Abstract
The recovery of valuable bioactive compounds from the main underutilised by-products of the food industry is one of the greatest challenges to be addressed in circular economy. Potato peels are the largest waste generated during potato processing. However, they could be a potential source of valuable bioactive compounds, such as polyphenols, that can be reused as natural antioxidants. Currently, environmentally benign enabling technologies and new types of non-toxic organic solvents for the extraction of bioactive compounds may dramatically improve the sustainability of these processes. This paper focuses on the potential inherent in the valorisation of violet potato peels (VPPs) by recovering antioxidants using natural deep eutectic solvents (NaDES) under ultrasound (US)- and microwave (MW)-assisted extraction. Both the enabling technologies provided performances that were superior to those of conventional extractions in terms of antioxidant activity determined by the DPPH· (2,2-diphenyl-1-picrylhydrazyl) assay. In particular, the most promising approach using NaDES is proven to be the acoustic cavitation with a Trolox eq. of 1874.0 mmolTE/gExtr (40 °C, 500 W, 30 min), vs. the 510.1 mmolTE/gExtr of hydroalcoholic extraction (80 °C, 4 h). The shelf-life of both hydroalcoholic and NaDES-VPPs extracts have been assessed over a period of 24 months, and found that NaDES granted a 5.6-fold shelf-life extension. Finally, the antiproliferative activity of both hydroalcoholic and NaDES-VPPs extracts was evaluated in vitro using the MTS assay on human tumour Caco-2 cells and normal human keratinocyte cells (HaCaT). In particular, NaDES-VPPs extracts exhibited a significantly more pronounced antiproliferative activity compared to the ethanolic extracts without a noteworthy difference between effects on the two cell lines.
1. Introduction
Potato (Solanum tuberosum) is one of the most widely grown vegetables in the world and the fourth largest crop after rice, wheat, and corn, according to the 2021 FAO estimates [1]. The potato processing industry generates large quantities of by-products, mainly potato peels (PPs), that pose a disposal problem for the potato industry as wet wastes are a source of plant spoilage and pathogenic infections [2,3,4].
However, in a circular approach to the green economy, these by-products have recently been exploited as livestock feed or fertiliser [5,6,7,8] and as a new resource to produce biofuels and biogas [9,10,11]. Moreover, in line with the concept of “One Health”, potato waste could represent an even more affordable and effective feedstock for the extraction of value-added compounds, such as dietary fibres, natural antioxidants, biopolymers, and natural food additives, in a circular perspective [12,13,14]. PPs contain a wide range of nutritionally interesting components, such as phenolic compounds (chlorogenic acids, flavonoids, etc.) [15], glycoalkaloids [16], cell wall polysaccharides, and dietary fibre [17], suggesting that this by-product could have a wide range of potential re-uses [18,19,20]. Moreover, some red- or purple-coloured genotypes, such as the Siècle or Vitelote varieties, have been found to contain high levels of anthocyanins [18,21,22]. Phenolic compounds are well known for their health-promoting activities, such as antioxidant and anti-inflammatory activity [20,23]. Conventional solid/liquid extractions (SLE) remain the most common method for extracting bioactive compounds and, in particular, polyphenols from PPs [18,19]. However, conventional extraction processes heavily rely on the use of organic solvents, long processing times, high temperatures, and high energy consumption, which can have a negative impact on both human health and the environment. To overcome these constraints, innovative processes for the recovery of polyphenols from agro-industrial wastes have been proposed and thoroughly investigated [24,25,26].
The most promising innovative extraction techniques documented in the literature include Microwave-Assisted Extraction (MAE) [27,28], Ultrasound-Assisted Extraction (UAE) [29,30], Supercritical Fluid Extraction (SFE) [31], Pulsed Electric Fields (PEF) [32,33], Pressurised-Liquid Extraction (PLE), and Subcritical Water Extraction (SWE) [34,35].
Among others, MAE is an eco-friendly technique capable of penetrating the vegetable matrix and interacting with polar components. MW allows for fast solvent heating and a high extraction efficiency in short times, as documented in the pioneering work of Singh et al. (2014) for the extraction of polyphenols from PPs [36]. Likewise, UAE is a versatile, flexible, and simple technique that requires relatively low capital investment and is scalable for commercial use [37]. It amplifies extraction by accelerating diffusion phenomena and improving solvent penetration and mass transfer. UAE has been demonstrated significant improvements in the recovery of polyphenol extracts from PPs, compared to conventional extraction methods, as reported by Kumari et al. (2017) [38].
Moreover, the use of green and sustainable solvents, in conjunction with the application of the abovementioned environmentally friendly technologies, represents a promising holistic approach for the development of “green” extraction processes within the United Nations development plan based on 17 sustainable goals (SDG) [39,40]. In this framework, the agri-food industries have strived to improve their process sustainability through strategies based on the implementation of emerging sustainable technologies with the use of green solvents to decrease the overall environmental impact and improve the process efficiency for transforming agri-food residues into high-value-added products [41,42].
In particular, replacing harmful solvents with more environmentally friendly alternatives is not trivial and, in some cases, new challenges and limitations may arise due to the different physicochemical properties of the new solvents under consideration [37]. Several alternatives have recently been introduced in the field of sustainable extraction, such as supercritical fluids, neoteric, bio-based, and supramolecular solvents [43].
Among the neoteric ones, deep eutectic solvents (DESs), including those of natural origin (NaDESs), composed of plant metabolites [44,45], have recently received the highest attention thanks to their low toxicity, biocompatibility, and recyclability [46,47]. Natural Deep Eutectic Solvents (NaDES) are eutectic mixtures usually produced by the complexation of a quaternary ammonium salt (a hydrogen bond acceptor (HBA) with a hydrogen bond donor (HBD). The most widely used HBA constituent is choline chloride (ChCl), an inexpensive salt, while the most commonly used HBDs are alcohols, carboxylic acids, sugars, amino acids, and urea [48]. A special advantage in using NaDES for the extraction of bioactive from residual biomass is their ability to permeate and modify biomass cell walls and tissues and facilitate the release of compounds [49]. NaDESs have shown great potential for emerging green extraction technologies, especially when coupled with dielectric heating in MAE, due to their capability to interact with the electromagnetic field of MW [50]. Moreover, NaDES are expected to be widely transferred to industry in the coming years [51].
The present study aimed to investigate the potential recovery of polyphenols from Violet PPs waste using MW and US as enabling extraction technologies in combination with sustainable solvents. The NaDES chosen for this work is the ChLA, an equimolar mixture of ChCl and lactic acid (LA). The antioxidant activity of the VPPs extracts was evaluated using the DPPH· scavenging activity (2,2-diphenyl-1-picrylhydrazyl assay). The results have been compared with those of conventional extractions, and the shelf-life of both hydroalcoholic and NaDES-PPs extracts has been evaluated over a period of 24 months. Finally, the biological activity of the NaDES-PPs extract was investigated in terms of its antiproliferative activity adopting the so-called MTS assay, both on human tumour Caco-2 cells and human skin HaCaT cells.
2. Materials and Methods
2.1. Biomass Material and Chemicals
The Solanum tuberosum L. cv Vitelotte peels used in this work were bought at the city market (Turin, Italy) and come from a biological culture. Before use, violet potato peels (VPP) were freeze-dried and milled using a laboratory blender (HGBTWTS360, Waring Blender, Stamford, USA). Sieving was applied to select <1000 µm granulometry (Giuliani, Turin, Italy).
All chemicals were purchased from Sigma-Aldrich and used without further purification. NaDES was obtained via heating: ChLA was prepared with equimolar ratios of choline chloride (ChCl) and lactic acid (LA) [52]. The two components were stirred and heated at 50 °C in a Xelsius reactor (LabTech, Bergamo, Italy) without adding water until a homogeneous liquid was formed. ChLA was finally collected for biomass extraction without further purification.
2.2. Conventional Extraction (Hydroalcoholic Extraction)
For the sake of comparison, conventional reflux extraction was performed with an EtOH hydroalcoholic solution. The result of this test was used as a benchmark [53]. In a typical extraction, 10 g of dry VPP (previously milled) was mixed inside a round-bottom flask with the correct amount of EtOH or hydroalcoholic solution at the 1:20 S/L ratio. The mixture was continuously stirred while reflux conditions were reached by means of an oil bath. After extraction, the solutions were vacuum filtered (25 μm filter paper pore size), and the matrices were thoroughly washed with fresh extraction solvent. The alcoholic fraction was removed using a rotary evaporator, and the crude extracts were then freeze-dried (LyoQuest–85, Telstar, Barcelona, Spain), and the dry material was analysed for antioxidant activity by means of DPPH· assay. For sake of comparison, the same procedure was applied, and the hydroalcoholic solution was replaced with ChLA. All the other parameters were kept unchanged. ChLA solutions were analysed for antioxidant activity. Every test was performed in triplicate, and results are reported as average value ± the standard deviation (S.D.).
2.3. Microwave-Assisted Extraction (MAE)
MAE was performed in a SynthWAVE reactor (Milestone Srl, Bergamo, Italy), a pressurisable multimode MW system that can work under an inert atmosphere (N2). Tests were performed by mixing 1 g of dry VPP (previously milled) with the desired solvent at the 1:20 S/L ratio. The protocol was applied to different solvent systems, namely, hydroalcoholic solutions (70:30 EtOH/H2O) and ChLA NaDES. Before each run, the system was purged with nitrogen three times to reduce oxygen-derived degradations. The reactor was finally pressurised with 5 bars of N2 to avoid solvent evaporation at the working temperature. All tests were performed at a maximum power of 1500 W of irradiation with a heating ramp of 5 min. The extractions have been carried out at different temperatures (80, 100, and 120 °C) and times (60, 30, and 5 min) with 650 rpm of magnetic stirring. The latter allows to avoid the generation of eventual hotspots in the extraction media due to MW irradiation. After the extraction, the solutions were vacuum filtered (25 μm filter paper pore size), and the matrices were thoroughly washed with fresh extraction solvent. Where necessary, the alcoholic fraction was removed by a rotary evaporator, and the crude extracts were then freeze-dried (LyoQuest–85, Telstar, Barcelona, Spain), and the dry material was analysed for antioxidant activity by means of DPPH· assay. ChLA solutions were analysed for antioxidant activity by means of DPPH· assay as such. Every test was performed in triplicate, and results are reported as average value.
2.4. Ultrasound-Assisted Extraction (UAE)
UAE extractions were performed using an immersion sonotrode (HNG-20500-SP, Hainertec, Suzhou, China), working at a frequency of 21 kHz, with 100 W and 500 W exploited during the screening. Extractions were carried out by mixing 5 g of dry VPP (previously milled) with the desired solvent at the 1:20 S/L ratio. The mixture was placed in a Pyrex® thimble and cooled by means of an ice bath. The temperature was measured throughout UAE and was maintained approx. at 40 °C to preserve cavitation efficiency [54]. The abovementioned protocol was applied to different solvent systems: hydroalcoholic solution (70:30 EtOH/H2O ratio) and ChLA NaDES. All the extraction performed with the US did not require stirring due to the peculiar mass-transfer enhancement of cavitation phenomena. After extraction, the solutions were vacuum filtered (25 μm filter paper pore size), and the matrices were thoroughly washed with fresh extraction solvent. Where necessary, the alcoholic fraction was removed using a rotary evaporator, the crude extracts were then freeze-dried (LyoQuest–85, Telstar, Barcelona, Spain) and the dry material was analysed for antioxidant activity by means of DPPH· assay. ChLA solutions were analysed for antioxidant activity by means of DPPH· assay. Every test was performed in triplicate, and results are reported as average value ± the S.D.
2.5. Antioxidant Activity—DPPH·Assay
The antioxidant activity of the extracts was evaluated following the method described by Brand-Williams et al. by using the stable free radical DPPH· (2,2-diphenyl-1-picrilidrazile) [55]. The DPPH· radical inhibition, caused by the VPP extracts and measured by the decolouration of the solution (from violet to colourless), was monitored and referred to a Trolox methanolic solution, considered an antioxidant standard. The EC50 (the extract concentration able to inhibit 50% of the DPPH· radical at equilibrium) was evaluated as the scavenging activity parameter. Different concentration solutions of the dry extracts were prepared by operating subsequent dilutions, and the absorbance was read at 515 nm (Cary 60 UV-vis spectrophotometer, Agilent Technologies, Santa Clara, CA, USA). Bobo Least Squares software (ver. 0.9.1.) was used to process the absorbance data obtained to define a proper Probit regression [56]. A blank containing only water and methanol was used to zero the instrument; a blank sample containing the dry extract, without the DPPH· radical, was used to evaluate the matrix effect; and a reference sample containing water and DPPH· radical was used to normalise the results and verify the reactive absorbance.
2.6. Cell Proliferation Assay
Cytotoxicity and antiproliferative activity were evaluated in vitro using the CellTiter 96® AQueous One Solution Cell Proliferation assay (briefly, MTS assay). Two human adherent cell lines were used for this test: cancer Caco-2 cells derived from the colorectal adenocarcinoma and normal human keratinocyte cells (HaCaT). The caco-2 cell line was cultivated in DMEM supplemented with 20% (v/v) FBS and 1% (v/v) antibiotic/antimitotic solution, and the HaCaT cell line was cultivated in DMEM supplemented with 5% (v/v) FBS and 1% (v/v) antibiotic/antimitotic solution. The cell lines were kept in BioLite Petri dishes (Thermo Fisher Scientific, Waltham, MA, USA) in an incubator with a humidified atmosphere and 5% v/v CO2 at 37 °C. Single tests on the antiproliferative activity and cytotoxicity of the extracts were performed in 96-well plates (Thermo Fisher Scientific, USA) that had been seeded with exponentially growing cells at an initial concentration of 3 × 104 cells per well in 100 μL of culture media. After 24 h of incubation, under cell cultivation conditions, the cells were treated with the extracts. The raw VPP were all diluted in the culture medium, then applied to the cells, resulting in final volume ratios of 0.5%, 2%, and 5% (v/v). Treatment lasted for 72 h in the incubator, followed by the MTS assay. The assay was carried out according to the manufacturer’s instructions with a few modifications. A 10 μL volume of MTS reagent was added to each well, and the cells were incubated for 3 h; the absorbance was measured at 492 nm on the microplate reader (Tecan, Männedorf, Switzerland). Cell viability percentage was expressed as the ratio between the absorbances of the treated versus nontreated control cells. The tests were performed in triplicate with four parallels for each volume ratio.
2.7. DCF-DA Assay
Reactive oxygen species (ROS) formation was determined spectrofluorimetrically by DCF-DA assay. HaCaT and Caco-2 cells were seeded in 96-well black plates at an initial concentration of 1 × 105 cells/mL and incubated for 24 h. The next day, cells were treated with VPP extracts (5%, v/v) for a further 20 h. Cellular oxidation was induced by adding 100 μM H2O2 and incubating for 4 h. Cells were washed with PBS, and 100 μL of 50 μM DCF-DA was added to each well. Plates were incubated for 30 min in the dark and subsequently read by spectrofluorometer (Carry Eclipse, Varian, Palo Alto, CA, USA) at λex = 485 ± 10 nm and λem = 530 ± 12 nm.
3. Results and Discussion
3.1. Hydroalcoholic Extraction
Hydroalcoholic solutions are usually considered as the elective solvent for polyphenols extraction due to their polarity and the chance to recover the ethanolic fraction by means of distillation.
3.1.1. Conventional Protocol—Hydroalcoholic Solution
In this first section, we screened different water/ethanol ratios, aiming to determine which one ensures the best performance in terms of dry yields. The latter was defined by solid residue quantification after solvent removal. In particular, according to the following equation: [(Dry Extract Weight)/(Matrix Weight)] × 100.
The tests were conducted with a conventional protocol (see Section 2.2) to evaluate only the effect owed to solvent composition. Results defined the 70:30 ratio as the most promising one, with an overall yield of 15.67% versus 5.69% and 10.53% of 99.8% and 50:50, respectively. The best extraction media was then selected for further screenings. The dry yield determination was selected as a preliminary test with the intention of defining a valorisation protocol that focuses not only on the extract activity but also on the process productivity, paving the way to a suitable valorisation protocol of a largely produced food residue.
To better understand the nature of the recoverable extract and its robustness, the conventional extraction was exploited, extending to 4 h for the procedure, investigating the antioxidant activity at RT and 80 °C. The results, reported in Table 1 and achieved with the optimised hydroalcoholic solution, are compared to the 1 h extraction.

Table 1.
Results of conventional extraction of violet potato peels in terms of temperature and time screening.
As partially expected, hot extraction with prolonged time affects the overall activity of the extract (161.1 vs. 339.3 µmolTE/gExtr, after 4 and 1 h, respectively), whilst low temperature could preserve this feature also after 4 h. This information confirms the thermolabile nature of the VPP bioactive fraction.
3.1.2. Technology Screening—Hydroalcoholic Solution
Starting from the gathered information, two of the main enabling technologies, namely, MW and US, were tested by exploiting the optimised EtOH:H2O 70:30 solution. According to the nature of the cavitation phenomena, behaving well at temperatures far from the solvent boiling point [54], the US-assisted samples have been collected at a temperature of ca. 40 °C. Thus, a comparison between conventional and MW-assisted protocols can only be indirect. For the latter, we adopted the lower extraction temperature, ascribable to the conventional extraction (Table 2).

Table 2.
Results of technological screening for violet potato peel extraction.
It is possible to hypothesise a different selectivity in the quality of the extract, where, apparently, the MW enhance the recovery of non-active metabolites, according to the DPPH· test. On the other hand, UAE appears to be the less effective method, with limited activity and yield. Hence, the conventional approach has been adopted as a reference benchmark for further tests since it leads to the best outcome.
With the aim to better exploit the key features of the enabling technologies addressed in this work, additional investigations with the hydroalcoholic system have been devoted to further characterise their behaviour.
3.1.3. US-Assisted Extraction (UAE)—Hydroalcoholic Solvent
To enhance the poor outcome achieved by means of UAE, extraction time and cavitation power have been evaluated, extending the first from 30 to 60 min and increasing the latter from 100 W to 500 W (See Table 3).

Table 3.
Results of cavitational parameters for ultrasound-assisted extraction of violet potato peels.
The results confirm that VPP extraction with acoustic cavitation is affected by some drawbacks from both dry yield and antioxidant activity points of view. Likely, stronger or prolonged treatments result in the degradation of metabolites, leading to condensation products with poor solubility. This behaviour, as an example, is typical of anthocyanins compounds [57]. This phenomenon is boosted by the presence of atmospheric oxygen in the extraction open vessel, as well. For this reason, attention has been moved to the MW-assisted procedure.
3.1.4. MW-Assisted Extraction (MAE)—Hydroalcoholic Solvent
Starting from the previous results (see Table 2), the VPP MAE screening with hydroalcoholic solvent was pursued by increasing the extraction time (60 min vs. 30 min, at 80 °C) and investigating a higher temperature for shorter extraction time (5 min, 120 °C), as well. An intermediate protocol was adopted as a control (30 min, 100 °C) (see Table 4).

Table 4.
Results of irradiation parameters for microwave-assisted extraction of violet potato peels.
According to the results reported in Table 4, it is possible to state that the milder treatment displays lower selectivity, with high extraction yield and low activity. On the other hand, the flash protocol at 120 °C results in the best outcome with the highest Trolox equivalents (337.1 µmolTE/gExtr), approximately matching the value of the conventional protocol, adopted as a benchmark (339.3 µmolTE/gExtr).
3.2. ChLA Extraction
The second section of this work is dedicated to exploring VPP extraction by means of the NaDES system, more precisely with ChLA. This choline chloride-based eutectic solvent has been selected due to previous experiences with polyphenols extraction, in addition to their stabilisation and biological activity [50]. For the sake of comparison, the screening followed the procedure adopted for the hydroalcoholic protocol. Hence, ChLA has been studied with conventional and unconventional techniques (UAE and MAE). It is worth noting that no dry yields are reported hereafter since the aim of this study is to explore the activity of formulates built by the combination of extract/NaDES as reported by several studies [58,59,60]. The isolation of metabolites recovered by means of NaDES usually requires the exploitation of additional purification steps (i.e., resin adsorption) that unavoidably increase the environmental and economic impact of the final product [61,62]. The development of a dedicated sustainable approach to separate the eutectic system from the bioactive will be addressed in future work.
3.2.1. Conventional Protocol—ChLA
Table 5 reports the first set of screening, running through the same protocol proposed in Table 1. The ChLA system shows the best activity for prolonged extraction under heating, on the contrary respect to the hydroalcoholic solvent (57.2% Trolox eq. increase vs. 47.5% Trolox eq. decrease, respectively). Thus, it confirmed the stabilising nature of the eutectic medium, which is able to protect the metabolites from thermal and oxidative degradation. The enhancement of product shelf life, already observed on other biomasses, will be investigated in a dedicated paragraph of this manuscript (see Section 3.3.1) [50,59].

Table 5.
Results of conventional NaDES extraction of violet potato peels in terms of temperature and time screening.
3.2.2. Technology Screening—ChLA
As performed for the EtOH/H2O system, the ChLA has been tested with a similar approach exploiting UAE and MAE (see Table 6).

Table 6.
Results of technological screening for violet potato peels NaDES extraction.
The eutectic solvent displayed a different trend again, in contrast with the results collected in Table 3, where, for the hydroalcoholic solvent, the conventional protocol remained the best-performing one. For ChLA compared with MAE, the simple heating protocol, it is possible to appreciate a 49.6% activity increase (as Trolox eq.), whilst UAE achieved a decrease of only 7.8%, in contrast to the 46.7% of EtOH/H2O. These results encouraged us to pursue the investigation of the ChLA features for VPP extraction.
3.2.3. US-Assisted Extraction (UAE)—ChLA
The UAE of VPP was further explored, modifying the applied power (up to 500 W) and the extraction time (see Table 7). It is necessary to state that, with respect to the hydroalcoholic solution (see Table 3), it was not possible to increase from 30 to 60 min the process due to the viscous nature of ChLA, which leads to system overheating. For this reason, the extraction protocol was investigated at 15 min.

Table 7.
Results of cavitational parameters for ultrasound-assisted NaDES extraction of violet potato peels.
The UAE carried out at 500 W for 30 min results in the best activity, reaching an overall Trolox eq. of 1874.0 mmolTE/gExtr, with a 5.7-fold increase if compared with the 15 min at the same power (325.5 mmolTE/gExtr) and a 6.8-fold increase respect to 100 W (274.5 mmolTE/gExtr at 30 min). Further considerations concerning technologies and solvent comparisons will be reported at the end of the next paragraph.
3.2.4. MW-Assisted Extraction (MAE)—ChLA
The VPP MAE with ChLA was performed by retracing the screening protocols proposed in Table 4 for EtOH/H2O, increasing the extraction time (60 min vs. 30 min, at 80 °C) and investigating a higher temperature for shorter extraction time (5 min, 120 °C), as well. An intermediate protocol was adopted as a control (30 min, 100 °C). The results are reported in Table 8.

Table 8.
Results of irradiation parameters for microwave-assisted NaDES extraction of violet potato peels.
Higher temperatures seem to compensate for the high viscosity of the system, allowing it to enhance the mass transfer. In fact, the extracts achieved at 100 °C and 120 °C exhibit approx. comparable activity (493.7 and 468.2 mmolTE/gExtr, respectively), even if the latter can count on only 5 min of treatment. However, the comparison between samples at 100 °C and 120 °C suggests that the viscosity reduction is not enough to produce an appreciable activity increment moving from 5 to 30 min, with a consequent kinetic limitation.
According to the technology screenings reported, the most promising approach with ChLA is proven to be the acoustic cavitation, with a Trolox eq. of 1874.0 mmolTE/gExtr (40 °C, 500 W, 30 min), vs. the 493.7 mmolTE/gExtr of MAE (120 °C, 5 min) and 510.1 mmolTE/gExtr of conventional protocol (80 °C, 4 h). The MW irradiation can assure a very quick protocol able to nearly reach the conventional benchmark in 5 min vs. 4 h. This result can be related to the partial increase in NaDES fluidity (and consequently, mass transfer). On the other hand, extending the treatment to 30 min, the US irradiation exhibits a dramatic extraction intensification. In addition, thanks to the stabilisation features of ChLA, the degradation issues observed for the hydroalcoholic solvent have been avoided.
3.3. ChLA Stabilization Effects
It is commonly accepted, due to a massive literature production, that one of the main responsible for the deep eutectic solvent features are the hydrogen bonds, causing their formation and framework arrangement [48,63,64]. Likely, they can help to solubilise a large range of compounds [65], enhance extractions [49], and act in synergy with several actives [66]. Another property ascribed to eutectic solvents is stabilising the molecules dissolved within [50,67]. Thus, we decided to address this point by exploring the VPP/ChLA extracts.
3.3.1. Shelf-Life
The ChLA sample exhibiting the best antioxidant activity (UAE, 30 min, 500 W) has been monitored across 2 years (sampling after 1, 3, 6, 12, 18, and 24 months, respectively, storage at 4 °C), tracking the variation in Trolox equivalents. For the sake of comparison, the same test was performed on the best sample achieved with the hydroalcoholic solvent (MAE, 5 min, 120 °C). The gathered data has been summarised as percentual degradation (normalising on the “0” sample) in Figure 1 and with Pareto charts in Figure 2A,B.
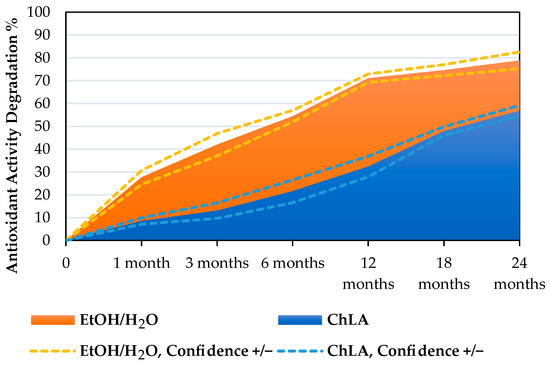
Figure 1.
Monitoring antioxidant activity of EtOH/H2O vs. ChLA systems over 24 months (degradation). Dashed lines represent confidence intervals resulting from SD.
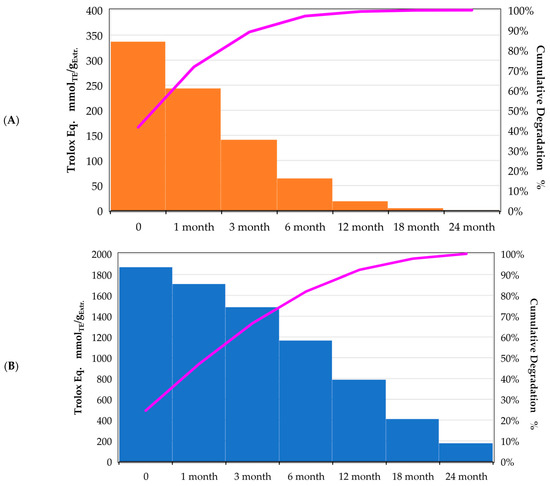
Figure 2.
Antioxidant activity reported as Pareto charts, monitored over 24 months; Purple line reports cumulative degradation; (A): hydroalcoholic solution; (B): ChLA.
From Figure 1, it is possible to appreciate the degradation trends of the two different solvent systems. The sample in EtOH/H2O displayed a higher activity reduction, losing approx. 30% after only 1 month and approaching ca. 70% in 12 months. The final degradation nearly reached 79%. The ChLA system, on the other hand, showed a milder abatement of antioxidant activity across the monitored period, reaching around 57% of the final degradation. To better evaluate the activity variations, the Trolox eq. have been organised by means of Pareto charts (see Figure 2A,B), where the purple line describes the cumulative variation trend.
In Figure 2A, the hydroalcoholic solution is characterised by a steep reduction in Trolox eq., reaching a sort of steady state after 6 months, where the degradation slows down. The antioxidant activity of the eutectic system (Figure 2B), on the contrary, is described by a milder slope without real stationarity even after 24 months.
These results support the enhancement of extracts’ shelf-life according to the antioxidant activity of VPP metabolites, in particular if compared to a common hydroalcoholic medium. It is possible to adopt the antioxidant activity half-life as a reference point, resulting in 4.12 months and 23.22 months for EtOH/H2O and ChLA, respectively. Hence, the exploitation of the NaDES granted a 5.6-fold shelf-life extension. To better understand the trend in the shelf-life of the VPPs extracts, preliminary quantification of the total anthocyanin content (TAC) in both the fresh and 24 months EtOH/H2O and ChLA extracts have been performed via colourimetric assay (see Appendix A.1 and Appendix A.2). Moreover, on ChLA extract a preliminary semi-quantitative anthocyanins detection via LC-MS analysis has been performed recording namely pelargonidin 3-rutinoside-5-glucoside (m/z: 741.22), pelargonidin 3-feruloylrutinoside-5-glucoside (m/z: 917.27), and petunidin 3-p-coumaroylrutinoside-5-glucoside (m/z: 933.27) (see Appendix A.3 and Appendix A.4).
3.3.2. Antioxidant Activity Modification: EtOH Addition and US Degradation Tests
After confirming the NaDES stabilisation features, the successive step was to verify how this feature can be affected by the variation of the H-bond strength (see Table 9). Hence, different aliquots of EtOH (1 and 10%) were added to a ChLA extract of “medium activity” (assuring the best range to evaluate both eventual increase and decrease in Trolox equivalents). For this purpose, the sample achieved by means of MW at 100 °C (30 min) was selected.

Table 9.
Antioxidant activity modification by EtOH addition.
Table 9 shows an increase in antioxidant activity, dependent on the addition of alcohol, with a maximum of +58.6%. This substantial enhancement is likely dependent on an interference of EtOH with the existing H-bond of ChLA presumably occupied in stabilising the extract bioactive [68,69]. This phenomenon was further studied by submitting the sample “ChLA (+10% w/v EtOH)” to US irradiation (30 min, as the extraction protocol), evaluating the modification caused by cavitation (see “ChLA Degradation Test (+10% w/v EtOH)” in Table 9). The choice fell on the acoustic treatment due to the effectiveness exhibited by this technique for VPP extraction. After the US processing, the sample displayed a considerable reduction in antioxidant activity, with a 47.1% loss (from 783.2 to 414.3 mmolTE/gExtr). It is possible to conclude that reducing the H-bond strength in ChLA results in enhancing the overall extract activity and, as a natural consequence, its proneness to degradation. An additional test was performed to explore this enhancement/destabilisation dualism, evaluating how a small addition of EtOH could effectively influence the quality of a VPP extract achieved by UAE. Table 10 reported the comparison between the best performing US/ChLA extraction (30 min, 500 W) and the same procedure carried out exploiting “ChLA (+1% w/v EtOH)” as a solvent medium.

Table 10.
Antioxidant activity modification by US degradation tests. Evaluation of EtOH effect.
As further evidence, the activity of the sample recovered by means of “ChLA (+1% w/v EtOH)” resulted strongly decreased, more than 77% in comparison to the pure ChLA system (431.3 vs. 1874.0 mmolTE/gExtr).
A first evaluation of extract composition and how sample aging could affect metabolites are addressed in Appendix A of this manuscript, where some preliminary tests on anthocyanins content and LC-MS analysis are reported.
3.4. Biological Activity
During this work, antioxidant features of different VPP extracts have been evaluated, in relation to the two main enabling technologies (MW and US), exploiting a conventional (EtOH/H2O) and an unconventional solvent (ChLA, a NaDES)
3.4.1. Antiproliferative Activity
First, the antiproliferative activity of the extracts was investigated on the Caco-2 cell line. This cell line derives from human colorectal adenocarcinoma and is often used as a model for the epithelial barrier of the intestine. Therefore, it was selected to test the extracts obtained for their potential use as a dietary supplement and/or nutraceutical. The bioactive obtained from the VPP could also serve as natural antioxidants for cosmetic products, so the effect of the extracts on the HaCaT cell line was investigated. HaCaT cells are derived from healthy human keratinocytes, which represent 95% of the epidermal cells and primarily have structural and barrier functions in the human skin. The antiproliferative activity was determined by MTS assay. The results are presented in Figure 3.
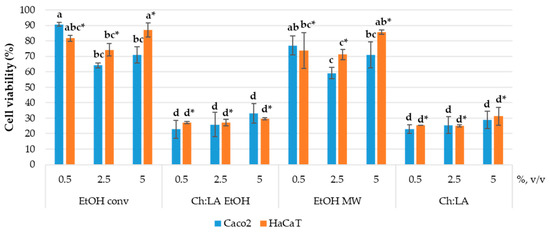
Figure 3.
Effect of VPP extracts in the final volume ratios of 0.5, 2.5, and 5% (v/v) on Caco-2 and HaCaT cell viability. Results are expressed as average values ± SD. Statistically different data (p < 0.05) are designated by lower-case letters (a–d or a*–d*, Caco-2 and HaCaT, respectively), according to volume ratios for each cell line.
Figure 3 confirmed that NaDES could enhance the biological activity of the extracts. In particular, extracts prepared in NaDES possess a significantly pronounced antiproliferative effect with respect to the ethanolic extracts. The antiproliferative effect is dose-independent in the case of EtOH and EtOH MW extracts, while both extracts prepared in NADES have shown no statistically significant among doses. This means that even the lowest concentration of NADES extracts ensures exceptional biological activity. Additionally, there is no difference between the effects on the two cell lines employed in this study. The decrease in cell viability is similar on Caco-2 and on HaCaT cells. Due to the observed antiproliferative activity of Ch:LA EtOH, EtOH MW, and Ch:LA extracts, their possible usage as nutraceuticals or in cosmetics could not be expected, at least not in tested volume ratios. Rather, it could be further assessed for possible anticancer activity on different human tumour cell lines, as already shown for different polyphenols extracts obtained by conventional extraction and by green approaches [50,70]. However, keeping in mind the complex relationship between the antioxidant activity of natural compounds and their anticancer activity, supplementation with such extracts requires careful consideration. It is well known that antioxidants may perform a role in cancer prevention by reducing oxidative stress and protecting DNA, but antioxidant supplementation in cancer patients undergoing treatment may interfere with the efficacy of these treatments. Furthermore, different natural compounds with antioxidant properties, obtained as plant extracts, can have diverse effects on cancer cells since they contain multiple bioactive components often characterised by distinct mechanisms of action.
3.4.2. In Vitro Antioxidant Activity
VPP extracts antioxidant activity was evaluated in vitro on both Caco-2 and HaCaT cell lines by DCF-DA assay. The cell lines were treated with 0.5 % v/v of extracts, the smallest volume ratio, which has a similar impact on cell viability as the higher concentrations. The performed in vitro assay evaluates if the added VPP extracts have antioxidant capacity to prevent ROS (reactive oxygen species) generation during induced oxidative stress in tested cell lines. After pre-treatment with VPP extracts, the oxidative stress was induced by the addition of H2O2 in the cultivation medium. Lower ROS generation means a higher antioxidant activity of tested extracts. The results are shown in Figure 4.
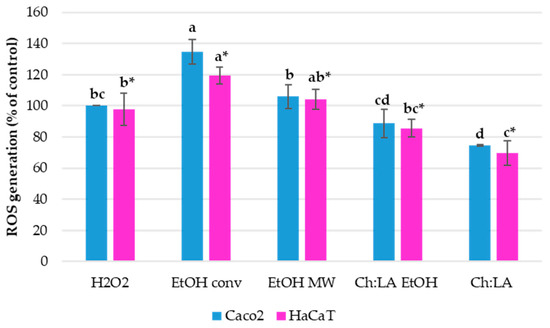
Figure 4.
VPP extracts’ effect on intracellular ROS formation in Caco-2 and HaCaT cells during H2O2-induced oxidative stress. Results are expressed as % of control cells. Results are expressed as average values ± SD. Statistically different data (p < 0.05) are designated by lower-case letters (a–d or a*–c*, Caco-2 and HaCaT, respectively).
Pre-treatment with ChLA and ChLA EtOH extracts have shown to decrease ROS generation after the induction of oxidative stress, i.e., can protect cells against the presence of intracellular ROS. Moreover, ChLA extract has shown a significantly higher antioxidant activity when compared to ChLA EtOH extract. Therefore, this extract could be used to prevent oxidative stress in organisms and thus prevent numerous correlated diseases. Other tested extracts did not have such a positive effect; moreover, it seems that the hydroalcoholic extracts fostered the generation of ROS, what is completely opposed to the DPPH· test result.
In particular, according to the DPPH· test (see Table 9), the hydroalcoholic extract achieved by means of MAE should exhibit the best antioxidant activity, but on the contrary, it slightly promoted ROS generation as determined by in vitro DCF-DA assay. The antioxidant activity of ChLA obtained extracts (with and without the EtOH addition) is in good correlation by both applied tests, which confirmed that the eutectic solvent has a higher antioxidant activity than ChLA + EtOH.
The VPP extracts prepared in NaDES showed an antiproliferative effect on the Caco-2 cell line, and HaCaT cell line, with no statistically significant differences among NADES extracts. This effect was much greater than in the extracts prepared in EtOH. Regarding the antioxidative activity, ChLA extract is shown to prevent ROS generation even stronger than ChLA EtOH extract.
Such, in a way, inconsistent results should definitely be further investigated, but it can be explained by a few facts already known in the literature. First, the exact phytochemical profile of the extracts, which was not assessed in this study, obtained by classical extraction and NaDES is definitely different [71]. Otherwise, plant extracts may contain compounds that can either promote or inhibit the formation of ROS, depending on the specific extract, the way of extraction and the conditions of exposure [72]. Finally, the metabolism of ethanol itself can promote the formation of ROS, which could lead to oxidative stress and related cellular damage. Therefore, the effects of (ethanol) plant extracts on ROS formation and oxidative stress are complex and depend on multiple factors, meaning further research is needed to better understand it.
4. General Remarks and Perspectives
Recovery of bioactive compounds from food waste not only mitigates environmental problems but also enhances the profitability of the food industry. To achieve this objective, it is necessary to apply innovative technologies that would allow to achieve higher extraction yields, preserving the properties of the recovered extracts. In this framework, the implementation of a biorefinery approach on potato peels as the main source could open future industrial applications that would allow the exhaustive exploitation of this by-product. Recently, a variety of new methodologies for PP management have been successfully applied, such as MAE, UAE, SFE, PEF, or SWE, as reported above. However, in practice, the idiosyncrasy of the agri-food industry and the lack of low-cost industrial equipment have limited the implementation of these technologies. For this reason, easily affordable hydroalcoholic extraction still remains the reference protocol against which each new extraction strategy should be compared, including that herein reported [53,73]. To better clarify the sustainable extraction approach adopted in this paper, a flow chart of the whole VPP valorisation process is reported in Figure 5, together with a comprehensive summary showing the major results recently documented in the literature concerning the antioxidant recovery from PP (Table 11).
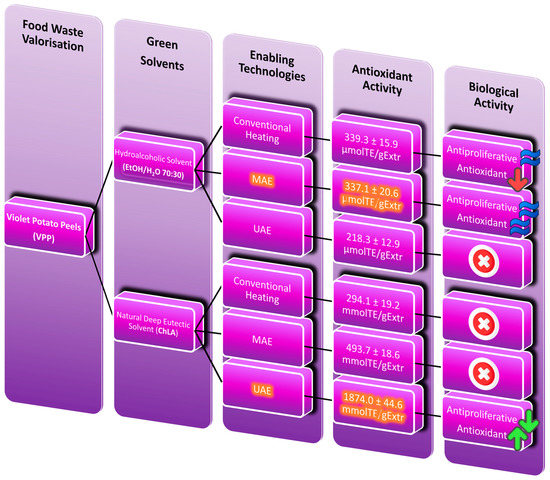
Figure 5.
Flowchart of the extraction strategy adopted in this work for the sustainable valorisation of VPP: technologies and solvent screening enabling the best-performing antioxidant activity. Highlighted squares represent the best performing samples.

Table 11.
Final comparison between extraction strategies for the recovery of bioactive compounds from potato peels.
The results documented in this study demonstrate that PP waste could be a good source for antioxidant recovery while contributing to the revalorisation of these agri-food by-products in a sustainable way. When the same violet potato variety was considered for the same purpose, the addition of unsustainable hydrochloric acid was required to stabilise the anthocyanin-rich extract [73], which was not necessary in this work due to the inherent acidity of the ChLA eutectic system used. Despite the potential of the aforementioned food waste and the attention that NADES are receiving in the scientific community, not many studies have been carried out so far concerning the sustainable extraction of phenolic compounds from these residues [18] compared to other biomass sources [77]. However, future research aiming to design effective and easily affordable technological strategies for the complete valorisation of these by-products would be necessary, both for the recovery of bioactive and for their applications.
5. Conclusions
The potato is one of the most widely produced vegetables in the world, and due to its extensive use in various industries, large amounts of potato waste are generated. So far, the huge potential of potato waste is underexploited. One of the most promising applications of PPs is the content of active metabolites, which can be extracted using different green technologies, leading to economic and environmental advantages.
In this study, the exploitation of a deep eutectic solvent, the ChLA, for the valorisation of VPPs proved to be crucial for both the recovery and furtherstabilisationn of target antioxidant compounds.
According to the reported technology screenings (UAE, MAE, and conventional heating), the most promising approach is proven to be acoustic cavitation. Indeed, in the presence of NaDES, UAE enabled 3.6 times higher Trolox equivalents per gram of ChLA extract in 30 min (40 °C, 500 W) than that obtained in 4 h with the hydroalcoholic protocol (1874.0 mmolTE/gExtr vs. 510.10 mmolTE/gExtr, respectively). On the other hand, with the same antioxidant power recorded for the VPP extracts (~500 mmolTE/gExtr), MAE proved to be effective in drastically shortening the extraction time up to 98%, compared to the conventional hydroalcoholic approach. Moreover, the exploitation of the NaDES granted a 5.6-fold shelf-life extension in terms of its antioxidant activity (monitored over a 24-moths period) if compared to the hydroalcoholic procedure. Finally, the in vitro antiproliferative and antioxidant activity of conventional and ChLA VPP extracts were determined by MTS and DCF-DA assay, respectively, on tumour (Caco-2) and normal human keratinocyte (HaCaT) cell lines. NaDES-extracts exhibited a significantly pronounced antiproliferative effect vs. ethanolic one. They are also the only ones featuring ROS generation decrease after oxidative stress induction in both selected cell lines, confirming that NaDES can enhance the biological activity of the VPP extracts. However, there is a strong need for in vitro and in vivo studies to help better understand the pharmacodynamic and pharmacokinetic properties of these bioactive compounds, helping for the development of new nutraceutical and/or pharmaceutical products as well. This comparative study could pave the way for the development of a synergistic process, combining enabling technologies with green solvents for the extraction of high-added-value products from waste biomass.
Author Contributions
Experimental data curation, G.G., G.C. (Giorgio Capaldi) and V.G.; writing—original draft preparation, G.G., E.C.G., K.R. and I.R.-R.; writing—review and editing, S.T., K.R., E.C.G. and G.C. (Giancarlo Cravotto). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the University of Turin (Ricerca Locale 2022).
Data Availability Statement
Data is contained within the article.
Acknowledgments
Authors thank LabTech (Bergamo) for the loan of the Xelsius reactor at the Department of Drug Science and Technology (Turin).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Preliminary sets of deeper extract characterisation have been performed, opening new perspectives for further studies and applications. For this purpose, a colourimetric assay for anthocyanin semi-quantitative detection and qualitative LC-MS analysis were adopted. Methods and first results have been shared hereafter.
Appendix A.1. Total Anthocyanin Content (TAC) Determination
The TAC in the extracts was determined according to the method reported by Ribéreau-Gayon et al. [78]. 100 μL of extract solution was placed in the test tubes and sequentially diluted with 60% v/v EtOH solution containing 0.1% v/v of HCl and 2 mL of 2% v/v HCl solution. In one parallel test, 400 μL of distilled water was added to the sample solution, and in the other, 400 μL of 15% w/v sodium bisulfite was added. The resulting solution was mixed thoroughly. After 15 min, the absorbance was measured at 520 nm, in a 1 cm quartz cuvette (Cary 60 UV-Vis spectrophotometer, Agilent Technologies, Santa Clara, CA, USA), on a, against a blank. The TAC was calculated using Equation (A1):
𝑇𝐴𝐶 = 875 × (𝐷1 − 𝐷2)
D1: absorbance of the control sample (diluted with distilled water); D2: absorbance of the bisulphite bleached sample.
TAC is expressed as anthocyanin weight over the weight of dry VPP (mg/g). All analyses were performed in triplicate, and results were expressed as the average.
Appendix A.2. Total Anthocyanin Content (TAC)—Shelf-Life
To better describe the extraction products and their shelf-life, as additional characterisation, TAC has been determined for the optimised sample, fresh and after 24 months of storage (see Table A1).

Table A1.
TAC quantification, ChLa vs. EtOH/H2O, fresh and after 24 months.
Table A1.
TAC quantification, ChLa vs. EtOH/H2O, fresh and after 24 months.
| Solvent | Storage | TAC (mg/g) | Degradation (%) |
|---|---|---|---|
| ChLa | Fresh | 1.95 | - |
| 24 months | 1.58 | 18.97 | |
| EtOH/H2O | Fresh | 2.49 | - |
| 24 months | 0.66 | 73.49 |
The results roughly follow the degradation onset reported in Figure 1 (18.97% vs. 73.49% for ChLa and EtOH/H2O, respectively). It is worth noting that, as an exception, the ChLA system appears to better stabilise the anthocyanins fraction, surely due to its acidic nature. Further studies on anthocyanin behaviours in this type of system will follow.
Appendix A.3. LC-MS Analysis
LC-MS analyses were carried out with a Waters system equipped with a Waters FractionLink sampler and two detectors: a diode array (wavelength set at 280, 335 and 515 nm) and a single quadrupole mass spectrometer (ESI+, Cone: 20.00 V; Capillary: 3.00 kV; Source T: 110 °C; Desolvation T: 220 °C, m/z range 100–1200). MassLynx V4.1 software was adopted for system management and data processing. The chromatographic separation was performed according to a previously reported protocol [27], with a Sinergy Hydro-RP column (Waters, 4 μm 80 Å, 250 × 4.6 mm) Flow: 1 mL/min; injection V: 20 μL. Eluents: A = water/CH3COOH 3%, B = ACN.
Appendix A.4. ChLa Extract: LC-MS Qualitative Determination
A preliminary LC-MS qualitative analysis was conducted, evaluating the optimised VPP extract (ChLa/US). Hereafter are reported the DAD chromatograms obtained monitoring at 280, 335 nm (Figure A1), and 515 nm (Figure A2). The latter was selected for anthocyanins detection.
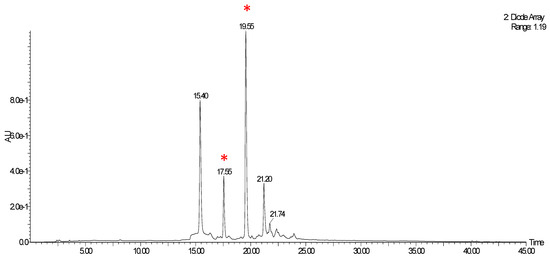
Figure A1.
DAD chromatogram of VPP extract (ChLa/US), 280 and 335 nm. Peaks matched with mass spectra are signalled with a red wildcard character.
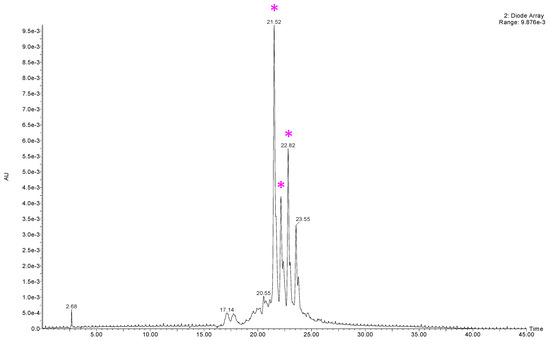
Figure A2.
DAD chromatogram of VPP extract (ChLa/US), 515 nm. Peaks matched with mass spectra are signalled with a purple wildcard character.
The above-reported chromatograms were further matched with mass detections, searching for the main compounds ascribed to VPP, according to the literature [78]. Two of the four main peaks in Figure A1 can be ascribed to chlorogenic acid isomer, with m/z: 354.3 (see Figure A3, red wildcard characters).
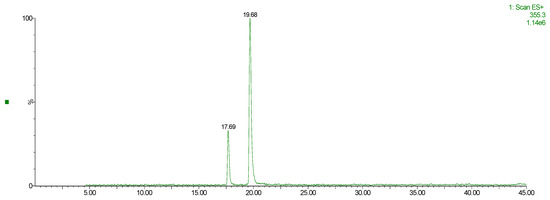
Figure A3.
ESI+ Mass Spectra Scan, 355.3 [M+] → Chlorogenic acid isomers.
Concerning the anthocyanins detection, three of the expected compounds [79] have been successfully detected (see Figure A4, purple wildcard characters), namely, pelargonidin 3-rutinoside-5-glucoside (m/z: 741.22), pelargonidin 3-feruloylrutinoside-5-glucoside (m/z: 917.27), and petunidin 3-p-coumaroylrutinoside-5-glucoside (m/z: 933.27).
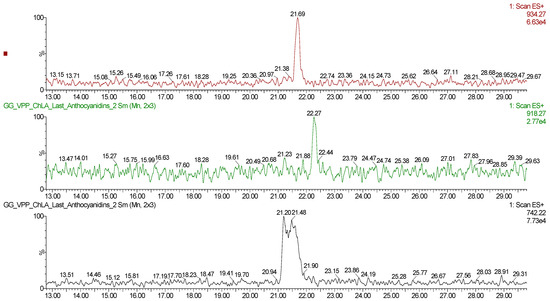
Figure A4.
ESI+ Mass Spectra Scan, 742.22 [M+] → pelargonidin 3-rutinoside-5-glucoside; 918.27 [M+] → pelargonidin 3-feruloylrutinoside-5-glucoside; 934.27 [M+] → petunidin 3-p-coumaroylrutinoside-5-glucoside.
These preliminary results encouraged the further development of analytical protocols for new studies, deepening the characterisation of metabolites recovered by means of ChLa from VPP, even considering the potential correlation between shelf-life extension and detected bioactive.
References
- Available online: http://fao.org/faostat/ (accessed on 20 April 2023).
- Oreopoulou, V.; Russ, W. Utilization of by-Products and Treatment of Waste in the Food Industry; Springer: New York, NY, USA, 2007. [Google Scholar]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Figueroa-Torres, G.; Azapagic, A. The extent of food waste generation in the UK and its environmental impacts. Sustain. Prod. Consum. 2021, 26, 532–547. [Google Scholar] [CrossRef]
- Tawila, M.A.; Omer, H.A.A.; Gad, S.M. Partial replacing of concentrate feed mixture by potato processing waste in sheep rations. Am.-Eurasia J. Agric. Environ. Sci. 2008, 4, 156–164. [Google Scholar]
- Maske, N.S.; Satyanarayan, S. Effect of special fish feed prepared using potato peels on fresh water fish labeorohita. J. Indus. Pollut. Control 2012, 29, 33–38. [Google Scholar] [CrossRef]
- Chimonyo, M. A review of the utility of potato by-products as a feed resource for smallholder pig production. Anim. Feed. Sci. Technol. 2017, 227, 107–117. [Google Scholar] [CrossRef]
- Pandit, N.; Ahmad, N.; Maheshwari, S. Vermicomposting biotechnology an eco-loving approach for recycling of solid organic wastes into valuable biofertilizers. J. Biofertil. Biopestic. 2012, 3, 1–8. [Google Scholar] [CrossRef]
- Arapoglou, D.; Varzakas, T.; Vlyssides, A.; Israilides, C. Ethanol production from potato peel waste (PPW). Waste Manag. 2010, 30, 1898–1902. [Google Scholar] [CrossRef]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef]
- Despoudi, S.; Bucatariu, C.; Otles, S.; Kartal, C. Chapter 1—Food Waste Management, Valorization, and Sustainability in the Food Industry. In Food Waste Recovery, 2nd ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 3–19. ISBN 9780128205631. [Google Scholar] [CrossRef]
- Lacy, P.; Rutqvist, J. Waste to Wealth—The Circular Economy Advantage; Palgrave Macmillan: London, UK, 2015. [Google Scholar] [CrossRef]
- Sales, F.C.V.; De Souza, M.; Trento, L.R.; Pereira, G.M.; Borchardt, M.; Milan, G.S. Food Waste in Distribution: Causes and Gaps to Be Filled. Sustainability 2023, 15, 3598. [Google Scholar] [CrossRef]
- Wu, D. Recycle technology for potato peel waste processing: A review. Procedia Environ. Sci. 2016, 31, 103–107. [Google Scholar] [CrossRef]
- Samotyja, U. Potato peel as a sustainable resource of natural antioxidants for the food industry. Potato Res. 2019, 62, 435–451. [Google Scholar] [CrossRef]
- Eltayeb, E.A.; Al-Sinani, S.S.; Khan, I.A. Determination of the Glycoalkaloids α-Solanine and α-Chaconine Levels in 18 Varieties of Potato (Solarium Tuberosum L.) Grown in Oman. Potato Res. 2003, 46, 57. [Google Scholar] [CrossRef]
- Scharf, R.; Wang, W.; Maycock, J.; Ho, P.; Chen, S.; Orfila, C. Valorisation of Potato (Solanum tuberosum) Peel Waste: Extraction of Fibre, Monosaccharides and Uronic Acids. Waste Biomass Valor. 2020, 11, 2123–2128. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Colletti, A.; Grillo, G.; Tabasso, S.; Cravotto, G. Emerging Processing Technologies for the Recovery of Valuable Bioactive Compounds from Potato Peels. Foods 2020, 3, 1598. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, S.; Milliron, H.; Han, Q. The Efficacy of Phenolic Compound Extraction from Potato Peel Waste. Processes 2022, 10, 2326. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds in potato (Solanum tuberosum L.) peel and their health-promoting activities. Int. J. Food Sci. Technol. 2020, 55, 2273–2281. [Google Scholar] [CrossRef]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato By-Products as a Source of Natural Chlorogenic Acids and Phenolic Compounds: Extraction, Characterization, and Antioxidant Capacity. Molecules 2021, 26, 177. [Google Scholar] [CrossRef] [PubMed]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic Compounds and Antioxidant Activities of Potato Cultivars with White, Yellow, Red and Purple Flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef]
- Arun, K.B.; Chandran, J.; Dhanya, R.; Krishna, P.; Jayamurthy, P.; Nisha, P. A comparative evaluation of antioxidant and antidiabetic potential of peel from young and matured potato. Food Biosci. 2015, 9, 36–46. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional Versus Green Extraction Techniques—A Comparative Perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Madhuresh, D. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 719–752. [Google Scholar] [CrossRef]
- Aimone, C.; Grillo, G.; Boffa, L.; Giovando, S.; Cravotto, G. Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Appl. Sci. 2023, 13, 2494. [Google Scholar] [CrossRef]
- Gunjevic, V.; Grillo, G.; Carnaroglio, D.; Binello, A.; Barge, A.; Cravotto, G. Selective recovery of terpenes, polyphenols and cannabinoids from Cannabis sativa L. inflorescences under microwaves. Ind. Crop. Prod. 2021, 162, 113247–113258. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Talarico, S.; Solarino, R.; Binello, A.; Cavaglià, G.; Bensaid, S.; Telysheva, G.; Cravotto, G. Batch and Flow Ultrasound-Assisted Extraction of Grape Stalks: Process Intensification Design up to a Multi-Kilo Scale. Antioxidants 2020, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Grillo, G.; Boffa, L.; Calcio, G.E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined Ultrasound and Pulsed Electric Fields in Continuous-Flow Industrial Olive-Oil Production. Foods 2022, 28, 3419. [Google Scholar] [CrossRef]
- Zhou, J.; Gullón, B.; Wang, M.; Gullón, P.; Lorenzo, J.M.; Barba, F.J. The Application of Supercritical Fluids Technology to Recover Healthy Valuable Compounds from Marine and Agricultural Food Processing By-Products: A Review. Processes 2021, 9, 357. [Google Scholar] [CrossRef]
- Velusamy, M.; Rajan, A.; Radhakrishnan, M. Valorisation of food processing wastes using PEF and its economic advances—Recent update. Int. J. Food Sci. Technol. 2023, 58, 2021–2041. [Google Scholar] [CrossRef]
- Frontuto, D.; Carullo, D.; Harrison, S.M.; Brunton, N.P.; Ferrari, G.; Lyng, J.G.; Pataro, G. Optimization of Pulsed Electric Fields-Assisted Extraction of Polyphenols from Potato Peels Using Response Surface Methodology. Food Bioprocess Technol. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Cravotto, C.; Grillo, G.; Binello, A.; Gallina, L.; Olivares-Vicente, M.; Herranz-López, M.; Micol, V.; Barrajón-Catalán, E.; Cravotto, G. Bioactive Antioxidant Compounds from Chestnut Peels through Semi-Industrial Subcritical Water Extraction. Antioxidants 2022, 11, 988. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D.A. Subcritical water extraction of phenolic compounds from potato peel. Food Res. Int. 2011, 44, 82452–82458. [Google Scholar] [CrossRef]
- Singh, A.; Nair, G.P.; Liplap, P.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Effect of Dielectric Properties of a Solvent-Water Mixture Used in Microwave-Assisted Extraction of Antioxidants from Potato Peels. Antioxidants 2014, 3, 99–113. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2018, 9, 147–154. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef]
- Clark, J.H.; Deswarte, F.E.I.; Farmer, T.J. The Integration of Green Chemistry into Future Biorefineries. Biofuels Bioprod. Biorefining 2009, 3, 72–90. [Google Scholar] [CrossRef]
- Glavič, P.; Pintarič, Z.N.; Bogataj, M. Process Design and Sustainable Development—A European Perspective. Processes 2021, 9, 148. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citruswastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging Green Techniques for the Extraction of Antioxidants from Agri-Food By-Products as Promising Ingredients for the Food Industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tabasso, S.; Grillo, G.; Cravotto, G.; Dreyer, T.; Schories, G.; Altenberg, S.; Lauberte, L.; Telysheva, G. Wheat straw lignin extraction with bio-based solvents using enabling technologies. Comptes Rendus Chem. 2018, 21, 563. [Google Scholar] [CrossRef]
- Grillo, G.; Calcio Gaudino, E.; Rosa, R.; Leonelli, C.; Timonina, A.; Grygiškis, S.; Tabasso, S.; Cravotto, G. Green Deep Eutectic Solvents for Microwave-Assisted Biomass Delignification and Valorisation. Molecules 2021, 26, 798. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Ćurko, N.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, J.; Huang, Z.; Guo, Y. Sustainable recovery and recycling of natural deep eutectic solvent for biomass fractionation via industrial membrane-based technique. Ind. Crops Prod. 2023, 194, 116351. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Radošević, K.; Ćurko, N.; Srček, V.G.; Bubalo, M.C.; Tomašević, M.; Ganić, K.K.; Redovniković, I.R. Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Grillo, G.; Gunjević, V.; Radošević, K.; Redovniković, I.R.; Cravotto, G. Deep Eutectic Solvents and Nonconventional Technologies for Blueberry-Peel Extraction: Kinetics, Anthocyanin Stability, and Antiproliferative Activity. Antioxidants 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural deep eutectic solvent as a unique solvent for valorisation of orange peel waste by the integrated biorefinery approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef]
- Vanda, H.; Verpoorte, R.; Klinkhamer, P.G.L.; Choi, Y.H. Natural Deep Eutectic Solvents: From Their Discovery to Their Applications. In Deep Eutectic Solvents: Synthesis, Properties, and Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020. [Google Scholar]
- Rodríguez Amado, I.; Franco, D.; Sánchez, M.; Zapata, C.; Vázquez, J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef]
- Lévêque, J.M.; Cravotto, G.; Delattre, F.; Cintas, P. Organic Sonochemistry, Challenges and Perspectives for the 21st Century; Springer Nature: Chem, Switzerland, 2018. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Locatelli, M.; Gindro, R.; Travaglia, F.; Coïsson, J.D.; Rinaldi, M.; Arlorio, M. Study of the DPPH—cavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009, 114, 889–897. [Google Scholar] [CrossRef]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef]
- Olivares, B.; Martínez, F.; Rivas, L.; Calderon, C.; Munita, J.M.; Campodonico, P.R. A Natural Deep Eutectic Solvent Formulated to Stabilize β-Lactam Antibiotics. Sci. Rep. 2018, 8, 14900. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Lecomte, J.; Upasani, R.; Chabi, B.; Bayrasy, C.; Baréa, B.; Jublanc, E.; Clarke, M.J.; Moore, D.J.; Crowther, J.; et al. Evaluation of the ROS Inhibiting Activity and Mitochondrial Targeting of Phenolic Compounds in Fibroblast Cells Model System and Enhancement of Efficiency by Natural Deep Eutectic Solvent (NADES) Formulation. Pharm. Res. 2017, 34, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Juan, E.; Rodríguez-Romero, C.; Fernández-Bolaños, J.; Florido, M.C.; Garcia-Borrego, A. Phenolic compounds from virgin olive oil obtained by natural deep eutectic solvent (NADES): Effect of the extraction and recovery conditions. J. Food Sci. Technol. 2021, 58, 552–561. [Google Scholar] [CrossRef]
- Ruesgas-Ramón, M.; Suárez-Quiroz, M.L.; González-Ríos, O.; Baréa, B.; Cazals, G.; Figueroa-Espinoza, M.C.; Durand, E. Bioactive Compounds from Cocoa Husk: Extraction, Analysis and Applications in Food Production Chain. J. Sci. Food Agric. 2020, 100, 798. [Google Scholar] [CrossRef]
- Rente, D.; Paiva, A.; Duarte, A.R. The Role of Hydrogen Bond Donor on the Extraction of Phenolic Compounds from Natural Matrices Using Deep Eutectic Systems. Molecules 2021, 26, 2336. [Google Scholar] [CrossRef]
- Kalhor, P.; Zheng, Y.-Z.; Ashraf, H.; Cao, B.; Yu, Z.-W. Influence of Hydration on the Structure and Interactions of Ethaline Deep-Eutectic Solvent: A Spectroscopic and Computational Study. ChemPhysChem 2020, 21, 995–1005. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sa-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Jeong, K.M.; Ko, J.; Zhao, J.; Jin, Y.; Yoo, D.E.; Han, S.Y.; Lee, J.L. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Alcalde, R.; Atilhan, M.; Aparicio, S. On the properties of (choline chloride + lactic acid) deep eutectic solvent with methanol mixtures. J. Mol. Liq. 2018, 272, 815–820. [Google Scholar] [CrossRef]
- Mannucci, G.; Busato, M.; Tofoni, A.; D’Angelo, P. Structural evolution of the butylated hydroxytoluene/menthol hydrophobic eutectic solvent upon methanol and ethanol cosolvent addition. J. Mol. Liq. 2023, 375, 121302. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Rente, D.; Bubalo, M.C.; Panić, M.; Paiva, A.; Caprin, B.; Redovniković, I.R.; Duarte, A.R.C. Review of deep eutectic systems from laboratory to industry, taking the application in the cosmetics industry as an example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.V.; Anil Kumar, N.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The Optimisation of Extraction of Antioxidants from Potato Peel by Pressurised Liquids. Food Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Benavides-Guerrero, R.; Revelo-Cuarán, Y.A.; Arango-Bedoya, O.; Osorio-Mora, O. Ultrasound-Assisted Extraction of Phenolic Compounds from Two Varieties of an Andean Native Potato (Solanum Phureja) and Evaluation of Their Antioxidant Activity. Inf. Tecnol. 2020, 21, 43–50. [Google Scholar] [CrossRef]
- Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.J.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels. Molecules 2011, 16, 2218–2232. [Google Scholar] [CrossRef]
- Palos-Hernández, A.; Gutiérrez Fernández, M.Y.; Escuadra Burrieza, J.; Pérez-Iglesias, J.L.; González-Paramás, A.M. Obtaining green extracts rich in phenolic compounds from underexploited food by-products using natural deep eutectic solvents. Opportunities and challenges. Sustain. Chem. Pharm. 2022, 29, 100773. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Stonestreet, E. Le dosage des tannins du vin rouge et la détermination de leur structure. Chim. Analitique 1966, 48, 188–196. [Google Scholar]
- Lewis, C.E.; Walker, J.R.L.; Lancaster, J.E.; Sutton, K.H. Determination of anthocyanins, flavonoids and phenolic acids in potatoes. I: Coloured cultivars of Solanum tuberosum L. J. Sci. Food Agric. 2018, 77, 45–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).