load, glycemic index and risk of cardiovascular diseases: Meta-analyses of prospective studies. Atherosclerosis 2012, 223, 491–496. [Google Scholar] [CrossRef]
- Zhu, F. Glycemic control in Chinese steamed bread: Strategies and opportunities. Trends Food Sci. Technol. 2019, 86, 252–259. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Weng, Y.M.; Yu, Z.R.; Wang, B.J. Substitution of wheat flour with wholegrain flours affects physical properties, sensory acceptance, and starch digestion of Chinese steam bread (Mantou). LWT Food Sci. Technol. 2017, 86, 571–576. [Google Scholar] [CrossRef]
- Zhu, F.; Chan, C. Effect of chia seed on glycemic response, texture, and sensory properties of Chinese steamed bread. LWT Food Sci. Technol. 2018, 98, 77–84. [Google Scholar] [CrossRef]
- Guo, D.; Yin, X.; Cheng, H.; Chen, J.; Ye, X. Fortification of Chinese steamed bread with Glycyrrhiza uralensis polysaccharides and evaluation of its quality and performance attributes. Foods 2022, 11, 2253. [Google Scholar] [CrossRef]
- Espin, S.; Gonzalez-Manzano, S.; Taco, V.; Poveda, C.; Ayuda-Duran, B.; Gonzalez-Paramas, A.M.; Santos-Buelga, C. Phenolic Composition and Antioxidant Capacity of Yellow and Purple-red Ecuadorian Cultivars of Tree Tomato (Solanum betaceum Cav.). Food Chem. 2016, 194, 1073–1080. [Google Scholar] [CrossRef]
- Abdul Mutalib, M.; Rahmat, A.; Ali, F.; Othman, F.; Ramasamy, R. Nutritional compositions and antiproliferative activities of different solvent fractions from ethanol extract of Cyphomandra betacea (tamarillo) fruit. Malays J. Med. Sci. 2017, 24, 19–32. [Google Scholar] [CrossRef]
- Muliarta, M.; Tirtayasa, K.; Prabawa, P.Y.; Wiryadana, K.A. Tamarillo consumption associated with increased acetylcholinesterase activity and improved oxidative stress markers in farmers exposed to daily pesticide-related activities in Baturiti, Bali, Indonesia. Open Access Maced. J. Med. Sci. 2020, 8, 244–250. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Tamarillo (Solanum betaceum): Chemical composition, biological properties, and product innovation. Trends Food Sci. Technol. 2020, 95, 45–58. [Google Scholar] [CrossRef]
- Diep, T.T.; Rush, E.C.; Yoo, M.J.Y. Tamarillo (Solanum betaceum Cav.): A review of physicochemical and bioactive properties and potential applications. Food Rev. Int. 2022, 38, 1343–1367. [Google Scholar] [CrossRef]
- Hu, C.; Gao, X.; Dou, K.; Zhu, C.; Zhou, Y.; Hu, Z. Physiological and Metabolic Changes in Tamarillo (Solanum betaceum) during Fruit Ripening. Molecules 2023, 28, 1800. [Google Scholar] [CrossRef]
- Abdul Kadir, N.A.A.; Rahmat, A.; Jaafar, H.Z.E. Protective effects of tamarillo (Cyphomandra betacea) extract against high fat diet induced obesity in sprague-dawley rats. J. Obes. 2015, 2015, 846041. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, R.M.; Ordóñez, A.A.L.; Sayago, J.E.; Nieva Moreno, M.I.; Isla, M.I. Antimicrobial activity of glycosidase inhibitory protein isolated from Cyphomandra betacea Sendt. fruit. Peptides 2006, 27, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Senthil Kumar, C.; Sivakumar, M.; Ruckmani, K. Microwave-assisted extraction of polysaccharides from Cyphomandra betacea and its biological activities. Int. J. Biol. Macromol. 2016, 92, 682–693. [Google Scholar]
- Orqueda, M.E.; Rivas, M.; Zampini, I.C.; Alberto, M.R.; Torres, S.; Cuello, S.; Sayago, J.; Thomas-Valdes, D.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; et al. Chemical and functional characterization of seed, pulp and skin powder from chilto (Solanum betaceum), an Argentine native fruit. Phenolic fractions affect key enzymes involved in metabolic syndrome and oxidative stress. Food Chem. 2017, 216, 70–79. [Google Scholar] [CrossRef]
- Suárez-Montenegro, Z.J.; Ballesteros-Vivas, D.; Gallego, R.; Valdés, A.; Sánchez-Martínez, J.D.; Parada-Alfonso, F.; Ibáñez, E.; Cifuentes, A. Neuroprotective potential of tamarillo (Cyphomandra betacea) epicarp extracts obtained by sustainable extraction process. Front. Nutr. 2021, 8, 769617. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, Y.; Adzahan, N.M.; Yusof, Y.A.; Muhammad, K. Effect of wall materials on the spray drying efficiency, powder properties and stability of bioactive compounds in tamarillo juice microencapsulation. Powder Technol. 2018, 328, 406–414. [Google Scholar] [CrossRef]
- Fernandino, C.M.; Nepomuceno, A.T.; Fonseca, H.C.; Bastos, R.A.; Lima, J.P. Physicochemical properties of tamarillo pulp (Solanum betaceum) and its applicability in the production of ice cream. Braz. J. Food Technol. 2021, 24, e2020090. [Google Scholar] [CrossRef]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Huang, Y.-X.; Lin, Z.-E. White Mantou (Knife Cut)—Making of Chinese Traditional Wheat Food—Ferment Dough; China Grain Products Research & Development Institute: New Taipei, Taiwan, 2007; Chapter 6, 6-1-1; pp. 31–33. [Google Scholar]
- AACC International. Approved Methods of the AACC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. Official Methods of Analysis. Method 985.29. Total Dietary Fiber in Foods, Enzymatic-Gravimetric Method, 17th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2003. [Google Scholar]
- Mau, J.L.; Lee, C.C.; Yang, C.W.; Chen, R.W.; Zhang, Q.F.; Lin, S.D. Physicochemical, antioxidant and sensory characteristics of bread partially substituted with aerial parts of sweet potato. LWT Food Sci. Technol. 2020, 117, 108602. [Google Scholar] [CrossRef]
- Lu, T.M.; Lee, C.C.; Mau, J.L.; Lin, S.D. Quality and antioxidant property of green tea sponge cake. Food Chem. 2010, 119, 1090–1095. [Google Scholar] [CrossRef]
- Mau, J.L.; Lee, C.C.; Chen, Y.P.; Lin, S.D. Physicochemical, antioxidant and sensory characteristics of chiffon cake prepared with black rice as replacement for wheat flour. LWT Food Sci. Technol. 2017, 75, 434–439. [Google Scholar] [CrossRef]
- Knockaert, G.; Lemmens, L.; Van Buggenhout, S.; Hendrickx, M.; Van Loey, A. Changes in β-carotene bioaccessibility and concentration during processing of carrot puree. Food Chem. 2012, 133, 60–67. [Google Scholar] [CrossRef]
- Attia, T.Z. Simultaneous determination of rutin and ascorbic acid mixture in their pure forms and combined dosage form. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 169, 82–86. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Germaine, K.A.; Samman, S.; Fryirs, C.G.; Griffiths, P.J.; Johnson, S.K.; Quail, K.J. Comparison of in vitro starch digestibility methods for predicting the glycaemic index of grain foods. J. Sci. Food Agric. 2008, 88, 652–658. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Robertson, J.A.; Eastwood, M.A. An examination of factors which may affect the water holding capacity of dietary fibre. Br. J. Nutr. 1981, 45, 83–88. [Google Scholar] [CrossRef]
- Smith, A.R. Color gamut transform pairs. ACM Siggraph Comput. Graph. 1978, 12, 12–19. [Google Scholar] [CrossRef]
- Song, K.Y.; Joung, K.Y.; Shin, S.Y.; Kim, Y.S. Effects of basil (Ocimum basilicum L.) seed mucilage substituted for fat source in sponge cake: Physicochemical, structural, and retrogradation properties. Ital. J. Food Sci. 2017, 29, 681–696. [Google Scholar]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L. Fruits and vegetables in the prevention of cellular oxidative damage. Am. J. Clin. Nutr. 2003, 78, 570S–578S. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.Y.; Sung, J.M.; Huang, P.W.; Lin, S.D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Kähkönen, M.; Heinonen, M. Antioxidant Activity of Anthocyanins and Their Aglycons. J. Agric. Food Chem. 2003, 51, 628–633. [Google Scholar] [CrossRef]
- Nuray, K.; Feryal, K. Changes of bioactive compounds and anti-oxidant activity during cold storage of carrot. Int. J. Food Sci. Technol. 2008, 43, 2019–2025. [Google Scholar]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Ahmed, J.; Almusallam, A.S.; Al-Salman, F.; AbdulRahman, M.H.; Al-Salem, E. Rheological properties of water insoluble date fiber incorporated wheat flour dough. LWT Food Sci. Technol. 2013, 51, 409–416. [Google Scholar] [CrossRef]
- Zhu, F.; Sun, J. Physicochemical and sensory properties of steamed bread fortified with purple sweet potato flour. Food Biosci. 2019, 30, 100411. [Google Scholar] [CrossRef]
- Wang, S.; Opassathavorn, A.; Zhu, F. Influence of quinoa flour on quality characteristics of cookie, bread, and Chinese steamed bread. J. Texture Stud. 2015, 46, 281–292. [Google Scholar] [CrossRef]
- Wang, S.; Khamchanxana, P.; Zhu, F.; Zhu, C.; Pan, J. Textural and sensory properties of steamed bread fortified with high-amylose maize starch. J. Texture Stud. 2017, 48, 3–8. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Relationship between instrumental parameters and sensory characteristics in gluten-free breads. Eur. Food Res. Technol. 2012, 235, 107–117. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42 (Suppl. 1), S46–S60. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, Y.; Björck, I.; Drews, A.; Tovar, J. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Eur. J. Clin. Nutr. 1992, 46, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.H.; Liu, K.L.; Chen, P.Y.; Ke, N.J.; Chen, J.J.; Sung, J.M.; Wu, Y.L.; Lin, S.D. Predicted glycemic index and glycemic index of rice varieties grown in Taiwan. Cereal Chem. 2016, 93, 150–155. [Google Scholar] [CrossRef]
- Stone, H.; Sidel, J.L. Sensory Evaluation Practices, 2nd ed.; Academic Press Inc.: San Diego, CA, USA, 1993; p. 234. [Google Scholar]

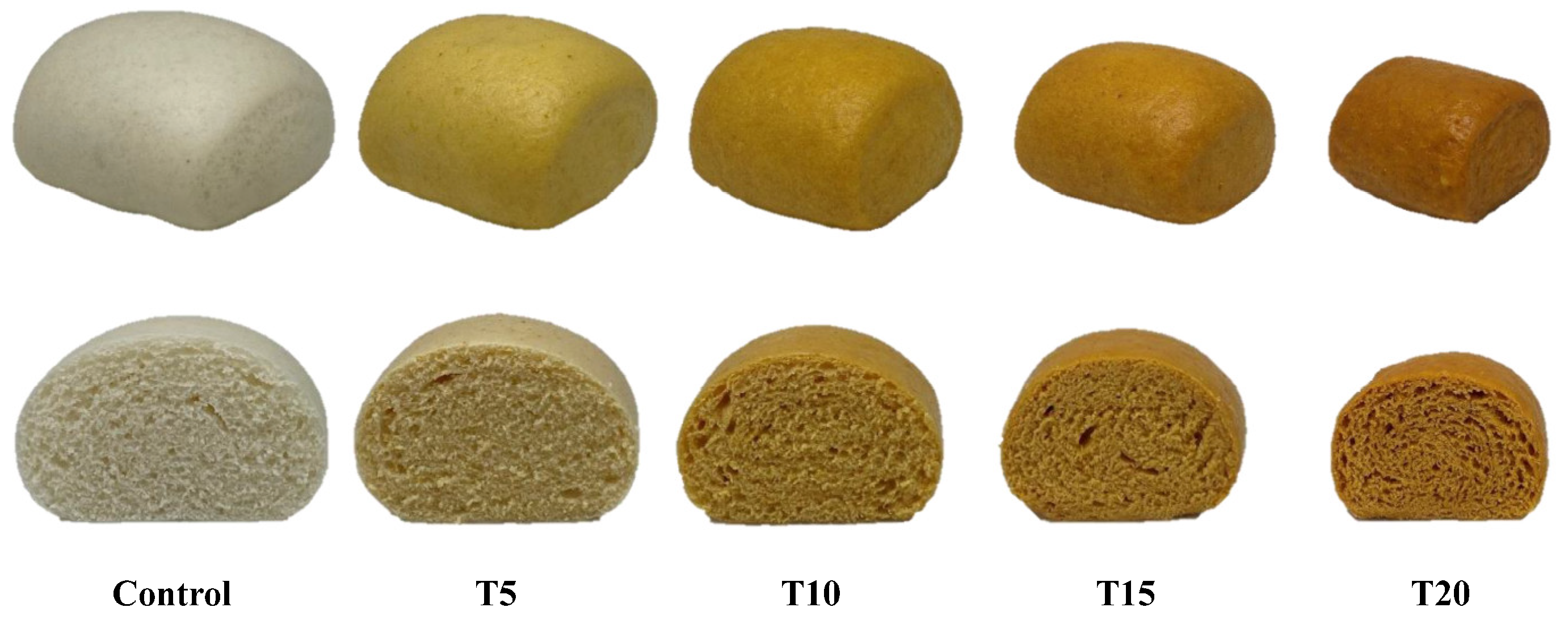
 ), T5 (
), T5 ( ), T10 (
), T10 ( ), T15 (
), T15 ( ), T20 (
), T20 ( ). Values of each hedonic scale were averaged from 90 replicates. Nine-point hedonic scale with 1, 5, and 9 representing extremely disliked, neither liked nor disliked, and extremely liked, respectively.
). Values of each hedonic scale were averaged from 90 replicates. Nine-point hedonic scale with 1, 5, and 9 representing extremely disliked, neither liked nor disliked, and extremely liked, respectively.
 ), T5 (
), T5 ( ), T10 (
), T10 ( ), T15 (
), T15 ( ), T20 (
), T20 ( ). Values of each hedonic scale were averaged from 90 replicates. Nine-point hedonic scale with 1, 5, and 9 representing extremely disliked, neither liked nor disliked, and extremely liked, respectively.
). Values of each hedonic scale were averaged from 90 replicates. Nine-point hedonic scale with 1, 5, and 9 representing extremely disliked, neither liked nor disliked, and extremely liked, respectively.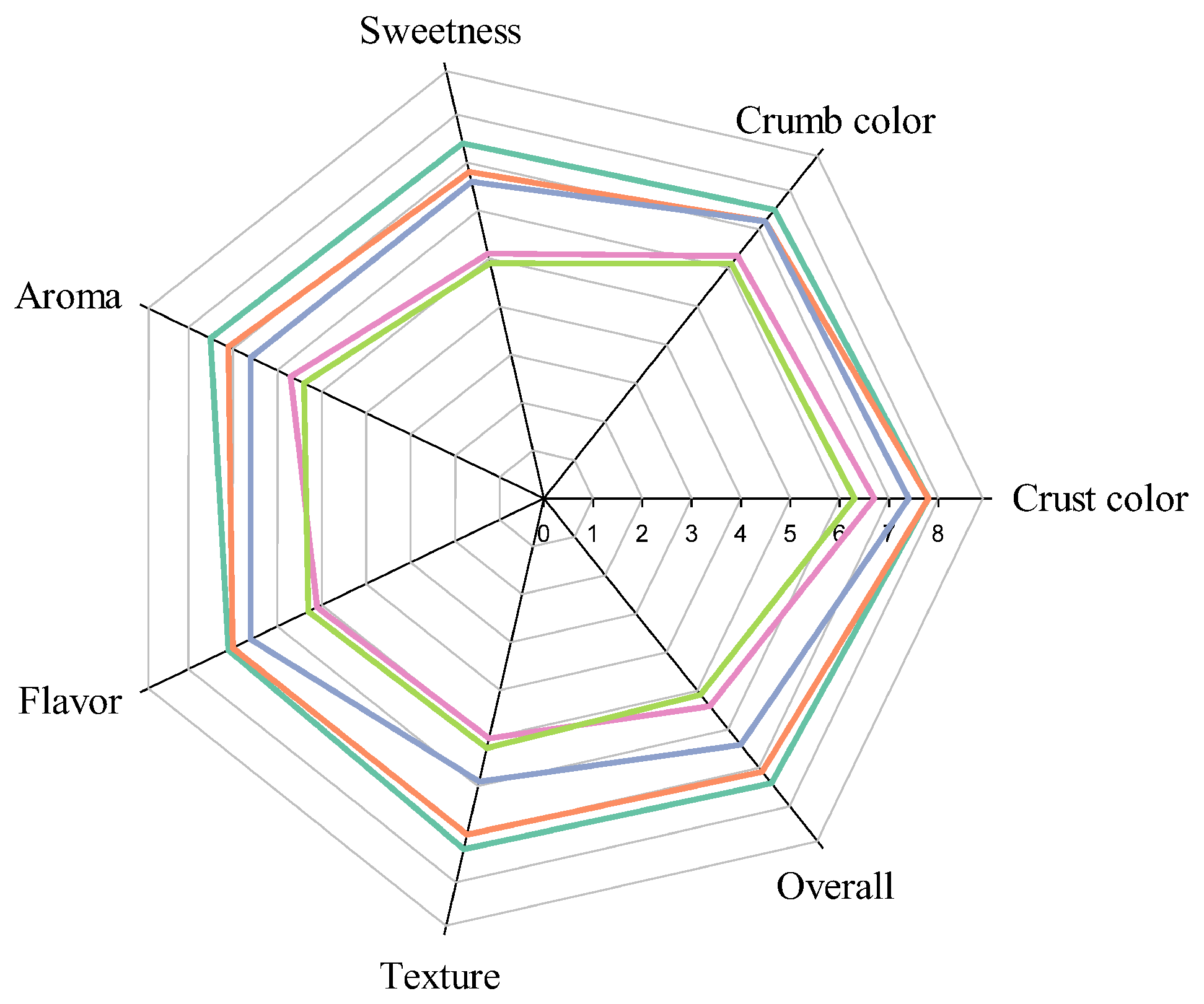

| Ingredient (%) | C 1 | T5 | T10 | T15 | T20 | T20-05 | T20-10 | T20-15 | T20-20 | T20-25 | T20-30 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wheat flour | middle protein flour | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 | 80 |
| low-protein flour | 20 | 15 | 10 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tamarillo powder | 0 | 5 | 10 | 15 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| Soybean powder | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Yeast | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |
| Sugar | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |
| Shortening | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Water | 55 | 55 | 55 | 55 | 55 | 60 | 65 | 70 | 75 | 80 | 85 | |
| Total | 165 | 165 | 165 | 165 | 165 | 170 | 175 | 180 | 185 | 190 | 195 | |
| TP 1 | MPF | LPF | |
|---|---|---|---|
| Moisture (g/100 g) | 1.28 ± 0.04 c2 | 6.94 ± 0.09 a | 6.20 ± 0.09 b |
| Crude fat (g/100 g) | 0.94 ± 0.03 a | 0.93 ± 0.02 a | 0.94 ± 0.02 a |
| Crude protein (g/100 g) | 10.51 ± 0.21 b | 11.07 ± 0.08 a | 8.15 ± 0.08 c |
| Crude ash (g/100 g) | 6.91 ± 0.01 a | 0.38 ± 0.01 c | 0.44 ± 0.01 b |
| Carbohydrate (g/100 g) | 80.36 ± 0.21 c | 80.68 ± 0.18 b | 84.27 ± 0.02 a |
| Total dietary fiber (g/100 g) | 36.45 ± 9.21 a | 1.93 ± 0.73 b | 2.07 ± 0.89 b |
| Water activity | 0.254 ± 0.002 c | 0.641 ± 0.005 a | 0.593 ± 0.006 b |
| Water holding capacity (g H2O absorbed/g sample) | 2.97 ± 0.01 a | 0.81 ± 0.01 b | 0.59 ± 0.01 c |
| Color property | |||
| L* | 53.50 ± 0.12 c | 93.11 ± 0.07 b | 93.98 ± 0.08 a |
| a* | 19.16 ± 0.12 a | 0.19 ± 0.04 b | 0.02 ± 0.03 b |
| b* | 21.91 ± 0.08 a | 8.61 ± 0.06 b | 7.26 ± 0.04 c |
| WI* | 44.99 ± 0.17 c | 88.97 ± 0.01 b | 90.57 ± 0.06 a |
| c* | 29.23 ± 0.12 a | 8.55 ± 0.06 b | 7.30 ± 0.04 c |
| h* (°) | 48.83 ± 0.01 c | 88.74 ± 0.01 b | 89.84 ± 0.01 a |
| ΔE | 45.99 ± 0.13 b | 47.23 ± 0.02 a | |
| TP 1 | MPF | LPF | |
|---|---|---|---|
| Yield (g extract/100 g powder) | 52.69 ± 0.86 a3 | 7.16 ± 0.16 b | 6.31 ± 0.38 b |
| Total phenols (mg GAE/g extract) 1 | 28.90 ± 0.20 a | 13.27 ± 0.24 b | 12.31 ± 0.51 c |
| Ascorbic acid (mg/g extract) | 3.25 ± 0.05 a | 0.32 ± 0.01 b | 0.31 ± 0.01 b |
| Total anthocyanins (μg C3GE/g extract) 1 | 316.35 ± 0.02 | nd 4 | nd |
| Total carotenoids (μg βCE/g extract) 1 | 12.68 ± 1.33 | nd | nd |
| EC50 value of antioxidant property (mg extract/mL) 2 | |||
| Scavenging ability of DPPH radicals | 0.29 ± 0.01 c | 3.84 ± 0.05 b | 4.13 ± 0.02 a |
| Reducing power | 0.95 ± 0.05 b | 46.27 ± 0.32 a | 46.35 ± 0.72 a |
| Control | T5 | T10 | T15 | T20 | |
|---|---|---|---|---|---|
| Weight (g) | 25.25 ± 0.10 a1 | 25.19 ± 0.19 a | 25.11 ± 0.10 a | 25.49 ± 0.34 a | 25.40 ± 0.11 a |
| Dimension | |||||
| Length (mm) | 59.15 ± 1.45 a | 58.90 ± 0.50 a | 45.90 ± 0.10 b | 43.20 ± 0.30 c | 43.70 ± 1.10 c |
| Width (mm) | 51.65 ± 0.65 a | 48.48 ± 0.60 b | 45.40 ± 0.30 c | 42.76 ± 0.25 d | 37.90 ± 0.6 e |
| Height (mm) | 32.65 ± 0.35 a | 32.11 ± 0.10 b | 31.25 ± 0.15 c | 31.05 ± 0.15 c | 28.75 ± 0.30 d |
| Spread ratio | 1.582 ± 0.004 a | 1.510 ± 0.034 b | 1.453 ± 0.023 c | 1.377 ± 0.021 d | 1.318 ± 0.023 e |
| Volume (mL) | 78.5 ± 0.7 a | 76.0 ± 0.1 b | 69.5 ± 0.7 c | 57.5 ± 0.7 d | 44.0 ± 1.4 e |
| Specific volume (mL/g) | 3.11 ± 0.03 a | 3.02 ± 0.02 a | 2.77 ± 0.02 b | 2.26 ± 0.05 c | 1.73 ± 0.05 d |
| Crust color property 2 | |||||
| L* | 82.83 ± 0.49 a | 76.12 ± 1.30 b | 68.41 ± 0.79 c | 64.09 ± 0.62 d | 59.15 ± 0.66 e |
| a* | −0.18 ± 0.08 e | 5.71 ± 0.14 d | 10.98 ± 0.25 c | 15.01 ± 0.44 b | 17.45 ± 0.59 a |
| b* | 11.15 ± 0.32 d | 25.77 ± 0.44 c | 34.18 ± 0.52 a | 34.95 ± 0.81 a | 31.36 ± 0.74 b |
| c* | 11.15 ± 0.32 d | 26.39 ± 0.43 c | 35.90 ± 0.48 b | 38.04 ± 0.75 a | 35.88 ± 0.88 b |
| h* (°) | 90.92 ± 0.26 a | 77.51 ± 0.20 b | 72.19 ± 0.26 c | 66.76 ± 0.67 d | 60.91 ± 0.29 e |
| ΔE | 17.12 ± 0.37 d | 29.38 ± 0.39 c | 33.89 ± 0.51 b | 35.77 ± 0.60 a | |
| Crumb color property | |||||
| L* | 79.82 ± 1.08 a | 75.14 ± 0.86 b | 68.74 ± 0.98 c | 67.34 ± 0.79 d | 60.62 ± 0.74 e |
| a* | −0.14 ± 0.01 e | 4.61 ± 0.15 d | 8.79 ± 0.17 c | 11.34 ± 0.19 b | 14.14 ± 0.40 a |
| b* | 12.17 ± 0.44 d | 24.62 ± 0.88 c | 30.83 ± 0.31 b | 31.94 ± 0.47 a | 30.77 ± 0.43 b |
| c* | 12.17 ± 0.44 d | 25.05 ± 0.88 c | 32.06 ± 0.31 b | 33.89 ± 0.47 a | 33.86 ± 0.47 a |
| h* (°) | 90.66 ± 0.16 a | 79.39 ± 0.26 b | 74.09 ± 0.11 c | 70.45 ± 0.31 d | 65.32 ± 0.21 e |
| ΔE | 14.12 ± 0.96 d | 23.47 ± 0.89 c | 26.14 ± 0.79 b | 30.33 ± 0.74 a | |
| Texture profile analysis | |||||
| Hardness (N) | 4.86 ± 0.12 e | 6.18 ± 0.30 d | 7.61 ± 0.20 c | 10.08 ± 0.23 b | 11.65 ± 0.41 a |
| Cohesiveness | 0.81 ± 0.01 a | 0.74 ± 0.01 b | 0.69 ± 0.01 c | 0.64 ± 0.01 d | 0.61 ± 0.02 e |
| Springiness | 0.99 ± 0.02 a | 0.93 ± 0.02 a | 0.86 ± 0.03 b | 0.80 ± 0.04 c | 0.76 ± 0.05 c |
| Gumminess (N) | 3.94 ± 0.08 e | 4.57 ± 0.41 d | 5.25 ± 0.03 c | 6.45 ± 0.16 b | 7.11 ± 0.46 a |
| Chewiness (N) | 3.89 ± 0.11 e | 4.25 ± 0.37 d | 4.52 ± 0.14 c | 5.16 ± 0.35 b | 5.40 ± 1.44 a |
| Resilience | 0.43 ± 0.01 a | 0.29 ± 0.01 b | 0.27 ± 0.02 c | 0.23 ± 0.01 d | 0.21 ± 0.02 d |
| Control | T5 | T10 | T15 | T20 | |
|---|---|---|---|---|---|
| Moisture (%) | 37.58 ± 0.07 ab1 | 37.66 ± 0.07 a | 37.48 ± 0.16 b | 37.25 ± 0.09 c | 37.02 ± 0.07 d |
| Crude ash (%) | 0.41 ± 0.01 e | 0.54 ± 0.01 d | 0.67 ± 0.03 c | 0.84 ± 0.01 b | 1.05 ± 0.01 a |
| Crude fat (%) | 0.61 ± 0.01 a | 0.61 ± 0.01 a | 0.61 ± 0.01 a | 0.62 ± 0.01 a | 0.62 ± 0.01 a |
| Crude protein (%) | 7.63 ± 0.04 c | 7.63 ± 0.05 c | 7.82 ± 0.11 b | 7.90 ± 0.17 b | 8.09 ± 0.04 a |
| Carbohydrate (%) | 53.77 ± 0.07 a | 53.56 ± 0.01 ab | 53.42 ± 0.171 b | 53.39 ± 0.184 b | 53.22 ± 0.07 b |
| Total dietary fiber (%) | 1.19 ± 0.47 e | 2.01 ± 0.53 d | 3.15 ± 0.091 c | 4.25 ± 0.073 b | 5.11 ± 0.602 a |
| Water activity | 0.984 ± 0.004 a | 0.982 ± 0.062 a | 0.975 ± 0.058 a | 0.965 ± 0.053 b | 0.964 ± 0.058 b |
| Control | T5 | T10 | T15 | T20 | |
|---|---|---|---|---|---|
| Yield (g extract/100 g powder) | 15.72 ± 0.11 e3 | 18.48 ± 0.08 d | 20.63 ± 0.93 c | 24.09 ± 0.06 b | 25.43 ± 0.12 a |
| Total phenols (mg GAE/g extract) 1 | 10.58 ± 0.25 e | 12.19 ± 0.54 d | 14.74 ± 0.05 c | 15.22 ± 0.08 b | 15.92 ± 0.06 a |
| Ascorbic acid (mg/g extract) | 0.241 ± 0.008 d | 0.384 ± 0.008 c | 0.546 ± 0.003 b | 0.621 ± 0.029 a | 0.627 ± 0.004 a |
| Total anthocyanins (μg C3GE/g extract) 1 | nd 4 | 243.1 ± 2.1 d | 265.5 ± 1.3 c | 357.1 ± 3.5 b | 389.3 ± 2.3 a |
| Total carotenoids (μg βCE/g extract) 1 | nd | 25.39 ± 0.48 c | 27.25 ± 0.23 b | 27.14 ± 0.16 b | 30.34 ± 0.23 a |
| EC50 value of antioxidant property (mg extract/mL) 2 | |||||
| Scavenging ability of DPPH radicals | 12.93 ± 0.06 a | 2.14 ± 0.10 b | 1.34 ± 0.12 b | 0.97 ± 0.04 b | 0.88 ± 0.05 b |
| Reducing power | 42.29 ± 0.38 a | 6.39 ± 0.21 b | 4.22 ± 0.18 c | 2.98 ± 0.72 d | 2.35 ± 0.04 e |
| Sample | pGI | GI Category 1 |
|---|---|---|
| Control | 97.28 ± 0.47 A2 | High |
| T5 | 94.82 ± 0.38 B | High |
| T10 | 89.72 ± 0.15 C | High |
| T15 | 86.37 ± 0.44 D | High |
| T20 | 80.02 ± 0.13 E | Medium |
| Control | T20 | T20-05 | T20-10 | T20-15 | T20-20 | T20-25 | T20-30 | |
|---|---|---|---|---|---|---|---|---|
| Weight (g) | 25.25 ± 0.10 a1 | 25.40 ± 0.63 a | 25.25 ± 0.33 a | 24.95 ± 0.26 a | 25.31 ± 0.24 a | 25.15 ± 0.32 a | 25.29 ± 0.48 a | 25.06 ± 0.40 a |
| Moisture (%) | 38.72 ± 0.20 d | 38.46 ± 0.57 d | 39.49 ± 0.14 c | 40.10 ± 0.82 c | 43.18 ± 0.39 b | 43.81 ± 0.13 b | 45.46 ± 0.33 a | 45.67 ± 0.17 a |
| Water activity | 0.961 ± 0.006 d | 0.950 ± 0.003 e | 0.954 ± 0.001 e | 0.960 ± 0.001 d | 0.963 ± 0.003 cd | 0.967 ± 0.001 bc | 0.970 ± 0.001 ab | 0.972 ± 0.002 a |
| Volume (mL) | 78.50 ± 0.50 a | 44.00 ± 1.00 e | 62.77 ± 0.58 d | 65.33 ± 0.58 c | 70.67 ± 0.29 b | 68.63 ± 0.29 b | 65.50 ± 0.5 c | 64.00 ± 0.05 cd |
| Specific volume (mL/g) | 3.11 ± 0.03 a | 1.73 ± 0.05 d | 2.49 ± 0.06 c | 2.62 ± 0.02 bc | 2.79 ± 0.04 b | 2.73 ± 0.11 b | 2.59 ± 0.07 bc | 2.55 ± 0.06 bc |
| Hedonic test | ||||||||
| Crust color | 7.8 ± 1.1 a | 6.3 ± 1.3 c | 6.3 ± 1.3 c | 6.3 ± 1.3 c | 7.6 ± 1.2 a | 7.3 ± 1.1 ab | 6.8 ± 1.3 bc | 6.5 ± 1.2 c |
| Crumb color | 7.3 ± 1.3 a | 6.0 ± 1.2 b | 6.0 ± 1.4 b | 6.2 ± 0.8 b | 7.0 ± 1.5 a | 6.9 ± 1.5 a | 6.2 ± 0.9 b | 6.1 ± 1.1 b |
| Sweetness | 7.2 ± 1.6 a | 5.5 ± 1.2 c | 5.8 ± 0.9 bc | 5.9 ± 1.1 bc | 6.0 ± 1.0 bc | 6.0 ± 1.1 bc | 6.6 ± 1.5 abc | 6.8 ± 1.6 ab |
| Aroma | 7.0 ± 1.5 a | 5.4 ± 1.2 b | 5.5 ± 1.2 b | 6.5 ± 1.0 a | 6.8 ± 1.4 a | 6.9 ± 1.3 a | 6.4 ± 1.0 a | 6.5 ± 1.1 a |
| Flavor | 7.1 ± 1.3 a | 5.4 ± 1.2 c | 5.7 ± 0.8 c | 5.9 ± 0.9 c | 6.9 ± 1.0 a | 6.9 ± 1.5 a | 6.8 ± 1.4 ab | 6.1 ± 1.3 bc |
| Texture | 7.6 ± 0.9 a | 5.4 ± 1.1 b | 6.3 ± 0.5 b | 6.6 ± 1.4 b | 7.6 ± 1.2 a | 7.8 ± 1.0 a | 7.8 ± 1.1 a | 7.6 ± 1.4 a |
| Overall | 7.6 ± 0.9 a | 5.6 ± 0.8 e | 6.8 ± 1.2 cd | 6.9 ± 0.9 bcd | 7.5 ± 0.7 ab | 7.4 ± 1.1 abc | 6.7 ± 1.2 d | 6.5 ± 1.0 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syu, P.-C.; Zhang, Q.-F.; Lin, S.-D. Physicochemical, Antioxidant, Sensory, and Starch Digestibility Properties of Steamed Bread Fortified with Tamarillo Powder. Foods 2023, 12, 2306. https://doi.org/10.3390/foods12122306
Syu P-C, Zhang Q-F, Lin S-D. Physicochemical, Antioxidant, Sensory, and Starch Digestibility Properties of Steamed Bread Fortified with Tamarillo Powder. Foods. 2023; 12(12):2306. https://doi.org/10.3390/foods12122306
Chicago/Turabian StyleSyu, Pei-Ci, Qi-Fang Zhang, and Sheng-Dun Lin. 2023. "Physicochemical, Antioxidant, Sensory, and Starch Digestibility Properties of Steamed Bread Fortified with Tamarillo Powder" Foods 12, no. 12: 2306. https://doi.org/10.3390/foods12122306
APA StyleSyu, P.-C., Zhang, Q.-F., & Lin, S.-D. (2023). Physicochemical, Antioxidant, Sensory, and Starch Digestibility Properties of Steamed Bread Fortified with Tamarillo Powder. Foods, 12(12), 2306. https://doi.org/10.3390/foods12122306





