Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Animal Experimental Design
2.3. Observation Index and Skin Damage Scores
2.4. Histological Analysis of Skin Tissue
2.5. Determination of Skin Lipids
2.6. Assessment of Antioxidant Activities in Skin Tissue
2.7. Cytokine Measurements Using ELISA Assay
2.8. Quantitative RT-PCR Analysis
2.9. Immunohistochemical Analysis
2.10. Statistical Analysis
3. Results
3.1. Determination of Naringenin and Apigenin Based on HPLC-MS/MS
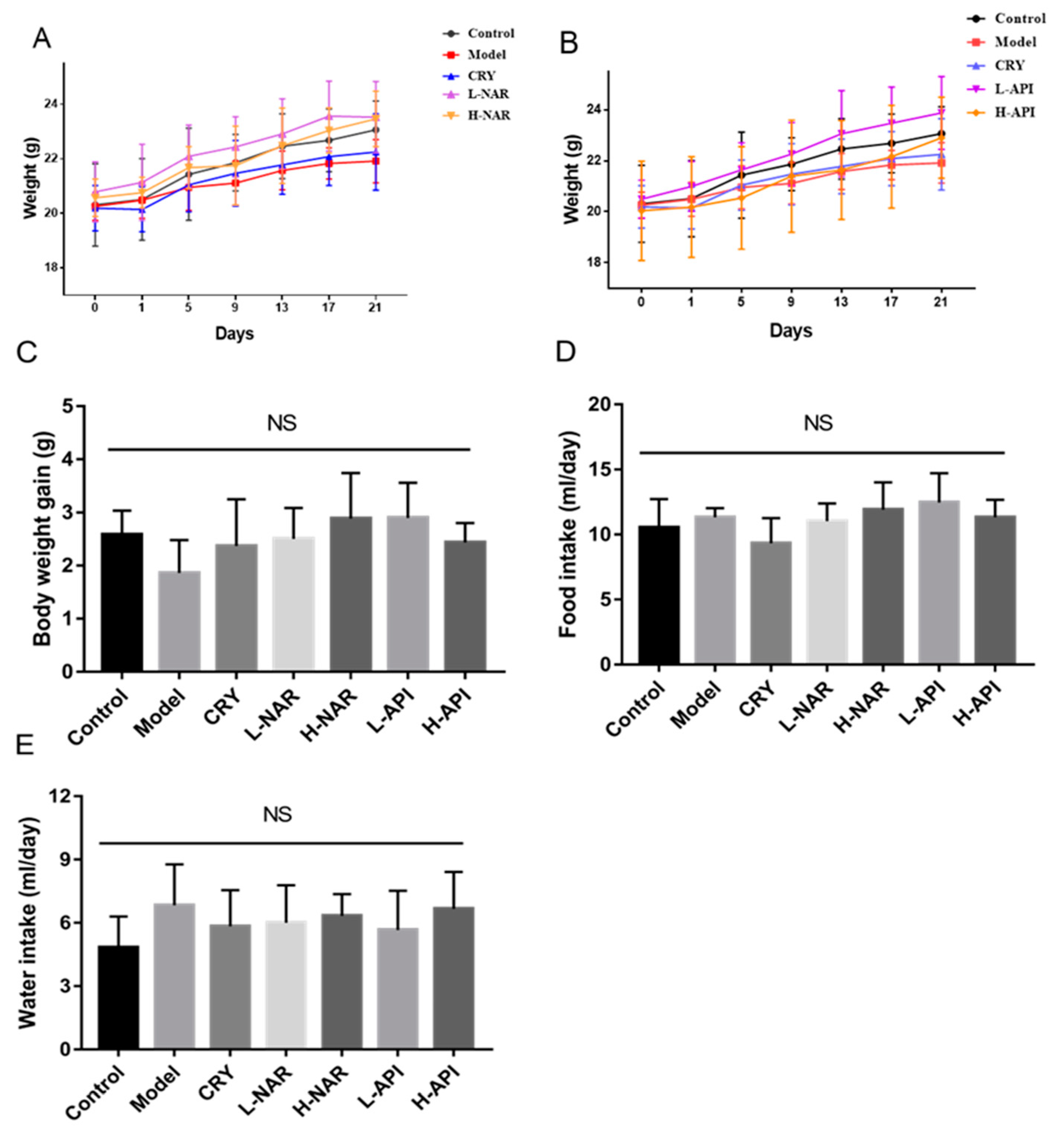
3.2. Effects of Naringenin and Apigenin on Body Weight and Food and Water Intake of Mice
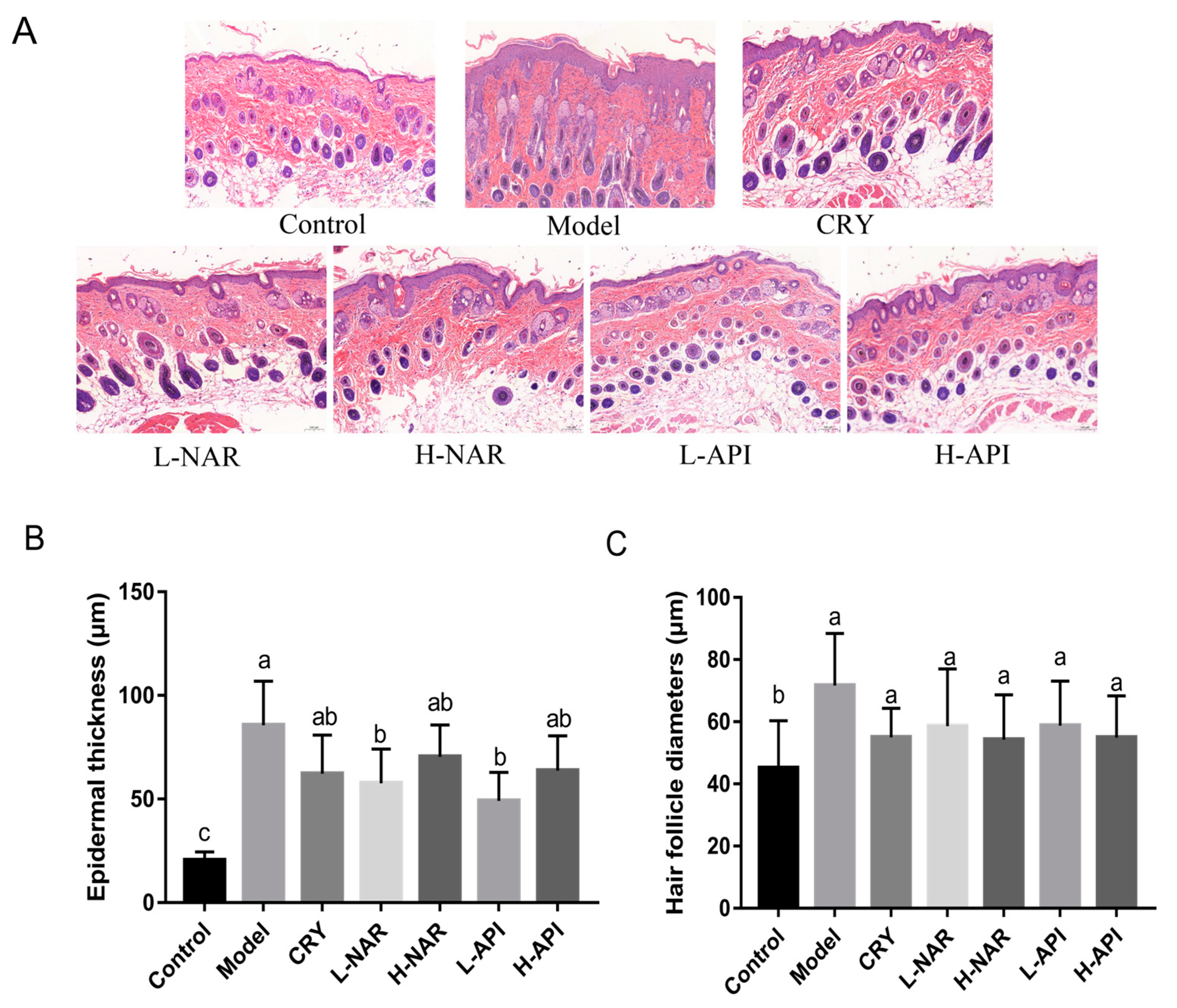
3.3. Pathological Morphology of Skin Tissues in Mice
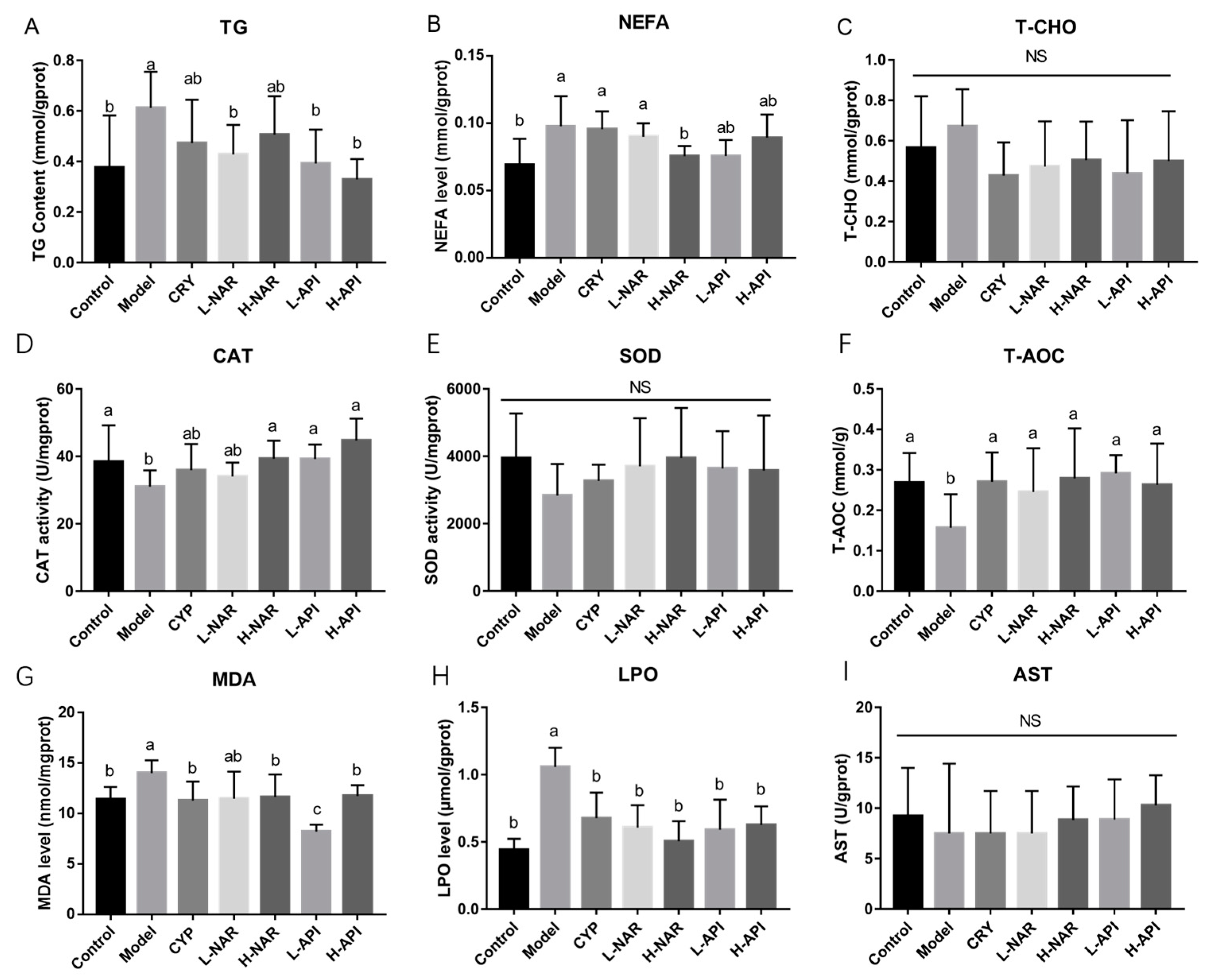
3.4. Decreased Skin Sebum Levels with Pretreatment with Naringenin and Apigenin
3.5. Improved Antioxidative System after Naringenin and Apigenin Supplementation
3.6. Prevention of Inflammatory Response with Naringenin and Apigenin Pretreatments
3.7. Effects of Naringenin and Apigenin on mRNA Levels Related to Antioxidation and Inflammation
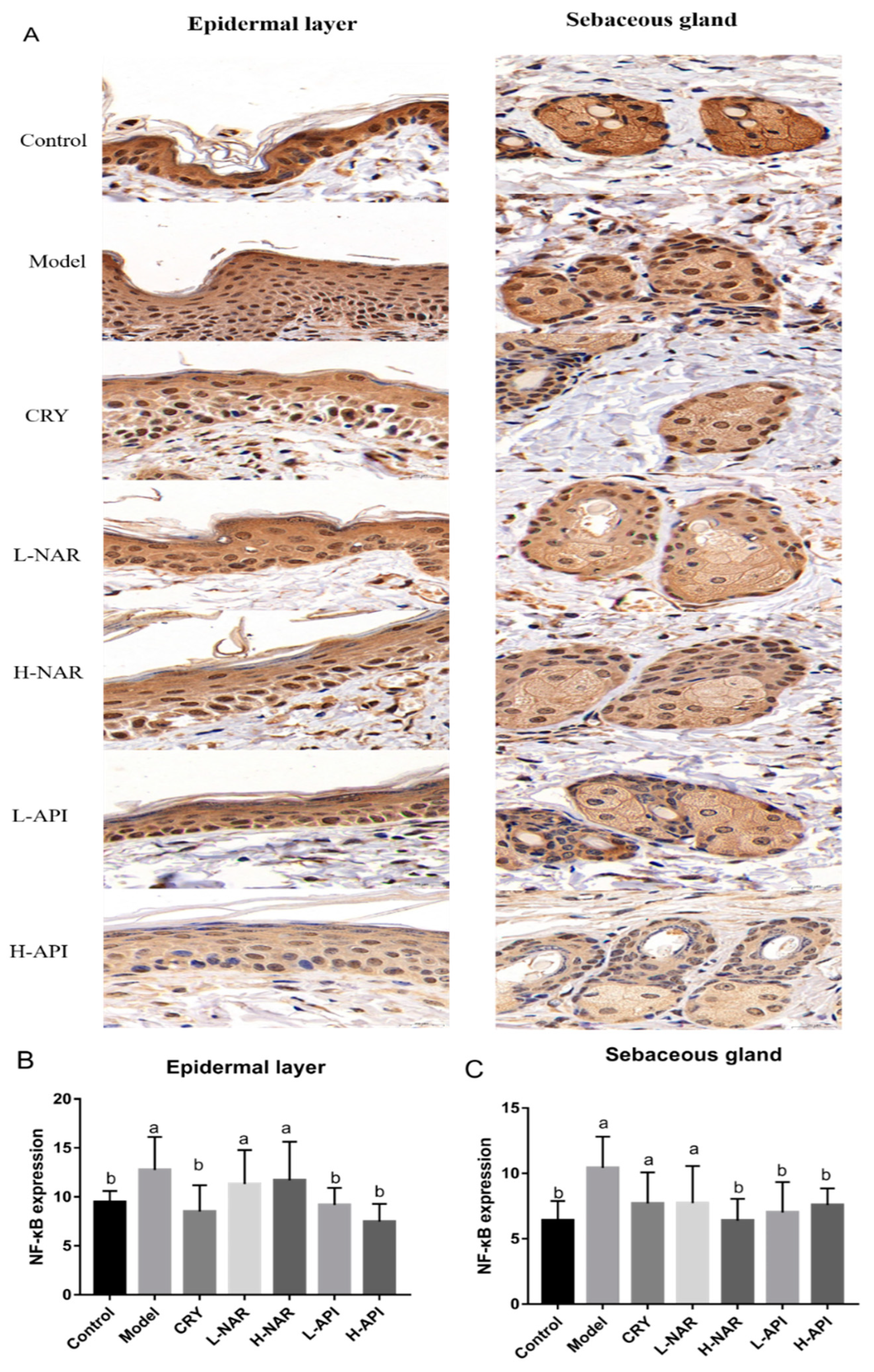
3.8. Effects of Naringenin and Apigenin on NF-κB Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, C.L.; Li, X.F. A Review of Oenanthe javanica (Blume) DC. as Traditional Medicinal Plant and Its Therapeutic Potential. Evid.-Based Complement. Altern. Med. 2019, 2019, 6495819. [Google Scholar] [CrossRef]
- Proteggente, A.R.; Saija, A.; De Pasquale, A.; Rice-Evans, C.A. The compositional characterisation and antioxidant activity of fresh juices from sicilian sweet orange (Citrus sinensis L. Osbeck) varieties. Free Radic. Res. 2003, 37, 681–687. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.S.; Lee, I.H.; Hong, J.T. Therapeutic potency of fermented field water-dropwort (Oenanthe javanica (Blume) DC.) in ethanol-induced liver injury. RSC Adv. 2020, 10, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef] [PubMed]
- Al-Roujayee, A.S. Naringenin improves the healing process of thermally-induced skin damage in rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Caviglione, C.V.; Vignoli, J.A.; Barbosa, D.S.; Baracat, M.M.; Georgetti, S.R.; Verri, W.A., Jr.; Casagrande, R. Naringenin Inhibits UVB Irradiation-Induced Inflammation and Oxidative Stress in the Skin of Hairless Mice. J. Nat. Prod. 2015, 78, 1647–1655. [Google Scholar] [CrossRef]
- Martinez, R.M.; Pinho-Ribeiro, F.A.; Steffen, V.S.; Silva, T.C.; Caviglione, C.V.; Bottura, C.; Fonseca, M.J.; Vicentini, F.T.; Vignoli, J.A.; Baracat, M.M.; et al. Topical Formulation Containing Naringenin: Efficacy against Ultraviolet B Irradiation-Induced Skin Inflammation and Oxidative Stress in Mice. PLoS ONE 2016, 11, e0146296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Bhan Tiku, A. Naringenin Suppresses Chemically Induced Skin Cancer in Two-Stage Skin Carcinogenesis Mouse Model. Nutr. Cancer 2020, 72, 976–983. [Google Scholar] [CrossRef]
- Hernandez-Aquino, E.; Muriel, P. Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J. Gastroenterol. 2018, 24, 1679–1707. [Google Scholar] [CrossRef]
- Sanaye, P.M.; Mojaveri, M.R.; Ahmadian, R.; Jahromi, M.S.; Bahramsoltani, R.J.P. Different mechanisms by which apigenin improves treatment of dermatological disorders Apigenin and its dermatological applications: A comprehensive review. Phytochemistry 2022, 203, 113390. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of Apigenin on RBL-2H3, RAW264.7, and HaCaT Cells: Anti-Allergic, Anti-Inflammatory, and Skin-Protective Activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lin, Y.; Liu, y.; Li, W.; He, J.; Fang, M.; Lin, D. Effects of Apigenin Treatment on Random Skin Flap Survival in Rats. Front. Pharmacol. 2021, 12, 625733. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging-From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Naidoo, K.; Birch-Machin, M. Oxidative Stress and Ageing: The Influence of Environmental Pollution, Sunlight and Diet on Skin. Cosmetics 2017, 4, 4. [Google Scholar] [CrossRef]
- Williams, H.C.; Dellavalle, R.P.; Garner, S. Acne vulgaris. Lancet 2012, 379, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.E.; Patzelt, A.; Knorr, F.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. One-year study on the variation of carotenoid antioxidant substances in living human skin: Influence of dietary supplementation and stress factors. J. Biomed. Opt. 2008, 13, 044028. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCdelta and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef]
- Katsuta, Y.; Iida, T.; Hasegawa, K.; Inomata, S.; Denda, M. Function of oleic acid on epidermal barrier and calcium influx into keratinocytes is associated with N-methyl D-aspartate-type glutamate receptors. Br. J. Dermatol. 2009, 160, 69–74. [Google Scholar] [CrossRef]
- Yang, Y.; Trevethan, M.; Wang, S.; Zhao, L. Beneficial Effects of Citrus Flavanones Naringin and Naringenin and Their Food Sources on Lipid Metabolism: An Update on Bioavailability, Pharmacokinetics, and Mechanisms. J. Nutr. Biochem. 2022, 104, 108967. [Google Scholar] [CrossRef]
- Ganai, S.A. Plant-derived flavone Apigenin: The small-molecule with promising activity against therapeutically resistant prostate cancer. Biomed. Pharmacother. 2017, 85, 47–56. [Google Scholar] [CrossRef]
- Guo, W.; Cui, S.; Tang, X.; Zhang, Q.; Zhao, J.; Mao, B.; Zhang, H. Intestinal Microbiomics and Metabolomics Insights into the Hepatoprotective Effects of Lactobacillus paracasei CCFM1222 Against the Acute Liver Injury in Mice. Probiotics Antimicrob. Proteins 2022, 1–15. [Google Scholar] [CrossRef]
- Kim, Y.R.; Kim, J.H.; Shin, H.J.; Choe, Y.B.; Ahn, K.J.; Lee, Y.W. Clinical Evaluation of a New-Formula Shampoo for Scalp Seborrheic Dermatitis Containing Extract of Rosa centifolia Petals and Epigallocatechin Gallate: A Randomized, Double-Blind, Controlled Study. Ann. Dermatol. 2014, 26, 733–738. [Google Scholar] [CrossRef]
- Su, L.; Su, Y.; An, Z.; Zhang, P.; Yue, Q.; Zhao, C.; Sun, X.; Zhang, S.; Liu, X.; Li, K.; et al. Fermentation products of Danshen relieved dextran sulfate sodium-induced experimental ulcerative colitis in mice. Sci. Rep. 2021, 11, 16210. [Google Scholar] [CrossRef]
- Ryu, A.R.; Kim, Y.-W.; Lee, M.-Y. Chlorin e6 and halogen light as a sebostatic photomedicine modulates linoleic acid-induced lipogenesis. Mol. Cell. Toxicol. 2018, 15, 49–56. [Google Scholar] [CrossRef]
- Torocsik, D.; Fazekas, F.; Poliska, S.; Gregus, A.; Janka, E.A.; Dull, K.; Szegedi, A.; Zouboulis, C.C.; Kovacs, D. Epidermal Growth Factor Modulates Palmitic Acid-Induced Inflammatory and Lipid Signaling Pathways in SZ95 Sebocytes. Front. Immunol. 2021, 12, 600017. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.C.; Guo, B.C.; Chen, C.H.; Hu, P.A.; Lee, T.S. Apigenin ameliorates hepatic lipid accumulation by activating the autophagy-mitochondria pathway. J. Food Drug Anal. 2021, 29, 240–254. [Google Scholar] [CrossRef]
- Kang, S.R.; Park, K.I.; Park, H.S.; Lee, D.H.; Kim, J.A.; Nagappan, A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Park, M.K.; et al. Anti-inflammatory effect of flavonoids isolated from Korea Citrus aurantium L. on lipopolysaccharide-induced mouse macrophage RAW 264.7 cells by blocking of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways. Food Chem. 2011, 129, 1721–1728. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.S.; Lee, Y.; Shin, M.H.; Cho, S.I.; Zouboulis, C.C.; Kim, M.K.; Lee, D.H.; Chung, J.H. Histone Deacetylase 1 Reduces Lipogenesis by Suppressing SREBP1 Transcription in Human Sebocyte Cell Line SZ95. Int. J. Mol. Sci. 2021, 22, 4477. [Google Scholar] [CrossRef]
- Ghosh, M.; Niyogi, S.; Bhattacharyya, M.; Adak, M.; Nayak, D.K.; Chakrabarti, S.; Chakrabarti, P. Ubiquitin Ligase COP1 Controls Hepatic Fat Metabolism by Targeting ATGL for Degradation. Diabetes 2016, 65, 3561–3572. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhao, Z.; Jiang, P.; Yu, H.; Xiao, H.; Yang, R. Identification of the bovine HSL gene expression profiles and its association with fatty acid composition and fat deposition traits. Meat Sci. 2017, 131, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, M.; Shen, M.; Li, Y.; Tan, S.; Shao, J.; Zhang, F.; Chen, A.; Wang, S.; Zheng, S. Oroxylin A regulates the turnover of lipid droplet via downregulating adipose triglyceride lipase (ATGL) in hepatic stellate cells. Life Sci. 2019, 238, 116934. [Google Scholar] [CrossRef]
- Shin, J.; Kim, K.P.; Ahn, H.Y.; Kim, B.; Cho, Y. Alterations in IL-4, IL-10 and IFN-gamma levels synergistically decrease lipid content and protein expression of FAS and mature SREBP-1 in human sebocytes. Arch. Dermatol. Res. 2019, 311, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Nyambuya, T.M.; Dludla, P.V.; Nkambule, B.B. Diet-Induced Obesity Promotes the Upregulation of Fas Expression on T-cells. Biology 2021, 10, 217. [Google Scholar] [CrossRef]
- Lovaszi, M.; Mattii, M.; Eyerich, K.; Gacsi, A.; Csanyi, E.; Kovacs, D.; Ruhl, R.; Szegedi, A.; Kemeny, L.; Stahle, M.; et al. Sebum lipids influence macrophage polarization and activation. Br. J. Dermatol. 2017, 177, 1671–1682. [Google Scholar] [CrossRef]
- Borda, L.J.; Perper, M.; Keri, J.E. Treatment of seborrheic dermatitis: A comprehensive review. J. Dermatolog. Treat. 2019, 30, 158–169. [Google Scholar] [CrossRef]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S.; Rawat, R. Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: The ‘future’ in dermatology therapeutics? Arch. Dermatol. Res. 2015, 307, 767–780. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kim, J.Y.; Jun, H.J.; Kim, S.J.; Lee, J.H.; Hoang, M.H.; Hwang, K.Y.; Um, S.J.; Chang, H.I.; Lee, S.J. The natural carotenoid astaxanthin, a PPAR-alpha agonist and PPAR-gamma antagonist, reduces hepatic lipid accumulation by rewiring the transcriptome in lipid-loaded hepatocytes. Mol. Nutr. Food Res. 2012, 56, 878–888. [Google Scholar] [CrossRef]
- Capitao, A.; Lopes-Marques, M.; Pascoa, I.; Ruivo, R.; Mendiratta, N.; Fonseca, E.; Castro, L.F.C.; Santos, M.M. The Echinodermata PPAR: Functional characterization and exploitation by the model lipid homeostasis regulator tributyltin. Environ. Pollut. 2020, 263, 114467. [Google Scholar] [CrossRef]
- Capitanio, B.; Lora, V.; Ludovici, M.; Sinagra, J.L.; Ottaviani, M.; Mastrofrancesco, A.; Ardigo, M.; Camera, E. Modulation of sebum oxidation and interleukin-1alpha levels associates with clinical improvement of mild comedonal acne. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1792–1797. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zhang, J.; Zheng, Z.; Wang, Z. Antioxidant Apigenin Relieves Age-Related Muscle Atrophy by Inhibiting Oxidative Stress and Hyperactive Mitophagy and Apoptosis in Skeletal Muscle of Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, Y.; Xiao, Z.; Wang, M.; Xu, M.; Wang, Z.; Wang, Y.; Zhuang, Z.; Yang, D.; Chen, G.; et al. Bicyclol ameliorates nonalcoholic fatty liver disease in mice via inhibiting MAPKs and NF-kappaB signaling pathways. Biomed. Pharmacother. 2021, 141, 111874. [Google Scholar] [CrossRef]
- Fang, F.; Xie, Z.; Quan, J.; Wei, X.; Wang, L.; Yang, L. Baicalin suppresses Propionibacterium acnes-induced skin inflammation by downregulating the NF-kappaB/MAPK signaling pathway and inhibiting activation of NLRP3 inflammasome. Braz. J. Med. Biol. Res. 2020, 53, e9949. [Google Scholar] [CrossRef]
- Adalsteinsson, J.A.; Kaushik, S.; Muzumdar, S.; Guttman-Yassky, E.; Ungar, J. An update on the microbiology, immunology and genetics of seborrheic dermatitis. Exp. Dermatol. 2020, 29, 481–489. [Google Scholar] [CrossRef]
- Caporali, S.; Levati, L.; Graziani, G.; Muzi, A.; Atzori, M.G.; Bonmassar, E.; Palmieri, G.; Ascierto, P.A.; D’Atri, S. NF-kappa B is activated in response to temozolomide in an AKT-dependent manner and confers protection against the growth suppressive effect of the drug. J. Transl. Med. 2012, 10, 252. [Google Scholar] [CrossRef]
- Khan, A.A.; Iadarola, M.; Yang, H.Y.; Dionne, R.A. Expression of COX-1 and COX-2 in a clinical model of acute inflammation. J. Pain. 2007, 8, 349–354. [Google Scholar] [CrossRef]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-kappaB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2020, 122, 109772. [Google Scholar] [CrossRef] [PubMed]
- Poligone, B.; Baldwin, A.S. Positive and negative regulation of NF-kappaB by COX-2: Roles of different prostaglandins. J. Biol. Chem. 2001, 276, 38658–38664. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.T.; Xiao, L.; Zhu, L.; Wang, Q.; Yan, T. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 2014, 37, 2085–2090. [Google Scholar] [CrossRef] [PubMed]


| Symptoms | Control | Model | CRY | L-NAR | H-NAR | L-API | H-API |
|---|---|---|---|---|---|---|---|
| Erythema | 0 | 1.38 ± 0.52 a | 0.43 ± 0.53 a | 0.25 ± 0.46 a | 0.75 ± 0.46 a | 0.25 ± 0.46 a | 0.25 ± 0.46 a |
| Scales | 0 | 2.25 ± 0.71 a | 2.14 ± 0.69 a | 1.13 ± 0.64 a | 1.50 ± 0.83 a | 1.00 ± 0.53 a | 1.50 ± 0.53 a |
| Erosion | 0 | 0.50 ± 0.53 a | 0.29 ± 0.49 a | 0.13 ± 0.35 a | 0.38 ± 0.74 a | 0.13 ± 0.35 a | 0.25 ± 0.46 a |
| Pruritus | 0 | 1.50 ± 0.53 a | 1.29 ± 0.49 a | 1.13 ± 0.35 a | 1.25 ± 0.46 a | 1.13 ± 0.35 a | 1.13 ± 0.35 a |
| Total | 0 | 5.63 ± 1.41 a | 4.14 ± 1.77 b | 2.63 ± 0.74 bc | 3.88 ± 1.13 bc | 2.38 ± 0.92 c | 3.25 ± 1.04 bc |
| Group | Control | Model | CRY | L-NAR | H-NAR | L-API | H-NAR | |
|---|---|---|---|---|---|---|---|---|
| Parameter | ||||||||
| Apparent symptoms | Damage scores | 0 | 5.63 ± 1.41 a | 4.14 ± 1.77 b | 2.63 ± 0.74 bc | 3.88 ± 1.13 bc | 2.38 ± 0.92 c | 3.25 ± 1.04 bc |
| Epidermal thickness (μm) | 20.5 ± 1.44 c | 85.68 ± 6.55 a | 62.37 ± 7.50 ab | 57.67 ± 5.45 b | 70.45 ± 5.84 ab | 63.85 ± 5.91 b | 49.24 ± 4.83 ab | |
| Hair follicle diameter (μm) | 45.09 ± 6.23 b | 71.73 ± 6.84 a | 55.07 ± 3.79 a | 58.63 ± 7.50 a | 50.97 ± 5.89 a | 51.63 ± 5.86 a | 58.79 ± 5.45 a | |
| Skin lipid expression | TG level | 0.38 ± 0.08 b | 0.61 ± 0.06 a | 0.47 ± 0.07 ab | 0.43 ± 0.05 b | 0.51 ± 0.06 ab | 0.39 ± 0.05 b | 0.33 ± 0.03 b |
| NEFA level | 0.069 ± 0.008 b | 0.098 ± 0.009 a | 0.095 ± 0.005 a | 0.090 ± 0.004 a | 0.075 ± 0.003 b | 0.076 ± 0.007 ab | 0.089 ± 0.007 ab | |
| T-CHO level | 0.57 ± 0.10 a | 0.67 ± 0.07 a | 0.43 ± 0.07 a | 0.47 ± 0.09 a | 0.50 ± 0.08 a | 0.44 ± 0.11 a | 0.50 ± 0.10 a | |
| Antioxidative expression | CAT level | 38.43 ± 4.08 a | 31 ± 1.84 b | 35.91 ± 2.94 ab | 34.09 ± 1.55 ab | 39.34 ± 2.01 a | 39.21 ± 1.62 a | 44.7 ± 2.47 a |
| SOD level | 3948 ± 541.2 a | 2839 ± 380.4 a | 3273 ± 195 a | 3701 ± 585.1 a | 3954 ± 605.3 a | 3641 ± 452.3 a | 3577 ± 666.9 a | |
| T-AOC level | 0.27 ± 0.03 a | 0.16 ± 0.03 b | 0.27 ± 0.03 a | 0.25 ± 0.04 a | 0.28 ± 0.04 a | 0.29 ± 0.02 a | 0.26 ± 0.04 a | |
| MDA level | 11.48 ± 0.49 b | 14.02 ± 0.52 a | 11.31 ± 0.77 b | 11.49 ± 1.10 ab | 11.68 ± 0.90 b | 8.23 ± 0.28 c | 11.75 ± 0.44 b | |
| LPO level | 0.44 ± 0.03 b | 1.06 ± 0.06 a | 0.68 ± 0.08 b | 0.61 ± 0.07 b | 0.51 ± 0.06 b | 0.59 ± 0.09 b | 0.63 ± 0.06 b | |
| AST level | 9.22 ± 1.81 a | 7.49 ± 2.83 a | 7.49 ± 1.49 a | 7.49 ± 1.49 a | 8.85 ± 1.18 a | 8.87 ± 1.41 a | 10.28 ± 1.06 a | |
| Inflammatory cytokines | Skin IL-6 (pg/mg protein) | 59.45 ± 5.43 c | 98.01 ± 5.50 a | 74.26 ± 6.55 b | 66.37 ± 6.74 a | 51.57 ± 2.68 bc | 23.24 ± 6.76 d | 43.87 ± 6.77 c |
| Skin IL-1β (pg/mg protein) | 179.8 ± 19.01 c | 328.2 ± 21.85 a | 250.7 ± 20.42 b | 298.2 ± 23.24 a | 176.4 ± 28.44 bc | 58.7 ± 6.36 d | 130.1 ± 21.26 c | |
| Skin TNF-α (pg/mg protein) | 72.49 ± 8.45 b | 116.8 ± 5.32 a | 88.58 ± 8.44 b | 77.96 ± 8.70 b | 78.63 ± 9.01 b | 36.7 ± 5.76 c | 63.57 ± 6.40 bc | |
| Skin IL-10 (pg/mg protein) | 60.22 ± 3.79 b | 36.76 ± 3.80 c | 62.90 ± 4.16 b | 95.04 ± 7.32 a | 60.43 ± 6.47 b | 43.83 ± 3.14 bc | 47.25 ± 3.41 bc | |
| Oxidative and lipid metabolism | SREBP-1 mRNA expression | 0.16 ± 0.05 b | 1.10 ± 0.24 a | 0.79 ± 0.06 ab | 0.77 ± 0.07 ab | 0.38 ± 0.20 b | 0.66 ± 0.15 ab | 0.57 ± 0.21 ab |
| Atgl mRNA expression | 0.46 ± 0.12 c | 1.16 ± 0.20 b | 2.10 ± 0.60 a | 2.21 ± 0.31 a | 2.16 ± 0.50 a | 1.88 ± 0.24 a | 1.27 ± 0.24 b | |
| PPARα mRNA expression | 0.42 ± 0.07 c | 1.24 ± 0.45 ab | 1.23 ± 0.18 b | 1.64 ± 0.65 ab | 2.53 ± 0.66 ab | 3.17 ± 0.55 a | 3.30 ± 0.72 a | |
| FAS mRNA expression | 1.05 ± 0.27 a | 2.07 ± 0.60 a | 1.05 ± 0.24 a | 2.07 ± 0.52 a | 2.24 ± 0.40 a | 2.16 ± 0.45 a | 1.69 ± 0.45 a | |
| Nrf2 mRNA expression | 1.38 ± 0.13 a | 0.62 ± 0.18 b | 1.34 ± 0.13 a | 0.87 ± 0.25 ab | 1.18 ± 0.24 ab | 1.75 ± 0.25 a | 1.57 ± 0.15 a | |
| Inflammation signaling pathway | Relative Akt mRNA expression | 1.46 ± 0.47 a | 1.06 ± 0.20 b | 1.30 ± 0.14 a | 1.83 ± 0.77 a | 1.76 ± 0.38 a | 1.36 ± 0.29 a | 1.36 ± 0.23 a |
| Relative COX-2 mRNA expression | 0.15 ± 0.08 c | 1.11 ± 0.02 a | 0.73 ± 0.14 ab | 0.45 ± 0.10 b | 0.53 ± 0.14 b | 0.45 ± 0.14 bc | 0.60 ± 0.11 ab | |
| Relative NF-κB mRNA expression | 0.11 ± 0.03 c | 1.08 ± 0.20 a | 0.90 ± 0.25 ab | 0.48 ± 0.11 b | 0.70 ± 0.09 b | 0.24 ± 0.07 bc | 0.39 ± 0.12 bc | |
| Relative IL-1β mRNA expression | 0.01 ± 0.003 c | 1.05 ± 0.27 a | 0.33 ± 0.16 ab | 0.36 ± 0.14 ab | 0.41 ± 0.15 ab | 0.11 ± 0.01 b | 0.26 ± 0.17 ab | |
| Relative IL-6 mRNA expression | 0.67 ± 0.14 ab | 1.06 ± 0.18 a | 0.64 ± 0.22 ab | 0.74 ± 0.24 a | 0.51 ± 0.17 ab | 0.26 ± 0.24 b | 0.74 ± 0.11 ab | |
| Relative IL-10 mRNA expression | 1.64 ± 0.07 a | 0.96 ± 0.16 b | 1.00 ± 0.26 ab | 0.65 ± 0.36 b | 0.51 ± 0.23 b | 1.42 ± 0.45 ab | 1.03 ± 0.33 ab | |
| Relative TNFα mRNA expression | 0.04 ± 0.01 b | 1.04 ± 0.16 a | 0.58 ± 0.21 ab | 0.32 ± 0.12 b | 0.40 ± 0.19 ab | 0.53 ± 0.20 ab | 0.50 ± 0.10 ab | |
| Immunohistochemical analysis | NF-κB expression in skin issues | 9.806 ± 0.76 b | 16.56 ± 0.62 a | 14.3 ± 0.65 b | 12.48 ± 0.84 b | 13.02 ± 0.84 b | 11.18 ± 0.88 b | 11.32 ± 1.07 b |
| NF-κB expression in the epidermal layer | 9.47 ± 0.46 b | 12.76 ± 1.37 a | 8.51 ± 1.10 b | 11.32 ± 1.42 a | 11.7 ± 1.61 a | 9.18 ± 0.71 b | 7.48 ± 0.74 b | |
| NF-κB expression in the sebaceous gland | 6.41 ± 0.60 b | 10.41 ± 0.98 a | 7.71 ± 1.38 a | 7.23 ± 1.16 a | 6.39 ± 0.68 b | 7.02 ± 0.95 b | 7.59 ± 0.52 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Zhang, H.; Cui, S. Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice. Foods 2023, 12, 2120. https://doi.org/10.3390/foods12112120
Li J, Mao B, Tang X, Zhang Q, Zhao J, Zhang H, Cui S. Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice. Foods. 2023; 12(11):2120. https://doi.org/10.3390/foods12112120
Chicago/Turabian StyleLi, Jie, Bingyong Mao, Xin Tang, Qiuxiang Zhang, Jianxin Zhao, Hao Zhang, and Shumao Cui. 2023. "Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice" Foods 12, no. 11: 2120. https://doi.org/10.3390/foods12112120
APA StyleLi, J., Mao, B., Tang, X., Zhang, Q., Zhao, J., Zhang, H., & Cui, S. (2023). Protective Effects of Naringenin and Apigenin in Ameliorating Skin Damage via Mediating the Nrf2 and NF-κB Pathways in Mice. Foods, 12(11), 2120. https://doi.org/10.3390/foods12112120






