Detection of Ciguatoxins and Tetrodotoxins in Seafood with Biosensors and Other Smart Bioanalytical Systems
Abstract
1. Introduction
2. Biorecognition Elements
2.1. Cells
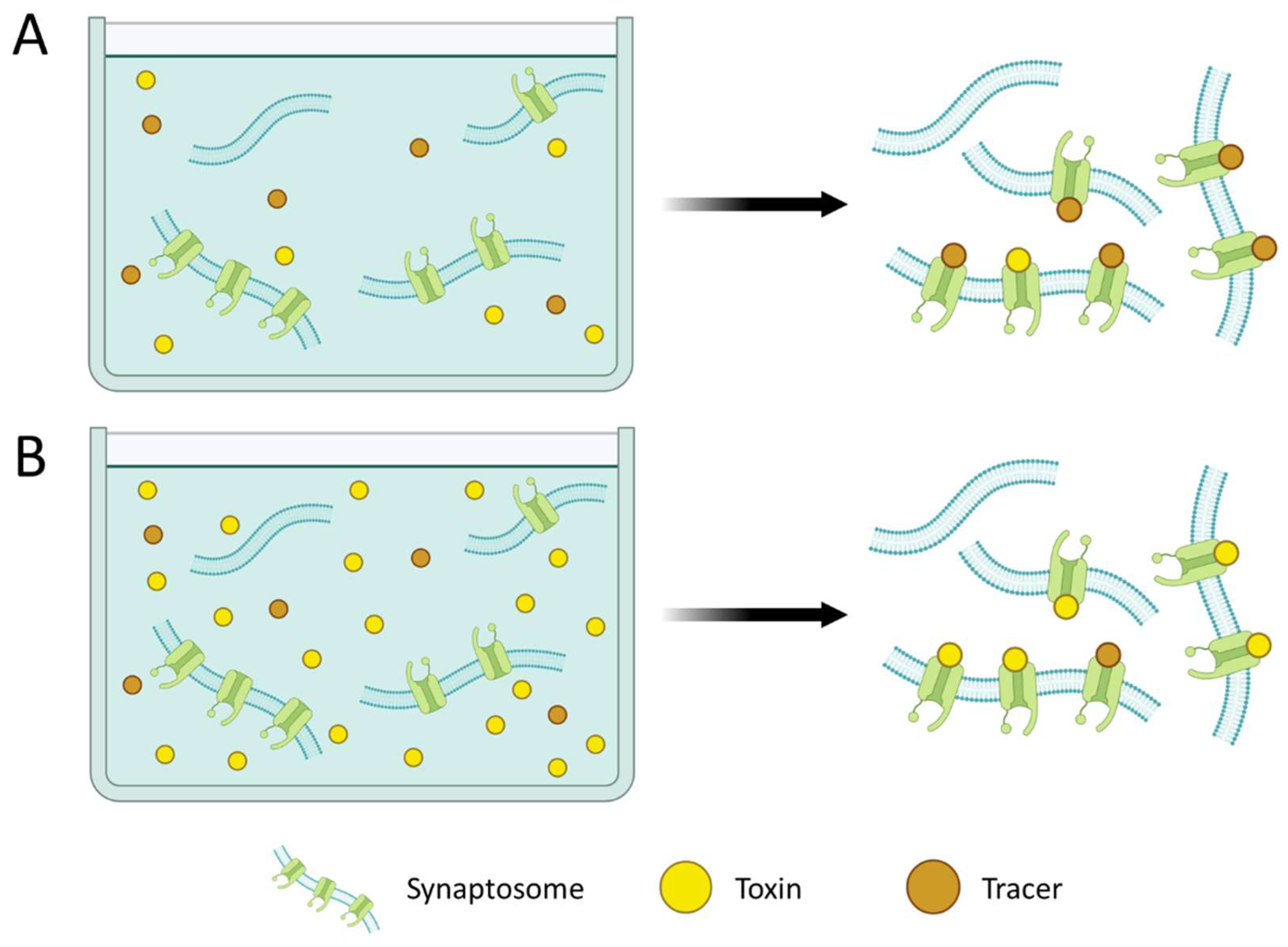
2.2. Receptors
2.3. Antibodies
2.4. Aptamers
3. Biosensors and Other Smart Bioanalytical Systems
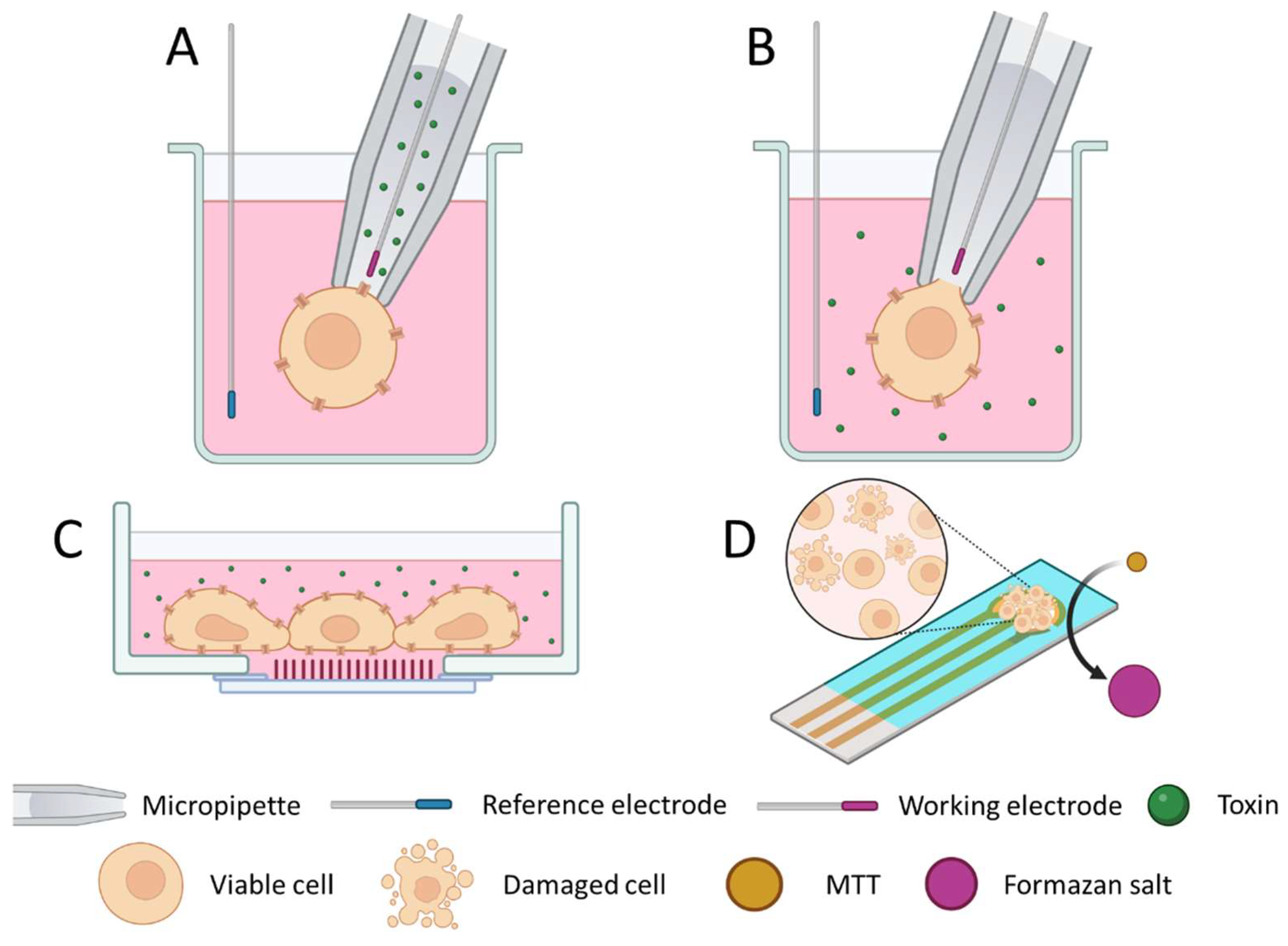
3.1. Cell-Based Biosensors
3.2. Receptor Binding Biosensors
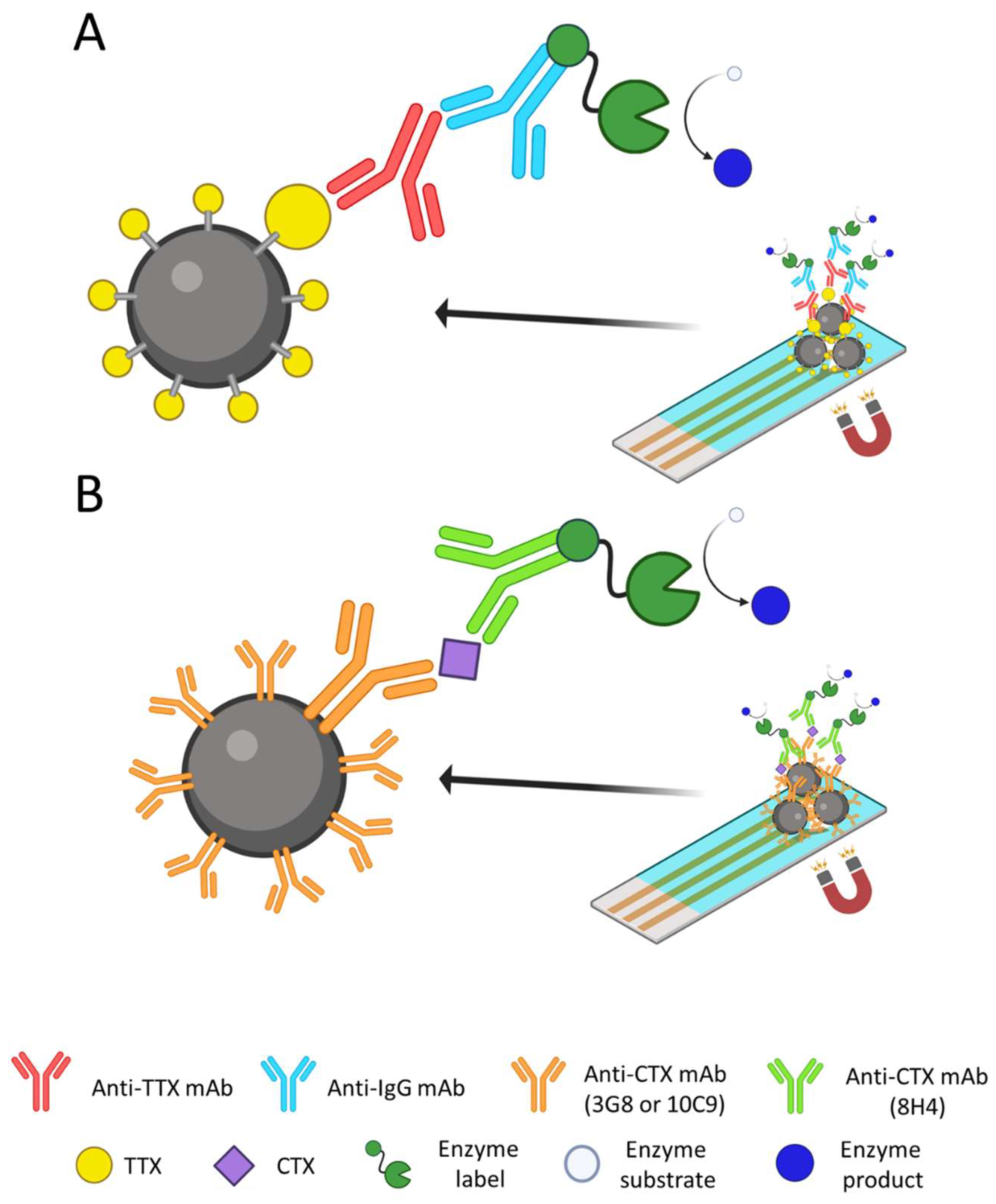
3.3. Immunosensors
3.4. Aptamer-Based Biosensors
4. Analysis of Samples
5. Comparison among Techniques
6. Miniaturization and Automation
7. Quality Control
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vilariño, N.; Louzao, M.C.; Abal, P.; Cagide, E.; Carrera, C.; Vieytes, M.R.; Botana, L.M. Human Poisoning from Marine Toxins: Unknowns for Optimal Consumer Protection. Toxins 2018, 10, 324. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Soliño, L.; Carnicer, O.; Diogène, J.; Campàs, M. Alternative Methods for the Detection of Emerging Marine Toxins: Biosensors, Biochemical Assays and Cell-Based Assays. Mar. Drugs 2014, 12, 5719–5763. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Gatti, C.M.I.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera Poisonings: A Global Review of Occurrences and Trends. Harmful Algae 2021, 102, 101873. [Google Scholar] [CrossRef] [PubMed]
- FAO. WHO Report of the Expert Meeting on Ciguatera Poisoning; Rome, 19–23 November 2018. Food Saf. Qual. No. 9; FAO: Rome, Italy, 2020. [Google Scholar]
- Jacques, Y.; Romey, G.; Cavey, M.T.; Kartalovski, B.; Lazdunski, M. Interaction of Pyrethroids with the Na+ Channel in Mammalian Neuronal Cells in Culture. Biochim. Biophys. Acta (BBA)-Biomembr. 1980, 600, 882–897. [Google Scholar] [CrossRef]
- L’Herondelle, K.; Talagas, M.; Mignen, O.; Misery, L.; Le Garrec, R. Neurological Disturbances of Ciguatera Poisoning: Clinical Features and Pathophysiological Basis. Cells 2020, 9, 2291. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Intoxicación Alimentaria Por Ciguatoxinas. Brotes y Casos Registrados Por El SVEICC* Según Fecha, Isla, Especie y Peso de Pescado Implicada y Confirmación de Presencia de Toxina. Canarias. 2008–2022. Available online: https://www3.gobiernodecanarias.org/sanidad/scs/content/3afef0ad-5ada-11ed-8f3c-13be511c2d56/CuadroBrotes_2008-2022.pdf (accessed on 28 April 2023).
- Costa, P.R.; Estevez, P.; Castro, D.; Soliño, L.; Gouveia, N.; Santos, C.; Rodrigues, S.M.; Leao, J.M.; Gago-Martínez, A. New Insights into the Occurrence and Toxin Profile of Ciguatoxins in Selvagens Islands (Madeira, Portugal). Toxins 2018, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Estevez, P.; Castro, D.; Pequeño-Valtierra, A.; Leao, J.M.; Vilariño, O.; Diogène, J.; Gago-Martínez, A. An Attempt to Characterize the Ciguatoxin Profile in Seriola fasciata Causing Ciguatera Fish Poisoning in Macaronesia. Toxins 2019, 11, 221. [Google Scholar] [CrossRef]
- Costa, P.R.; Estévez, P.; Soliño, L.; Castro, D.; Rodrigues, S.M.; Timoteo, V.; Leao-Martins, J.M.; Santos, C.; Gouveia, N.; Diogène, J.; et al. An Update on Ciguatoxins and CTX-like Toxicity in Fish from Different Trophic Levels of the Selvagens Islands (NE Atlantic, Madeira, Portugal). Toxins 2021, 13, 580. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F.; Caillaud, A.; Diogène, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus Sp. Nov. (Dinophyceae), a Benthic Toxic Dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 2011, 11, 10–22. [Google Scholar] [CrossRef]
- Rodríguez, F.; Fraga, S.; Ramilo, I.; Rial, P.; Figueroa, R.I.; Riobó, P.; Bravo, I. Canary Islands (NE Atlantic) as a Biodiversity ‘Hotspot’ of Gambierdiscus: Implications for Future Trends of Ciguatera in the Area. Harmful Algae 2017, 67, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Bravo, I.; Rodriguez, F.; Ramilo, I.; Rial, P.; Fraga, S. Ciguatera-Causing Dinoflagellate Gambierdiscus Spp. (Dinophyceae) in a Subtropical Region of North Atlantic Ocean (Canary Islands): Morphological Characterization and Biogeography. Toxins 2019, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Tudó, À.; Gaiani, G.; Varela, M.R.; Tsumuraya, T.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Diogène, J. Further Advance of Gambierdiscus Species in the Canary Islands, with the First Report of Gambierdiscus belizeanus. Toxins 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Reverté, L.; Toldrà, A.; Andree, K.B.; Fraga, S.; de Falco, G.; Campàs, M.; Diogène, J. Assessment of Cytotoxicity in Ten Strains of Gambierdiscus australes from Macaronesian Islands by Neuro-2a Cell-Based Assays. J. Appl. Phycol. 2018, 30, 2447–2461. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Kretzschmar, A.L.; Kaufmann, M.J.; Murray, S.A. Morphological and Molecular Phylogenetic Identification and Record Verification of Gambierdiscus excentricus (Dinophyceae) from Madeira Island (NE Atlantic Ocean). Mar. Biodivers. Rec. 2019, 12, 16. [Google Scholar] [CrossRef]
- Aligizaki, K.; Nikolaidis, G. Morphological Identification of Two Tropical Dinoflagellates of the Genera Gambierdiscus and Sinophysis in the Mediterranean Sea. J. Biol. Res. 2008, 9, 75–82. [Google Scholar]
- Tudó, À.; Toldrà, A.; Rey, M.; Todolí, I.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Sureda, F.X.; Diogène, J. Gambierdiscus and Fukuyoa as Potential Indicators of Ciguatera Risk in the Balearic Islands. Harmful Algae 2020, 99, 101913. [Google Scholar] [CrossRef]
- Gaiani, G.; Cucchi, F.; Toldrà, A.; Andree, K.B.; Rey, M.; Tsumuraya, T.; O’Sullivan, C.K.; Diogène, J.; Campàs, M. Electrochemical Biosensor for the Dual Detection of Gambierdiscus australes and Gambierdiscus excentricus in Field Samples. First Report of G. excentricus in the Balearic Islands. Sci. Total Environ. 2022, 806, 150915. [Google Scholar] [CrossRef]
- US FDA. Fish and Fishery Products Hazards and Controls Guidance; Department of Health and Human Services, Public Health Services, Food and Drug Administration Center for Food Safety and Applied Nutrition, Office of Food Safety: College Park, MA, USA, 2022. Available online: https://www.fda.gov/media/80637/download (accessed on 28 April 2023).
- Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02004R0853-20230215 (accessed on 28 April 2023).
- Suehiro, M. Historical Review of Medical and Chemical Research on Globefish Toxin, Tetrodotoxin. Rev. Hist. Pharm. 1996, 44, 379–380. [Google Scholar] [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An Updated Review of Tetrodotoxin and Its Peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef]
- Biessy, L.; Boundy, M.J.; Smith, K.F.; Harwood, D.T.; Hawes, I.; Wood, S.A. Tetrodotoxin in Marine Bivalves and Edible Gastropods: A Mini-Review. Chemosphere 2019, 236, 124404. [Google Scholar] [CrossRef] [PubMed]
- Narahashi, T. Tetrodotoxin: A Brief History. Proc. Jpn. Acad. Ser. B 2008, 84, 147–154. [Google Scholar] [CrossRef]
- How, C.K.; Chern, C.H.; Huang, Y.C.; Wang, L.M.; Lee, C.H. Tetrodotoxin Poisoning. Am. J. Emerg. Med. 2003, 21, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the Pufferfish Toxin Tetrodotoxin in European Bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First Detection of Tetrodotoxin in Greek Shellfish by UPLC-MS/MS Potentially Linked to the Presence of the Dinoflagellate Prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef] [PubMed]
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.L.A.P. First Report on the Occurrence of Tetrodotoxins in Bivalve Mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New Gastropod Vectors and Tetrodotoxin Potential Expansion in Temperate Waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.; Alfonso, A.; Vale, C.; Alfonso, C.; Vale, P.; Tellez, A.; Botana, L.M. First Toxicity Report of Tetrodotoxin and 5,6,11-TrideoxyTTX in the Trumpet Shell Charonia lampas lampas in Europe. Anal. Chem. 2008, 80, 5622–5629. [Google Scholar] [CrossRef]
- Leão, J.M.; Lozano-Leon, A.; Giráldez, J.; Vilariño, Ó.; Gago-Martínez, A. Preliminary Results on the Evaluation of the Occurrence of Tetrodotoxin Associated to Marine Vibrio spp. in Bivalves from the Galician Rias (Northwest of Spain). Mar. Drugs 2018, 16, 81. [Google Scholar] [CrossRef]
- Dell’Aversano, C.; Tartaglione, L.; Polito, G.; Dean, K.; Giacobbe, M.; Casabianca, S.; Capellacci, S.; Penna, A.; Turner, A.D. First Detection of Tetrodotoxin and High Levels of Paralytic Shellfish Poisoning Toxins in Shellfish from Sicily (Italy) by Three Different Analytical Methods. Chemosphere 2019, 215, 881–892. [Google Scholar] [CrossRef]
- Bordin, P.; Dall’Ara, S.; Tartaglione, L.; Antonelli, P.; Calfapietra, A.; Varriale, F.; Guiatti, D.; Milandri, A.; Dell’Aversano, C.; Arcangeli, G.; et al. First Occurrence of Tetrodotoxins in Bivalve Mollusks from Northern Adriatic Sea (Italy). Food Control 2021, 120, 107510. [Google Scholar] [CrossRef]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Réveillon, D.; Savar, V.; Schaefer, E.; Chevé, J.; Halm-Lemeille, M.P.; Hervio-Heath, D.; Travers, M.A.; Abadie, E.; Rolland, J.L.; Hess, P. Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins 2021, 13, 740. [Google Scholar] [CrossRef]
- Noguchi, T.; Ebesu, J.S.M. Puffer Poisoning: Epidemiology and Treatment. J. Toxicol. Toxin Rev. 2001, 20, 1–10. [Google Scholar] [CrossRef]
- Regulation (EC) No 854/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Rules for the Organisation of Official Controls on Products of Animal Origin Intended for Human Consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32004R0854 (accessed on 28 April 2023).
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for Public Health Related to the Presence of Tetrodotoxin (TTX) and TTX Analogues in Marine Bivalves and Gastropods. EFSA J. 2017, 15, e04752. [Google Scholar]
- Munday, R. Toxicology of Seafood Toxins: Animal Studies and Mechanisms of Action. In Comprehensive Analytical Chemistry: Recent Advances in the Analysis of Marine Toxins; Elsevier: Amsterdam, The Netherlands, 2017; Volume 78, pp. 211–225. [Google Scholar]
- Suzuki, T.; Uchida, H.; Watanabe, R. LC/MS Analysis of Marine Toxins. In Comprehensive Analytical Chemistry: Recent Advances in the Analysis of Marine Toxins; Elsevier: Amsterdam, The Netherlands, 2017; Volume 78, pp. 137–192. [Google Scholar]
- Vilariño, N.; Louzao, M.C.; Vieytes, M.R.; Botana, L.M. Biological Methods for Marine Toxin Detection. Anal. Bioanal. Chem. 2010, 397, 1673–1681. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 15/2011 of 10 January 2011 Amending Regulation (EC) No 2074/2005 as Regards Recognised Testing Methods for Detecting Marine Biotoxins in Live Bivalve Molluscs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32011R0015 (accessed on 28 April 2023).
- Abal, P.; Louzao, M.C.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Acute Toxicity Assessment: Macroscopic and Ultrastructural Effects in Mice Treated with Oral Tetrodotoxin. Toxins 2019, 11, 305. [Google Scholar] [CrossRef]
- Munday, R.; Murray, S.; Rhodes, L.L.; Larsson, M.E.; Harwood, D.T. Ciguatoxins and Maitotoxins in Extracts of Sixteen Gambierdiscus Isolates and One Fukuyoa Isolate from the South Pacific and Their Toxicity to Mice by Intraperitoneal and Oral Administration. Mar. Drugs 2017, 15, 208. [Google Scholar] [CrossRef] [PubMed]
- Abal, P.; Louzao, M.C.; Antelo, A.; Alvarez, M.; Cagide, E.; Vilariño, N.; Vieytes, M.R.; Botana, L.M. Acute Oral Toxicity of Tetrodotoxin in Mice: Determination of Lethal Dose 50 (LD50) and No Observed Adverse Effect Level (NOAEL). Toxins 2017, 9, 75. [Google Scholar] [CrossRef]
- Fessard, V. Cytotoxicity Assays: Identification of Toxins and Mechanism of Action. In Comprehensive Analytical Chemistry: Recent Advances in the Analysis of Marine Toxins; Elsevier: Amsterdam, The Netherlands, 2017; Volume 78, pp. 231–275. [Google Scholar]
- Kogure, K.; Tamplin, M.L.; Simidu, U.; Colwell, R.R. A Tissue Culture Assay for Tetrodotoxin, Saxitoxin and Related Toxins. Toxicon 1988, 26, 191–197. [Google Scholar] [CrossRef]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerforg, J.M.; Wekell, M.M. Tetrazolium-Based Cell Bioassay for Neurotoxins Active on Voltage-Sensitive Sodium Channels: Semiautomated Assay for Saxitoxins, Brevetoxins, and Ciguatoxins. Anal. Biochem. 1993, 214, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Manger, R.L.; Leja, L.S.; Lee, S.Y.; Hungerford, J.M.; Hokama, Y.; Dickey, R.W.; Granade, H.R.; Lewis, R.; Yasumoto, T.; Wekell, M.M. Detection of Sodium Channel Toxins: Directed Cytotoxicity Assays of Purified Ciguatoxins, Brevetoxins, Saxitoxins, and Seafood Extracts. J. AOAC Int. 1995, 78, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Vale, C.; Antelo, A.; Hirama, M.; Yamashita, S.; Vieytes, M.R.; Botana, L.M. Differential Effects of Ciguatoxin and Maitotoxin in Primary Cultures of Cortical Neurons. Chem. Res. Toxicol. 2014, 27, 1387–1400. [Google Scholar] [CrossRef] [PubMed]
- Toh, M.F.; Brooks, J.M.; Strassmaier, T.; Haedo, R.J.; Puryear, C.B.; Roth, B.L.; Ouk, K.; Pin, S.S. Application of High-Throughput Automated Patch-Clamp Electrophysiology to Study Voltage-Gated Ion Channel Function in Primary Cortical Cultures. SLAS Discov. Adv. Sci. Drug Discov. 2020, 25, 447–457. [Google Scholar] [CrossRef]
- Pierre, O.; Fouchard, M.; Le Goux, N.; Buscaglia, P.; Leschiera, R.; Lewis, R.J.; Mignen, O.; Fluhr, J.W.; Misery, L.; Le Garrec, R. Pacific-Ciguatoxin-2 and Brevetoxin-1 Induce the Sensitization of Sensory Receptors Mediating Pain and Pruritus in Sensory Neurons. Mar. Drugs 2021, 19, 387. [Google Scholar] [CrossRef]
- Tukker, A.M.; Vrolijk, M.F.; van Kleef, R.G.D.M.; Sijm, D.T.H.M.; Westerink, R.H.S. Mixture Effects of Tetrodotoxin (TTX) and Drugs Targeting Voltage-Gated Sodium Channels on Spontaneous Neuronal Activity in Vitro. Toxicol. Lett. 2023, 373, 53–61. [Google Scholar] [CrossRef]
- LePage, K.T.; Dickey, R.W.; Gerwick, W.H.; Jester, E.L.; Murray, T.F. On the Use of Neuro-2a Neuroblastoma Cells Versus Intact Neurons in Primary Culture for Neurotoxicity Studies. Crit. Rev. Neurobiol. 2005, 17, 27–50. [Google Scholar] [CrossRef]
- Noreng, S.; Li, T.; Payandeh, J. Structural Pharmacology of Voltage-Gated Sodium Channels. J. Mol. Biol. 2021, 433, 166967. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. Overview of the Voltage-Gated Sodium Channel Family. Genome Biol. 2003, 4, 207. [Google Scholar] [CrossRef]
- Ji, Y. Toxins That Affect Voltage-Gated Sodium Channels. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2018; Volume 246, pp. 187–207. [Google Scholar]
- Inserra, M.C.; Israel, M.R.; Caldwell, A.; Castro, J.; Deuis, J.R.; Harrington, A.M.; Keramidas, A.; Garcia-Caraballo, S.; Maddern, J.; Erickson, A.; et al. Multiple Sodium Channel Isoforms Mediate the Pathological Effects of Pacific Ciguatoxin-1. Sci. Rep. 2017, 7, 42810. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, M.; Hendriksen, C.F.M. Critical Steps in the Production of Polyclonal and Monoclonal Antibodies: Evaluation and Recommendations. ILAR J. 2005, 46, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Kawatsu, K.; Hamano, Y.; Yoda, T.; Terano, Y.; Shibata, T. Rapid and Highly Sensitive Enzyme Immunoassay for Quantitative Determination of Tetrodotoxin. Jpn. J. Med. Sci. Biol. 1997, 50, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Tsumoto, K. Hybridoma Technologies for Antibody Production. Immunotherapy 2011, 3, 371–380. [Google Scholar] [CrossRef]
- Frenzel, A.; Hust, M.; Schirrmann, T. Expression of Recombinant Antibodies. Front. Immunol. 2013, 4, 217. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Hussain, B.; Yüce, M. Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef]
- Lyu, C.; Khan, I.M.; Wang, Z. Capture-SELEX for Aptamer Selection: A Short Review. Talanta 2021, 229, 122274. [Google Scholar] [CrossRef]
- Qi, S.; Duan, N.; Khan, I.M.; Dong, X.; Zhang, Y.; Wu, S.; Wang, Z. Strategies to Manipulate the Performance of Aptamers in SELEX, Post-SELEX and Microenvironment. Biotechnol. Adv. 2022, 55, 107902. [Google Scholar] [CrossRef]
- Spiga, F.M.; Maietta, P.; Guiducci, C. More DNA-Aptamers for Small Drugs: A Capture-SELEX Coupled with Surface Plasmon Resonance and High-Throughput Sequencing. ACS Comb. Sci. 2015, 17, 326–333. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Q. Cell-Based Biosensors: Principles and Applications; Artech House: London, UK, 2010. [Google Scholar]
- Liu, Q.; Wu, C.; Cai, H.; Hu, N.; Zhou, J.; Wang, P. Cell-Based Biosensors and Their Application in Biomedicine. Chem. Rev. 2014, 114, 6423–6461. [Google Scholar] [CrossRef]
- Alkassar, M.; Leonardo, S.; Diogène, J.; Campàs, M. Immobilisation of Neuro-2a Cells on Electrodes and Electrochemical Detection of MTT Formazan Crystals to Assess Their Viability. Bioelectrochemistry 2022, 148, 108274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Huang, G.; Wang, S.; Wu, J.; Lee, W.G.; Chen, Y.; Xu, F.; Lu, T. Advances in Cell-Based Biosensors Using Three-Dimensional Cell-Encapsulating Hydrogels. Biotechnol. J. 2011, 6, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. The Action Potential in Mammalian Central Neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liao, C.; Liu, S.; Xia, T.; Jiang, G. Nanotechnology: New Opportunities for the Development of Patch-clamps. J. Nanobiotechnology 2021, 19, 97. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; Xiong, P.; Yan, X.; Liao, C.; Jiang, G. Application of Electrophysiological Technique in Toxicological Study: From Manual to Automated Patch-Clamp Recording. TrAC Trends Anal. Chem. 2020, 133, 116082. [Google Scholar] [CrossRef]
- Raposo-Garcia, S.; Louzao, M.C.; Fuwa, H.; Sasaki, M.; Vale, C.; Botana, L.M. Determination of the Toxicity Equivalency Factors for Ciguatoxins Using Human Sodium Channels. Food Chem. Toxicol. 2022, 160, 112812. [Google Scholar] [CrossRef]
- Noguchi, A.; Ikegaya, Y.; Matsumoto, N. In Vivo Whole-Cell Patch-Clamp Methods: Recent Technical Progress and Future Perspectives. Sensors 2021, 21, 1448. [Google Scholar] [CrossRef]
- Hong, B.; He, J.; Le, Q.; Bai, K.; Chen, Y.; Huang, W. Combination Formulation of Tetrodotoxin and Lidocaine as a Potential Therapy for Severe Arrhythmias. Mar. Drugs 2019, 17, 685. [Google Scholar] [CrossRef]
- Suk, H.J.; Boyden, E.S.; van Welie, I. Advances in the Automation of Whole-Cell Patch Clamp Technology. J. Neurosci. Methods 2019, 326, 108357. [Google Scholar] [CrossRef]
- Pancrazio, J.J.; Gray, S.A.; Shubin, Y.S.; Kulagina, N.; Cuttino, D.S.; Shaffer, K.M.; Eisemann, K.; Curran, A.; Zim, B.; Gross, G.W.; et al. A Portable Microelectrode Array Recording System Incorporating Cultured Neuronal Networks for Neurotoxin Detection. Biosens. Bioelectron. 2003, 18, 1339–1347. [Google Scholar] [CrossRef]
- Charkhkar, H.; Knaack, G.L.; Gnade, B.E.; Keefer, E.W.; Pancrazio, J.J. Development and Demonstration of a Disposable Low-Cost Microelectrode Array for Cultured Neuronal Network Recording. Sens. Actuators B Chem. 2012, 161, 655–660. [Google Scholar] [CrossRef]
- Nicolas, J.; Hendriksen, P.J.M.; van Kleef, R.G.D.M.; de Groot, A.; Bovee, T.F.H.; Rietjens, I.M.C.M.; Westerink, R.H.S. Detection of Marine Neurotoxins in Food Safety Testing Using a Multielectrode Array. Mol. Nutr. Food Res. 2014, 58, 2369–2378. [Google Scholar] [CrossRef] [PubMed]
- IAEA-TECDOC-1729. Detection of Harmful Algal Toxins Using the Radioligand Receptor Binding Assay. Available online: https://www.iaea.org/publications/10480/detection-of-harmful-algal-toxins-using-the-radioligand-receptor-binding-assay (accessed on 28 April 2023).
- Díaz-Asencio, L.; Clausing, R.J.; Rañada, M.L.; Alonso-Hernández, C.M.; Dechraoui Bottein, M.Y. A Radioligand Receptor Binding Assay for Ciguatoxin Monitoring in Environmental Samples: Method Development and Determination of Quality Control Criteria. J. Environ. Radioact. 2018, 192, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Fouc, M.T.; Chinain, M. Ciguatera Risk Assessment in Two Toxic Sites of French Polynesia Using the Receptor-Binding Assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef]
- Rivera, V.R.; Poli, M.A.; Bignami, G.S.; Rivera, V.R.; Poli, M.A.; Bignami, G.S. Prophylaxis and Treatment with a Monoclonal Antibody of Tetrodotoxin Poisoing in Mice. Toxicon 1995, 33, 1231–1237. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; McCall, J.R.; Bourdelais, A.J.; Baden, D.G.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Flores Quintana, H.A.F.; et al. Fluorescent Receptor Binding Assay for Detecting Ciguatoxins in Fish. PLoS ONE 2016, 11, 153348. [Google Scholar] [CrossRef]
- Brevetoxin/Ciguatoxin RBA Test Kit. Available online: https://www.seatoxresearch.com/products/brevetoxin-ciguatoxin-test-kit/ (accessed on 28 April 2023).
- Campàs, M.; Alkassar, M.; Gaiani, G.; Leonardo, S.; Rambla-Alegre, M.; Diogène, J. The Wide Spectrum of Methods Available to Study Marine Neurotoxins. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2021; Volume 6, pp. 275–315. [Google Scholar]
- Kreuzer, M.P.; Pravda, M.; O’Sullivan, C.K.; Guilbault, G.G. Novel Electrochemical Immunosensors for Seafood Toxin Analysis. Toxicon 2002, 40, 1267–1274. [Google Scholar] [CrossRef]
- Reverté, L.; De La Iglesia, P.; Del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of Tetrodotoxins in Puffer Fish by a Self-Assembled Monolayer-Based Immunoassay and Comparison with Surface Plasmon Resonance, LC-MS/MS, and Mouse Bioassay. Anal. Chem. 2015, 87, 10839–10847. [Google Scholar] [CrossRef]
- Reverté, L.; Campbell, K.; Rambla-Alegre, M.; Elliott, C.T.; Diogène, J.; Campàs, M. Immunosensor Array Platforms Based on Self-Assembled Dithiols for the Electrochemical Detection of Tetrodotoxins in Puffer Fish. Anal. Chim. Acta 2017, 989, 95–103. [Google Scholar] [CrossRef]
- Rambla-Alegre, M.; Reverté, L.; del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Izquierdo-Muñoz, A.; et al. Evaluation of Tetrodotoxins in Puffer Fish Caught along the Mediterranean Coast of Spain. Toxin Profile of Lagocephalus sceleratus. Environ. Res. 2017, 158, 1–6. [Google Scholar] [CrossRef]
- Reverté, L.; Rambla-Alegre, M.; Leonardo, S.; Bellés, C.; Campbell, K.; Elliott, C.T.; Gerssen, A.; Klijnstra, M.D.; Diogène, J.; Campàs, M. Development and Validation of a Maleimide-Based Enzyme-Linked Immunosorbent Assay for the Detection of Tetrodotoxin in Oysters and Mussels. Talanta 2018, 176, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Gaiani, G.; O’sullivan, C.K.; Campàs, M. Magnetic Beads in Marine Toxin Detection: A Review. Magnetochemistry 2019, 5, 62. [Google Scholar] [CrossRef]
- Campàs, M.; Reverté, J.; Rambla-Alegre, M.; Campbell, K.; Gerssen, A.; Diogène, J. A Fast Magnetic Bead-Based Colorimetric Immunoassay for the Detection of Tetrodotoxins in Shellfish. Food Chem. Toxicol. 2020, 140, 111315. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, S.; Kiparissis, S.; Rambla-Alegre, M.; Almarza, S.; Roque, A.; Andree, K.B.; Christidis, A.; Flores, C.; Caixach, J.; Campbell, K.; et al. Detection of Tetrodotoxins in Juvenile Pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the North Aegean Sea (Greece) by an Electrochemical Magnetic Bead-Based Immunosensing Tool. Food Chem. 2019, 290, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Tsumuraya, T.; Fujii, I.; Hirama, M. Preparation of Anti-Ciguatoxin Monoclonal Antibodies Using Synthetic Haptens: Sandwich ELISA Detection of Ciguatoxins. J. AOAC Int. 2014, 97, 373–379. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Takeuchi, K.; Yamashita, S.; Fujii, I.; Hirama, M. Development of a Monoclonal Antibody against the Left Wing of Ciguatoxin CTX1B: Thiol Strategy and Detection Using a Sandwich ELISA. Toxicon 2012, 60, 348–357. [Google Scholar] [CrossRef]
- Oguri, H.; Hirama, M.; Tsumuraya, T.; Fujii, I.; Maruyama, M.; Uehara, H.; Nagumo, Y. Synthesis-Based Approach toward Direct Sandwich Immunoassay for Ciguatoxin CTX3C. J. Am. Chem. Soc. 2003, 125, 7608–7612. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Fujii, I.; Inoue, M.; Tatami, A.; Miyazaki, K.; Hirama, M. Production of Monoclonal Antibodies for Sandwich Immunoassay Detection of Ciguatoxin 51-HydroxyCTX3C. Toxicon 2006, 48, 287–294. [Google Scholar] [CrossRef]
- Leonardo, S.; Gaiani, G.; Tsumuraya, T.; Hirama, M.; Turquet, J.; Sagristà, N.; Rambla-Alegre, M.; Flores, C.; Caixach, J.; Diogène, J.; et al. Addressing the Analytical Challenges for the Detection of Ciguatoxins Using an Electrochemical Biosensor. Anal. Chem. 2020, 92, 4858–4865. [Google Scholar] [CrossRef]
- Gaiani, G.; Leonardo, S.; Tudó Toldrà, A.; Rey, M.; Andree, K.B.; Tsumuraya, T.; Hirama, M.; Diogène, J.; O’Sullivan, C.K.; Alcaraz, C.; et al. Rapid Detection of Ciguatoxins in Gambierdiscus and Fukuyoa with Immunosensing Tools. Ecotoxicol. Environ. Saf. 2020, 204, 111004. [Google Scholar] [CrossRef]
- Campàs, M.; Leonardo, S.; Oshiro, N.; Kuniyoshi, K.; Tsumuraya, T.; Hirama, M.; Diogène, J. A Smartphone-Controlled Amperometric Immunosensor for the Detection of Pacific Ciguatoxins in Fish. Food Chem. 2022, 374, 131687. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.D.; Ladd, J.; Etheridge, S.; Deeds, J.; Hall, S.; Jiang, S. Quantitative Detection of Tetrodotoxin (TTX) by a Surface Plasmon Resonance (SPR) Sensor. Sens. Actuators B Chem. 2008, 130, 120–128. [Google Scholar] [CrossRef]
- Taylor, A.D.; Vaisocherová, H.; Deeds, J.; DeGrasse, S.; Jiang, S. Tetrodotoxin Detection by a Surface Plasmon Resonance Sensor in Pufferfish Matrices and Urine. J. Sens. 2011, 2011, 601704. [Google Scholar] [CrossRef]
- Yakes, B.J.; Deeds, J.; White, K.; DeGrasse, S.L. Evaluation of Surface Plasmon Resonance Biosensors for Detection of Tetrodotoxin in Food Matrices and Comparison to Analytical Methods. J. Agric. Food Chem. 2011, 59, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.; Barnes, P.; Haughey, S.A.; Higgins, C.; Kawatsu, K.; Vasconcelos, V.; Elliott, C.T. Development and Single Laboratory Validation of an Optical Biosensor Assay for Tetrodotoxin Detection as a Tool to Combat Emerging Risks in European Seafood Rapid Detection in Food and Feed. Anal. Bioanal. Chem. 2013, 405, 7753–7763. [Google Scholar] [CrossRef]
- Reverté, L.; Campàs, M.; Yakes, B.J.; Deeds, J.R.; Katikou, P.; Kawatsu, K.; Lochhead, M.; Elliott, C.T.; Campbell, K. Tetrodotoxin Detection in Puffer Fish by a Sensitive Planar Waveguide Immunosensor. Sens. Actuators B Chem. 2017, 253, 967–976. [Google Scholar] [CrossRef]
- Shao, B.Y.; Gao, X.; Yang, F.; Chen, W.B.; Miao, T.Y.; Peng, J. Screening and Structure Analysis of the Aptamer Against Tetrodotoxin. J. Chin. Inst. Food Sci. Technol. 2012, 2, 347–351. [Google Scholar]
- Shao, B.Y.; Chen, B.; Chen, W.B.; Yang, F.; Miao, T.Y.; Peng, J. Preparation and Application of Tetrodotoxin DNA Aptamer. Food Sci. 2014, 35, 205–208. [Google Scholar]
- Lan, Y.; Qin, G.; Wei, Y.; Dong, C.; Wang, L. Highly Sensitive Analysis of Tetrodotoxin Based on Free-Label Fluorescence Aptamer Sensing System. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 411–418. [Google Scholar] [CrossRef]
- Lan, Y.; Qin, G.; Wei, Y.; Wang, L.; Dong, C. Exonuclease I-Assisted Fluorescence Aptasensor for Tetrodotoxin. Ecotoxicol. Environ. Saf. 2020, 194, 110417. [Google Scholar] [CrossRef]
- Dou, X.; Xu, S.; Jiang, Y.; Ding, Z.; Xie, J. Aptamers-Functionalized Nanoscale MOFs for Saxitoxin and Tetrodotoxin Sensing in Sea Foods through FRET. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2023, 284, 121827. [Google Scholar] [CrossRef] [PubMed]
- Shkembi, X.; Skouridou, V.; Svobodova, M.; Leonardo, S.; Bashammakh, A.S.; Alyoubi, A.O.; Campàs, M.; O′Sullivan, C.K. Hybrid Antibody-Aptamer Assay for Detection of Tetrodotoxin in Pufferfish. Anal. Chem. 2021, 93, 14810–14819. [Google Scholar] [CrossRef] [PubMed]
- Fomo, G.; Waryo, T.T.; Sunday, C.E.; Baleg, A.A.; Baker, P.G.; Iwuoha, E.I. Aptameric Recognition-Modulated Electroactivity of Poly(4-Styrenesolfonic Acid)-Doped Polyaniline Films for Single-Shot Detection of Tetrodotoxin. Sensors 2015, 15, 22547–22560. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, D.I.; Magarlamov, T.Y. An Overview of the Anatomical Distribution of Tetrodotoxin in Animals. Toxins 2022, 14, 576. [Google Scholar] [CrossRef] [PubMed]
- Campàs, M.; Leonardo, S.; Rambla-Alegre, M.; Sagristà, N.; Vaya, R.; Diogène, J.; Torréns, M.; Fragoso, A. Cyclodextrin Polymer Clean-up Method for the Detection of Ciguatoxins in Fish with Cell-Based Assays. Food Chem. 2023, 401, 134196. [Google Scholar] [CrossRef]
- Devlin, R.; Campbell, K.; Kawatsu, K.; Elliott, C. Studies in the Use of Magnetic Microspheres for Immunoaffinity Extraction of Paralytic Shellfish Poisoning Toxins from Shellfish. Toxins 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Leonardo, S.; Rambla-Alegre, M.; Samdal, I.A.; Miles, C.O.; Kilcoyne, J.; Diogène, J.; O’Sullivan, C.K.; Campàs, M. Immunorecognition Magnetic Supports for the Development of an Electrochemical Immunoassay for Azaspiracid Detection in Mussels. Biosens. Bioelectron. 2017, 92, 200–206. [Google Scholar] [CrossRef]
- FUJIFILM Wako Pure Chemical Corporation. Available online: https://labchem-wako.fujifilm.com (accessed on 13 May 2023).
- Laboratorio CIFGA, S.A. Available online: https://cifga.com (accessed on 13 May 2023).
- National Research Council Canada. Available online: https://shop-magasin.nrc-cnrc.gc.ca (accessed on 13 May 2023).

| Biosensor Type | Cell-Based Biosensors (CBBs) | Receptor Binding Biosensors (RBBs) | Immunosensors | Aptamer-Based Biosensors |
|---|---|---|---|---|
| Biorecognition element | Cells (primary cultures or immortal cell lines) | Receptors (synaptosomes) | Antibodies (mono-clonal or polyclonal) | Aptamers (RNA or DNA) |
| Approach | Toxicological | Structural | Structural | Structural |
| Supports | Micropipettes, patch clamp chips, micro-electrode arrays (MEAs), electrodes | RBB not yet developed; only receptor-binding assays (RBAs) on microtiter plates | Electodes, magnetic beads, surface plasmon resonance (SPR) chips, planar waveguide nanoarray chips | Electrodes |
| Sensitivity | High | Medium | High | High |
| Toxins | CTXs and TTXs | Only RBAs for CTXs and TTXs | CTXs and TTXs | TTXs |
| Samples | Fishes (CTXs and TTXs) and spiked mussels (TTXs) | Only RBAs for fishes (CTXs) and microalgae (CTXs) | Fishes (CTXs and TTXs), microalgae (CTXs), and shellfish (TTXs) | Only aptamer-based assay for fishes (TTXs) and shellfish (TTXs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reverté, J.; Alkassar, M.; Diogène, J.; Campàs, M. Detection of Ciguatoxins and Tetrodotoxins in Seafood with Biosensors and Other Smart Bioanalytical Systems. Foods 2023, 12, 2043. https://doi.org/10.3390/foods12102043
Reverté J, Alkassar M, Diogène J, Campàs M. Detection of Ciguatoxins and Tetrodotoxins in Seafood with Biosensors and Other Smart Bioanalytical Systems. Foods. 2023; 12(10):2043. https://doi.org/10.3390/foods12102043
Chicago/Turabian StyleReverté, Jaume, Mounira Alkassar, Jorge Diogène, and Mònica Campàs. 2023. "Detection of Ciguatoxins and Tetrodotoxins in Seafood with Biosensors and Other Smart Bioanalytical Systems" Foods 12, no. 10: 2043. https://doi.org/10.3390/foods12102043
APA StyleReverté, J., Alkassar, M., Diogène, J., & Campàs, M. (2023). Detection of Ciguatoxins and Tetrodotoxins in Seafood with Biosensors and Other Smart Bioanalytical Systems. Foods, 12(10), 2043. https://doi.org/10.3390/foods12102043









