IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection
Abstract
1. Introduction
2. Materials and Methods
- The e-nose system measures the emissions of volatile organic compounds (VOCs) using the gas sensors array every 6 h.
- The beef quality is assessed daily by counting the microbial populations of Pseudomonas spp., Lactic Acid Bacteria (LAB), and aerobic bacteria, and performing Salmonella detection and pH levels measurements.
- Two shelf-life experimental runs were carried out for each storage temperature (4 °C and 21 °C) to verify the microbiological quantification results and validate the precision of the shelf-life estimation and gas concentration measurements.
2.1. Sample Preparation
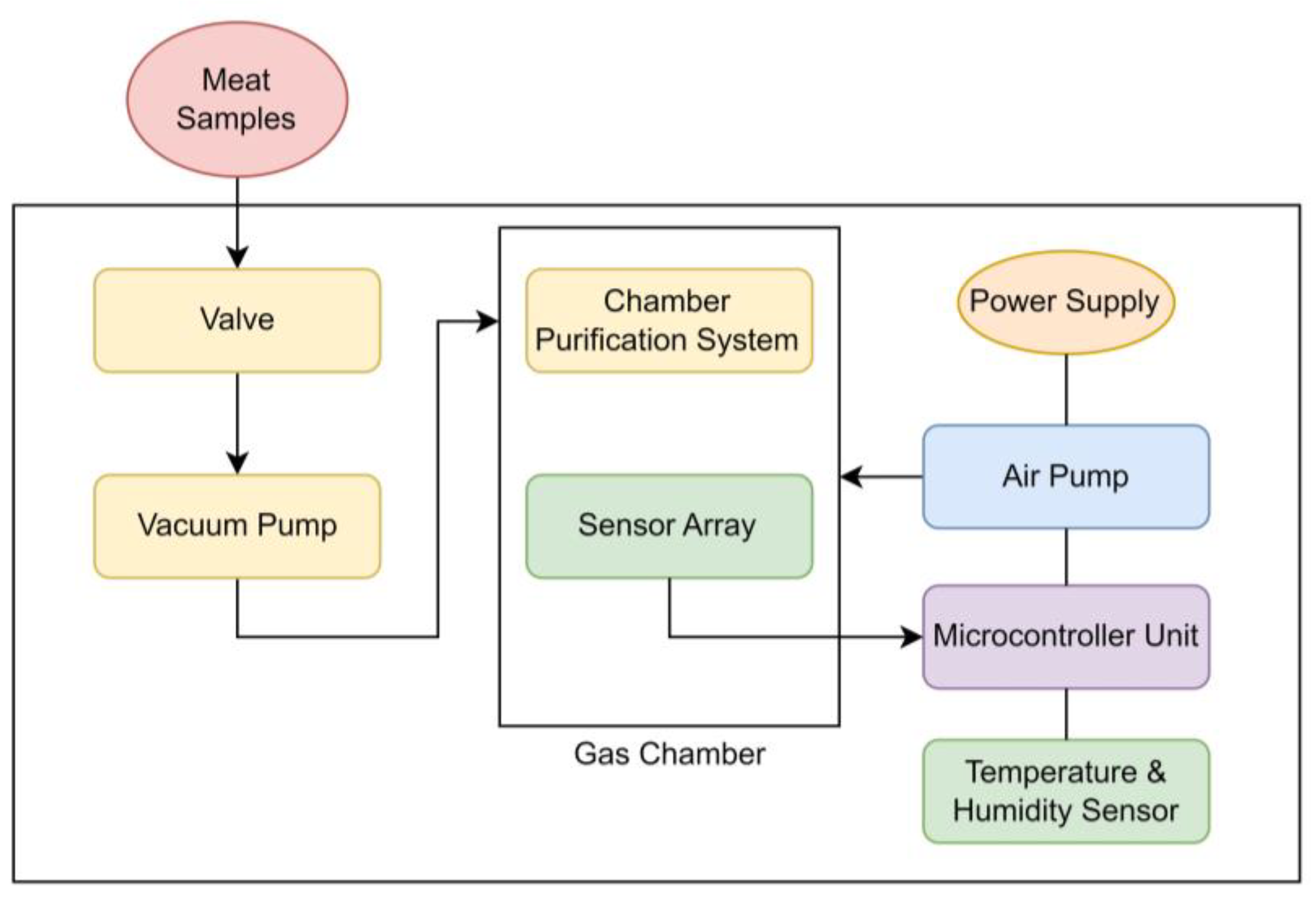
2.2. Quality Monitoring System
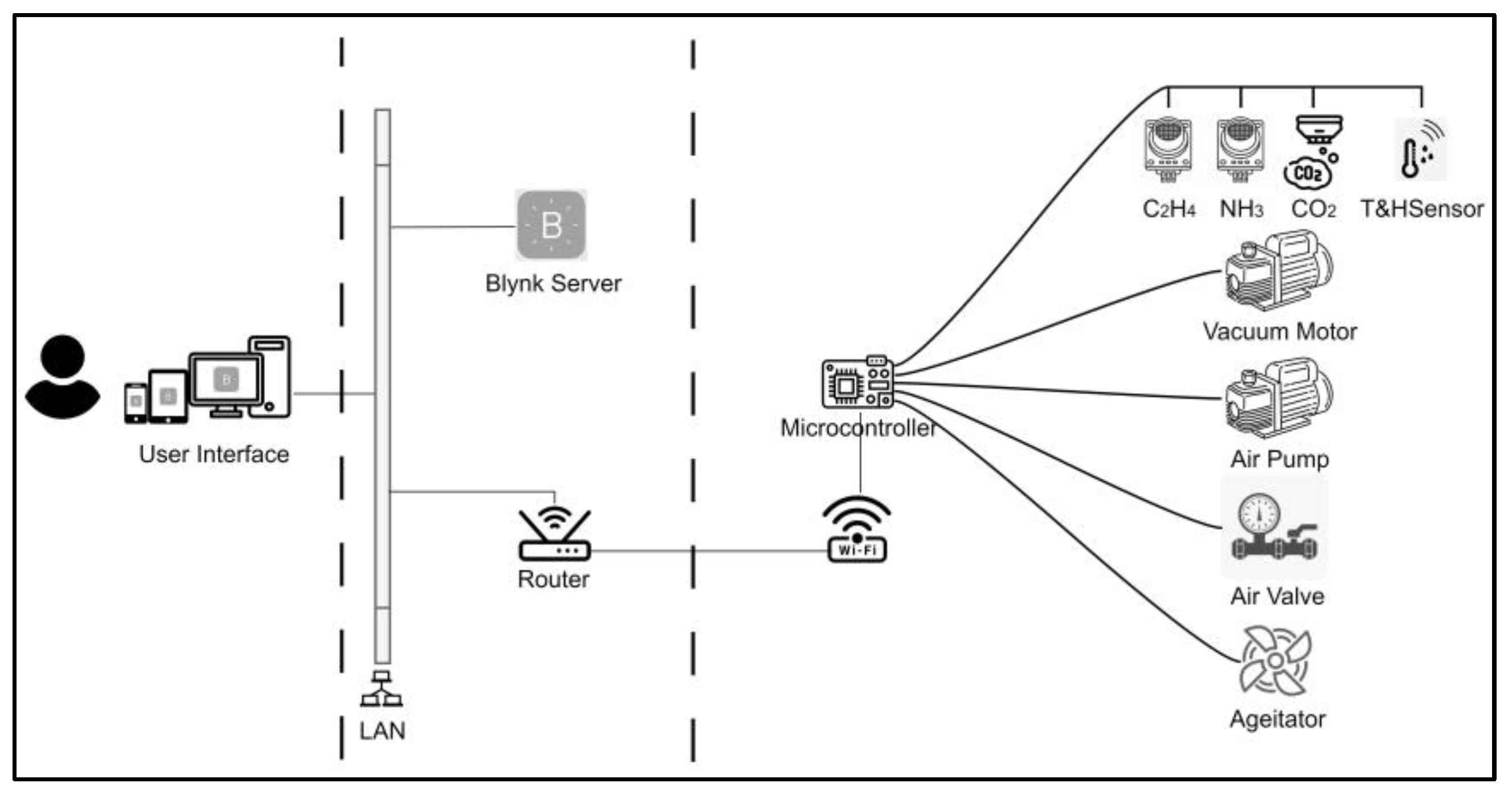
2.2.1. The Architecture Design of the IoT Monitoring System
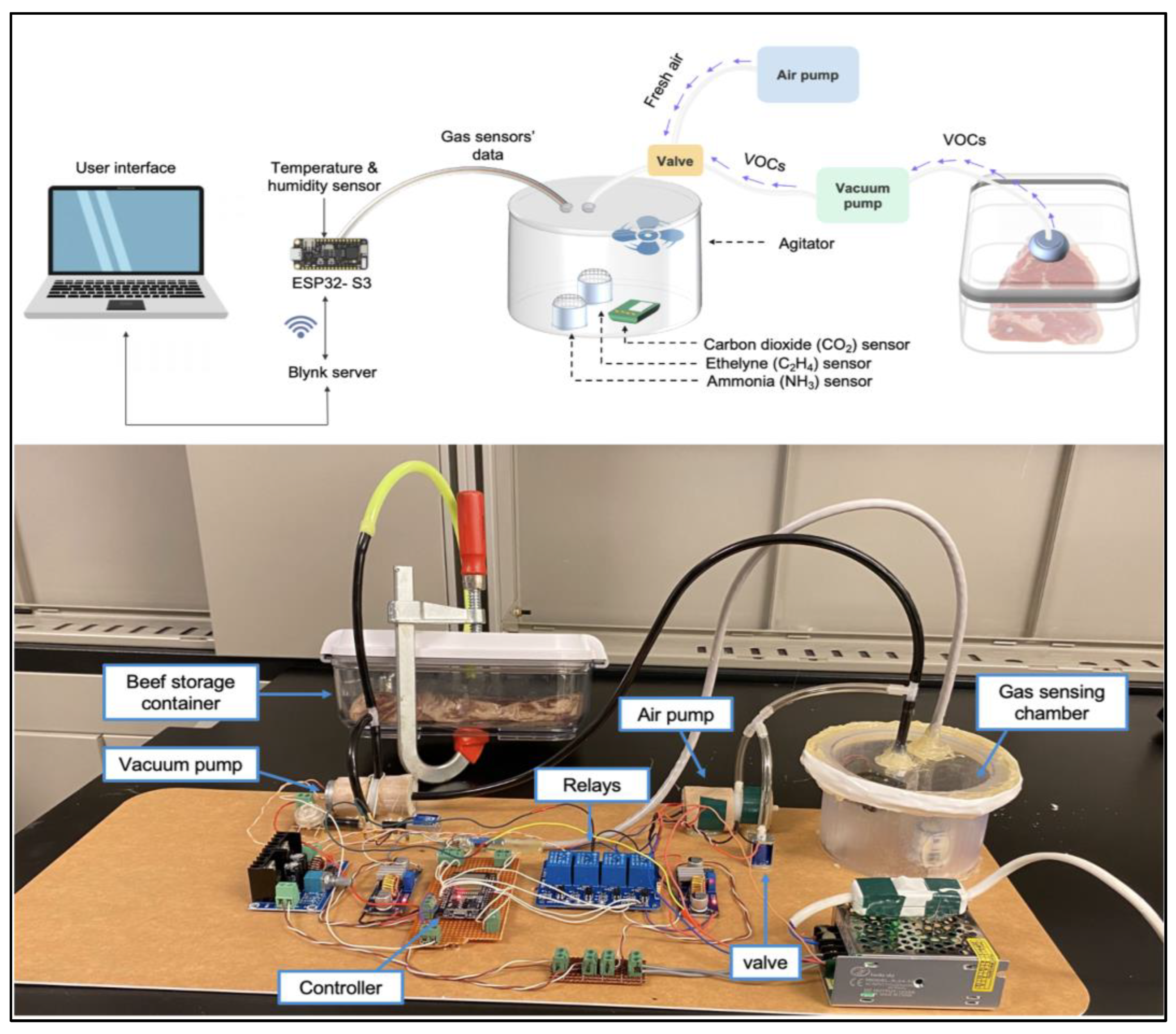
2.2.2. The E-Nose System Architecture and Components
2.2.3. The E-Nose Block Diagram
2.3. Shelf Life and Quality Assessment
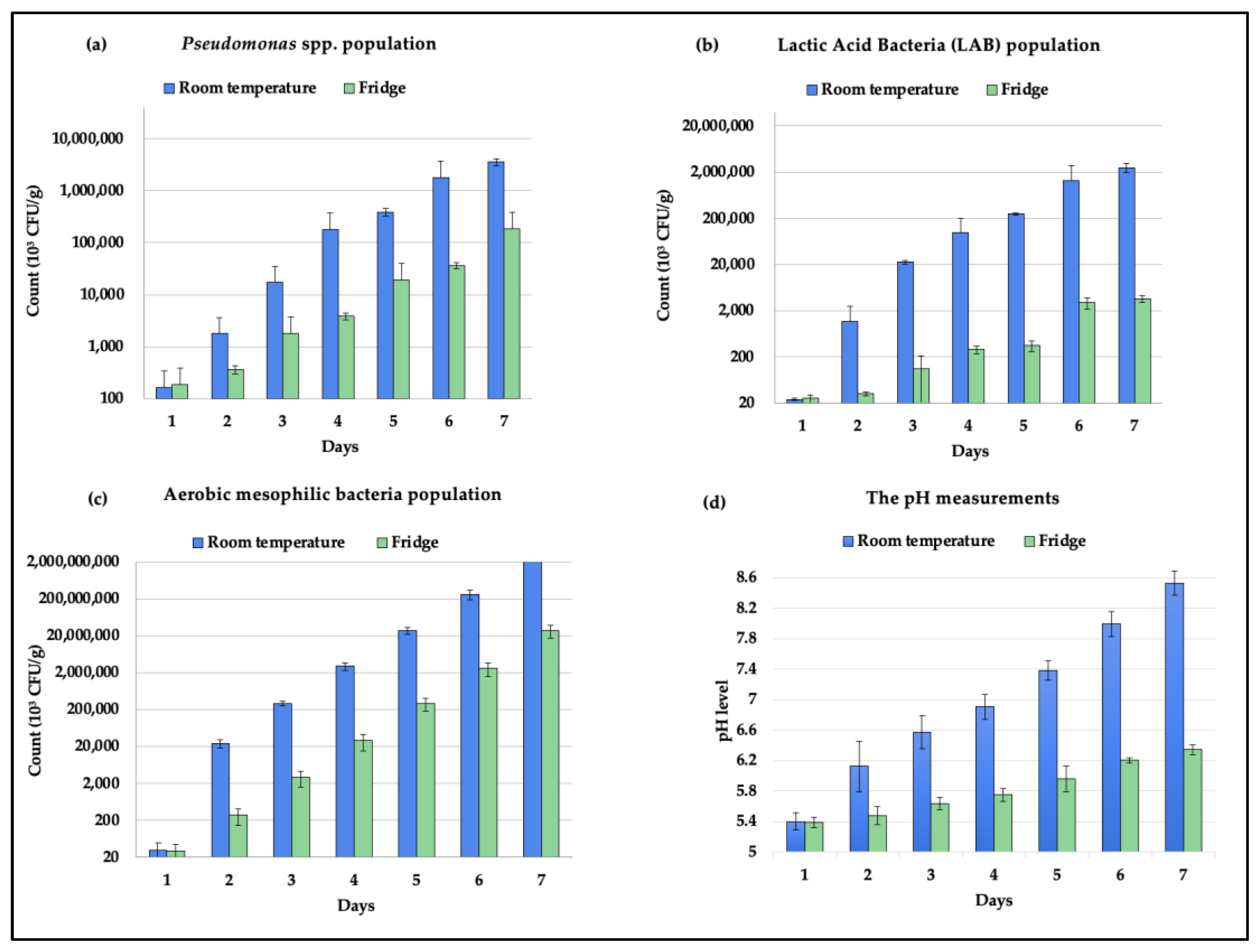
2.3.1. Microbial Population Quantification
2.3.2. pH Measurement
2.4. Statistical Analysis
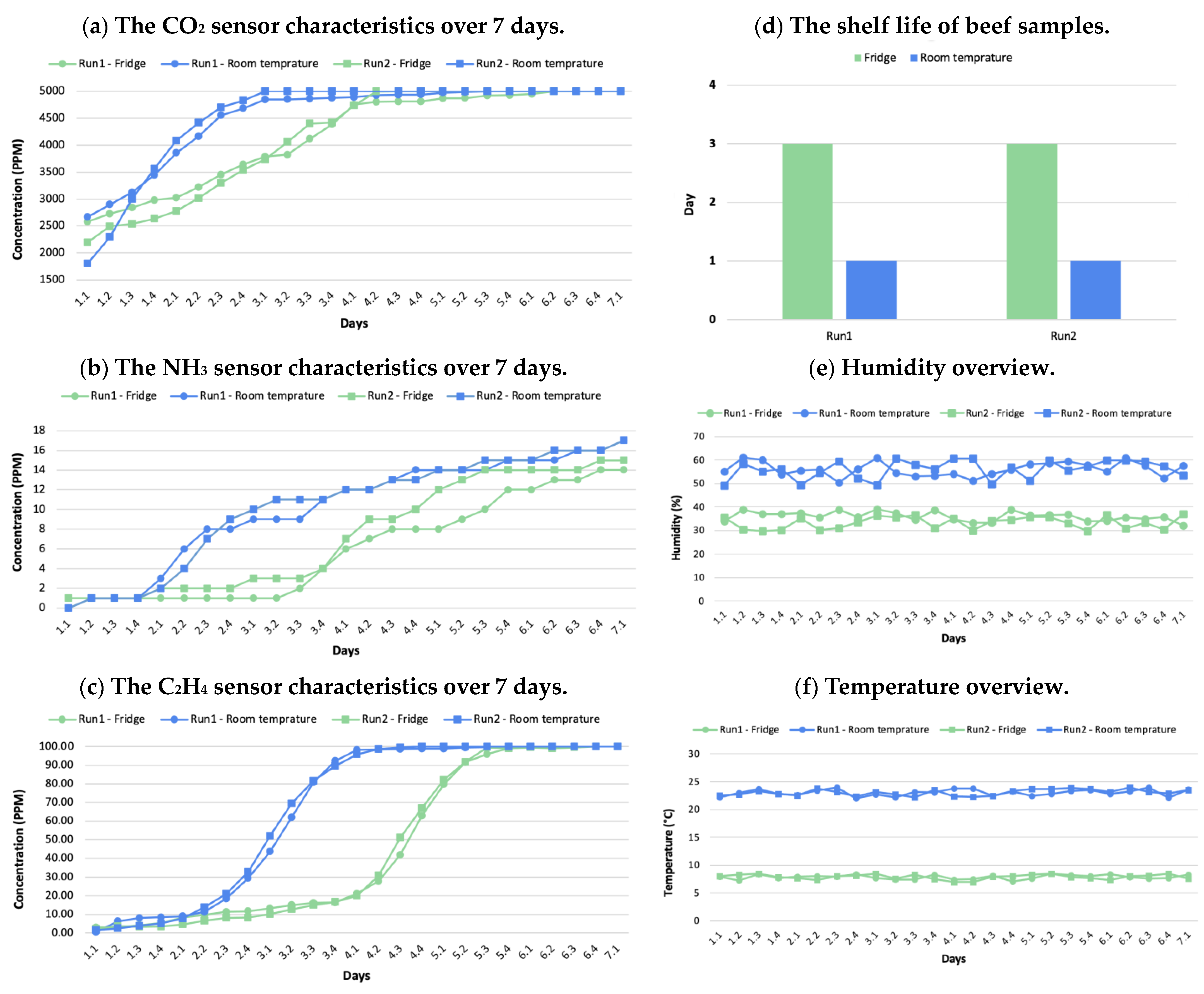
3. Results
3.1. Quality and Shelf Life Assessment
3.2. Electronic Nose System
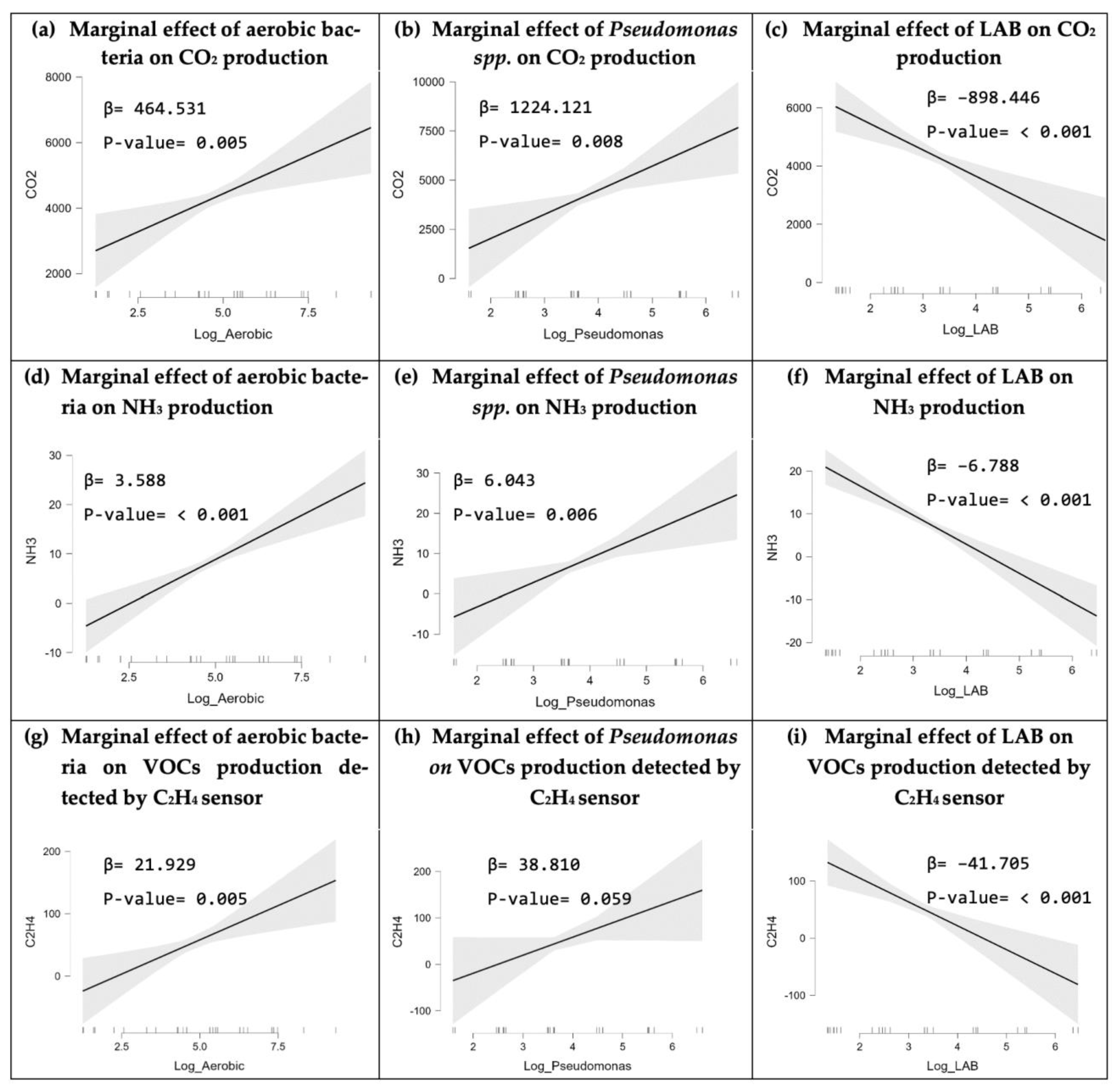
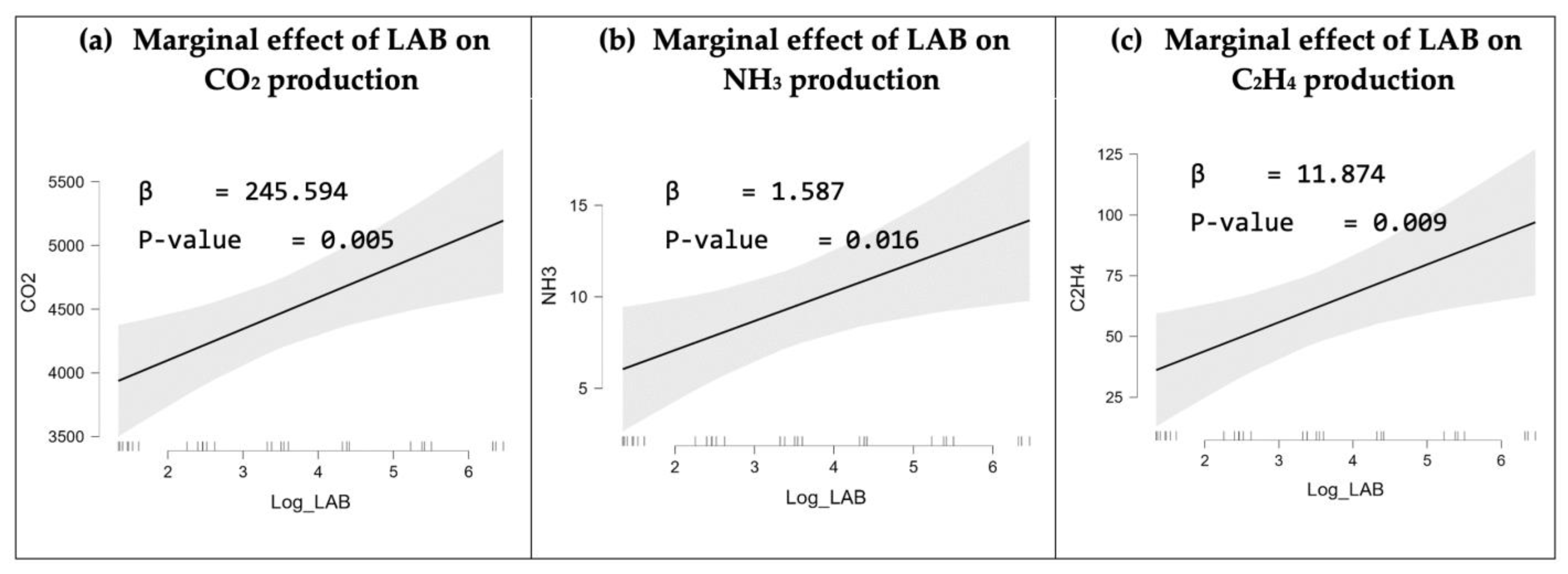
3.3. Statistical Analysis
3.3.1. Linear Regression Result of Bacterial Growth on CO2 Production
3.3.2. Linear Regression Result of Bacterial Growth on NH3 Production
3.3.3. Linear Regression Result of Bacterial Growth on VOCs Detected by C2H4 Sensor
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- U. N. E. Programme. Food Waste Index Report. 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 25 February 2023).
- Kustar, A.; Patino-Echeverri, D. A review of environmental life cycle assessments of diets: Plant-based solutions are truly sustainable, even in the form of fast foods. Sustainability 2021, 13, 9926. [Google Scholar] [CrossRef]
- Costantini, M.; Vazquez-Rowe, I.; Manzardo, A.; Bacenetti, J. Environmental impact assessment of beef cattle production in semi-intensive systems in Paraguay. Sustain. Prod. Consump. 2021, 27, 269–281. [Google Scholar] [CrossRef]
- Damdam, A.N.; Alzahrani, A.; Salah, L.; Salama, K.N. Effects of UV-C Irradiation and Vacuum Sealing on the Shelf-Life of Beef, Chicken and Salmon Fillets. Foods 2023, 12, 606. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding food loss and waste—Why are we losing and wasting food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Stenmarck, Â.; Jensen, C.; Quested, T.; Moates, G.; Buksti, M.; Cseh, B.; Juul, S.; Parry, A.; Politano, A.; Redlingshofer, B.; et al. Estimates of European Food Waste Levels; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Wickramasinghe, N.N.; Ravensdale, J.; Coorey, R.; Chandry, S.P.; Dykes, G.A. The predominance of psychrotrophic pseudomonads on aerobically stored chilled red meat. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.G.; Jin, S.K.; Hur, S.J. Microbial changes under packaging conditions during transport and comparison between sampling methods of beef. J. Anim. Sci. Technol. 2019, 61, 47–53. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; He, J.; Li, L.; Shen, M.; Tan, X.; Min, H.; Zheng, L. Wireless sensor network for real-time perishable food supply chain management. Comput. Electron. Agric. 2015, 110, 196–207. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef]
- Ajaykumar, V.; Mandal, P.K. Modern concept and detection of spoilage in meat and meat products. In Meat Quality Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 335–349. [Google Scholar]
- Fletcher, B.; Mullane, K.; Platts, P.; Todd, E.; Power, A.; Roberts, J.; Chapman, J.; Cozzolino, D.; Chandra, S. Advances in meat spoilage detection: A short focus on rapid methods and technologies. CyTA-J. Food 2018, 16, 1037–1044. [Google Scholar] [CrossRef]
- Lohumi, S.; Lee, S.; Lee, H.; Cho, B.-K. A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends Food Sci. Technol. 2015, 46, 85–98. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, K.; Park, Y. A simple and rapid method for detecting living microorganisms in food using laser speckle decorrelation. arXiv 2016, arXiv:1603.07343. [Google Scholar]
- Jung, Y.; Min, J.; Choi, J.; Bang, J.; Jeong, S.; Pyun, K.R.; Ahn, J.; Cho, Y.; Hong, S.; Hong, S.; et al. Smart paper electronics by laser-induced graphene for biodegradable real-time food spoilage monitoring. Appl. Mater. Today 2022, 29, 101589. [Google Scholar] [CrossRef]
- Dong, D.; Jiao, L.; Li, C.; Zhao, C. Rapid and real-time analysis of volatile compounds released from food using infrared and laser spectroscopy. TrAC Trends Anal. Chem. 2019, 110, 410–416. [Google Scholar] [CrossRef]
- Poghossian, A.; Geissler, H.; Schöning, M.J. Rapid methods and sensors for milk quality monitoring and spoilage detection. Biosens. Bioelectron. 2019, 140, 111272. [Google Scholar] [CrossRef] [PubMed]
- Nowshad, F.; Khan, M.S. Electronic tongue for food safety and quality assessment. In Techniques to Measure Food Safety and Quality; Springer: Berlin/Heidelberg, Germany, 2021; pp. 229–247. [Google Scholar]
- Kaur, G.; Bhari, R.; Kumar, K. Electronic noses and tongue-based sensor systems in food science. In Nanosensing and Bioanalytical Technologies in Food Quality Control; Springer: Berlin/Heidelberg, Germany, 2022; pp. 357–384. [Google Scholar]
- Shi, J.; Xiao, Y.; Jia, C.; Zhang, H.; Gan, Z.; Li, X.; Yang, M.; Yin, Y.; Zhang, G.; Hao, J.; et al. Physiological and biochemical changes during fruit maturation and ripening in highbush blueberry (Vaccinium corymbosum). Food Chem. 2022, 410, 135299. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, Y. Gas sensors for volatile compounds analysis in muscle foods: A review. TrAC Trends Anal. Chem. 2020, 126, 115877. [Google Scholar] [CrossRef]
- Thakur, M.; Ragavan, K. Biosensors in food processing. J. Food Sci. Technol. 2013, 50, 625–641. [Google Scholar] [CrossRef]
- Loudiyi, M.; Temiz, H.T.; Sahar, A.; Haseeb Ahmad, M.; Boukria, O.; Hassoun, A.; Aït-Kaddour, A. Spectroscopic techniques for monitoring changes in the quality of milk and other dairy products during processing and storage. Crit. Rev. Food Sci. Nutr. 2022, 62, 3063–3087. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Saber, O.; Kumar, S. Gases in food production and monitoring: Recent advances in target chemiresistive gas sensors. Chemosensors 2022, 10, 338. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Principles and recent advances in electronic nose for quality inspection of agricultural and food products. Trends Food Sci. Technol. 2020, 99, 1–10. [Google Scholar]
- Wang, M.; Gao, F.; Wu, Q.; Zhang, J.; Xue, Y.; Wan, H.; Wang, P. Real-time assessment of food freshness in refrigerators based on a miniaturized electronic nose. Anal. Methods 2018, 10, 4741–4749. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Mohtasebi, S.S.; Ghasemi-Varnamkhasti, M.; Ahmadi, H.; Lozano, J. Development and application of a new low cost electronic nose for the ripeness monitoring of banana using computational techniques (PCA, LDA, SIMCA, and SVM). Czech J. Food Sci. 2014, 32, 538–548. [Google Scholar] [CrossRef]
- Rivai, M.; Budiman, F.; Purwanto, D.; Al Baid, M.S.A.; Tukadi; Aulia, D. Discrimination of durian ripeness level using gas sensors and neural network. Procedia Comput. Sci. 2022, 197, 677–684. [Google Scholar] [CrossRef]
- Rivai, M.; Budiman, F.; Purwanto, D.; Simamora, J. Meat freshness identification system using gas sensor array and color sensor in conjunction with neural network pattern recognition. J. Theor. Appl. Inf. Technol. 2018, 96, 12. [Google Scholar]
- Weston, M.; Geng, S.; Chandrawati, R. Food sensors: Challenges and opportunities. Adv. Mater. Technol. 2021, 6, 2001242. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, G.; Nejad, M.B.; Shahrokhabadi, M.H. Polymer-based gas sensors to detect meat spoilage: A review. React. Funct. Polym. 2021, 165, 104962. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, S.; Wang, X.; Huang, J.; Pan, W.; Zhang, J.; Meteku, B.E.; Zeng, J. UV illumination-enhanced ultrasensitive ammonia gas sensor based on (001) TiO2/MXene heterostructure for food spoilage detection. J. Hazard. Mater. 2022, 423, 127160. [Google Scholar] [CrossRef] [PubMed]
- Matindoust, S.; Baghaei-Nejad, M.; Abadi, M.H.S.; Zou, Z.; Zheng, L.-R. Food quality and safety monitoring using gas sensor array in intelligent packaging. Sens. Rev. 2016, 36, 169–183. [Google Scholar] [CrossRef]
- Falagan, N.; Terry, L.A. Recent advances in controlled and modified atmosphere of fresh produce. Johns. Matthey Technol. Rev. 2018, 62, 107–117. [Google Scholar] [CrossRef]
- da Costa, T.P.; Gillespie, J.; Cama-Moncunill, X.; Ward, S.; Condell, J.; Ramanathan, R.; Murphy, F. A systematic review of real-time monitoring technologies and its potential application to reduce food loss and waste: Key elements of food supply chains and IoT technologies. Sustainability 2023, 15, 614. (In English) [Google Scholar] [CrossRef]
- Reese, K.L.; Rasley, A.; Avila, J.R.; Jones, A.D.; Frank, M. Metabolic profiling of volatile organic compounds (VOCs) emitted by the pathogens Francisella tularensis and Bacillus anthracis in liquid culture. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, J.; Shi, H.; Tang, C.; Zhang, R. Characteristics of volatile organic compounds produced from five pathogenic bacteria by headspace-solid phase micro-extraction/gas chromatography-mass spectrometry. J. Basic Microb. 2017, 57, 228–237. (In English) [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G.M. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. (In English) [Google Scholar] [CrossRef]
- Böhme, K.; Cremonesi, P.; Severgnini, M.; Villa, T.G.; Fernández-No, I.C.; Velázquez, J.B.; Castiglioni, B.; Calo-Mata, P. Detection of food spoilage and pathogenic bacteria based on ligation detection reaction coupled to flow-through hybridization on membranes. Biomed. Res. Int. 2014, 2014, 156323. (In English) [Google Scholar] [CrossRef]
- Paniel, N.; Noguer, T. Detection of salmonella in food matrices, from conventional methods to recent aptamer-sensing technologies. Foods 2019, 8, 371. (In English) [Google Scholar] [CrossRef]
- Brezmes, J.; Fructuoso, M.; Llobet, E.; Vilanova, X.; Recasens, I.; Orts, J.; Saiz, G.; Correig, X. Evaluation of an electronic nose to assess fruit ripeness. IEEE Sens. J. 2005, 5, 97–108. [Google Scholar] [CrossRef]
- Ma, L.; Wang, L.; Chen, R.; Chang, K.; Wang, S.; Hu, X.; Sun, X.; Lu, Z.; Sun, H.; Guo, Q.; et al. A low cost compact measurement system constructed using a smart electrochemical sensor for the real-time discrimination of fruit ripening. Sensors 2016, 16, 501. [Google Scholar] [CrossRef]
- Putra, M.A.; Rivai, M.; Arifin, A. Milk assessment using potentiometric and gas sensors in conjunction with neural network. In Proceedings of the 2018 International Seminar on Intelligent Technology and Its Applications (ISITIA), Bali, Indonesia, 30–31 August 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 409–412. [Google Scholar]
- Winsen Co. Electrochemical Ethylene Gas Sensor ME3-C2H4. 9 July 2021. Available online: https://www.winsen-sensor.com/d/files/me3-c2h4-0-100ppm(ver1_1)-manual.pdf (accessed on 12 March 2023).
- Nastiti, P.; Bintoro, N.; Karyadi, J.; Rahayoe, S.; Nugroho, D. Classification of freshness levels and prediction of changes in evolution of NH3 and H2S gases from chicken meat during storage at room temperature. J. Tek. Pertan. Lampung J. Agric. Eng. 2022, 11, 90–98. [Google Scholar] [CrossRef]
- Pavase, T.R.; Lin, H.; Shaikh, Q.-U.-A.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Edita, R.; Darius, G.; Vinauskienė, R.; Eisinaitė, V.; Balčiūnas, G.; Dobilienė, J.; Tamkutė, L. Rapid evaluation of fresh chicken meat quality by electronic nose. Czech J. Food Sci. 2018, 36, 420–426. [Google Scholar]
- Hernández-Macedo, M.; Contreras-Castillo, C.; Tsai, S.; Da Cruz, S.; Sarantopoulos, C.; Padula, M.; Dias, C. Gases and volatile compounds associated with micro-organisms in blown pack spoilage of Brazilian vacuum-packed beef. Lett. Appl. Microbiol. 2012, 55, 467–475. (In English) [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.-J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. (In English) [Google Scholar] [CrossRef] [PubMed]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Met, A.; Yesilcubuk, N.S. Comparison of two volatile sampling techniques based on different loading factors in determination of volatile organic compounds released from spoiled raw beef. Food Anal. Methods 2017, 10, 2311–2324. (In English) [Google Scholar] [CrossRef]
| Sensor | Detection Range | Accuracy | Response Time | Operating Temperatures |
|---|---|---|---|---|
| ZE03-NH3 | 1–100 ppm | ±1 ppm | ≤150 s | From 0 °C to 50 °C |
| ZE03-C2H4 | 0–100 ppm | ±0.1 ppm | ≤30 s | From 0 °C to 50 °C |
| MH-Z19C | 400–5000 ppm | ±1 ppm | ≤120 s | From −10 °C to 50 °C |
| AM2302 | From −40 °C to 80 °C and 0–100% RH | ±0.5 °C and ±0.3% RH | ≤5 s | From −40 °C to 80 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damdam, A.N.; Ozay, L.O.; Ozcan, C.K.; Alzahrani, A.; Helabi, R.; Salama, K.N. IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection. Foods 2023, 12, 2227. https://doi.org/10.3390/foods12112227
Damdam AN, Ozay LO, Ozcan CK, Alzahrani A, Helabi R, Salama KN. IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection. Foods. 2023; 12(11):2227. https://doi.org/10.3390/foods12112227
Chicago/Turabian StyleDamdam, Asrar Nabil, Levent Osman Ozay, Cagri Kaan Ozcan, Ashwaq Alzahrani, Raghad Helabi, and Kahled Nabil Salama. 2023. "IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection" Foods 12, no. 11: 2227. https://doi.org/10.3390/foods12112227
APA StyleDamdam, A. N., Ozay, L. O., Ozcan, C. K., Alzahrani, A., Helabi, R., & Salama, K. N. (2023). IoT-Enabled Electronic Nose System for Beef Quality Monitoring and Spoilage Detection. Foods, 12(11), 2227. https://doi.org/10.3390/foods12112227







