Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Sample Preparation
2.3. Detection of Active Components in BR Active Fractions
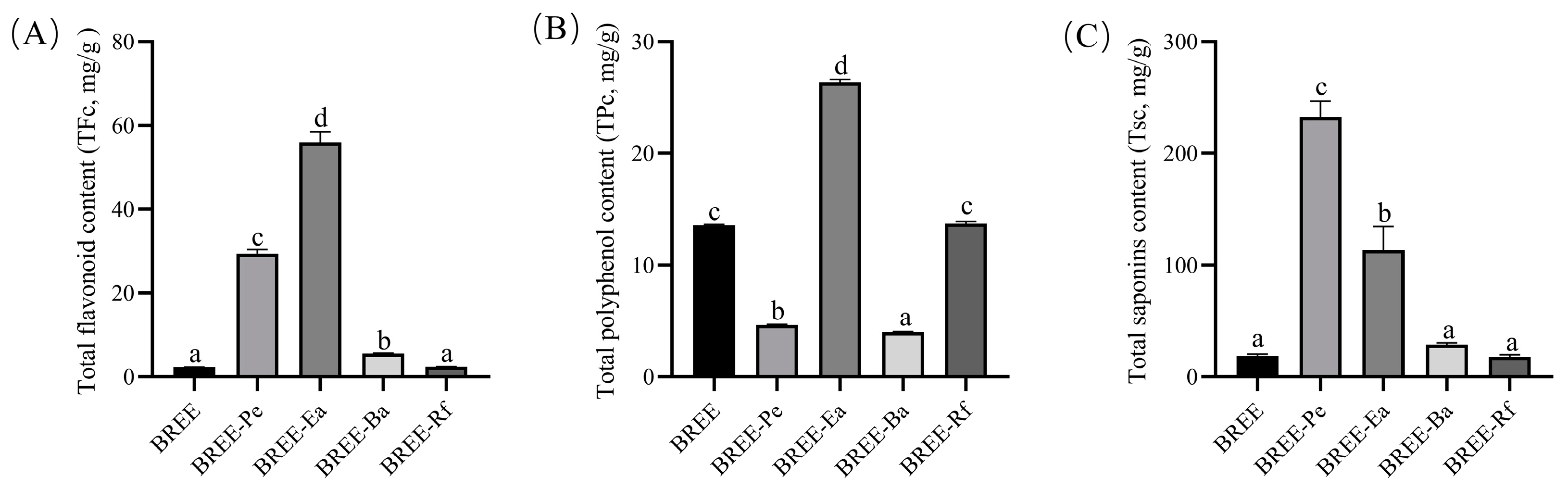
2.3.1. Total Flavonoid Content (TFc)
2.3.2. Total Polyphenol Content (TPc)
2.3.3. Total Saponin Content (TSc)
2.4. Antioxidant Activity
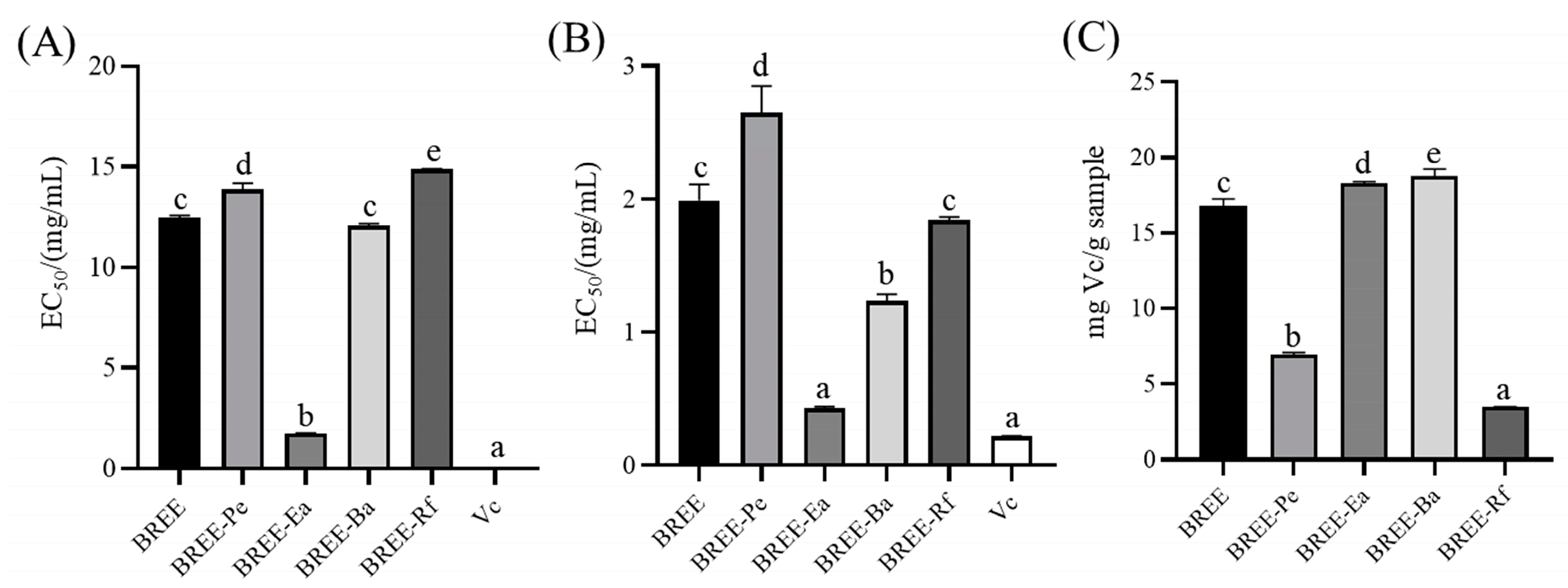
2.4.1. DPPH Method
2.4.2. ABTS Assay
2.4.3. Reducing Power
2.5. Cell Culture and Treatment
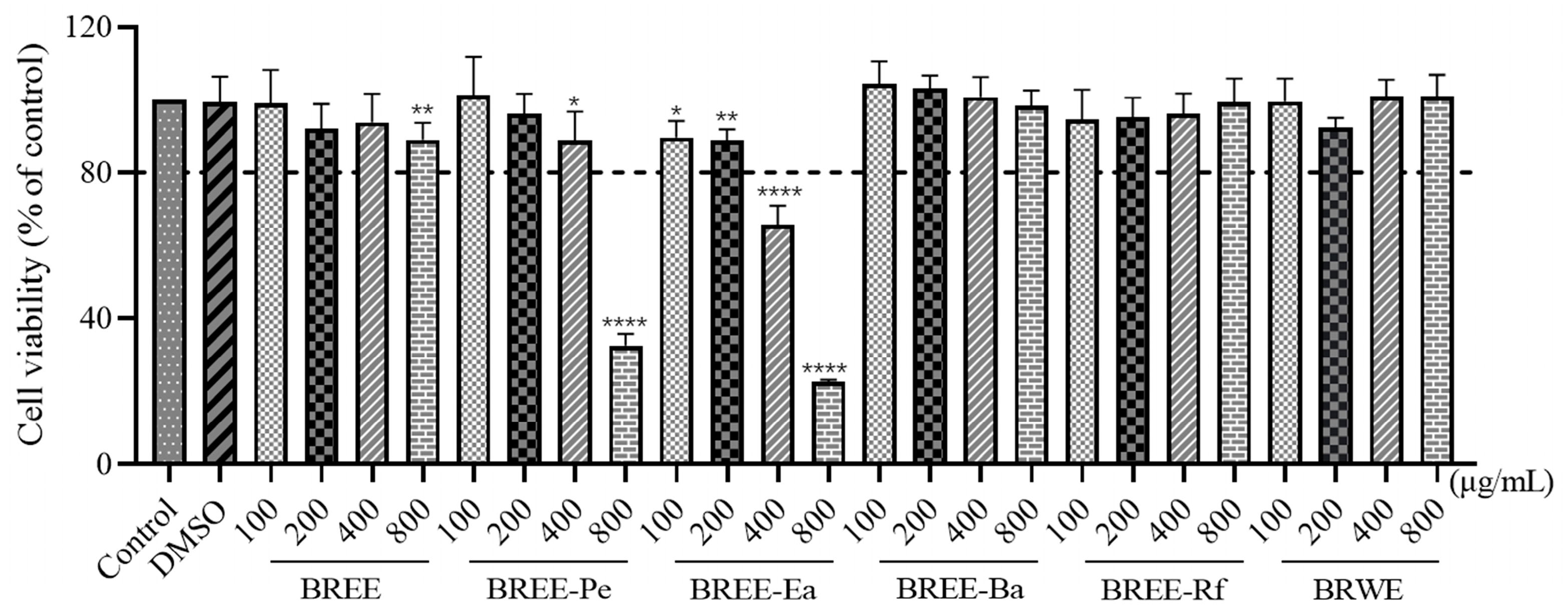
2.6. Cell Viability Assay
2.7. Detection of Intracellular ROS Accumulation in PC12 Cells
2.8. Determination of MDA, SOD, GSH-Px, and LDH in H2O2-Induced PC12 Cells
2.9. UPLC-MS Analysis of BREE-Ea
2.10. Statistical Analysis
3. Results and Discussion
3.1. Detection of Active Components in BR Active Fractions
3.2. Antioxidant Activity
3.3. Cytoprotective Activity on PC12 Cell Viability
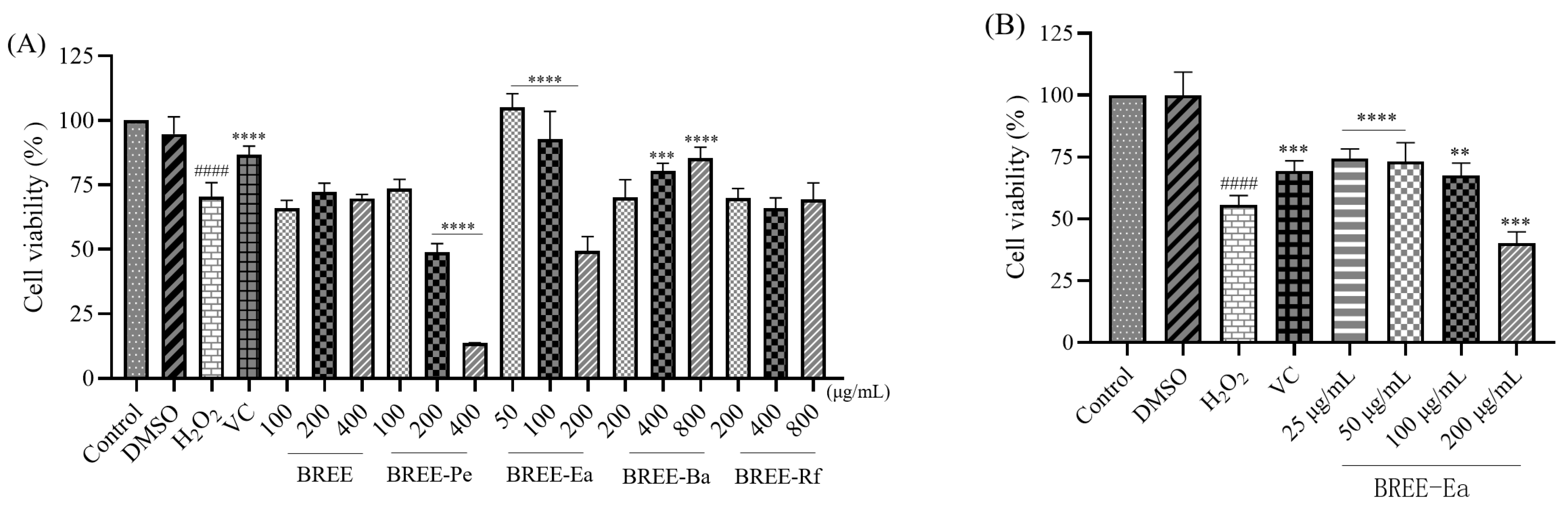
3.4. Cytoprotective Activity on H2O2-Induced PC12 Cell Viability
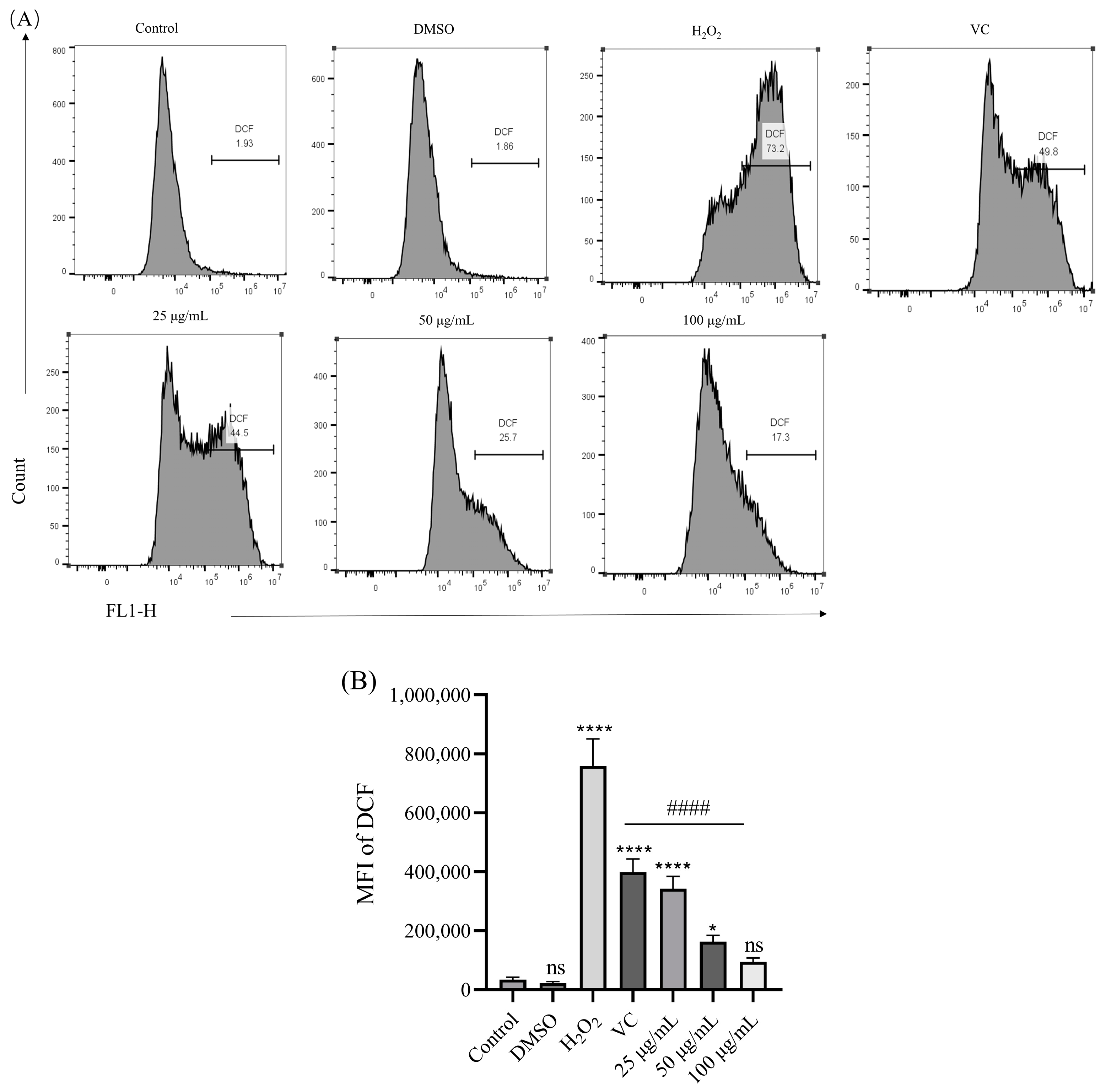
3.5. Effect of BREE-Ea on H2O2-Induced ROS Production
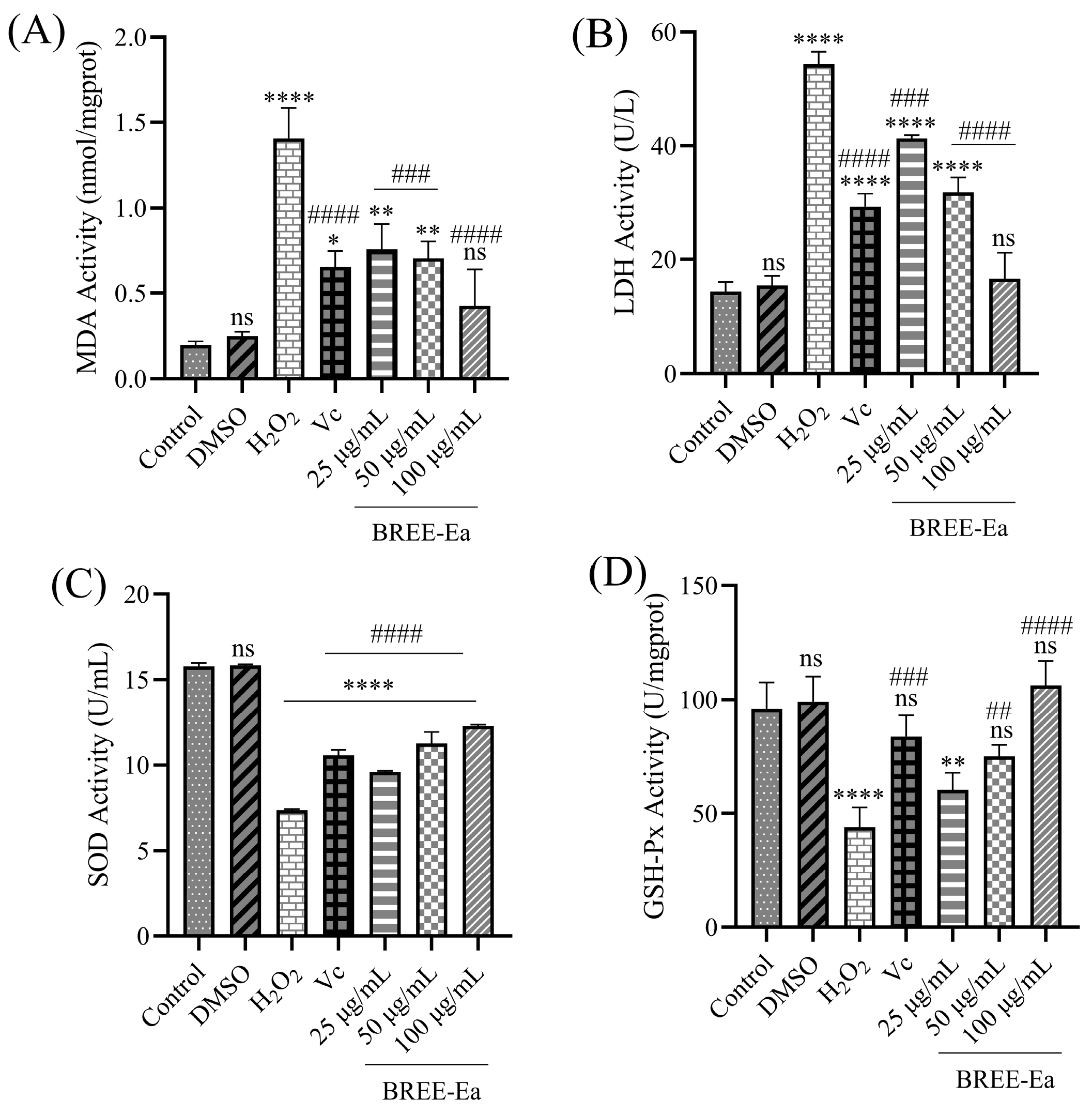
3.6. Effect of BREE-Ea on H2O2-Induced MDA, SOD, LDH, and GSH-Px Activity in PC12 Cells
3.7. Chemical Composition of BBRE-Ea
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging; Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Takaki, A.; Oyama, A.; Adachi, T.; Wada, N.; Onishi, H.; Okada, H. Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 2020, 12, 1576. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Author Correction: Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, K.; Ohta, Y.; Inufusa, H.; Loon, A.F.N.; Abe, K. Prevention of Cognitive Decline in Alzheimer’s Disease by Novel Antioxidative Supplements. Int. J. Mol. Sci. 2020, 21, 1974. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X. Antioxidant Therapies for Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2012, 18, 228–237. [Google Scholar] [CrossRef]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of Natural Antioxidants in Neurodegenerative Disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Su, D.; Pi, N.; Ma, C.; Gao, P. Composition Analysis and Anti-Hypoxia Activity of Polysaccharide from Brassica Rapa L. Int. J. Biol. Macromol. 2010, 47, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, G.; Peng, Y. A Critical Review on Phytochemical Profile and Biological Effects of Turnip (Brassica Rapa L.). Front. Nutr. 2021, 8, 721733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, C.; Cheng, Y.; Guo, Y.; Qian, H.; Liu, Y. Brassica Rapa L. (Tibetan Turnip) Prevents Sleep-Deprivation Induced Cognitive Deficits Via the Inhibition of Neuroinflammation and Mitochondrial Depolarization. Food Funct. 2022, 13, 10610. [Google Scholar] [CrossRef]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous Vegetables and Human Cancer Risk: Epidemiologic Evidence and Mechanistic Basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef]
- Johnson, I.T. Cruciferous Vegetables and Risk of Cancers of the Gastrointestinal Tract. Mol. Nutr. Food Res. 2018, 62, e1701000. [Google Scholar] [CrossRef] [PubMed]
- Eunyoung, H.; Gun-Hee, K. Anticancer and Antimicrobial Activities of β-Phenylethyl Isothiocyanate in Brassica Rapa L. Food Sci. Technol. Res. 2008, 14, 377–382. [Google Scholar]
- Liu, L.; Liu, C.; Hua, H.; Zhao, W.; Zhu, H.; Cheng, Y.; Guo, Y.; Qian, H. Effect of Polysaccharides from Tibetan Turnip (Brassica Rapa L.) on the Gut Microbiome after in Vitro Fermentation and in Vivo Metabolism. Food Funct. 2022, 13, 3063–3076. [Google Scholar] [CrossRef]
- Hua, H.; Zhu, H.; Liu, C.; Zhang, W.; Li, J.; Hu, B.; Guo, Y.; Cheng, Y.; Pi, F.; Xie, Y.; et al. Bioactive Compound from the Tibetan Turnip (Brassica Rapa L.) Elicited Anti-Hypoxia Effects in Ogd/R-Injured Ht22 Cells by Activating the Pi3k/Akt Pathway. Food Funct. 2021, 12, 2901–2913. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Baek, N.I.; Chung, H.G.; Bang, M.H.; Jeong, T.S.; Lee, K.T.; Kang, Y.J.; Lee, M.K.; Kim, H.J.; Yeo, J.; et al. Effects of the Ethanol Extract of the Roots of Brassica Rapa on Glucose and Lipid Metabolism in C57bl/Ksj-Db/Db Mice. Clin. Nutr. 2008, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Fernandes, C.; Carvalho, R.; Bennett, R.N.; Saavedra, M.J.; Rosa, E.A. Seasonal Effects on Bioactive Compounds and Antioxidant Capacity of Six Economically Important Brassica Vegetables. Molecules 2011, 16, 6816. [Google Scholar] [CrossRef]
- Fernandes, F.; Valentão, P.; Sousa, C.; Pereira, J.A.; Seabra, R.M.; Andrade, P.B. Chemical and Antioxidative Assessment of Dietary Turnip ( Brassica Rapa Var. Rapa L.). Food Chem. 2007, 105, 1003–1010. [Google Scholar] [CrossRef]
- Chu, B.; Chen, C.; Li, J.; Chen, X.; Li, Y.; Tang, W.; Jin, L.; Zhang, Y. Effects of Tibetan Turnip (Brassica Rapa L.) on Promoting Hypoxia-Tolerance in Healthy Humans. J. Ethnopharmacol. 2017, 195, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, J.; Chen, Y.; Chen, C.; Chu, B.; Zhang, Y. P-Coumaric Acid as a Prophylactic Measure against Normobaric Hypoxia Induced Pulmonary Edema in Mice. Life Sci. 2018, 211, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant Capacity and Phenolic Content of Selected Tropical Fruits from Malaysia, Extracted with Different Solvents. Food Chem. 2008, 115, 785–788. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Hu, T.; Guo, Y.Y.; Zhou, Q.F.; Zhong, X.K.; Zhu, L.; Piao, J.H.; Chen, J.; Jiang, J.G. Optimization of Ultrasonic-Assisted Extraction of Total Saponins from Eclipta Prostrasta L. Using Response Surface Methodology. J. Food Sci. 2012, 77, C975–C982. [Google Scholar] [CrossRef]
- Cao, W.; Wang, C.; Mayhesumu, X.; Pan, L.; Dang, Y.; Yili, A.; Abuduwaili, A.; Mansur, S. Isolation, Structural Elucidation, Antioxidant and Hypoglycemic Activity of Polysaccharides of Brassica Rapa L. Molecules 2022, 27, 3002. [Google Scholar] [CrossRef]
- Miao, J.; Li, X.; Zhao, C.; Gao, X.; Wang, Y.; Gao, W. Active Compounds, Antioxidant Activity and Alpha-Glucosidase Inhibitory Activity of Different Varieties of Chaenomeles Fruits. Food Chem. 2018, 248, 330–339. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, C.; Li, X.; Chen, X.; Mao, X.; Huang, H.; Wang, T.; Gao, W. Chemical Composition and Bioactivities of Two Common Chaenomeles Fruits in China: Chaenomeles Speciosa and Chaenomeles Sinensis. J. Food Sci. 2016, 81, H2049–H2058. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2′,7′-Dichlorodihydrofluorescein as a Fluorescent Probe for Reactive Oxygen Species Measurement: Forty Years of Application and Controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Li, R.L.; Zhang, Q.; Liu, J.; Sun, J.Y.; He, L.Y.; Duan, H.X.; Peng, W.; Wu, C.J. Hydroxy-Alpha-Sanshool Possesses Protective Potentials on H2o2-Stimulated Pc12 Cells by Suppression of Oxidative Stress-Induced Apoptosis through Regulation of Pi3k/Akt Signal Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 3481758. [Google Scholar] [PubMed]
- Asraoui, F.; Kounnoun, A.; Cadi, H.E.; Cacciola, F.; Majdoub, Y.O.E.; Alibrando, F.; Mandolfino, F.; Dugo, P.; Mondello, L.; Louajri, A. Phytochemical Investigation and Antioxidant Activity of Globularia Alypum L. Molecules 2021, 26, 759. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Ma, H.; Fan, P.; Gao, R.; Jia, Z. Antioxidant Potential, Total Phenolic and Total Flavonoid Contents of Rhododendron Anthopogonoides and Its Protective Effect on Hypoxia-Induced Injury in Pc12 Cells. BMC Complement. Altern. Med. 2015, 15, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef]
- Boudouda, H.B.; Zeghib, A.; Karioti, A.; Bilia, A.R.; Ozturk, M.; Aouni, M.; Kabouche, A.; Kabouche, Z. Antibacterial, Antioxidant, Anti-Cholinesterase Potential and Flavonol Glycosides of Biscutella Raphanifolia (Brassicaceae). Pak. J. Pharm. Sci. 2015, 28, 153–158. [Google Scholar]
- Sen, S.; De, B.; Devanna, N.; Chakraborty, R. Total Phenolic, Total Flavonoid Content; Antioxidant Capacity of the Leaves of Meyna Spinosa Roxb., an Indian Medicinal Plant. Chin. J. Nat. Med. 2013, 11, 149–157. [Google Scholar] [CrossRef]
- Curcic, M.G.; Stankovic, M.S.; Radojevic, I.D.; Stefanovic, O.D.; Comic, L.R.; Topuzovic, M.D.; Djacic, D.S.; Markovic, S.D. Biological Effects, Total Phenolic Content and Flavonoid Concentrations of Fragrant Yellow Onion (Allium Flavum L.). Med. Chem. 2012, 8, 46–51. [Google Scholar] [CrossRef]
- Li, N.; Wen, L.; Li, T.; Yang, H.; Qiao, M.; Wang, T.; Song, L.; Huang, X.; Li, M.; Bukyei, E.; et al. Alleviating Effects of Black Soybean Peptide on Oxidative Stress Injury Induced by Lead in Pc12 Cells Via Keap1/Nrf2/Txnip Signaling Pathway. Nutrients 2022, 14, 3102. [Google Scholar] [CrossRef]
- Jang, J.H.; Surh, Y.J. Protective Effects of Resveratrol on Hydrogen Peroxide-Induced Apoptosis in Rat Pheochromocytoma (Pc12) Cells. Mutat. Res. 2001, 496, 181–190. [Google Scholar] [CrossRef]
- Duan, L.H.; Li, M.; Wang, C.B.; Wang, Q.M.; Liu, Q.Q.; Shang, W.F.; Shen, Y.J.; Lin, Z.H.; Sun, T.Y.; Wu, Z.Z.; et al. Protective Effects of Organic Extracts of Alpinia Oxyphylla against Hydrogen Peroxide-Induced Cytotoxicity in Pc12 Cells. Neural Regen. Res. 2020, 15, 682–689. [Google Scholar]
- Demirci, S.; Kutluhan, S.; Naziroglu, M.; Uguz, A.C.; Yurekli, V.A.; Demirci, K. Effects of Selenium and Topiramate on Cytosolic Ca(2+) Influx and Oxidative Stress in Neuronal Pc12 Cells. Neurochem. Res. 2013, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Sai, Y.; Wu, Q.; Le, W.; Ye, F.; Li, Y.; Dong, Z. Rotenone-Induced Pc12 Cell Toxicity Is Caused by Oxidative Stress Resulting from Altered Dopamine Metabolism. Toxicol. Vitro 2008, 22, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.P.; Lu, Y.H.; Wei, D.Z. Protective Effects of a Flavonoid-Rich Extract of Hypericum Perforatum L. Against Hydrogen Peroxide-Induced Apoptosis in Pc12 Cells. Phytother. Res. 2010, 24 (Suppl. S1), S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, S.H.; Bai, L.X.; Li, J.Y. The Protective Effect of Aspirin Eugenol Ester on Oxidative Stress to Pc12 Cells Stimulated with H(2)O(2) through Regulating Pi3k/Akt Signal Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 5527475. [Google Scholar] [CrossRef]
- Mutungi, M.M.; Muema, F.W.; Kimutai, F.; Xu, Y.B.; Zhang, H.; Chen, G.L.; Guo, M.Q. Antioxidant and Antiproliferative Potentials of Ficus Glumosa and Its Bioactive Polyphenol Metabolites. Pharmaceuticals 2021, 14, 266. [Google Scholar] [CrossRef]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; Da Cunha, E.F.F.; Ramalho, T.C. Future Therapeutic Perspectives into the Alzheimer’s Disease Targeting the Oxidative Stress Hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative Stress Hypothesis in Alzheimer’s Disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative Stress and the Amyloid Beta Peptide in Alzheimer’s Disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides Purified from Cordyceps Cicadae Protects Pc12 Cells against Glutamate-Induced Oxidative Damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Qiao, S.; Liu, R.; Lv, C.; Miao, Y.; Yue, M.; Tao, Y.; Wei, Z.; Xia, Y.; Dai, Y. Bergenin Impedes the Generation of Extracellular Matrix in Glomerular Mesangial Cells and Ameliorates Diabetic Nephropathy in Mice by Inhibiting Oxidative Stress Via the Mtor/Beta-Trcp/Nrf2 Pathway. Free Radic. Biol. Med. 2019, 145, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Phenolic Profiles and Antioxidant Activities in Selected Drought-Tolerant Leafy Vegetable Amaranth. Sci. Rep. 2020, 10, 18287. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, a Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Siddique, Y.H. Role of Flavonoids in Neurodegenerative Disorders with Special Emphasis on Tangeritin. CNS Neurol. Disord. Drug Targets 2019, 18, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef]
- Al-Edresi, S.; Alsalahat, I.; Freeman, S.; Aojula, H.; Penny, J. Resveratrol-Mediated Cleavage of Amyloid Beta(1-42) Peptide: Potential Relevance to Alzheimer’s Disease. Neurobiol. Aging 2020, 94, 24–33. [Google Scholar] [CrossRef]
- Kim, G.R.; Kim, E.N.; Park, K.J.; Kim, K.H.; Jeong, G.S. Inhibitory Effect of Lgs and Ode Isolated from the Twigs of Syringa Oblata Subsp. Dilatata on Rankl-Induced Osteoclastogenesis in Macrophage Cells. Molecules 2021, 26, 1779. [Google Scholar] [CrossRef]
- Ilgisonis, E.V.; Shalina, R.; Kasum-Zade, N.; Burkova, K.G.; Trifonova, O.P.; Maslov, D.L.; Kaysheva, A.L.; Markin, S.S. Metabolomic Markers for Predicting Preeclampsia in the First Trimester of Pregnancy: A Retrospective Study. Molecules 2022, 27, 2475. [Google Scholar] [CrossRef]
- Wieczorek, Z.; Zimecki, M.; Trojnar, J. Immunomodulatory Activity of a Potent Thymopentin Analog: Disulphide Bridged Beta-Mercaptopropionyl-Arginyl-Lysyl-Aspartyl-Valyl-Tyrosyl-Cysteine Amide. Pol. J. Pharmacol. 1996, 48, 31–38. [Google Scholar]
- Fiore, A.; Murray, P.J. Tryptophan and Indole Metabolism in Immune Regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Jeong, S.J.; Miyamoto, T.; Inagaki, M.; Kim, Y.C.; Higuchi, R. Rotundines a-C, Three Novel Sesquiterpene Alkaloids from Cyperus Rotundus. J. Nat. Prod. 2000, 63, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Macias, F.A.; Torres, A.; Galindo, J.L.; Varela, R.M.; Alvarez, J.A.; Molinillo, J.M. Bioactive Terpenoids from Sunflower Leaves Cv. Peredovick. Phytochemistry 2002, 61, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, C.; Gao, Y.; Li, D.; Huang, D.; Chen, Z.; Zhao, X.; Huang, Q.; Wu, D.; Lai, T.; et al. Bergenin-Activated Sirt1 Inhibits Tnf-Alpha-Induced Proinflammatory Response by Blocking the Nf-Kappab Signaling Pathway. Pulm. Pharmacol. Ther. 2020, 62, 101921. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chang, Z.; Lu, Q.; Chen, X.; Najafi, M. Nobiletin as an Inducer of Programmed Cell Death in Cancer: A Review. Apoptosis 2022, 27, 297–310. [Google Scholar] [CrossRef]
- Morrow, N.M.; Trzaskalski, N.A.; Hanson, A.A.; Fadzeyeva, E.; Telford, D.E.; Chhoker, S.S.; Sutherland, B.G.; Edwards, J.Y.; Huff, M.W.; Mulvihill, E.E. Nobiletin Prevents High-Fat Diet-Induced Dysregulation of Intestinal Lipid Metabolism and Attenuates Postprandial Lipemia. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 127–144. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J.; et al. Tangeretin Suppresses Osteoarthritis Progression Via the Nrf2/Nf-Kappab and Mapk/Nf-Kappab Signaling Pathways. Phytomedicine 2022, 98, 153928. [Google Scholar] [CrossRef]
- Bao, J.; Liang, Z.; Gong, X.; Zhao, Y.; Wu, M.; Liu, W.; Tu, C.; Wang, X.; Shu, X. Tangeretin Inhibits Bace1 Activity and Attenuates Cognitive Impairments in Ad Model Mice. J. Agric. Food Chem. 2022, 70, 1536–1546. [Google Scholar] [CrossRef]
- Ishida, T.; Nishiumi, S.; Tanahashi, T.; Yamasaki, A.; Yamazaki, A.; Akashi, T.; Miki, I.; Kondo, Y.; Inoue, J.; Kawauchi, S.; et al. Linoleoyl Ethanolamide Reduces Lipopolysaccharide-Induced Inflammation in Macrophages and Ameliorates 2,4-Dinitrofluorobenzene-Induced Contact Dermatitis in Mice. Eur. J. Pharmacol. 2013, 699, 6–13. [Google Scholar] [CrossRef]
- Su, Q.; Krai, P.; Goetz, M.; Cassera, M.B.; Kingston, D.G. Antiplasmodial Isoflavanes and Pterocarpans from Apoplanesia Paniculata. Planta Med. 2015, 81, 1128–1132. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, X.; Yao, Y.Z.; Wang, B.; Wei, C.Q.; Zhang, M.; Huang, F. Characterization of the Aroma-Active Compounds in Commercial Fragrant Rapeseed Oils Via Monolithic Material Sorptive Extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef]
- Wang, H.; Wang, F.; Wu, S.; Liu, Z.; Li, T.; Mao, L.; Zhang, J.; Li, C.; Liu, C.; Yang, Y. Traditional Herbal Medicine-Derived Sulforaphene Promotes Mitophagic Cell Death in Lymphoma Cells through Crm1-Mediated P62/Sqstm1 Accumulation and Ampk Activation. Chem. Biol. Interact. 2018, 281, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A Review of Pharmacological Effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Mao, K.; Gao, J.; Chitrakar, B.; Sadiq, F.A.; Wang, Z.; Wu, J.; Xu, C.; Sang, Y. Pear Pomace Soluble Dietary Fiber Ameliorates the Negative Effects of High-Fat Diet in Mice by Regulating the Gut Microbiota and Associated Metabolites. Front. Nutr. 2022, 9, 1025511. [Google Scholar] [CrossRef] [PubMed]
- Yanagita, T.; Han, S.Y.; Wang, Y.M.; Tsuruta, Y.; Anno, T. Cycloalliin, a Cyclic Sulfur Imino Acid, Reduces Serum Triacylglycerol in Rats. Nutrition 2003, 19, 140–143. [Google Scholar] [CrossRef]
- Weiss, T.; Bruning, T.; Bolt, H.M. Dephenylation of the Rubber Chemical N-Phenyl-2-Naphthylamine to Carcinogenic 2-Naphthylamine: A Classical Problem Revisited. Crit. Rev. Toxicol. 2007, 37, 553–566. [Google Scholar] [CrossRef]
- Lim, H.; Kwon, Y.S.; Kim, D.; Lee, J.; Kim, H.P. Flavonoids from Scutellaria Baicalensis Inhibit Senescence-Associated Secretory Phenotype Production by Interrupting Ikappabzeta/C/Ebpbeta Pathway: Inhibition of Age-Related Inflammation. Phytomedicine 2020, 76, 153255. [Google Scholar] [CrossRef]
- Camagna, M.; Ojika, M.; Takemoto, D. Detoxification of the Solanaceous Phytoalexins Rishitin, Lubimin, Oxylubimin and Solavetivone Via a Cytochrome P450 Oxygenase. Plant. Signal. Behav. 2020, 15, 1707348. [Google Scholar] [CrossRef]
- Said, H.M. Biotin: Biochemical, Physiological and Clinical Aspects. Subcell. Biochem. 2012, 56, 1–19. [Google Scholar]
- Shao, W.; Wang, X.; Liu, Z.; Song, X.; Wang, F.; Liu, X.; Yu, Z. Cyperotundone Combined with Adriamycin Induces Apoptosis in Mcf-7 and Mcf-7/Adr Cancer Cells by Ros Generation and Nrf2/Are Signaling Pathway. Sci. Rep. 2023, 13, 1384–1395. [Google Scholar] [CrossRef]
- Mayengbam, S.; Raposo, S.; Aliani, M.; House, J.D. Oral Exposure to the Anti-Pyridoxine Compound 1-Amino D-Proline Further Perturbs Homocysteine Metabolism through the Transsulfuration Pathway in Moderately Vitamin B(6) Deficient Rats. J. Nutr. Biochem. 2015, 26, 241–249. [Google Scholar] [CrossRef]
- Muhammad, D.; Lalun, N.; Bobichon, H.; Le Magrex Debar, E.; Gangloff, S.C.; Nour, M.; Voutquenne-Nazabadioko, L. Triterpenoids from the Leaves of Alphitonia Xerocarpus Baill and Their Biological Activity. Phytochemistry 2016, 129, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Jalilian, F.; Moieni-Arya, M.; Hosseinzadeh, L.; Shokoohinia, Y. Oxypeucedanin and Isoimperatorin Extracted from Prangos Ferulacea (L.) Lindl Protect Pc12 Pheochromocytoma Cells from Oxidative Stress and Apoptosis Induced by Doxorubicin. Res. Pharm. Sci. 2022, 17, 12–21. [Google Scholar] [PubMed]
- Li, N.; Yang, L.; Zuo, H. Arborinine Suppresses Ovarian Cancer Development through Inhibition of Lsd1. Life Sci. 2022, 291, 120275. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Lim, Y.H. Resveratrol Antibacterial Activity against Escherichia Coli Is Mediated by Z-Ring Formation Inhibition Via Suppression of Ftsz Expression. Sci. Rep. 2015, 5, 10029–10038. [Google Scholar] [CrossRef]
- Mendez-Lopez, L.F.; Caboni, P.; Arredondo-Espinoza, E.; Carrizales-Castillo, J.J.J.; Balderas-Renteria, I.; Camacho-Corona, M.D.R. Bioassay-Guided Identification of the Antiproliferative Compounds of Cissus Trifoliata and the Transcriptomic Effect of Resveratrol in Prostate Cancer Pc3 Cells. Molecules 2021, 26, 2200. [Google Scholar] [CrossRef]
- Kowalska, E.; Kozik, A. The Genes and Enzymes Involved in the Biosynthesis of Thiamin and Thiamin Diphosphate in Yeasts. Cell. Mol. Biol. Lett. 2008, 13, 271–282. [Google Scholar] [CrossRef]
- Feng, P.; Li, Q.; Liu, L.; Wang, S.; Wu, Z.; Tao, Y.; Huang, P.; Wang, P. Crocetin Prolongs Recovery Period of Dss-Induced Colitis Via Altering Intestinal Microbiome and Increasing Intestinal Permeability. Int. J. Mol. Sci. 2022, 23, 3832. [Google Scholar] [CrossRef]
- Tan, R.X.; Wolfender, J.L.; Ma, W.G.; Zhang, L.X.; Hostettmann, K. Secoiridoids and Antifungal Aromatic Acids from Gentiana Algida. Phytochemistry 1996, 41, 111–116. [Google Scholar] [CrossRef]
- Martin, S.A.; Brash, A.R.; Murphy, R.C. The Discovery and Early Structural Studies of Arachidonic Acid. J. Lipid Res. 2016, 57, 1126–1132. [Google Scholar] [CrossRef]
- Gorica, E.; Calderone, V. Arachidonic Acid Derivatives and Neuroinflammation. CNS Neurol. Disord. Drug Targets 2022, 21, 118–129. [Google Scholar] [CrossRef]
- Ito, H.; Sun, X.L.; Watanabe, M.; Okamoto, M.; Hatano, T. Chlorogenic Acid and Its Metabolite M-Coumaric Acid Evoke Neurite Outgrowth in Hippocampal Neuronal Cells. Biosci. Biotechnol. Biochem. 2008, 72, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Widmeier, E.; Airik, M.; Hugo, H.; Schapiro, D.; Wedel, J.; Ghosh, C.C.; Nakayama, M.; Schneider, R.; Awad, A.M.; Nag, A.; et al. Treatment with 2,4-Dihydroxybenzoic Acid Prevents Fsgs Progression and Renal Fibrosis in Podocyte-Specific Coq6 Knockout Mice. J. Am. Soc. Nephrol. 2019, 30, 393–405. [Google Scholar] [CrossRef]
- Lang, A.; Heckl, C.; Vogeser, M.; Stauch, T.; Homann, C.; Hennig, G.; Sroka, R.; Stepp, H. Rapid Spectrophotometric Quantification of Urinary Porphyrins and Porphobilinogen as Screening Tool for Attacks of Acute Porphyria. J. Biomed. Opt. 2018, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Omar, H.A.; Kulp, S.K.; Chen, C.S. Pharmacological Exploitation of Indole-3-Carbinol to Develop Potent Antitumor Agents. Mini Rev. Med. Chem. 2010, 10, 398–404. [Google Scholar] [CrossRef]
- Fang, J.G.; Lu, M.; Chen, Z.H.; Zhu, H.H.; Li, Y.; Yang, L.; Wu, L.M.; Liu, Z.L. Antioxidant Effects of Resveratrol and Its Analogues against the Free-Radical-Induced Peroxidation of Linoleic Acid in Micelles. Chemistry 2002, 8, 4191–4198. [Google Scholar] [CrossRef] [PubMed]
- Day, Z.I.; Mayfosh, A.J.; Giel, M.C.; Hong, Y.; Williams, S.A.; Santavanond, J.P.; Rau, T.F.; Poon, I.K.; Hulett, M.D. Novel Formulation of Undecylenic Acid Induces Tumor Cell Apoptosis. Int. J. Mol. Sci. 2022, 23, 14170. [Google Scholar] [CrossRef]
- Masyita, A.; Salim, E.; Asri, R.M.; Nainu, F.; Hori, A.; Yulianty, R.; Hatta, M.; Rifai, Y.; Kuraishi, T. Molecular Modeling and Phenoloxidase Inhibitory Activity of Arbutin and Arbutin Undecylenic Acid Ester. Biochem. Biophys. Res. Commun. 2021, 547, 75–81. [Google Scholar] [CrossRef]
- Mostafa, H.H.A.; Wang, H.; Song, J.; Li, X. Effects of Genotypes and Explants on Garlic Callus Production and Endogenous Hormones. Sci. Rep. 2020, 10, 4867–4877. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chiu, C.C.; Wu, C.P.; Chou, Y.T.; Wang, H.M. Enhancements of Skin Cell Proliferations and Migrations Via 6-Dehydrogingerdione. J. Agric. Food Chem. 2013, 61, 1349–1356. [Google Scholar] [CrossRef]
- Huang, S.H.; Lee, C.H.; Wang, H.M.; Chang, Y.W.; Lin, C.Y.; Chen, C.Y.; Chen, Y.H. 6-Dehydrogingerdione Restrains Lipopolysaccharide-Induced Inflammatory Responses in Raw 264.7 Macrophages. J. Agric. Food Chem. 2014, 62, 9171–9179. [Google Scholar] [CrossRef]
- Yao, J.; Ge, C.; Duan, D.; Zhang, B.; Cui, X.; Peng, S.; Liu, Y.; Fang, J. Activation of the Phase Ii Enzymes for Neuroprotection by Ginger Active Constituent 6-Dehydrogingerdione in Pc12 Cells. J. Agric. Food Chem. 2014, 62, 5507–5518. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.O.; Jin, W.J.; Kim, B.; Kim, H.H.; Lee, Z.H. Myristoleic Acid Inhibits Osteoclast Formation and Bone Resorption by Suppressing the Rankl Activation of Src and Pyk2. Eur. J. Pharmacol. 2015, 768, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Zhang, C.; Dong, M.; Jiang, J.; Xu, H.; Yan, C.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L.; et al. Myristoleic Acid Produced by Enterococci Reduces Obesity through Brown Adipose Tissue Activation. Gut 2020, 69, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.S.; Liao, W.T.; Kuo, C.J.; Chou, C.H.; Wu, C.J.; Wang, H.M. Phthalic Acid Chemical Probes Synthesized for Protein-Protein Interaction Analysis. Int. J. Mol. Sci. 2013, 14, 12914. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xiao, S.; Cai, Q.; Miao, J.; Li, J. Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L. Foods 2023, 12, 2075. https://doi.org/10.3390/foods12102075
Wang J, Xiao S, Cai Q, Miao J, Li J. Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L. Foods. 2023; 12(10):2075. https://doi.org/10.3390/foods12102075
Chicago/Turabian StyleWang, Jin, Shuang Xiao, Qi Cai, Jing Miao, and Jinyao Li. 2023. "Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L." Foods 12, no. 10: 2075. https://doi.org/10.3390/foods12102075
APA StyleWang, J., Xiao, S., Cai, Q., Miao, J., & Li, J. (2023). Antioxidant Capacity and Protective Effects on H2O2-Induced Oxidative Damage in PC12 Cells of the Active Fraction of Brassica rapa L. Foods, 12(10), 2075. https://doi.org/10.3390/foods12102075






