Effects of Mild Thermal Processing and Storage Conditions on the Quality Attributes and Shelf Life of Truffles (Terfezia claveryi)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
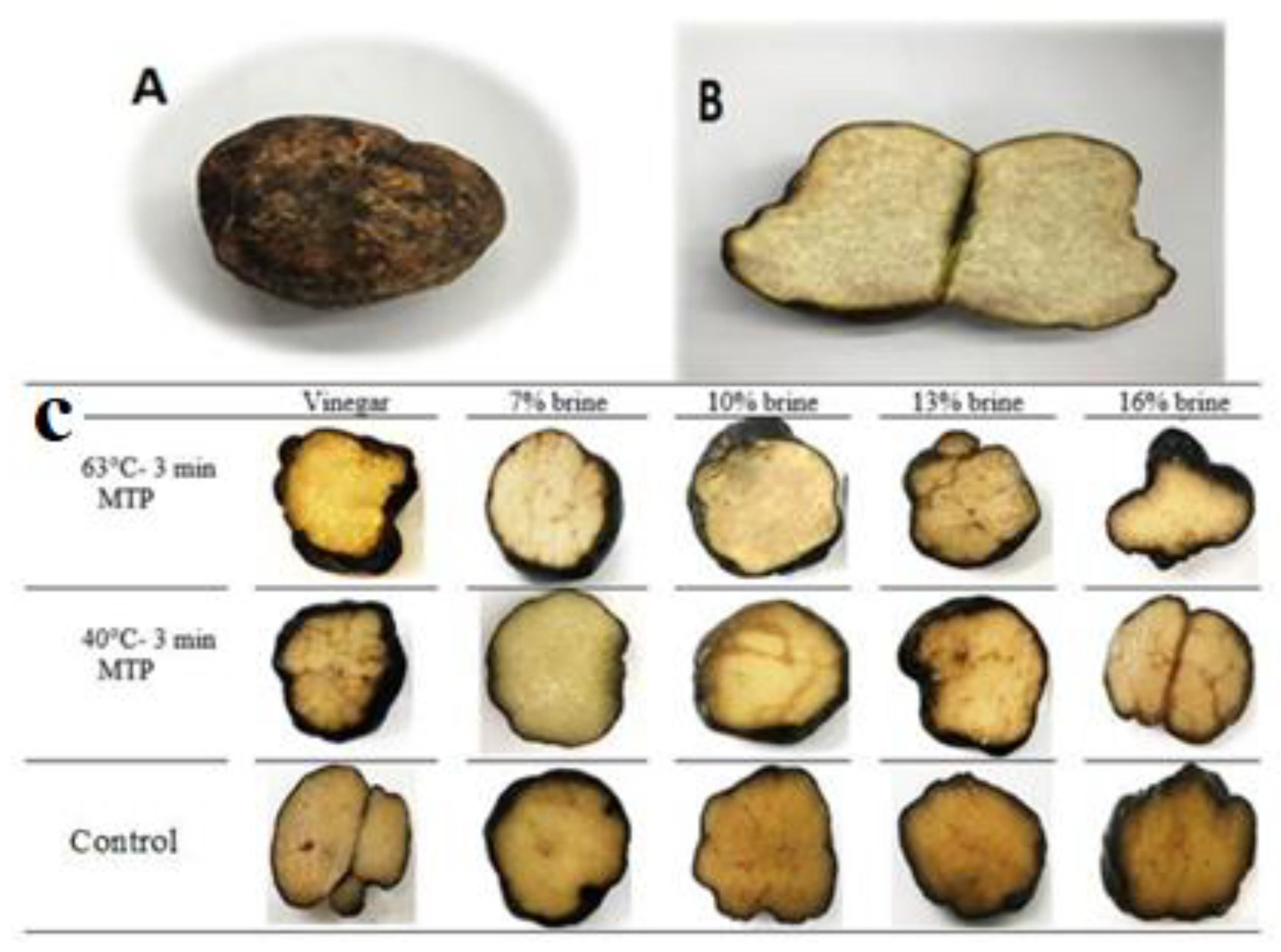
Treatments and Storage Conditions
2.3. Proximate Composition of Fresh Truffles
2.4. Determination of the Phenolic Composition of T. claveryi
2.5. Analysis of Ascorbic Acid
2.6. Weight Loss Percentage
2.7. Firmness Analysis
2.8. Microbial Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Composition of T. claveryi
3.2. Influence of Treatments on the Weight Loss
3.3. Influence of Treatments on Phenolic Compounds
3.4. Influence of Treatments on the Texture
3.5. Influence of Treatments on the Ascorbic Acid
3.6. Microbiological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benucci, G.M.N.; Bonito, G.M. The truffle microbiome: Species and geography effects on bacteria associated with fruiting bodies of hypogeous Pezizales. Microb. Ecol. 2016, 72, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Khabar, L. Mediterranean Basin: North Africa. In Desert Truffles; Springer: Berlin/Heidelberg, Germany, 2014; pp. 143–158. [Google Scholar] [CrossRef]
- Vahdatzadeh, M.; Splivallo, R. Improving truffle mycelium flavour through strain selection targeting volatiles of the Ehrlich pathway. Sci. Rep. 2018, 8, 9304. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Khalid, S.; Mubarak, M.S. Potential health benefits of natural products derived from truffles: A review. Trends Food Sci. Technol. 2017, 70, 1–8. [Google Scholar] [CrossRef]
- Alirezalu, K.; Azadmard-Damirchi, S.; Achachlouei, B.F.; Hesari, J.; Emaratpardaz, J.; Tavakolian, R. Physicochemical Properties and Nutritional Composition of Black Truffles Grown in Iran. Chem. Nat. Compd. 2016, 52, 290–293. [Google Scholar] [CrossRef]
- Campo, E.; Marco, P.; Oria, R.; Blanco, D.; Venturini, M.E. What is the best method for preserving the genuine black truffle (Tuber melanosporum) aroma? An olfactometric and sensory approach. LWT 2017, 80, 84–91. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Picariello, G.; Coppola, R.; Reale, A.; Di Luccia, A. Evaluation of gamma rays influence on some biochemical and microbiological aspects in black truffles. Food Chem. 2007, 103, 344–354. [Google Scholar] [CrossRef]
- Tirillini, B.; Maggi, F.; Venanzoni, R.; Angelini, P. Enhanced duration of truffle sauce preservation due to addition of linoleic acid. J. Food Qual. 2019, 2019, 8421898. [Google Scholar] [CrossRef]
- Halpin, B.; Lee, C. Effect of blanching on enzyme activity and quality changes in green peas. J. Food Sci. 1987, 52, 1002–1005. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Reale, A. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms 2020, 8, 1176. [Google Scholar] [CrossRef]
- Behera, S.S.; El Sheikha, A.F.; Hammami, R.; Kumar, A. Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits? J. Func. Foods 2020, 70, 103971. [Google Scholar] [CrossRef]
- Mills-Gray, S. Pickling Basics—In a Pickle. Nutrition and Health; UME: Columbia, MO, USA, 2015; pp. 1–4. [Google Scholar]
- AOAC. Official Methods of Analysis; Williams, S., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
- Bouatia, M.; Touré, H.; Cheikh, A.; Eljaoudi, R.; Rahali, Y.; Idrissi, O.M.B.; Khabar, L.; Draoui, M. Analysis of nutrient and antinutrient content of the truffle (Tirmania pinoyi) from Morocco. Int. Food Res. J. 2018, 25, 174–178. [Google Scholar]
- Lister, C.E.; Lancaster, J.E.; Sutton, K.H.; Walker, J.R. Developmental changes in the concentration and composition of flavonoids in skin of a red and a green apple cultivar. J. Sci. Food Agric. 1994, 64, 155–161. [Google Scholar] [CrossRef]
- Nurhidayati, U.A.; Ali, U.; Murwani, I. Yield and quality of cabbage (Brassica oleracea L. var. Capitata) under organic growing media using vermicompost and earthworm Pontoscolex corethrurus inoculation. Agric. Agric. Sci. 2016, 11, 5–13. [Google Scholar] [CrossRef]
- Gao, M.; Feng, L.; Jiang, T. Browning inhibition and quality preservation of button mushroom (Agaricus bisporus) by essential oils fumigation treatment. Food Chem. 2014, 149, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Padilla-Zakour, O.I. High Pressure Processing vs. Thermal Pasteurization of Whole Concord Grape Puree: Effect on Nutritional Value, Quality Parameters and Refrigerated Shelf Life. Foods 2021, 10, 2608. [Google Scholar] [CrossRef]
- Bokhary, H.; Parvez, S. Chemical composition of desert truffles Terfezia claveryi. J. Food Comp. Anal. 1993, 6, 285–293. [Google Scholar] [CrossRef]
- Wahiba, B.; Wafaà, T.; Asmaà, K.; Bouziane, A.; Mohammed, B. Nutritional and antioxidant profile of red truffles (Terfezia claveryi) and white truffle (Tirmania nivea) from southwestern of Algeria. Der. Pharm. Lett. 2016, 8, 134–141. [Google Scholar]
- AL-RUQAIE, I.M. Effect of different treatment processes and preservation methods on the quality of truffles: I. Conventional methods (drying/freezing). J. Food Proc. Pres. 2006, 30, 335–351. [Google Scholar] [CrossRef]
- Kagan-Zur, V.; Roth-Bejerano, N. Studying the brown desert truffles of Israel. Israel J. Plant Sci. 2008, 56, 309–314. [Google Scholar] [CrossRef]
- Murcia, M.A.; Martínez-Tomé, M.; Vera, A.; Morte, A.; Gutierrez, A.; Honrubia, M.; Jiménez, A.M. Effect of industrial processing on desert truffles Terfezia claveryi Chatin and Picoa juniperi Vittadini): Proximate composition and fatty acids. J. Sci. Food Agri. 2003, 83, 535–541. [Google Scholar] [CrossRef]
- Vahdani, M.; Rastegar, S.; Rahimizadeh, M.; Ahmadi, M.; Karmostaji, A. Physicochemical characteristics, phenolic profile, mineral and carbohydrate contents of two truffle species. Mag. Int. Sci. Agric. Technol. 2017, 19, 1091–1101. [Google Scholar]
- Ammarellou, A. Protein profile analysis of desert truffle (Terfezia claveryi Chatin). J. Food Agri. Env. 2007, 5, 62. [Google Scholar]
- Fan, K.; Zhang, M.; Fan, D.; Jiang, F. Effect of carbon dots with chitosan coating on microorganisms and storage quality of modified-atmosphere-packaged fresh-cut cucumber. J. Sci. Food. Agri. 2019, 99, 6032–6041. [Google Scholar] [CrossRef] [PubMed]
- Kıvrak, İ. Analytical methods applied to assess chemical composition, nutritional value and in vitro bioactivities of Terfezia olbiensis and Terfezia claveryi from Turkey. Food Anal. Methods 2015, 8, 1279–1293. [Google Scholar] [CrossRef]
- Parmar, N.; Singh, N.; Kaur, A.; Virdi, A.S.; Thakur, S. Effect of canning on color, protein and phenolic profile of grains from kidney bean, field pea and chickpea. Food Res. Int. 2016, 89, 526–532. [Google Scholar] [CrossRef]
- Basiri, S.; Gheybi, F. The effect of osmotic dehydration and various methods of drying pumpkin on quality and color of final product. J. Food Res. 2019, 29, 141–153. [Google Scholar]
- Tanaka, A.; Hoshino, E. Similarities between the thermal inactivation kinetics of Bacillus amyloliquefaciensα-amylase in an aqueous solution of sodium dodecyl sulphate and the kinetics in the solution of anionic-phospholipid vesicles. Biotechnol. Appl. Biochem. 2003, 38, 175–181. [Google Scholar] [CrossRef]
- Imaizumi, T.; Tanaka, F.; Uchino, T. Effects of mild heating treatment on texture degradation and peroxidase inactivation of carrot under pasteurization conditions. J. Food Eng. 2019, 257, 19–25. [Google Scholar] [CrossRef]
- Burdurlu, H.S.; Koca, N.; Karadeniz, F. Degradation of vitamin C in citrus juice concentrates during storage. J. Food Eng. 2006, 74, 211–216. [Google Scholar] [CrossRef]
- Yalim, S.; Özdemır, Y. Effects of preparation procedures on ascorbic acid retention in pickled hot peppers. Int. J. Food Sci. Nut. 2003, 54, 291–296. [Google Scholar] [CrossRef]
- Grzelakowska, A.; Cieślewicz, J.; Łudzińska, M. The dynamics of vitamin C content in fresh and processed cucumber (Cucumis sativus L.)/Dynamika zmian zawartości witaminy C w ogórku świeżym (Cucumis sativus L.) i jego przetworach. Chem. Didact. Ecol. Metrol. 2013, 18, 97–102. [Google Scholar] [CrossRef]
- Orsat, V.; Gariepy, Y.; Raghavan, G.; Lyew, D. Radio-frequency treatment for ready-to-eat fresh carrots. Int. Food Res. J. 2001, 34, 527–536. [Google Scholar] [CrossRef]
- Shweta, R.; Geeta, I.; Ganessin, A. Comparative evaluation of antimicrobial properties of piperidine and apple cider vinegar on Streptococcus mutansand in-silico demonstration of the mechanism of action. RGUHS J. Dent. Sci. 2021, 13, 42–50. [Google Scholar]
| Factor | Value |
|---|---|
| Weight (g) | 71.5 ± 0.5 |
| Volume (cm3) | 75.5 ±0.4 |
| Length (cm) | 6.5 ± 0.3 |
| Diameter (cm) | 4.5 ± 0.2 |
| Moisture (% in DW) | 74.3 ± 0.5 |
| Protein (% in DW) | 11.7 ± 0.5 |
| Carbohydrates (mg 100 g−1 DW) | 14.6 ± 0.5 |
| Vitamin C (mg/100 g) | 4.2 ± 0.8 |
| Fat (% in DW) | 1.4 ± 0.5 |
| Storage Days (D) | ||||||
|---|---|---|---|---|---|---|
| Preservation Method | Brine (Salt%) | 1 | 40 | 80 | 120 | 160 |
| Control | 5% vinegar | 0.01 Eb | 3.09 Dd | 5.92 Cd | 8.29 Bf | 10.2 Af |
| 7% | 0.01 Eb | 3.49 Dcd | 7.01 Cc | 10.14 Be | 13.41 Ae | |
| 10% | 0.01 Eb | 3.62 Dcd | 7.84 Cc | 12.27 Bd | 15.79 Ad | |
| 13% | 0.01 Eb | 4.29 Dbc | 9.20 Cb | 14.44 Bc | 18.65 Ac | |
| 16% | 0.01 Eb | 7.60 Da | 15.85 Ca | 24.49 Ba | 33.84 Aa | |
| MTP 40 °C-3 min | 5% vinegar | 0.008 Ec | 2.19 Dd | 4.04 Cef | 6.39 Bg | 8.19 Ag |
| 7% | 0.008 Ec | 2.53 Dcd | 4.72 Ce | 7.29 Bf | 9.64 Af | |
| 10% | 0.009 Ec | 2.60 Dc | 5.15 Cde | 8.14 Bf | 10.41 Af | |
| 13% | 1.01 Ea | 3.59 Dc | 5.64 Cd | 12.21 Bd | 15.5 Ad | |
| 16% | 1.09 Ea | 4.84 Db | 10.34 Cb | 19.84 Bb | 26.77 Ab | |
| MTP 63 °C-3 min | 5% vinegar | 0.003 Ec | 1.46 Dd | 3.57 Cf | 5.19 Bg | 6.37 Ah |
| 7% | 0.005 Ec | 1.62 Dd | 3.68 Cf | 5.44 Bg | 6.74 Ah | |
| 10% | 0.005 Ec | 2.09 Dd | 3.98 Cf | 6.27 Bg | 7.14 Ag | |
| 13% | 0.008 Ec | 2.53 Dcd | 4.72 Ce | 7.29 Bf | 9.01 Af | |
| 16% | 0.009 Ec | 4.69 Db | 6.7 Ccd | 8.14 Bf | 10.25 Af | |
| Time | Treatment | Catechin | p-Coumaric Acid | Ferulic Acid | Chlorogenic Acid | Syringic Acid | p-Hydroxy Benzoic Acid | Rutin | Proto Catechuic Acid | Eugenol | Gentisic Acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | 8.29 A** | 14.48 A | 12.15 A | 24.67 A | 2.47 A | 25.49 A | 5.58 A | 26.67 A | 8.45 A | 26.40 A | ||
| A * | 5.70 BC | 9.10 CD | 8.05 C | 19.25 CD | 1.75 D | 18.54 D | 3.18 CD | 19.87 C | 6.11 C | 16.70 DE | ||
| 5% vinegar | B | 5.45 C | 8.29 D | 7.10 D | 17.48 E | 1.00 I | 16.20 G | 2.88 D | 16.45 F | 5.03 E | 14.55 G | |
| C | 6.15 B | 10.18 B | 9.40 B | 20.85 B | 2.12 B | 20.05 B | 4.10 B | 22.20 B | 6.80 B | 20.35 B | ||
| A | 4.64 CD | 6.83 F | 6.50 DE | 16.20 FG | 1.64 DE | 17.64 F | 2.30 E | 18.30 D | 5.85 D | 14.78 G | ||
| 7% Brine | B | 3.20 E | 5.35 I | 5.48 EF | 13.10 H | 0.96 I | 14.18 I | 1.96 EF | 15.64 G | 4.94 E | 13.40 H | |
| C | 5.65 BC | 8.45 D | 7.87 CD | 20.18 BC | 2.05 C | 19.40 C | 3.70 BC | 21.80 B | 6.54 C | 18.65 CD | ||
| A | 3.75 E | 6.10 FG | 6.15 E | 15.35 G | 1.20 H | 15.40 H | 2.18 E | 17.65 E | 5.67 DE | 14.25 GH | ||
| Day 160 | 10% Brine | B | 2.48 F | 5.18 GH | 5.12 F | 12.49 I | 0.58 J | 12.55 K | 1.40 F | 14.80 GH | 4.48 FG | 12.18 I |
| C | 5.25 BC | 8.10 DE | 7.68 CD | 19.46 C | 1.87 CD | 19.18 CD | 3.45 C | 21.18 BC | 6.30 C | 18.45 CD | ||
| A | 3.64 E | 5.84 G | 5.45 EF | 14.97 GH | 1.10 H | 13.45 J | 2.06 E | 16.30 F | 5.10 E | 12.40 I | ||
| 13% Brine | B | 1.83 G | 4.96 I | 4.55 FG | 11.87 J | 0.35 J | 11.80 L | 1.26 F | 11.28 I | 4.12 G | 12.28 I | |
| C | 4.67 CD | 8.03 DE | 7.40 D | 19.16 CD | 1.63 DE | 18.96 CD | 3.28 CD | 20.15 C | 6.18 C | 17.36 D | ||
| A | 2.15 F | 5.15 GH | 4.98 FG | 14.40 GH | 0.45 J | 12.15 KL | 1.43 F | 14.84 GH | 4.65 F | 12.19 I | ||
| 16% Brine | B | 1.10 H | 4.80 I | 4.10 G | 11.25 J | ND | 10.50 M | 1.10 FG | 10.15 I | 3.30 H | 11.85 J | |
| C | 3.28 E | 7.30 E | 6.80 DE | 18.38 D | 1.50 F | 18.80 CD | 2.65 DE | 19.43 CD | 5.80 D | 16.80 DE |
| Storage Days (D) | ||||||
|---|---|---|---|---|---|---|
| Preservation Method | Brine (Salt%) | 1 | 40 | 80 | 120 | 160 |
| Control | 5% vinegar | 6.78 Aa | 4.78 Bbc | 3.69 Bbc | 2.48 Cd | 1.5 Dd |
| 7% | 6.78 Aa | 4.0 Bc | 2.88 Ccd | 2.29 Dd | 1.19 Ed | |
| 10% | 6.78 Aa | 3.79 Bc | 2.63 Ccd | 1.83 Dde | 0.96 Ed | |
| 13% | 6.78 Aa | 3.68 Bc | 2.23 Cd | 1.19 De | 0.91 Dd | |
| 16% | 6.78 Aa | 3.41 Bd | 1.9 Cd | 0.93 De | 0.23 De | |
| MTP 40 °C-3 min | 5% vinegar | 6.78 Aa | 5.81 Bab | 4.29 Cb | 3.69 Dbc | 3.35 Dbc |
| 7% | 6.81 Aa | 5.38 Bb | 4.08 Cb | 3.56 Dbc | 3.18 Dbc | |
| 10% | 6.79 A | 4.63 Bb | 3.78 Cbc | 2.43 Dd | 2.0 Dd | |
| 13% | 6.73 Aab | 4.13 Bcd | 2.66 Ccd | 2.23 CDd | 1.83 Dd | |
| 16% | 6.76 Aa | 4.03 Bc | 2.19 Cd | 1.78 CDde | 1.41 Dd | |
| MTP 63 °C-3 min | 5% vinegar | 6.76 Aa | 6.21 ABa | 5.8 Ba | 5.33 BCa | 4.92 Ca |
| 7% | 6.73 Aab | 5.78 Bab | 5.38 BCab | 4.96 Cab | 4.74 Ca | |
| 10% | 6.76 Aa | 5.7 Bab | 5.08 BCab | 4.31 Cb | 3.71 Db | |
| 13% | 6.69 Ab | 5.11 Bb | 4.39 Cb | 3.82 Dbc | 3.22 Dbc | |
| 16% | 6.73 Aab | 4.1 Bc | 3.48 Cc | 3.09 Cc | 2.53 Dc | |
| Storage Time (Day) | ||||||
|---|---|---|---|---|---|---|
| Preservation Method | Brine (Salt%) | 1 | 40 | 80 | 120 | 160 |
| Control | 5% vinegar | 4.43 Aa | 3.48 Ba | 2.83 Ca | 2.41 CDa | 1.66 Da |
| 7% | 4.41 Aa | 3.5 Ba | 2.68 Ca | 2.24 CDa | 1.51 Da | |
| 10% | 4.38 Aa | 3.33 Ba | 2.38 Cab | 2.03 Ca | 1.18 Da | |
| 13% | 4.41 Aa | 3.08 Bab | 2.13 Cb | 1.88 CDab | 1.1 Dab | |
| 16% | 4.38 Aa | 3.03 Bab | 2.01 Cb | 1.78 CDab | 1.03 Da | |
| MTP 40 °C-3 min | 5% vinegar | 4.48 Aa | 3.28 Bab | 2.44 Cab | 2.10 Da | 1.38 Ea |
| 7% | 4.48 Aa | 3.13 Bab | 2.28 Cab | 1.98 Dab | 1.23 Ea | |
| 10% | 4.48 Aab | 3.10 Bab | 2.13 Cb | 1.76 Dab | 1.04 Ea | |
| 13% | 4.48 Aa | 3.08 Bab | 2.00 Cb | 1.61 Db | 0.92 Eb | |
| 16% | 4.48 Aa | 2.82 Bb | 1.92 Cbc | 1.48 CDb | 0.81 Db | |
| MTP 63 °C-3 min | 5% vinegar | 3.78 Ab | 3.13 Bab | 2.30 Cab | 1.94 CDab | 1.33 Da |
| 7% | 3.38 Ac | 3.03 Aab | 2.16 Bb | 1.72 BCab | 0.98 Cb | |
| 10% | 3.34 Ac | 2.78 Bb | 2.02 Cb | 1.33 Db | 0.92 Db | |
| 13% | 3.36 Ac | 2.43 Bb | 1.81 BCbc | 1.13 Cb | 0.76 Db | |
| 16% | 3.40 Ac | 2.18 Bc | 1.68 BCc | 0.96 Cb | 0.63 Cb | |
| Storage Days (D) | ||||||
|---|---|---|---|---|---|---|
| Preservation Method Temperature | Brine (Salt%) | 1 | 40 | 80 | 120 | 160 |
| Control | 5% vinegar | 4.08 Da | 4.16 Db | 4.78 Cbc | 5.28 Bc | 7.53 Ab |
| 16% | 4.13 Da | 4.22 Db | 5.29 Cab | 5.98 Bb | 8.83 Ab | |
| 13% | 4.11 Ea | 4.26 Dab | 5.43 Cab | 6.18 Bb | 8.65 Ab | |
| 10% | 4.16 Ea | 4.78 Da | 5.83 Ca | 6.78 Bab | 8.38 Ab | |
| 7% | 4.13 Ea | 4.92 Da | 6.13 Ca | 7.33 Ba | 8.23 Aa | |
| MTP 40 °C | 5% vinegar | 2.08 Ca | 2.53 BCb | 3.05 Bbc | 3.86 ABc | 4.15 Ad |
| 16% | 2.96 Ca | 3.92 Ba | 4.46 Bab | 4.84 Ab | 5.78 Ac | |
| 13% | 2.88 Ca | 3.78 BCa | 4.33 Bab | 4.66 ABbc | 5.78 Ac | |
| 10% | 2.76 Ca | 3.63 Bab | 4.18 BCc | 4.58 ABbc | 5.55 Ac | |
| 7% | 2.63 Ba | 3.56 Bab | 3.96 Bc | 4.24 ABbc | 5.51 Ac | |
| MTP 63 °C | 5% vinegar | 0.88 Cb | 1.52 Bb | 2.23 Bb | 2.86 ABd | 3.03 Ad |
| 16% | 1.26 Cb | 2.73 Ba | 3.50 ABab | 3.73 Abc | 3.83 Acd | |
| 13% | 1.24 Cb | 2.46 Bab | 3.26 ABab | 3.68 ABc | 3.78 Acd | |
| 10% | 1.16 Cb | 2.33 Bb | 3.15 ABb | 3.58 Ac | 3.73 Ad | |
| 7% | 1.13 Cb | 2.18 Bb | 3.03 ABbc | 3.53 Ac | 3.70 Ad | |
| Storage Days (D) | |||||||
|---|---|---|---|---|---|---|---|
| Preservation Method Temperature | Brine (Salt%) | 1 | 40 | 80 | 120 | 160 | |
| Control | 5% vinegar | 3.10 Da | 3.66 Db | 3.95 Cbc | 4.00 Bc | 4.15 Ab | |
| 16% | 3.13 Ea | 3.98 Da | 4.84 Ca | 4.73 Ba | 4.85 Aa | ||
| 13% | 3.16 Ea0 | 3.98 Da | 4.63 Ca | 4.68 Bab | 4.78 Ab | ||
| 10% | 3.11 Ea | 3.96 Dab | 4.45 Cab | 4.56 Bb | 4.68 Ab | ||
| 7% | 3.13 Da | 3.92 Db | 4.15 Cab | 4.38 Bb | 4.63 Ab | ||
| MTP 40 °C | 5% vinegar | 0.63 Ca | 0.70 BCb | 0.76 Bbc | 0.86 ABc | 0.92 Ad | |
| 16% | 0.95 Ca | 0.99 Ba | 1.16 Bab | 1.24 Ab | 1.28 Ac | ||
| 13% | 0.88 Ca | 0.95 BCa | 0.98 Bab | 1.06 ABbc | 1.12 Ac | ||
| 10% | 0.76 Ca | 0.83 Bab | 0.86 BCc | 0.98 ABbc | 1.06 Ac | ||
| 7% | 0.68 Ba | 0.76 Bab | 0.85 Bc | 0.91 ABbc | 0.95 Ac | ||
| MTP 63 °C | 5% vinegar | 0.45 Cb | 0.50 Bb | 0.56 Bb | 0.65 ABd | 0.68 Ad | |
| 16% | 0.65 Cb | 0.73 Ba | 0.77 ABab | 0.82 Abc | 0.88 Acd | ||
| 13% | 0.63 Cb | 0.66 Bab | 0.74 ABab | 0.78 ABc | 0.85 Acd | ||
| 10% | 0.56 Cb | 0.60 Bb | 0.65 ABb | 0.68 Ac | 0.78 Ad | ||
| 7% | 0.53 Cb | 0.58 Bb | 0.63 ABbc | 0.67 Ac | 0.75 Ad | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daei, B.; Azadmard-Damirchi, S.; Javadi, A.; Torbati, M. Effects of Mild Thermal Processing and Storage Conditions on the Quality Attributes and Shelf Life of Truffles (Terfezia claveryi). Foods 2023, 12, 2212. https://doi.org/10.3390/foods12112212
Daei B, Azadmard-Damirchi S, Javadi A, Torbati M. Effects of Mild Thermal Processing and Storage Conditions on the Quality Attributes and Shelf Life of Truffles (Terfezia claveryi). Foods. 2023; 12(11):2212. https://doi.org/10.3390/foods12112212
Chicago/Turabian StyleDaei, Bahareh, Sodeif Azadmard-Damirchi, Afshin Javadi, and Mohammadali Torbati. 2023. "Effects of Mild Thermal Processing and Storage Conditions on the Quality Attributes and Shelf Life of Truffles (Terfezia claveryi)" Foods 12, no. 11: 2212. https://doi.org/10.3390/foods12112212
APA StyleDaei, B., Azadmard-Damirchi, S., Javadi, A., & Torbati, M. (2023). Effects of Mild Thermal Processing and Storage Conditions on the Quality Attributes and Shelf Life of Truffles (Terfezia claveryi). Foods, 12(11), 2212. https://doi.org/10.3390/foods12112212







