Effects of Puffing, Acid, and High Hydrostatic Pressure Treatments on Ginsenoside Profile and Antioxidant Capacity of Mountain-Cultivated Panax ginseng
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Puffing Treatment
2.4. HHP and Acid Treatment
2.5. Extraction Yield
2.6. Crude Saponin Content
2.7. Ginsenoside Profile
2.8. Functional Properties
2.8.1. Total Flavonoid Content (TFC)
2.8.2. Total Phenolic Content (TPC)
2.8.3. DPPH Radical Scavenging Capacity
2.9. Acidic Polysaccharides
2.10. Statistical Analysis
3. Results and Discussion
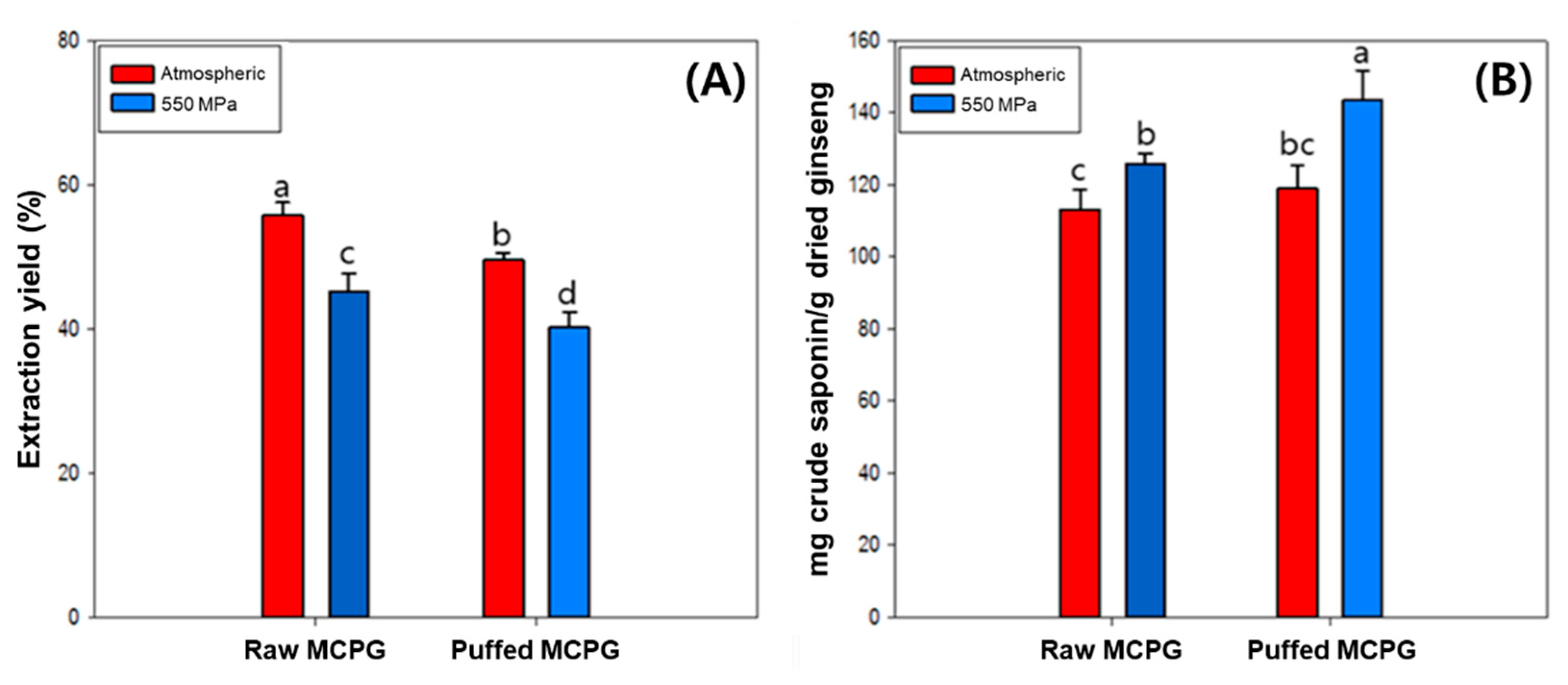
3.1. Extraction Yield and Crude Saponin Content
3.2. Ginsenoside Profile
3.3. Functional Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jie, Y.H.; Cammisuli, S.; Baggiolini, M. Immunomodulatory effects of Panax ginseng CA Meyer in the mouse. Agents Actions 1984, 15, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Park, E.-M.; Kim, D.-H.; Jung, K.; Jung, J.-S.; Lee, E.-J.; Hyun, J.-W.; Kang, J.L.; Kim, H.-S. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J. Neuroimmunol. 2009, 209, 40–49. [Google Scholar] [PubMed]
- Choi, Y.E.; Kim, Y.S.; Yi, M.J.; Park, W.G.; Yi, J.S.; Chun, S.R.; Han, S.S.; Lee, S.J. Physiological and chemical characteristics of field- and mountain-cultivated ginseng roots. J. Plant Biol. 2007, 50, 198–205. [Google Scholar] [CrossRef]
- Choi, G.-S.; Shin, J.-S.; Kim, W.; Baik, M.-Y. Increases in Ginsenoside Rg3, Compound K, and Antioxidant Activity of Cultivated Wild Panax Ginseng (CWPG) by Puffing. Foods 2022, 11, 2936. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jeon, S.-J.; Youn, S.J.; Lee, H.; Park, Y.-J.; Kim, D.-O.; Kim, B.-Y.; Kim, W.; Baik, M.-Y. Enhancement of minor ginsenosides contents and antioxidant capacity of american and canadian ginsengs (Panax quinquefolius) by puffing. Antioxidants 2019, 8, 527. [Google Scholar] [CrossRef]
- Liu, T.-G.; Huang, Y.; Cui, D.-D.; Huang, X.-B.; Mao, S.-H.; Ji, L.-L.; Song, H.-B.; Yi, C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer 2009, 9, 250. [Google Scholar]
- Sharma, A.; Lee, H.-J. Ginsenoside compound K: Insights into recent studies on pharmacokinetics and health-promoting activities. Biomolecules 2020, 10, 1028. [Google Scholar] [CrossRef]
- Choi, K.; Kim, M.; Ryu, J.; Choi, C. Ginsenosides compound K and Rh2 inhibit tumor necrosis factor-α-induced activation of the NF-κB and JNK pathways in human astroglial cells. Neurosci. Lett. 2007, 421, 37–41. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Wang, X.; Si, H. Anti-adipogenic effects and mechanisms of ginsenoside Rg3 in pre-adipocytes and obese mice. Front. Pharmacol. 2017, 8, 113. [Google Scholar] [CrossRef]
- Tian, J.; Fu, F.; Geng, M.; Jiang, Y.; Yang, J.; Jiang, W.; Wang, C.; Liu, K. Neuroprotective effect of 20 (S)-ginsenoside Rg3 on cerebral ischemia in rats. Neurosci. Lett. 2005, 374, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xia, J.; Wang, C.-Z.; Zhang, J.-Q.; Ruan, C.-C.; Sun, G.-Z.; Yuan, C.-S. Remarkable impact of acidic ginsenosides and organic acids on ginsenoside transformation from fresh ginseng to red ginseng. J. Agric. Food Chem. 2016, 64, 5389–5399. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, H.; Xu, L.; Deng, Y.; Xu, J.; Zhao, Y. Effects of different extraction methods in pharmacopoeia on the content and structure transformation of ginsenosides. Molecules 2022, 27, 4347. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, Y.S.; Kwak, Y.S.; Song, Y.B.; Kim, Y.S.; Park, J.D. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP). Planta Med. 2004, 70, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-Y.; Song, J.-Y.; Yun, Y.-S.; Yang, H.-O.; Rhee, D.-K.; Pyo, S. Immunostimulating effects of acidic polysaccharides extract of Panax ginseng on macrophage function. Immunopharmacol. Immunotoxicol. 2002, 24, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, Y.-S.; Jung, I.-S.; Park, S.-Y.; Chung, H.-Y.; Lee, I.-R.; Yun, Y.-S. Acidic polysaccharide from Panax ginseng, ginsan, induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Planta Med. 1998, 64, 110–115. [Google Scholar] [CrossRef]
- Kwak, Y.-S.; Shin, H.-J.; Song, Y.-B.; Kyung, J.-S.; Wee, J.-J.; Park, J.-D. Effect of oral administration of red ginseng acidic polysaccharide (RGAP) on the tumor growth inhibition. J. Ginseng Res. 2005, 29, 176–181. [Google Scholar]
- Bae, E.-A.; Han, M.J.; Kim, E.-J.; Kim, D.-H. Transformation of ginseng saponins to ginsenoside Rh 2 by acids and human intestinal bacteria and biological activities of their transformants. Arch. Pharmacal Res. 2004, 27, 61–67. [Google Scholar] [CrossRef]
- Han, B.; Park, M.; Han, Y.; Woo, L.; Sankawa, U.; Yahara, S.; Tanaka, O. Degradation of ginseng saponins under mild acidic conditions. Planta Med. 1982, 44, 146–149. [Google Scholar] [CrossRef]
- An, Y.-E.; Ahn, S.-C.; Yang, D.-C.; Park, S.-J.; Kim, B.-Y.; Baik, M.-Y. Chemical conversion of ginsenosides in puffed red ginseng. LWT—Food Sci. Technol. 2011, 44, 370–374. [Google Scholar] [CrossRef]
- Holm, J.; Björck, I.; Asp, N.-G.; Sjöberg, L.-B.; Lundquist, I. Starch availability in vitro and in vivo after flaking, steam-cooking and popping of wheat. J. Cereal Sci. 1985, 3, 193–206. [Google Scholar] [CrossRef]
- Rendueles, E.; Omer, M.K.; Alvseike, O.; Alonso-Calleja, C.; Capita, R.; Prieto, M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT—Food Sci. Technol. 2011, 44, 1251–1260. [Google Scholar] [CrossRef]
- Rivalain, N.; Roquain, J.; Boiron, J.-M.; Maurel, J.-P.; Largeteau, A.; Ivanovic, Z.; Demazeau, G. High hydrostatic pressure treatment for the inactivation of Staphylococcus aureus in human blood plasma. New Biotechnol. 2012, 29, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Patil, G.R.; Singh, A.K. High hydrostatic pressure technology in dairy processing: A review. J. Food Sci. Technol. 2011, 48, 260–268. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Meng, F.; Zhang, S.; Liu, Z. Effects of ultrahigh pressure extraction conditions on yields and antioxidant activity of ginsenoside from ginseng. Sep. Purif. Technol. 2009, 66, 340–346. [Google Scholar] [CrossRef]
- Shouqin, Z.; Junjie, Z.; Changzhen, W. Novel high pressure extraction technology. Int. J. Pharm. 2004, 278, 471–474. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Do, J.-H.; Lee, H.-O.; Lee, S.-K.; Jang, J.-K.; Lee, S.-D.; Sung, H.-S. Panax ginseng, its Extraction Condition and Stability. Korean J. Ginseng Sci. 1993, 17, 139–144. [Google Scholar]
- Shouqin, Z.; Ruizhan, C.; Changzheng, W. Experiment study on ultrahigh pressure extraction of ginsenosides. J. Food Eng. 2007, 79, 1–5. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ahn, S.-C.; Choi, S.-W.; Hur, N.-Y.; Kim, B.-Y.; Baik, M.-Y. Changes in effective components of ginseng by puffing. Appl. Biol. Chem. 2008, 51, 188–193. [Google Scholar]
- Choi, Y.; Lee, S.; Chun, J.; Lee, H.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Liu, M.; Zhang, L. A study on maillard reaction and its products during processing of red ginseng. China J. Chin. Mater. Med. 1999, 24, 274–278, 318. [Google Scholar]
- Kato, H.; Lee, I.E.; Van Chuyen, N.; Kim, S.B.; Hayase, F. Inhibition of nitrosamine formation by nondialyzable melanoidins. Agric. Biol. Chem. 1987, 51, 1333–1338. [Google Scholar]
- Kim, Y.S.; Kang, K.S.; Kim, S.I. Study on antitumor and immunomodulating activities of polysaccharide fractions from Panax ginseng: Comparison of effects of neutral and acidic polysaccharide fraction. Arch. Pharmacal Res. 1990, 13, 330–337. [Google Scholar] [CrossRef]
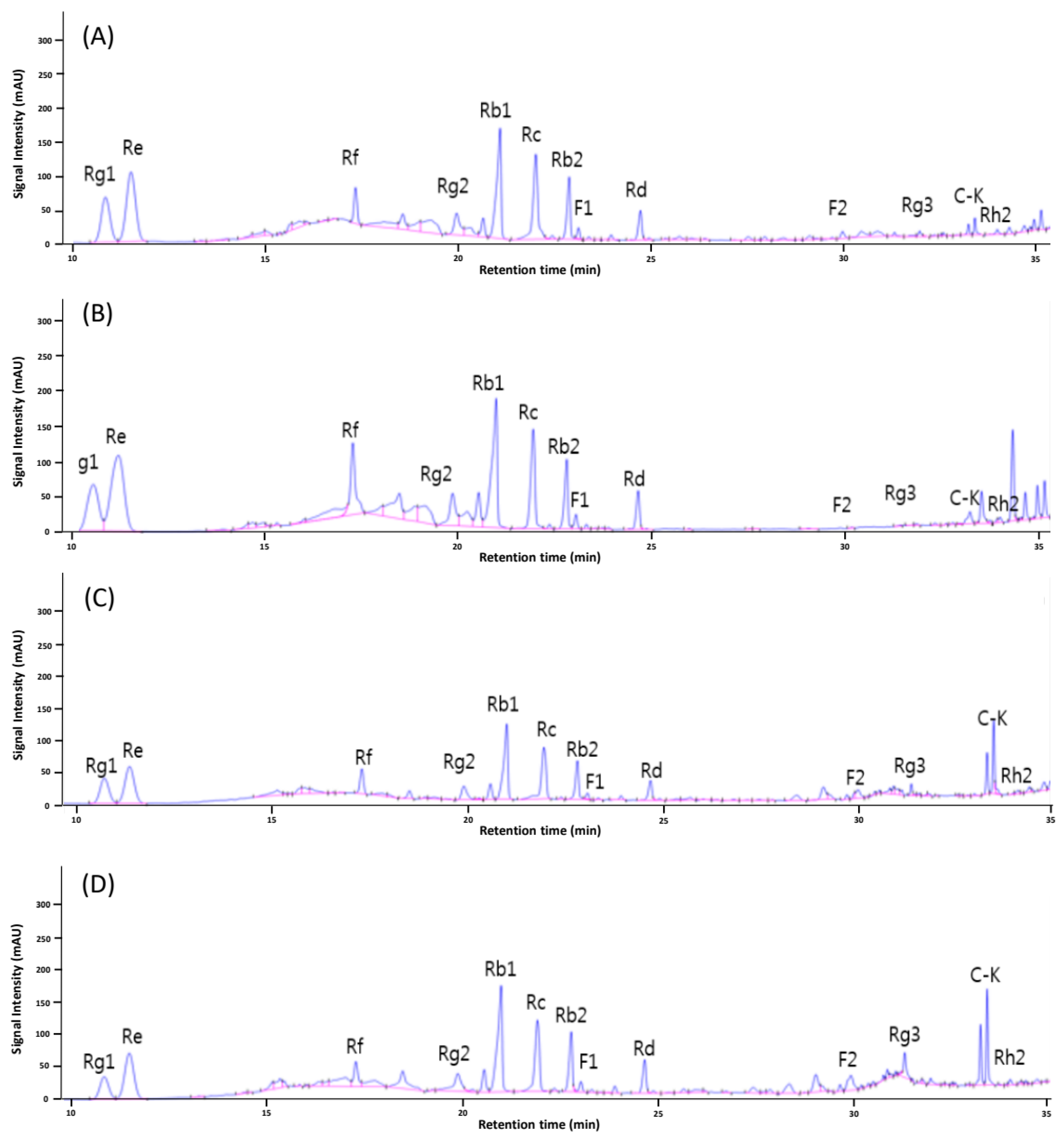
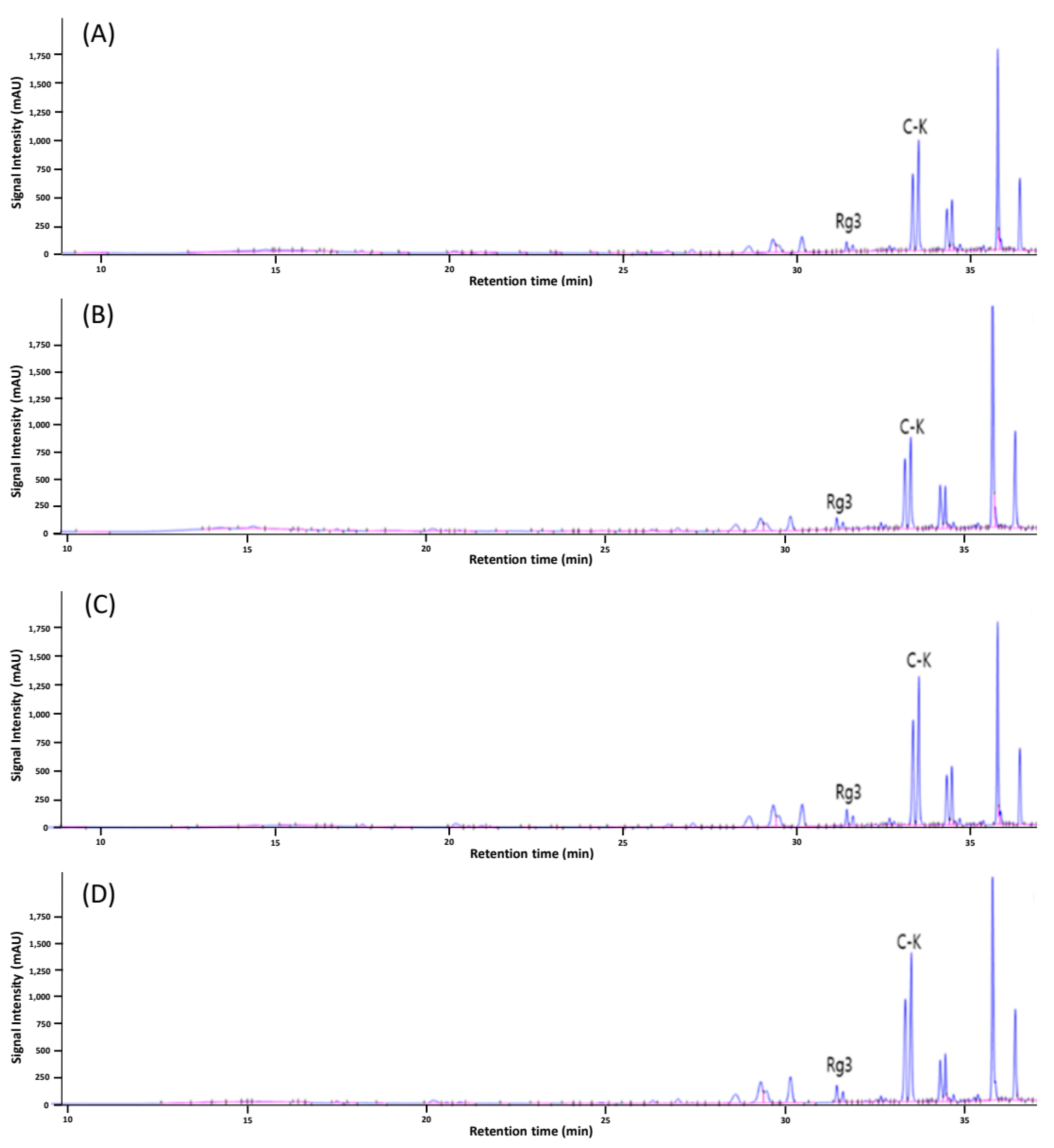

| Rg1 | Re | Rf | Rg2 | Rb1 | Rc | Rb2 | F1 | Rd | F2 | Rg3 | C-K ** | Rh2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 2.31 ± 0.29 B * | 5.31 ± 0.89 B | 2.43 ± 0.35 AB | 0.97 ± 0.07 B | 5.29 ± 0.35 AB | 3.00 ± 0.79 A | 3.28 ± 0.13 A | 0.17 ± 0.01 D | 0.78 ± 0.01 AB | 0.04 ± 0.01 D | 0.13 ± 0.02 F | 0.16 ± 0.02 F | 0.06 ± 0.09 B |
| H-MCPG | 3.75 ± 0.06 A | 6.21 ± 0.26 A | 2.13 ± 0.40 AB | 1.11 ± 0.25 AB | 5.52 ± 0.45 A | 3.45 ± 0.28 A | 3.25 ± 0.10 A | 0.30 ± 0.30 C | 0.70 ± 0.08 C | 0.06 ± 0.02 BCD | 0.20 ± 0.02 E | 0.15 ± 0.01 F | 0.03 ± 0.00 B |
| A-MCPG | 1.49 ± 0.12 D | 3.23 ± 0.35 C | 1.63 ± 0.12 C | 0.66 ± 0.03 C | 4.77 ± 0.70 BC | 2.25 ± 0.31 B | 2.31 ± 0.50 B | 0.52 ± 0.04 A | 0.57 ± 0.02 B | 0.27 ± 0.00 A | 0.33 ± 0.03 D | 0.76 ± 0.06 E | 0.14 ± 0.01 A |
| HA-MCPG | 1.87 ± 0.10 C | 3.52 ± 0.28 C | 1.93 ± 0.17 BC | 1.22 ± 0.12 A | 4.52 ± 0.34 C | 3.21 ± 0.35 A | 1.90 ± 0.10 C | 0.38 ± 0.02 B | 0.85 ± 0.12 A | 0.06 ± 0.01 BCD | 0.36 ± 0.02 D | 0.93 ± 0.03 E | 0.02 ± 0.00 B |
| P-MCPG | nd *** | 0.05 ± 0.00 D | 0.29 ± 0.01 D | 0.58 ± 0.03 C | 0.10 ± 0.02 D | 0.05 ± 0.00 C | 0.09 ± 0.00 D | nd *** | 0.04 ± 0.01 F | 0.05 ± 0.00 CD | 0.70 ± 0.04 C | 6.44 ± 0.33 C | 0.04 ± 0.00 B |
| PH-MCPG | nd *** | nd *** | 0.3 ± 0.02 D | 0.63 ± 0.05 C | 0.05 ± 0.00 D | 0.05 ± 0.00 C | 0.11 ± 0.02 D | nd *** | 0.12 ± 0.02 EF | 0.04 ± 0.00 D | 0.81 ± 0.03 B | 5.84 ± 0.15 D | 0.06 ± 0.01 B |
| PA-MCPG | nd *** | 0.09 ± 0.00 D | 0.37 ± 0.02 D | 0.93 ± 0.03 B | 0.17 ± 0.01 D | 0.11 ± 0.00 C | 0.14 ± 0.01 D | nd *** | 0.13 ± 0.02 E | 0.07 ± 0.00 B | 1.31 ± 0.50 A | 10.25 ± 0.10 B | 0.04 ± 0.00 B |
| PHA-MCPG | nd *** | 0.06 ± 0.01 D | 0.36 ± 0.00 D | 0.95 ± 0.01 B | 0.10 ± 0.02 D | 0.09 ± 0.01 C | 0.11 ± 0.00 D | 0.02 ± 0.00 E | 0.25 ± 0.01 D | 0.06 ± 0.00 BC | 1.36 ± 0.02 A | 10.57 ± 0.25 A | 0.04 ± 0.00 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Shin, J.-S.; Kim, W.; Lee, H.; Baik, M.-Y. Effects of Puffing, Acid, and High Hydrostatic Pressure Treatments on Ginsenoside Profile and Antioxidant Capacity of Mountain-Cultivated Panax ginseng. Foods 2023, 12, 2174. https://doi.org/10.3390/foods12112174
Kim J-H, Shin J-S, Kim W, Lee H, Baik M-Y. Effects of Puffing, Acid, and High Hydrostatic Pressure Treatments on Ginsenoside Profile and Antioxidant Capacity of Mountain-Cultivated Panax ginseng. Foods. 2023; 12(11):2174. https://doi.org/10.3390/foods12112174
Chicago/Turabian StyleKim, Jang-Hwan, Jae-Sung Shin, Wooki Kim, Hyungjae Lee, and Moo-Yeol Baik. 2023. "Effects of Puffing, Acid, and High Hydrostatic Pressure Treatments on Ginsenoside Profile and Antioxidant Capacity of Mountain-Cultivated Panax ginseng" Foods 12, no. 11: 2174. https://doi.org/10.3390/foods12112174
APA StyleKim, J.-H., Shin, J.-S., Kim, W., Lee, H., & Baik, M.-Y. (2023). Effects of Puffing, Acid, and High Hydrostatic Pressure Treatments on Ginsenoside Profile and Antioxidant Capacity of Mountain-Cultivated Panax ginseng. Foods, 12(11), 2174. https://doi.org/10.3390/foods12112174






