Application of Persimmon Pectin with Promising Emulsification Properties as an Acidified Milk Drinks Stabilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AMDs
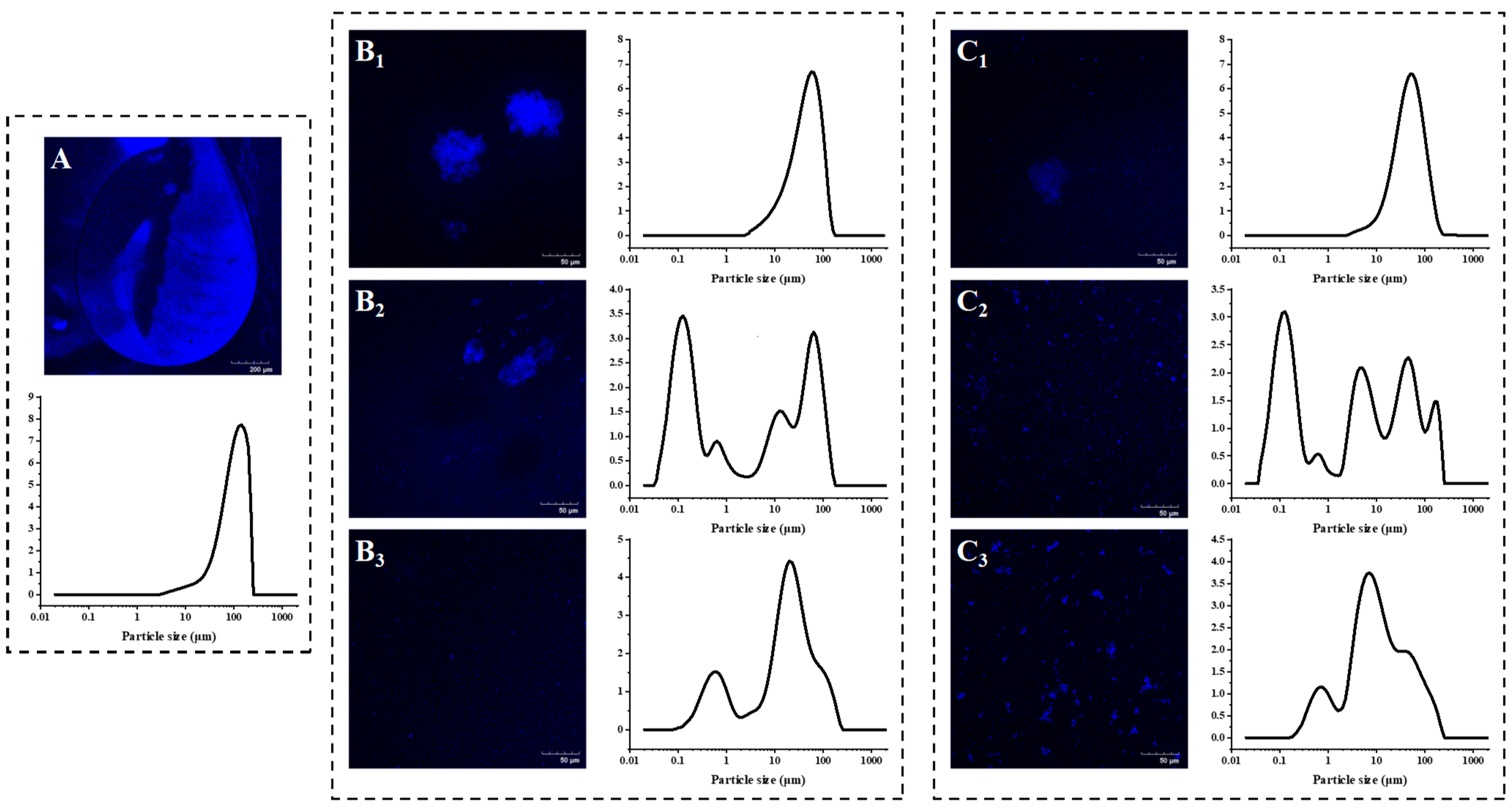
2.3. Particle Size Characterization
2.4. Micromorphology Observation
2.5. Zeta Potential and Sedimentation Measurement
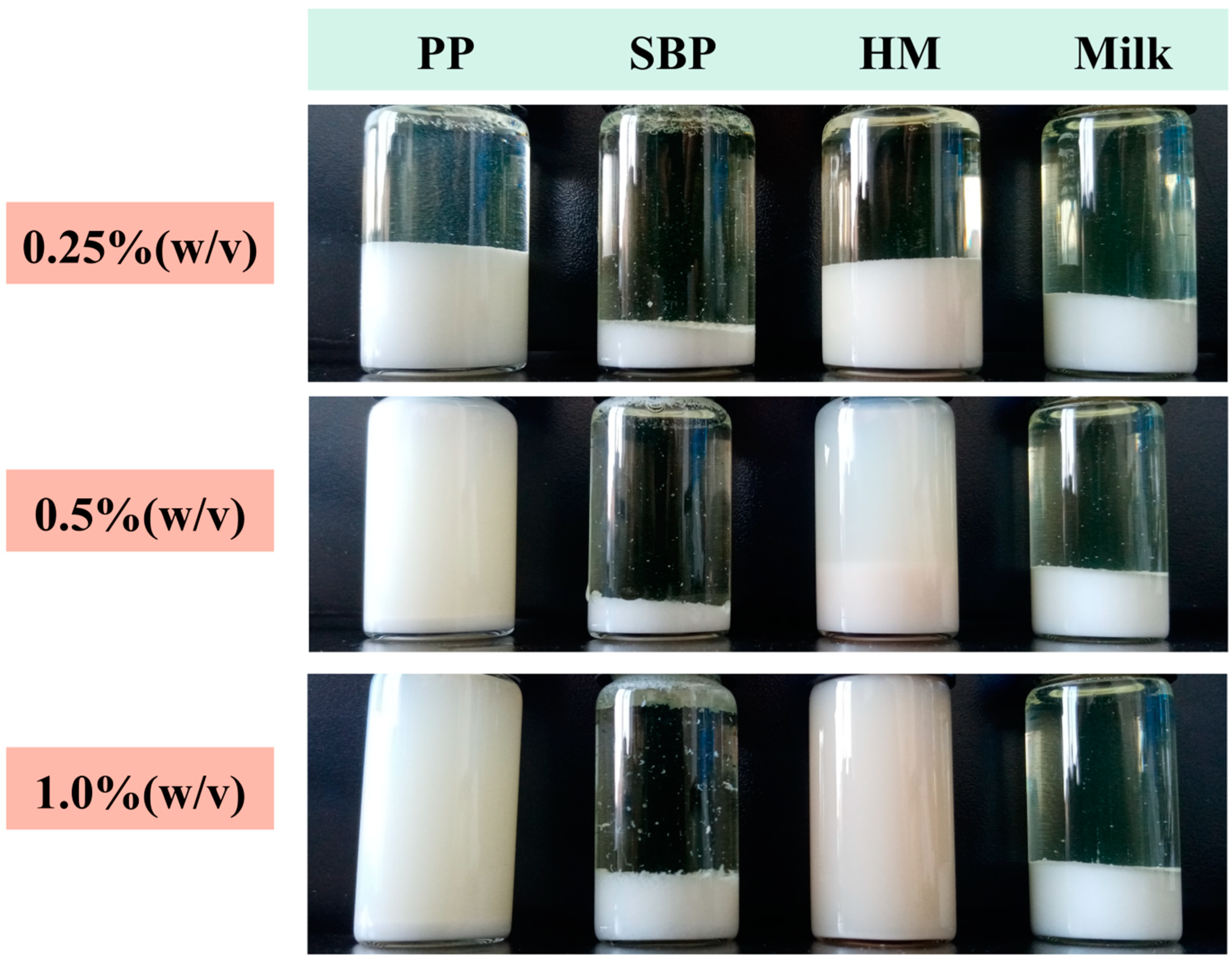
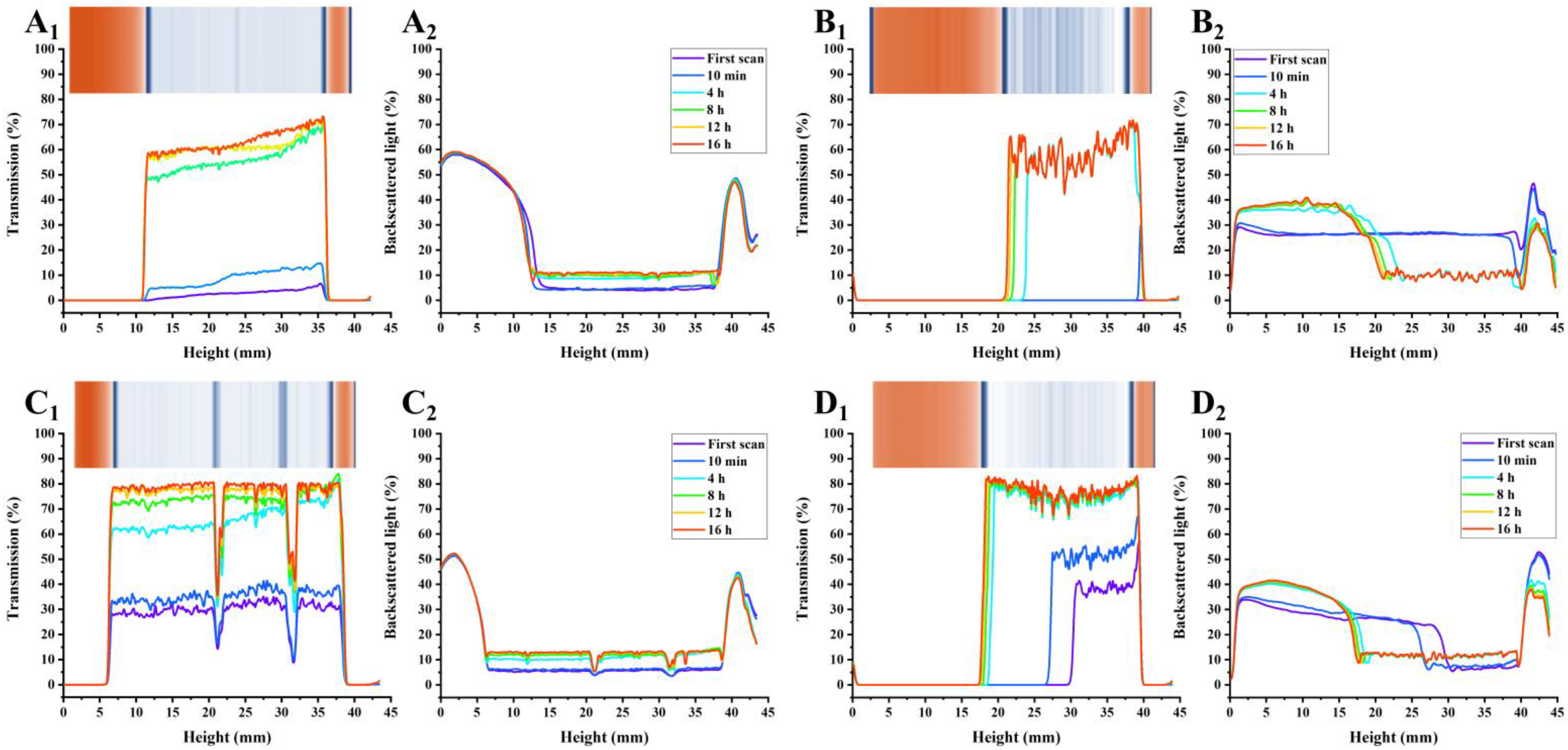
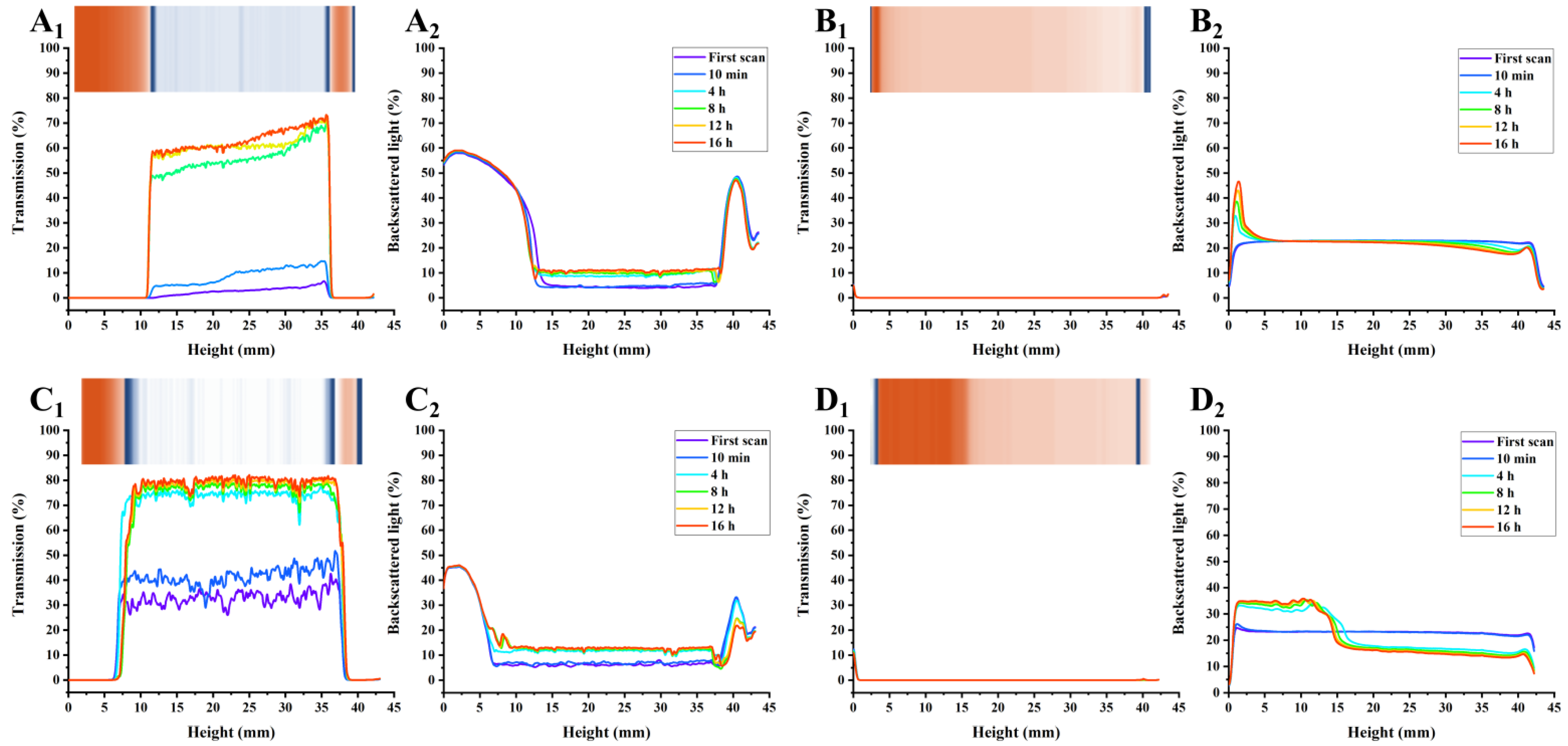
2.6. Stability Evaluation of AMDs
2.6.1. Storage Stability Evaluation of AMDs
2.6.2. Physical Stability Evaluation of AMDs
2.7. Data Analysis
3. Results
3.1. PP-Stabilzied AMDs Exhibited Smaller Particle Size and Narrower Size Distribution Compared with Commercial Pectins
3.2. PP-Stabilized AMDs Exhibited Strong Electrostatic Repulsion Than SBP and Comparable to HMP
3.3. PP Exhibited Lower Sedimentation Rate Compared with Commercial Pectins
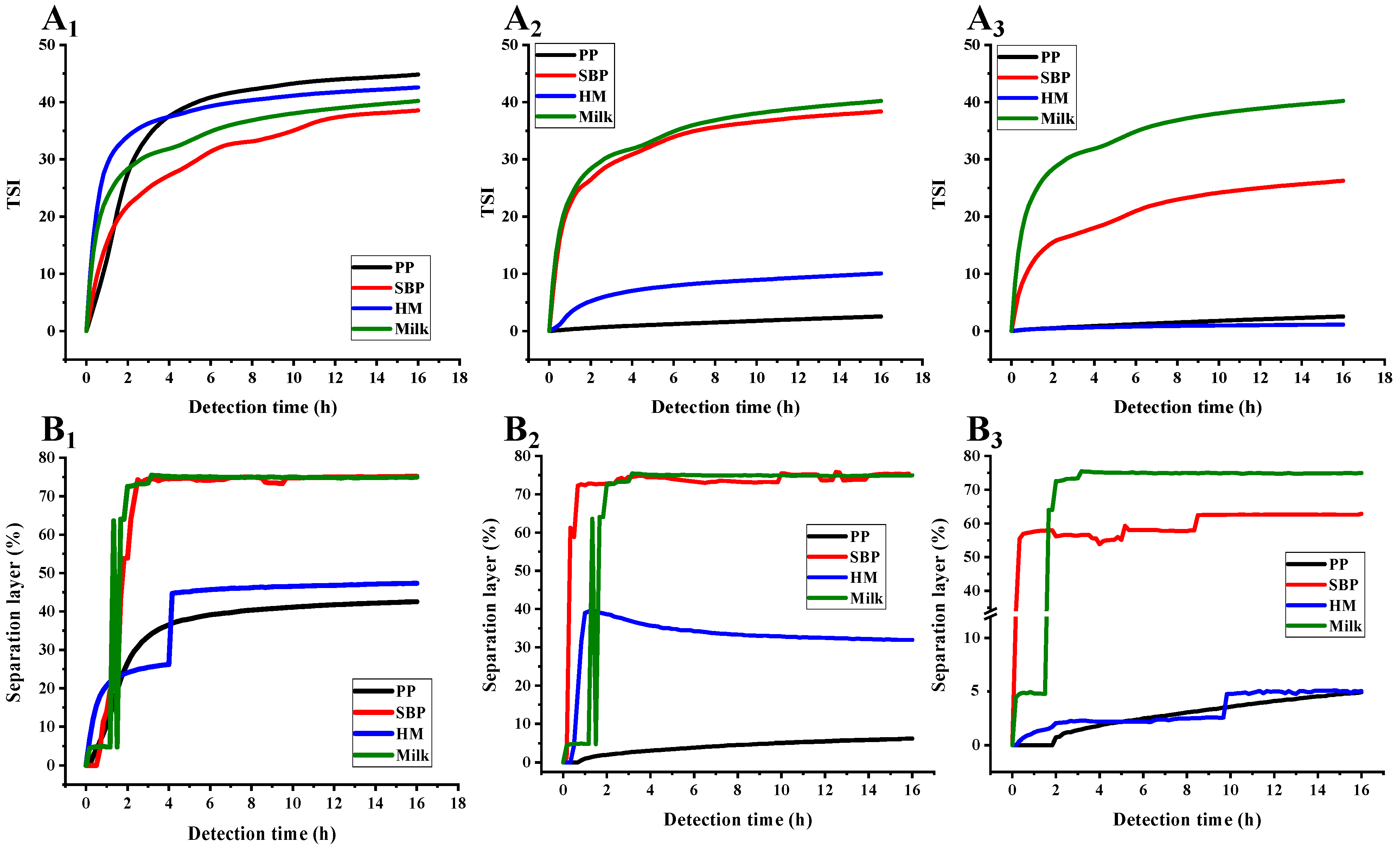
3.4. PP Exhibited Higher AMDs Storage Stability Compared with Commercial Pectins
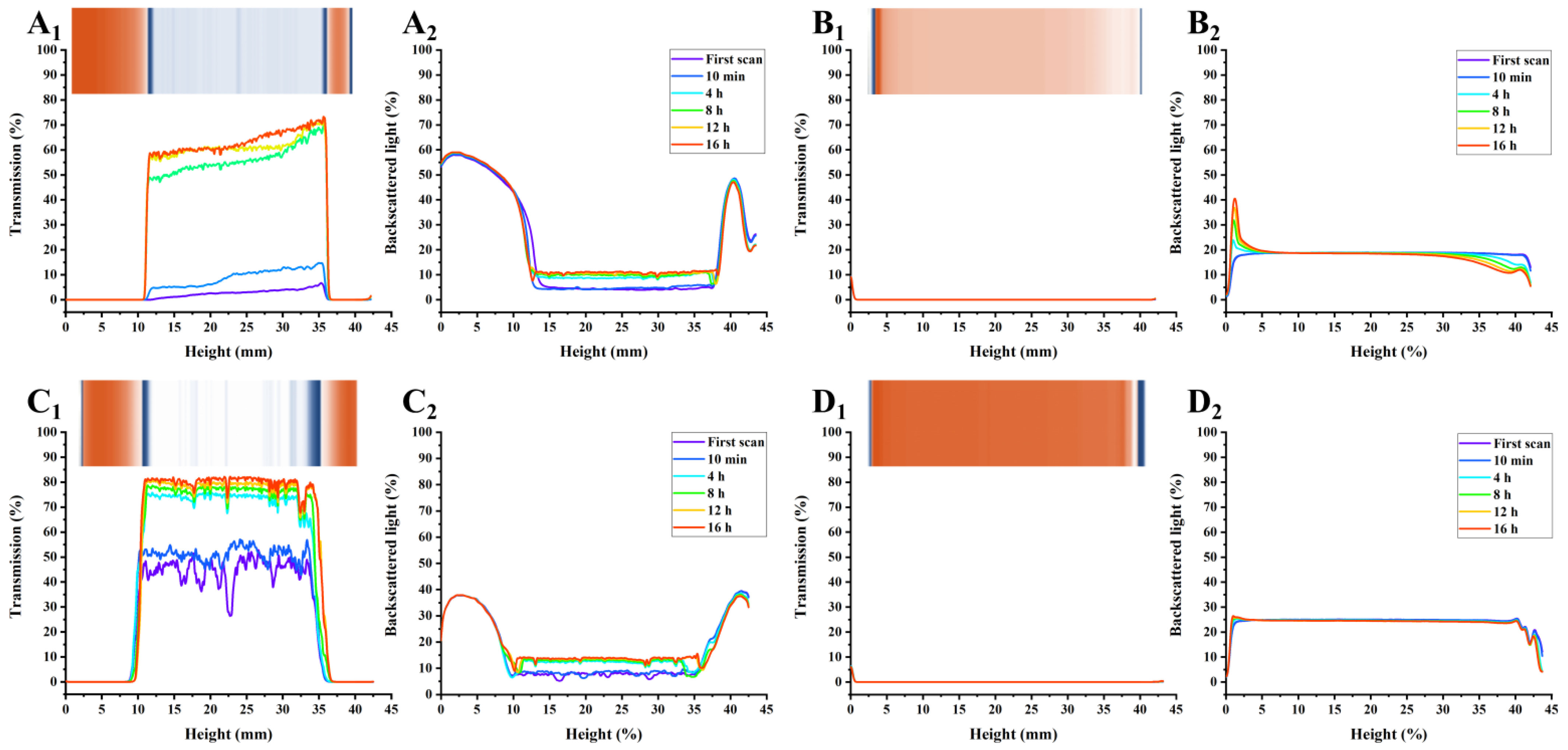
3.5. PP Exhibited Higher AMDs Stability Potential Based on Multi-Light Scattering Compared with Commercial Pectins
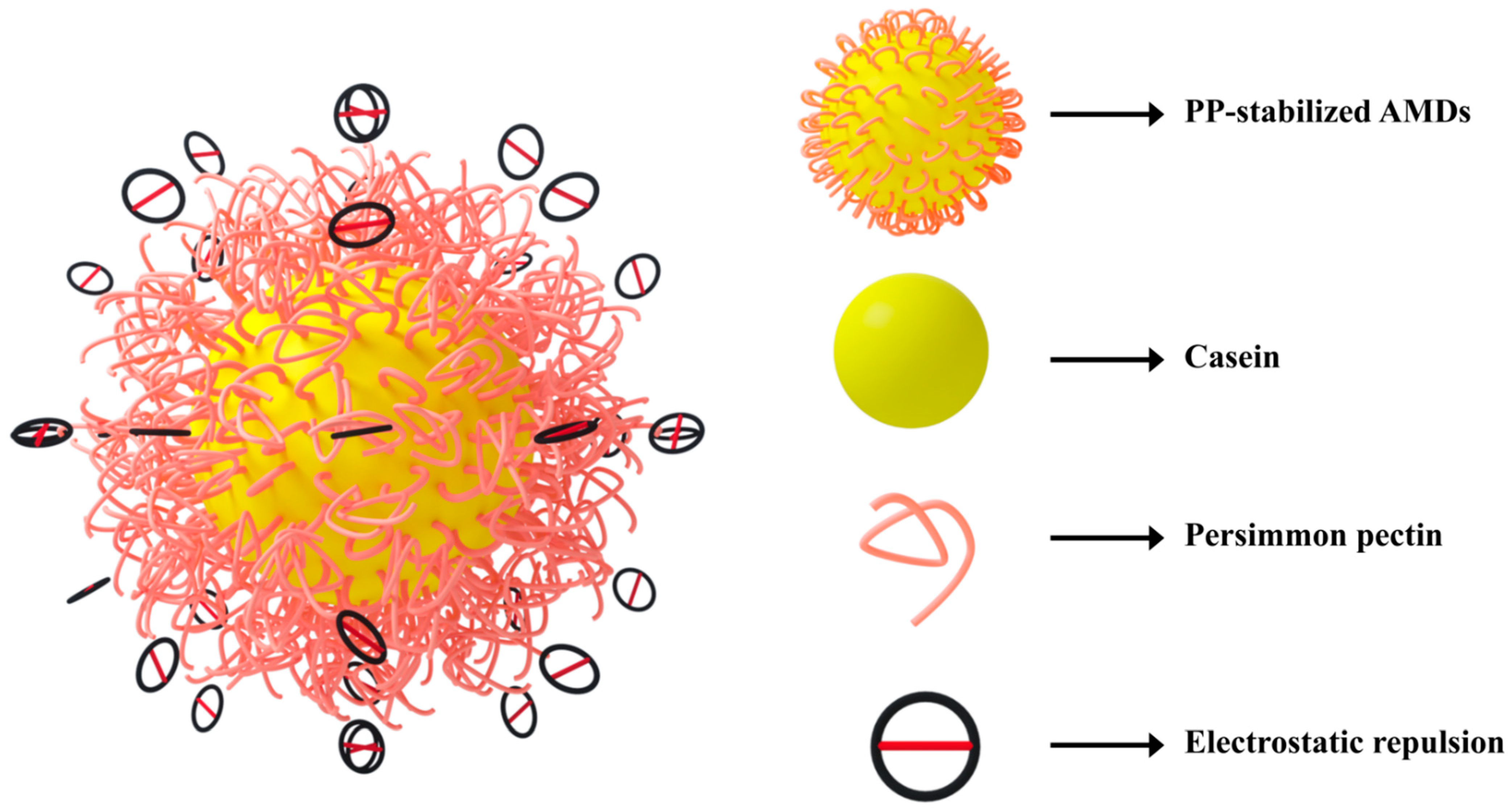
3.6. Potential Mechanism of PP as a Stabilizer for AMDs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Z.; Wu, J.; Du, B.; Zhang, H. Impact of distribution of carboxymethyl substituents in the stabilizer of carboxymethyl cellulose on the stability of acidified milk drinks. Food Hydrocoll. 2018, 76, 150–157. [Google Scholar] [CrossRef]
- Mocanu, M.; Rolin, C.; Mohammadifar, M.A.; Mohammadi, R.; Bahrami, R. The effect of sodium hexametaphosphate on the efficiency of pectin in stabilizing acidified milk drinks. Food Hydrocoll. 2021, 118, 106767. [Google Scholar] [CrossRef]
- Zhao, R.; Qi, J.; Liu, Q.; Zeng, W.; Yang, X. Fractionation and characterization of soluble soybean polysaccharide esterified of octenyl succinic anhydride and its effect as a stabilizer in acidified milk drinks. Food Hydrocoll. 2018, 85, 215–221. [Google Scholar] [CrossRef]
- Corredig, M.; Sharafbafi, N.; Kristo, E. Polysaccharide–protein interactions in dairy matrices, control and design of structures. Food Hydrocoll. 2011, 25, 1833–1841. [Google Scholar] [CrossRef]
- Cheng, M.; Qi, J.; Feng, J.; Cao, J.; Wang, J.; Yang, X. Pea soluble polysaccharides obtained from two enzyme-assisted extraction methods and their application as acidified milk drinks stabilizers. Food Res. Int. 2018, 109, 544–551. [Google Scholar] [CrossRef]
- Cai, Z.; Wei, Y.; Guo, Y.; Ma, A.; Zhang, H. Influence of the degree of esterification of soluble soybean polysaccharide on the stability of acidified milk drinks. Food Hydrocoll. 2020, 108, 106052. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, Y.; Cai, Z.; Hou, B.; Zhang, H. Stability of acidified milk drinks induced by various polysaccharide stabilizers: A review. Food Hydrocoll. 2021, 118, 106814. [Google Scholar] [CrossRef]
- Joudaki, H.; Mousavi, M.; Safari, M.; Razavi, S.H.; Emam-Djomeh, Z.; Gharibzahedi, S.M.T. A practical optimization on salt/high-methoxyl pectin interaction to design a stable formulation for Doogh. Carbohydr. Polym. 2013, 97, 376–383. [Google Scholar] [CrossRef]
- Bouhoute, M.; Taarji, N.; Vodo, S.; Kobayashi, I.; Zahar, M.; Isoda, H.; Nakajima, M.; Neves, M.A. Formation and stability of emulsions using crude extracts as natural emulsifiers from Argan shells. Colloids Surf. A Physicochem. Eng. Asp. 2020, 591, 124536. [Google Scholar] [CrossRef]
- Ozturk, B.; McClements, D.J. Progress in natural emulsifiers for utilization in food emulsions. Curr. Opin. Food Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Dominiak, M.; Søndergaard, K.M.; Wichmann, J.; Vidal-Melgosa, S.; Willats, W.G.; Meyer, A.S.; Mikkelsen, J.D. Application of enzymes for efficient extraction, modification, and development of functional properties of lime pectin. Food Hydrocoll. 2014, 40, 273–282. [Google Scholar] [CrossRef]
- Kermani, Z.J.; Shpigelman, A.; Pham, H.T.T.; Van Loey, A.M.; Hendrickx, M.E. Functional properties of citric acid extracted mango peel pectin as related to its chemical structure. Food Hydrocoll. 2015, 44, 424–434. [Google Scholar] [CrossRef]
- Naqash, F.; Masoodi, F.; Rather, S.A.; Wani, S.; Gani, A. Emerging concepts in the nutraceutical and functional properties of pectin—A Review. Carbohydr. Polym. 2017, 168, 227–239. [Google Scholar] [CrossRef]
- Ngouémazong, E.D.; Christiaens, S.; Shpigelman, A.; Van Loey, A.; Hendrickx, M. The emulsifying and emulsion-stabilizing properties of pectin: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 705–718. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Pectin at the oil-water interface: Relationship of molecular composition and structure to functionality. Food Hydrocoll. 2017, 68, 211–218. [Google Scholar] [CrossRef]
- Guo, X.; Meng, H.; Tang, Q.; Pan, R.; Zhu, S.; Yu, S. Effects of the precipitation pH on the ethanolic precipitation of sugar beet pectins. Food Hydrocoll. 2016, 52, 431–437. [Google Scholar] [CrossRef]
- Sun, W.; Yang, W.; Zheng, Y.; Zhang, H.; Fang, H.; Liu, D.; Kong, X.; Chen, S.; Ye, X.; Tian, J. Effect of potato pulp pectic polysaccharide on the stability of acidified milk drinks. Molecules 2020, 25, 5632. [Google Scholar] [CrossRef]
- Jensen, S.; Rolin, C.; Ipsen, R. Stabilisation of acidified skimmed milk with HM pectin. Food Hydrocoll. 2010, 24, 291–299. [Google Scholar] [CrossRef]
- Liang, L.; Luo, Y. Casein and pectin: Structures, interactions, and applications. Trends Food Sci. Technol. 2020, 97, 391–403. [Google Scholar]
- Liu, J.; Nakamura, A.; Corredig, M. Addition of pectin and soy soluble polysaccharide affects the particle size distribution of casein suspensions prepared from acidified skim milk. J. Agric. Food Chem. 2006, 54, 6241–6246. [Google Scholar] [CrossRef]
- Jia, Y.; Khalifa, I.; Hu, L.; Zhu, W.; Li, J.; Li, K.; Li, C. Influence of three different drying techniques on persimmon chips’ characteristics: A comparison study among hot-air, combined hot-air-microwave, and vacuum-freeze drying techniques. Food Bioprod. Process. 2019, 118, 67–76. [Google Scholar] [CrossRef]
- Mamet, T.; Ge, Z.; Zhang, Y.; Li, C. Interactions between highly galloylated persimmon tannins and pectins. Int. J. Biol. Macromol. 2018, 106, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Du, J.; Li, K.; Li, C. Emulsification mechanism of persimmon pectin with promising emulsification capability and stability. Food Hydrocoll. 2022, 131, 107727. [Google Scholar] [CrossRef]
- Jia, Y.; Khalifa, I.; Dang, M.; Zhang, Y.; Zhu, L.; Zhao, M.; Li, K.; Li, C. Confirmation and understanding the potential emulsifying characterization of persimmon pectin: From structural to diverse rheological aspects. Food Hydrocoll. 2022, 131, 107738. [Google Scholar] [CrossRef]
- Calhoun, W.; Maeta, H.; Roy, S.; Bali, L.; Bali, S. Sensitive real-time measurement of the refractive index and attenuation coefficient of milk and milk-cream mixtures. J. Dairy Sci. 2010, 93, 3497–3504. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Tao, R.; Li, R.; Wang, P.; Ma, A.; Zhang, H. Comparative studies on the stabilization of pea protein dispersions by using various polysaccharides. Food Hydrocoll. 2020, 98, 105233. [Google Scholar] [CrossRef]
- Karimi, N.; Sani, A.M.; Pourahmad, R. Influence of carboxy methyl cellulose (CMC) and pectin on rheological, physical stability and sensory properties of milk and concentrated jujuba mixture. J. Food Meas. Charact. 2016, 10, 396–404. [Google Scholar] [CrossRef]
- Sejersen, M.T.; Salomonsen, T.; Ipsen, R.; Clark, R.; Rolin, C.; Engelsen, S.B. Zeta potential of pectin-stabilised casein aggregates in acidified milk drinks. Int. Dairy J. 2007, 17, 302–307. [Google Scholar] [CrossRef]
- Verkempinck, S.; Kyomugasho, C.; Salvia-Trujillo, L.; Denis, S.; Bourgeois, M.; Van Loey, A.; Hendrickx, M.; Grauwet, T. Emulsion stabilizing properties of citrus pectin and its interactions with conventional emulsifiers in oil-in-water emulsions. Food Hydrocoll. 2018, 85, 144–157. [Google Scholar] [CrossRef]
| Control | PP | SBP | HMP | Control | PP | SBP | HMP | ||
|---|---|---|---|---|---|---|---|---|---|
| Concentrations | D32 (μm) | Concentrations | D43 (μm) | ||||||
| 0.25% (w/v) | 64.41 ± 1.66 | 32.81 ± 2.83↓ aA | 81.77 ± 1.93↑ bA | 29.51 ± 1.22↓ cA | 0.25% (w/v) | 113.20 ± 1.90 | 55.52 ± 5.60↓ aA | 184.49 ± 5.53↑ bA | 62.61 ± 13.04↓ aA |
| 0.5% (w/v) | 0.28 ± 0.10↓ aB | 84.35 ± 2.62↑ bB | 0.35 ± 0.11↓ aB | 0.5% (w/v) | 28.79 ± 4.62↓ aB | 135.86 ± 2.01↑ bB | 33.15 ± 6.76↓ aB | ||
| 1.0% (w/v) | 3.97 ± 1.02↓ aC | 83.47 ± 1.40↑ bAB | 1.97 ± 0.76↓ cC | 1.0% (w/v) | 27.05 ± 5.43↓ aB | 111.94 ± 47.90↑ bB | 29.06 ± 5.64↓ aB | ||
| Control | PP | SBP | HMP | Control | PP | SBP | HMP | |
|---|---|---|---|---|---|---|---|---|
| Concentrations | Zeta Potential (mV) | Sedimentation Rate (%) | ||||||
| 0.25% (w/v) | 1.70 ± 0.54 | −19.86 ± 1.18↓ aA | −5.14 ± 1.15↓ bA | −18.52 ± 2.61↓ aA | 34.03 ± 0.01 | 30.78 ± 2.54↓ aA | 37.20 ± 1.48↑ bA | 28.84 ± 3.39↓ aA |
| 0.5% (w/v) | −25.11 ± 0.88↓ aB | −5.98 ± 1.36↓ bA | −24.74 ± 1.26↓ aB | 19.00 ± 4.58↓ aB | 35.00 ± 1.00↑ bAB | 20.33 ± 1.53↓ aB | ||
| 1.0% (w/v) | −26.80 ± 0.94↓ aB | −22.71 ± 2.81↓ bB | −28.63 ± 0.43↓ cC | 16.74 ± 2.65↓ aB | 31.75 ± 2.08↓ bB | 17.54 ± 1.65↓ aB | ||
| Concentrations | PP (Fresh) | PP (Storage) | SBP (Fresh) | SBP (Storage) | HMP (Fresh) | HMP (Storage) |
|---|---|---|---|---|---|---|
| 0.25% (w/v) | 32.81 ± 2.83 | 37.41 ± 4.96 a | 81.77 ± 1.93 | 85.40 ± 8.93 b | 29.51 ± 1.22 | 30.20 ± 1.60 a |
| 0.5% (w/v) | 0.28 ± 0.10 | 6.74 ± 0.87 *a | 84.35 ± 2.62 | 123.38 ± 2.10 *b | 0.35 ± 0.11 | 13.47 ± 0.50 *c |
| 1.0% (w/v) | 3.97 ± 1.02 | 8.07 ± 0.33 *a | 83.47 ± 1.40 | 226.79 ± 12.64 *b | 1.97 ± 0.76 | 11.67 ± 1.03 *a |
| Concentrations | PP (Fresh) | PP (Storage) | SBP (Fresh) | SBP (Storage) | HMP (Fresh) | HMP (Storage) |
|---|---|---|---|---|---|---|
| 0.25% (w/v) | 55.52 ± 5.60 | 69.25 ± 4.03 *a | 184.49 ± 5.53 | 248.99 ± 68.30 *b | 62.61 ± 13.04 | 54.48 ± 2.91 a |
| 0.5% (w/v) | 28.79 ± 4.62 | 21.84 ± 6.26 a | 135.86 ± 2.01 | 300.10 ± 20.31 *b | 33.15 ± 6.76 | 34.85 ± 1.38 a |
| 1.0% (w/v) | 27.05 ± 5.43 | 14.77 ± 2.81 *a | 111.94 ± 47.90 | 551.74 ± 34.44 *b | 29.06 ± 5.64 | 38.23 ± 3.96 *c |
| Concentrations | PP (Fresh) | PP (Storage) | SBP (Fresh) | SBP (Storage) | HMP (Fresh) | HMP (Storage) |
|---|---|---|---|---|---|---|
| 0.25% (w/v) | −19.86 ± 1.18 | −13.77 ± 3.33 a | −5.14 ± 1.15 | −1.97 ± 0.44 *b | −18.52 ± 2.61 | −10.80 ± 3.31 *a |
| 0.5% (w/v) | −25.11 ± 0.88 | −24.94 ± 0.30 a | −5.98 ± 1.36 | −1.81 ± 0.70 *b | −25.98 ± 1.36 | −25.12 ± 1.22 a |
| 1.0% (w/v) | −26.80 ± 0.94 | −25.92 ± 0.89 a | −22.71 ± 2.81 | −18.20 ± 0.89 *b | −28.63 ± 0.43 | −26.79 ± 0.81 *a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Jia, Y.; Zhang, X.; Zhang, Y.; Dang, M.; Li, C. Application of Persimmon Pectin with Promising Emulsification Properties as an Acidified Milk Drinks Stabilizer. Foods 2023, 12, 2042. https://doi.org/10.3390/foods12102042
Hu L, Jia Y, Zhang X, Zhang Y, Dang M, Li C. Application of Persimmon Pectin with Promising Emulsification Properties as an Acidified Milk Drinks Stabilizer. Foods. 2023; 12(10):2042. https://doi.org/10.3390/foods12102042
Chicago/Turabian StyleHu, Lanlan, Yangyang Jia, Xiaoxiao Zhang, Yajie Zhang, Meizhu Dang, and Chunmei Li. 2023. "Application of Persimmon Pectin with Promising Emulsification Properties as an Acidified Milk Drinks Stabilizer" Foods 12, no. 10: 2042. https://doi.org/10.3390/foods12102042
APA StyleHu, L., Jia, Y., Zhang, X., Zhang, Y., Dang, M., & Li, C. (2023). Application of Persimmon Pectin with Promising Emulsification Properties as an Acidified Milk Drinks Stabilizer. Foods, 12(10), 2042. https://doi.org/10.3390/foods12102042





