Assessment of Aspartame (E951) Occurrence in Selected Foods and Beverages on the German Market 2000–2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Request under the Consumer Information Act
2.2. Data Description and Analysis Methods
2.3. Data Analysis and Selection of Food Items for Inclusion: Regrouping and Prioritizing Foods
2.4. Application of Artificial Intelligence Tools
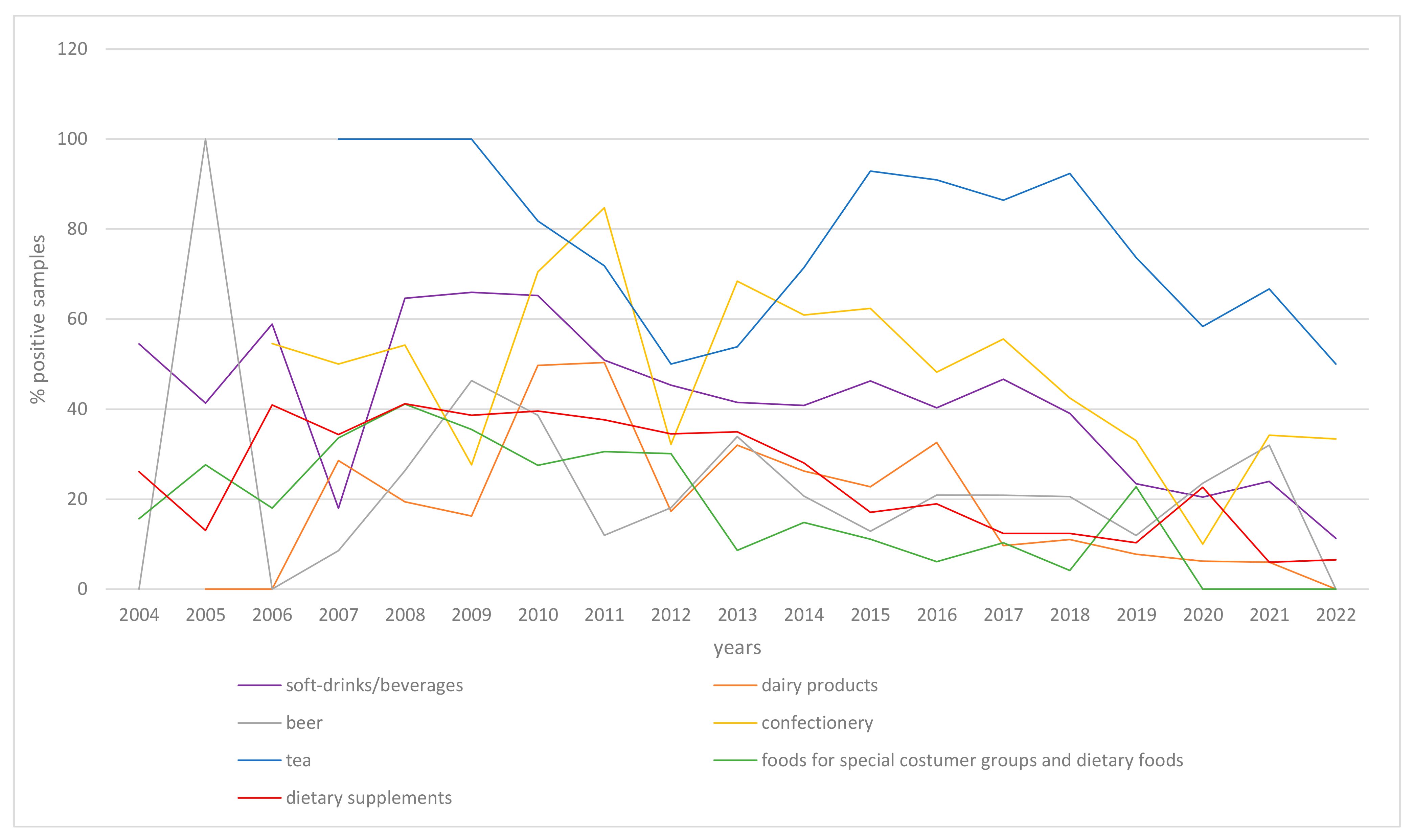
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Product Group | Product Subgroups Contained in the Product Group |
|---|---|
| Flavored milk drinks | Whey with other added food |
| Whey blend products from whey with fruit/fruit preparation | |
| Buttermilk products with fruit preparation | |
| Soft drink with added dairy products | |
| Soft drinks | Apple juice beverage |
| Orange juice beverage | |
| Orange lemonade | |
| Citrus lemonade | |
| Cola lemonade | |
| Bitter Lemon | |
| Tonic-Water | |
| Effervescent cold drink with flavor (raspberry, cherry, woodruff, citrus) | |
| Diet soft drinks | Fruit juice reduced in calorific value |
| Lemonade reduced in calorific value | |
| Cola lemonade reduced in calorific value | |
| Lemonade containing quinine and/or containing bitter substances, reduced in calorific value | |
| Bitter Lemon reduced in calorific value | |
| Effervescent cold drink reduced in calorific value | |
| Energy drink reduced in calorific value | |
| Non-alcoholic drinks for diabetics excl. fruit nectar | |
| Mixed beer drinks | Full beer without wheat beer with clear lemonade (1:1) “Alsterwasser” |
| Beer with sugars | |
| Candies | Hard caramel unfilled |
| Hard caramel unfilled reduced in calorific value | |
| Pressings | |
| Chewing gum | Chewing gum |
| Chewing gum coated | |
| Powdered drink bases | Tea extract with lemon extracts or lemon flavoring |
| Other Tea extracts | |
| Beverage powder with tea extract | |
| Sports foods (with protein and amino acids) | Food for intense muscular effort, especially for athletes |
| Protein concentrate incl. proteins/protein hydrolysates/amino acid mixture | |
| Dietary supplements containing carnitine | |
| Fiber supplements | Fiber concentrates |
References
- WHO. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)—Aspartame. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/62 (accessed on 3 April 2023).
- International Agency for Research on Cancer. Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024; 25–27 March 2019. France: Monographs on the Evaluation of Carcinogenic Risks to Humans. 2019. Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/10/IARCMonographs-AGReport-Priorities_2020-2024.pdf (accessed on 3 April 2023).
- IARC Monographs Priorities Group. Advisory Group recommendations on priorities for the IARC Monographs. Lancet Oncol. 2019, 20, 763–764. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Bertrand, K.A.; Birmann, B.M.; Sampson, L.; Willett, W.C.; Feskanich, D. Consumption of artificial sweetener–and sugar-containing soda and risk of lymphoma and leukemia in men and women. Am. J. Clin. Nutr. 2012, 96, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Wang, F.; Holly, E.A. Sweets, sweetened beverages, and risk of pancreatic cancer in a large popu-lation-based case-control study. Cancer Causes Control 2009, 20, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Andreatta, M.M.; Muñoz, S.E.; Lantieri, M.J.; Eynard, A.R.; Navarro, A. Artificial sweetener consumption and urinary tract tumors in Cordoba, Argentina. Prev. Med. 2008, 47, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Debras, C.; Chazelas, E.; Srour, B.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022, 19, e1003950. [Google Scholar] [CrossRef] [PubMed]
- Soffritti, M.; Padovani, M.; Tibaldi, E.; Falcioni, L.; Manservisi, F.; Belpoggi, F. The carcinogenic effects of aspartame: The urgent need for regulatory re-evaluation. Am. J. Ind. Med. 2014, 57, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Kamenickova, A.; Pecova, M.; Bachleda, P.; Dvorak, Z. Effects of artificial sweeteners on the AhR- and GR-dependent CYP1A1 expression in primary human hepatocytes and human cancer cells. Toxicol. In Vitro 2013, 27, 2283–2288. [Google Scholar] [CrossRef]
- Saunders, F.J.; Pautsch, W.F.; Nutting, E.F. The biological properties of aspartame. III. Examination for endocrine-like activities. J. Environ. Pathol. Toxicol. 1980, 3, 363–373. [Google Scholar] [PubMed]
- Çadirci, K.; Özdemir Tozlu, Ö.; Türkez, H.; Mardinoğlu, A. The in vitro cytotoxic, genotoxic, and oxidative damage potentials of the oral artificial sweetener aspartame on cultured human blood cells. Turk. J. Med. Sci. 2020, 50, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius Commission. Request for Information and Comments on the Priority List of Substances Proposed for Evaluation by JECFA. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/fr/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FCircular%252520Letters%252FCL%2525202021-81%252Fcl21_81e.pdf (accessed on 3 April 2023).
- International Agency for Research on Cancer. IARC Monographs—Volume 134—Call for Data. Available online: https://monographs.iarc.who.int/iarc-monographs-volume-134-call-for-data/ (accessed on 3 April 2023).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Food Additives List of Substances Scheduled for Evaluation and Request for Data. Available online: https://cdn.who.int/media/docs/default-source/food-safety/jecfa/call-for-data/jecfa96-call-for-data.pdf?sfvrsn=41389585_3 (accessed on 3 April 2023).
- International Organization for Standardization. ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization (ISO): Geneva, Switzerland, 2017. Available online: https://www.iso.org/standard/66912.html (accessed on 3 April 2023).
- Roth, M.; Hartmann, S.; Renner, R.; Hörtig, W. Risikoorientiertes Probenmanagement in Baden-Württemberg. Deut. Lebensm. Rundsch. 2007, 103, 45–52. [Google Scholar] [CrossRef]
- Open Knowledge Foundation Deutschland, e.V. Aspartam. Available online: https://fragdenstaat.de/anfrage/aspartam/ (accessed on 28 March 2023).
- Verbraucherinformationsgesetz in der Fassung der Bekanntmachung vom 17. Oktober 2012 (BGBl. I S. 2166, 2725), das Zuletzt durch Artikel 8 des Gesetzes vom 27. Juli 2021 (BGBl. I S. 3146) Geändert Worden ist. Available online: https://www.gesetze-im-internet.de/vig/BJNR255810007.html (accessed on 28 March 2023).
- Lebensmittel-und Futtermittelgesetzbuch in der Fassung der Bekanntmachung vom 15. September 2021 (BGBl. I S. 4253; 2022 I S. 28), das Zuletzt durch Artikel 2 Absatz 6 des Gesetzes vom 20. Dezember 2022 (BGBl. I S. 2752) Geändert Worden ist. Available online: https://www.gesetze-im-internet.de/lfgb/BJNR261810005.html (accessed on 3 April 2023).
- DIN EN 12856:1999-07; Lebensmittel—Bestimmung von Acesulfam-K, Aspartam und Saccharin—Hochleistungs-flüssigchromatographisches Verfahren; Deutsche Fassung EN_12856:1999. Beuth Verlag GmbH: Berlin, Germany, 1999. [CrossRef]
- Maes, P.; Monakhova, Y.B.; Kuballa, T.; Reusch, H.; Lachenmeier, D.W. Qualitative and quantitative control of carbonated cola beverages using ¹H NMR spectroscopy. J. Agric. Food Chem. 2012, 60, 2778–2784. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Ioannidou, S.; European Food Safety Authority. FoodEx2 maintenance 2020. EFSA Supp. Publ. 2021, 18, 6507E. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union. 2008, 354, 16–33. [Google Scholar]
- Bundesinstitut für Risikobewertung. Süßungsmittel: Mehrheit der Studien bestätigt Keine Gesundheitsbeeinträchtigung—Allerdings ist die Studienlage Unzureichend: Stellungnahme Nr. 004/2023 des BfR vom 07. Februar 2023 (Bewertungsstand 23. September 2019); Bundesinstitut für Risikobewertung: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food. Scientific Opinion on the re-evaluation of aspartame (E 951) as a food additive. EFSA J. 2013, 11, 3496. [Google Scholar] [CrossRef]
- Bär, A.; Biermann, C. Intake of intense sweeteners in Germany. Z. Ernahrungswiss. 1992, 31, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Brandt, P. Bundesweiter Überwachungsplan. In Berichte zur Lebensmittelsicherheit 2006; BVL-Reporte, Volume 2,3; Birkhäuser: Basel, Switzerland, 2008. [Google Scholar] [CrossRef]
- Hartmann, F. Zusatzstoffe in Getränken. In Berichte zur Lebensmittelsicherheit 2007; BVL-Reporte, Volume 3,3; Birkhäuser: Basel, Switzerland, 2007. [Google Scholar] [CrossRef]
- Hanewinkel-Meshkini, S. Süßstoffe in Süßwaren ohne Zuckerzusatz. In Berichte zur Lebensmittelsicherheit 2008; Brandt, P., Ed.; BVL-Reporte, Volume 4,5; Birkhäuser: Basel, Switzerland, 2009. [Google Scholar] [CrossRef]
- BVL. Monitoring 2015. Available online: https://www.bvl.bund.de/SharedDocs/Downloads/01_Lebensmittel/01_lm_mon_dokumente/01_Monitoring_Berichte/2015_lm_monitoring_bericht.pdf?__blob=publicationFile&v=6 (accessed on 3 April 2023).
- Van Vliet, K.; Melis, E.S.; de Blaauw, P.; van Dam, E.; Maatman, R.G.H.J.; Abeln, D.; van Spronsen, F.J.; Heiner-Fokkema, M.R. Aspartame and Phe-containing degradation products in soft drinks across Europe. Nutrients 2020, 12, 1887. [Google Scholar] [CrossRef]
- Bundesinstitut für Risikobewertung. Alternativen zu Zucker: Wie viel Süßungsmittel Steckt in Erfrischungsgetränken? Stellungnahme Nr. 006/2023 des BfR vom 07. Februar 2023; Bundesinstitut für Risikobewertung: Berlin, Germany, 2023. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monographs Preamble. Available online: https://monographs.iarc.who.int/wp-content/uploads/2019/07/Preamble-2019.pdf (accessed on 3 April 2023).
| Product Group | Total Number of Samples | Frequency of Aspartame-Positive Samples [%] |
|---|---|---|
| Diet soft drinks | 2783 | 72.7 |
| Soft drinks | 1167 | 49.2 |
| Candies | 603 | 56.2 |
| Chewing gum | 312 | 77.2 |
| Sports foods (with protein and amino acids) | 297 | 42.1 |
| Flavored milk drinks | 268 | 78.0 |
| Powdered drink bases | 195 | 83.6 |
| Mixed beer drinks | 58 | 69.0 |
| Fiber supplements | 20 | 55.0 |
| Product Group | Number of Quantifiable Samples | Unit | Mean | Median | Maximum | Standard Deviation | 95th Percentile | EU Maximum Level [23] |
|---|---|---|---|---|---|---|---|---|
| Chewing gum | 241 | mg/kg | 1543 | 1369 | 4617 | 1042 | 3649 | 2500 d 5500 e |
| Sports foods (with protein and amino acids) | 125 | mg/kg | 1453 | 1030 | 6615 | 1461 | 5002 | 2000 f 5000 f,g |
| Fiber supplements | 11 | mg/kg | 1248 | 1276 | 1469 | 175 | 1460 | 2000 f 5000 f,g |
| Powdered drink bases a | 162 | mg/kg | 1068 | 1133 | 4861 | 672 | 1600 | 600 h |
| Candies | 339 | mg/kg | 473 | 440 | 3096 | 332 | 890 | 2000 i 6000 j |
| Diet soft drinks | 2021 | mg/L | 91 | 60 | 970 | 101 | 335 | 600 |
| Soft drinks b | 574 | mg/L | 59 | 34 | 531 | 74 | 203 | 600 |
| Flavored milk drinks c | 207 | mg/kg | 48 | 47 | 90 | 17 | 79 | 1000 |
| Mixed beer drinks | 40 | mg/L | 24 | 26 | 55 | 15 | 42 | 600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schorb, S.; Gleiss, K.; Wedekind, R.; Suonio, E.; Kull, A.-K.; Kuntz, M.; Walch, S.G.; Lachenmeier, D.W. Assessment of Aspartame (E951) Occurrence in Selected Foods and Beverages on the German Market 2000–2022. Foods 2023, 12, 2156. https://doi.org/10.3390/foods12112156
Schorb S, Gleiss K, Wedekind R, Suonio E, Kull A-K, Kuntz M, Walch SG, Lachenmeier DW. Assessment of Aspartame (E951) Occurrence in Selected Foods and Beverages on the German Market 2000–2022. Foods. 2023; 12(11):2156. https://doi.org/10.3390/foods12112156
Chicago/Turabian StyleSchorb, Sydney, Katharina Gleiss, Roland Wedekind, Eero Suonio, Ann-Kathrin Kull, Marcel Kuntz, Stephan G. Walch, and Dirk W. Lachenmeier. 2023. "Assessment of Aspartame (E951) Occurrence in Selected Foods and Beverages on the German Market 2000–2022" Foods 12, no. 11: 2156. https://doi.org/10.3390/foods12112156
APA StyleSchorb, S., Gleiss, K., Wedekind, R., Suonio, E., Kull, A.-K., Kuntz, M., Walch, S. G., & Lachenmeier, D. W. (2023). Assessment of Aspartame (E951) Occurrence in Selected Foods and Beverages on the German Market 2000–2022. Foods, 12(11), 2156. https://doi.org/10.3390/foods12112156






