Changes in Phytochemical Content, Antioxidant Activity, and Anti-Inflammatory Properties of Cudrania tricuspidata Fruits Treated by Roasting
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Samples and Heat Treatment
2.2. Measurement of Color
2.3. Preparation of Roasted Fruit Extract
2.4. Analysis of DPPH Antioxidant Activity
2.5. Analysis of Total Polyphenol and Flavonoid Content
2.6. MTT Assay
2.7. Levels of ROS
2.8. Determination of NO Levels
2.9. β-Hexosaminidase Analysis
2.10. Phytochemicals Extraction and Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Roasting on the Color of C. tricuspidata Fruits
3.2. Effect of Roasting Conditions on the DPPH Antioxidant Activity
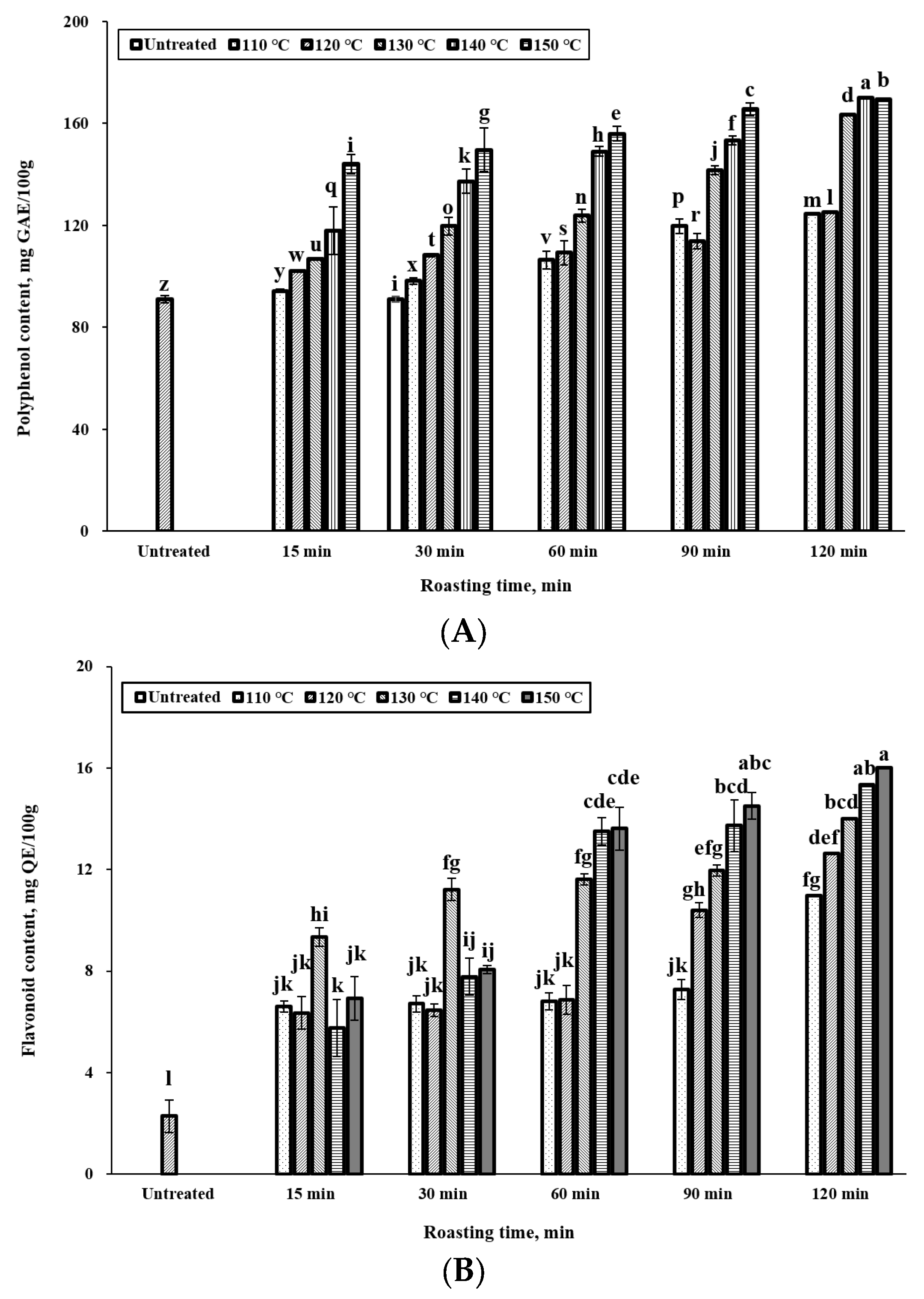
3.3. Effect of Roasting Conditions on Polyphenol and Flavonoid Content
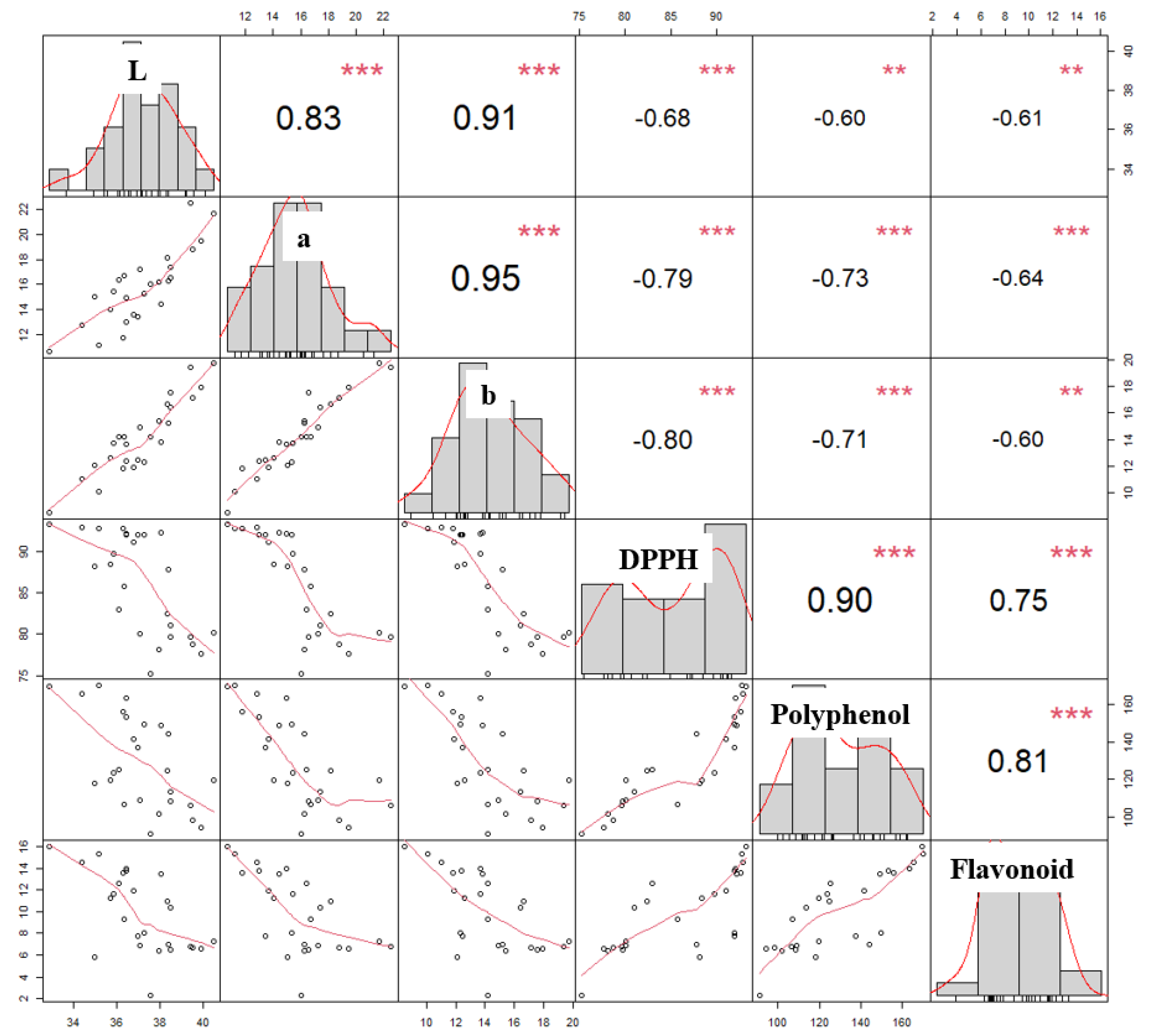
3.4. Relationships Amongst Color, DPPH, Polyphenol Content, and Flavonoid Content of Roasted C. tricuspidata Fruit
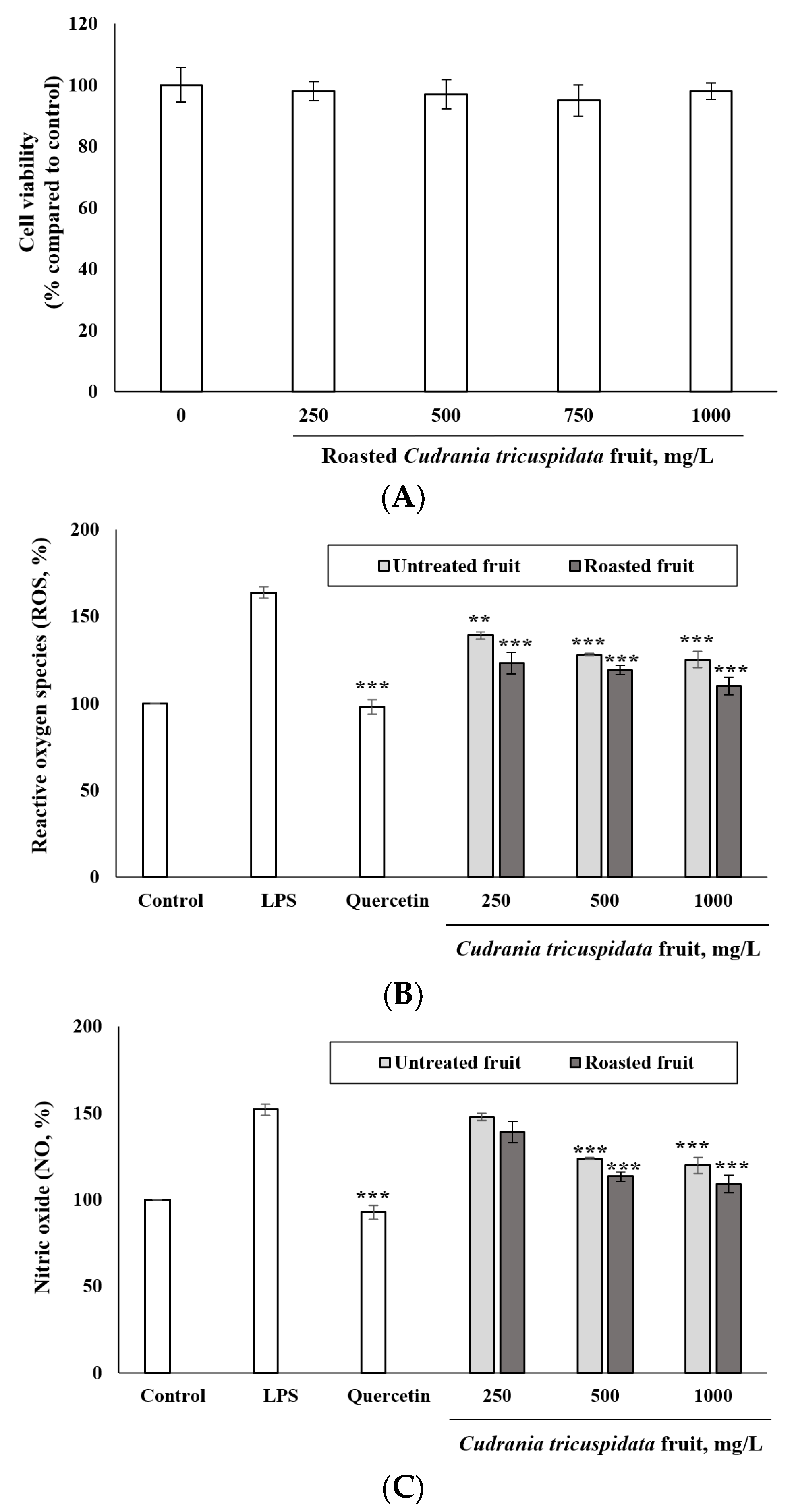
3.5. Inhibitory Effect of Roasted C. tricuspidata Fruits on ROS and NO Production
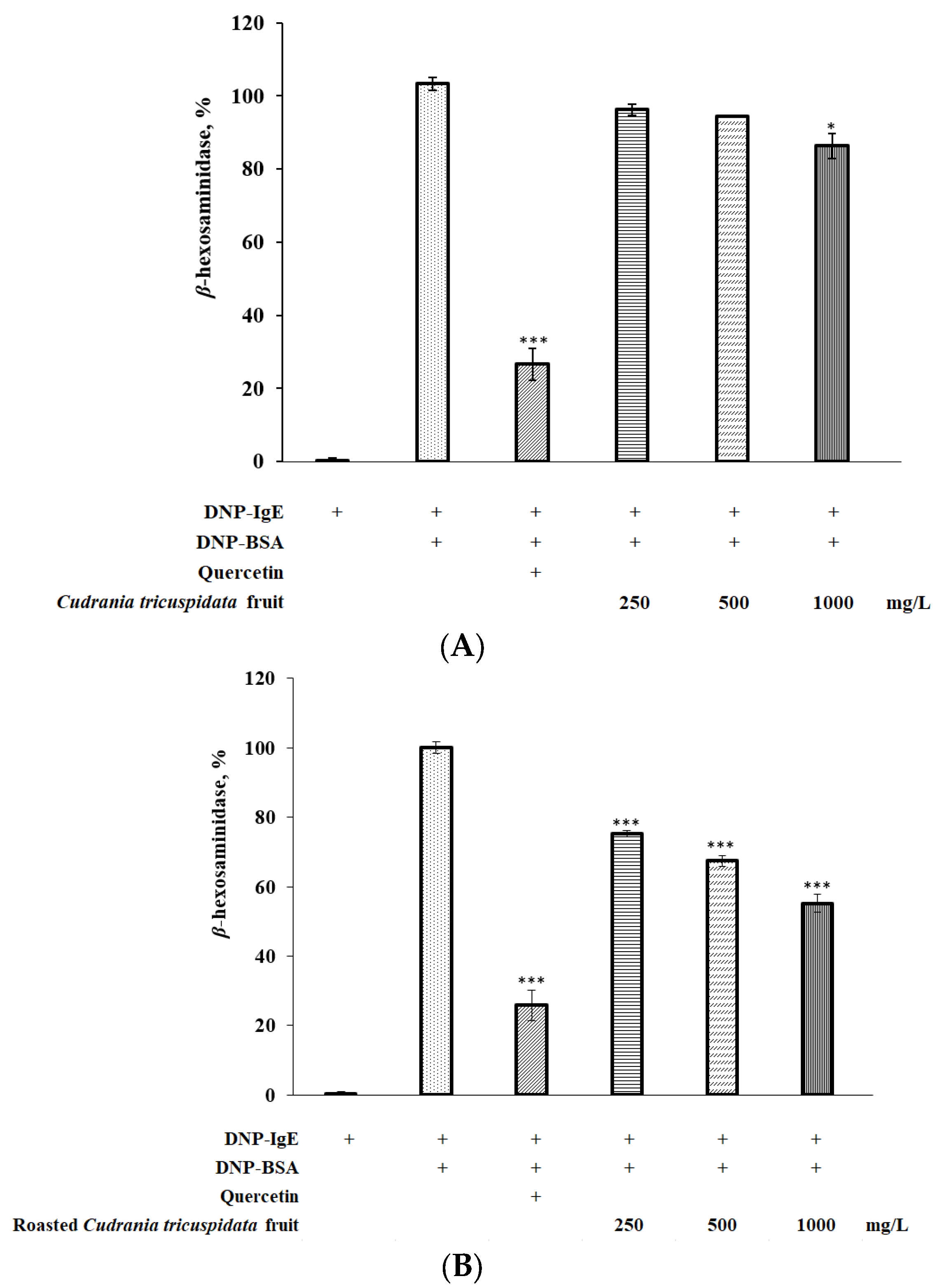
3.6. Effect of Roasted C. tricuspidata Fruit on β-Hexosaminidase in RBL-2H3 Cells
3.7. Changes in Phytochemical Content of Fruits Caused by Roasting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parke, D.V.; Lewis, D.F.V. Safety aspects of food preservatives. Food Addit. Contam. 1992, 9, 561–577. [Google Scholar] [CrossRef]
- Novais, C.; Molina, A.K.; Abreu, R.M.; Santo-Buelga, C.; Ferreira, I.C.; Pereira, C.; Barros, L. Natural food colorants and preservatives: A review, a demand, and a challenge. J. Agric. Food Chem. 2022, 70, 2789–2805. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Xin, L.T.; Yue, S.J.; Fan, Y.C.; Wu, J.S.; Yan, D.; Guan, H.S.; Wang, C.Y. Cudrania tricuspidata: An updated review on ethnomedicine, phytochemistry and pharmacology. RSC Adv. 2017, 7, 31807–31832. [Google Scholar] [CrossRef]
- Shin, J.H.; Rhee, M.H.; Kwon, H.W. Antiplatelet effect of cudraxanthone L isolated from Cudrania tricuspidata via inhibition of phosphoproteins. Nat. Prod. Sci. 2020, 26, 295–302. [Google Scholar]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Jin, Q.; Yang, J.; Ma, L.; Cai, J.; Li, J. Comparison of polyphenol profile and inhibitory activities against oxidation and α-glucosidase in mulberry (Genus Morus) cultivars from China. J. Food Sci. 2015, 80, C2440–C2451. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Dong, Q.; Ding, K. Structure elucidation and immunomodulatory activity in vitro of a xylan from roots of Cudrania tricuspidata. Food Chem. 2014, 152, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Ling, B.; Tang, J.; Kong, F.; Mitcham, E.J.; Wang, S. Kinetics of food quality changes during thermal processing: A review. Food Bioprocess Technol. 2015, 8, 343–358. [Google Scholar] [CrossRef]
- Pellegrini, G.; Annosi, M.C.; Contò, F.; Fiore, M. What are the conflicting tensions in an Italian cooperative and how do members manage them? Business goals’, integrated management, and reduction of waste within a fruit and vegetables supply chain. Sustainability 2020, 12, 3050. [Google Scholar] [CrossRef]
- Serafini, M.; Miglio, C.; Peluso, I.; Petrosino, T. Modulation of plasma non enzimatic antioxidant capacity (NEAC) by plant foods: The role of polyphenol. Curr. Top. Med. Chem. 2011, 11, 1821–1846. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Massini, R.; Nicoli, M.; Cassarà, A.; Lerici, C. Study on physical and physico-chemical changes of coffee beans during roasting. note 1. Ital. J. Food Sci. 1990, 2, 123–130. [Google Scholar]
- Bolek, S.; Ozdemir, M. Optimization of roasting conditions of Pistacia terebinthus in a fluidized bed roaster. LWT Food Sci. Technol. 2017, 80, 67–75. [Google Scholar] [CrossRef]
- Uysal, N.; Sumnu, G.; Sahin, S. Optimization of microwave–infrared roasting of hazelnut. J. Food Eng. 2009, 90, 255–261. [Google Scholar] [CrossRef]
- Pittia, P.; Dalla Rosa, M.; Lerici, C. Textural changes of coffee beans as affected by roasting conditions. LWT Food Sci. Technol. 2001, 34, 168–175. [Google Scholar] [CrossRef]
- Özdemir, M.; Devres, O. Analysis of color development during roasting of hazelnuts using response surface methodology. J. Food Eng. 2000, 45, 17–24. [Google Scholar] [CrossRef]
- Wellner, A.; Huettl, C.; Henle, T. Formation of Maillard reaction products during heat treatment of carrots. J. Agric. Food Chem. 2011, 59, 7992–7998. [Google Scholar] [CrossRef]
- Coghe, S.; Gheeraert, B.; Michiels, A.; Delvaux, F.R. Development of Maillard reaction related characteristics during malt roasting. J. Inst. Brew. 2006, 112, 148–156. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Sabu, M.C.; Pak-Dek, M.S. Phenolic compounds and antioxidant activity of peanut’s skin, hull, raw kernel and roasted kernel flour. Pak. J. Bot. 2011, 43, 1635–1642. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Walker, L.T.; Ogutu, S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). Lebensm.-Wiss. Technol. 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Iqbal, S. Effect of different cooking methods on the antioxidant activity of some vegetables from Pakistan. Int. J. Food Sci. Technol. 2008, 43, 560–567. [Google Scholar] [CrossRef]
- Bastola, K.P.; Guragain, Y.N.; Bhadriraju, V.; Vadlani, P.V. Evaluation of standards and interfering compounds in the determination of phenolics by Folin-Ciocalteu assay method for effective bioprocessing of biomass. Am. J. Anal. Chem. 2017, 8, 416–431. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Park, M.; Lee, K.G. Effect of roasting temperature and time on volatile compounds, total polyphenols, total flavonoids, and lignan of omija (Schisandra chinensis Baillon) fruit extract. Food Chem. 2021, 338, 127836. [Google Scholar] [CrossRef]
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, M.A. Antioxidant activities of Sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, A.; de Wit, M.; Osthoff, G.; Hugo, A. Relationship and correlation between antioxidant content and capacity, processing method and fruit colour of cactus pear fruit. Food Bioprocess Technol. 2018, 11, 1527–1535. [Google Scholar] [CrossRef]
- Kavitha, C.; Kuna, A. Effect of processing on antioxidant properties of ber (Zizyphus mauritiana) Fruit. Int. J. Sci. Res. 2014, 3, 2019–2025. [Google Scholar]
- Goh, S.G.; Noranizan, M.; Leong, C.M.; Sew, C.C.; Sobhi, B. Effect of thermal and ultraviolet treatments on the stability of antioxidant compounds in single strength pineapple juice throughout refrigerated storage. Int. Food Res. J. 2012, 19, 1131–1136. [Google Scholar]
- Sharma, K. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. J. Food Drug Anal. 2015, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zainol, M.K.; Abdul-Hamid, A.; Abu Bakar, F.; Pak Dek, S. Effect of different drying methods on the degradation of selected flavonoids in Centella asiatica. Int. Food Res. J. 2009, 16, 531–537. [Google Scholar]
- Price, K.R.; Bacon, J.R.; Rhodes, M.J.C. Effect of storage and domestic processing on the content and composition of flavonol glucosides in onion (Allium cepa). J. Agric. Food Chem. 1997, 45, 938–942. [Google Scholar] [CrossRef]
- Karou, D.; Mamoudou, H.D.; Simpore, J.; Traore, A.S. Antioxidant and antibacterial activities of polyphenols from ethnomedicinal plants of Burkina Faso. Afr. J. Biotechnol. 2005, 4, 823–828. [Google Scholar]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compost. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Roy, M.K.; Koide, M.; Rao, T.P.; Okubo, T.; Ogasawara, Y.; Juneja, L.R. ORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: Relationship between total polyphenol and individual catechin content. Int. J. Food Sci. Nutr. 2010, 61, 109–124. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W.; Waniska, R.D.; Rooney, W.L. Phenolic compounds and antioxidant activity of sorghum grains of varying genotypes. J. Agric. Food Chem. 2005, 53, 6813–6818. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef] [PubMed]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent. Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Zuidema, M.Y.; Hannink, M.; Zhang, C. Effects of interventions on oxidative stress and inflammation of cardiovascular diseases. World J. Cardiol. 2011, 3, 18–24. [Google Scholar] [CrossRef]
- Nagmoti, D.M.; Khatri, D.K.; Juvekar, P.R.; Juvekar, A.R. Antioxidant activity free radical-scavenging potential of Pithecellobium dulce Benth seed extracts. Free Radic. Res. 2012, 2, 37–43. [Google Scholar] [CrossRef]
- Konopka, I.; Tańska, M.; Faron, A.; Czaplicki, S. Release of free ferulic acid and changes in antioxidant properties during the wheat and rye bread making process. Food Sci. Biotechnol. 2014, 23, 831–840. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, G.W.; Oh, H.; Yoo, S.Y.; Kim, Y.O.; Oh, M.S. Influence of roasting on the antioxidant activity of small black soybean (Glycine max L. Merrill). Lebensm.-Wiss. Technol. 2011, 44, 992–998. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, I.; Kobayashi, M.; Hamada, T.; Tsuda, K.; Goto, H.; Imaizumi, K.; Nozawa, A.; Sugimoto, A.; Kakuda, T. Heat-epimerized tea catechins rich in gallocatechin gallate and catechin gallate are more effective to inhibit cholesterol absorption than tea catechins rich in epigallocatechin gallate and epicatechin gallate. J. Agric. Food Chem. 2003, 51, 7303–7307. [Google Scholar] [CrossRef] [PubMed]
- Ow, Y.Y.; Stupans, I. Gallic acid and gallic acid derivatives: Effects on drug metabolizing enzymes. Curr. Drug Metab. 2003, 4, 241–248. [Google Scholar] [CrossRef]
- Jeong, C.H.; Choi, G.N.; Kim, J.H.; Kwak, J.H.; Heo, H.J.; Shim, K.H.; Cho, B.R.; Bae, Y.I.; Choi, J.S. In vitro antioxidative activities and phenolic composition of hot water extract from different parts of Cudrania tricuspidata. Prev. Nutr. Food Sci. 2009, 14, 283–289. [Google Scholar] [CrossRef]

| Temp., °C | Time, min | ||||
|---|---|---|---|---|---|
| 15 | 30 | 60 | 90 | 120 | |
| 110 |  |  |  |  |  |
| 120 |  |  |  |  |  |
| 130 |  |  |  |  |  |
| 140 |  |  |  |  |  |
| 150 |  |  |  |  |  |
| T (°C) | T (min) | DPPH Antioxidant Activity |
|---|---|---|
| Untreated | - | 75.29 ± 0.28 e (1) |
| 110 | 15 | 77.66 ± 0.64 d |
| 30 | 78.73 ± 1.18 ed | |
| 60 | 79.68 ± 0.55 c | |
| 90 | 80.14 ± 0.41 c | |
| 120 | 82.42 ± 1.54 b | |
| 120 | 15 | 78.17 ± 1.56 d |
| 30 | 79.74 ± 1.17 cd | |
| 60 | 80.05 ± 0.87 cd | |
| 90 | 81.02 ± 0.39 c | |
| 120 | 82.97 ± 1.67 b | |
| 130 | 15 | 85.72 ± 5.92 c |
| 30 | 88.40 ± 4.74 bc | |
| 60 | 89.72 ± 3.45 bc | |
| 90 | 91.04 ± 1.56 bc | |
| 120 | 92.11 ± 1.12 b | |
| 140 | 15 | 88.17 ± 6.00 c |
| 30 | 91.97 ± 0.45 bc | |
| 60 | 92.21 ± 0.62 bc | |
| 90 | 91.92 ± 1.43 bc | |
| 120 | 92.73 ± 0.39 b | |
| 150 | 15 | 87.82 ± 6.35 c |
| 30 | 91.94 ± 0.55 bc | |
| 60 | 92.67 ± 0.73 b | |
| 90 | 92.83 ± 0.70 b | |
| 120 | 93.22 ± 0.41 b | |
| Ascorbic acid | - | 98.18 ± 0.06 a |
| Phytochemical Content, μg/g | Unroasted | Roasted (1) | Class |
|---|---|---|---|
| Epigallocatechin | 400.1 ± 3.2 (2) | 568.2 ± 2.5 | Flavan-3-ol derivatives |
| Catechin | 311.8 ± 3.8 | 452.6 ± 3.5 | Flavan-3-ol derivatives |
| Epicatechin | 110.7 ± 2.5 | 284.3 ± 3.0 | Flavan-3-ol derivatives |
| Gallic acid | 66.5 ± 1.5 | 97.1 ± 3.4 | Phenolic acid derivatives |
| Tannic acid | 27.8 ± 0.7 | 46.6 ± 2.6 | Phenolic acid derivatives |
| Vanillic acid | 68.4 ± 1.2 | 95.2 ± 1.5 | Phenolic acid derivatives |
| Caffeic acid | 64.1 ± 0.1 | 92.2 ± 1.7 | Phenolic acid derivatives |
| p-Coumaric acid | 44.9 ± 1.7 | 58.2 ± 0.1 | Phenolic acid derivatives |
| Ferulic acid | 38.9 ± 2.6 | 43.5 ± 0.2 | Phenolic acid derivatives |
| Rutin | 47.5 ± 0.2 | 64.7 ± 1.2 | Flavanol derivatives |
| Quercetin | 20.1 ± 0.0 | 31.9 ± 0.1 | Flavanol derivatives |
| Kaempferol | 13.9 ± 0.1 | 2.4 ± 0.0 | Flavanol derivatives |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, S.Y.; Jung, J.Y.; Yang, J.-K. Changes in Phytochemical Content, Antioxidant Activity, and Anti-Inflammatory Properties of Cudrania tricuspidata Fruits Treated by Roasting. Foods 2023, 12, 2146. https://doi.org/10.3390/foods12112146
Ha SY, Jung JY, Yang J-K. Changes in Phytochemical Content, Antioxidant Activity, and Anti-Inflammatory Properties of Cudrania tricuspidata Fruits Treated by Roasting. Foods. 2023; 12(11):2146. https://doi.org/10.3390/foods12112146
Chicago/Turabian StyleHa, Si Young, Ji Young Jung, and Jae-Kyung Yang. 2023. "Changes in Phytochemical Content, Antioxidant Activity, and Anti-Inflammatory Properties of Cudrania tricuspidata Fruits Treated by Roasting" Foods 12, no. 11: 2146. https://doi.org/10.3390/foods12112146
APA StyleHa, S. Y., Jung, J. Y., & Yang, J.-K. (2023). Changes in Phytochemical Content, Antioxidant Activity, and Anti-Inflammatory Properties of Cudrania tricuspidata Fruits Treated by Roasting. Foods, 12(11), 2146. https://doi.org/10.3390/foods12112146






