Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom
Abstract
1. Introduction
2. Methodology of the Review
3. Pleurotus ostreatus and Its Potential
3.1. Oyster Mushroom and Agro-Waste Management
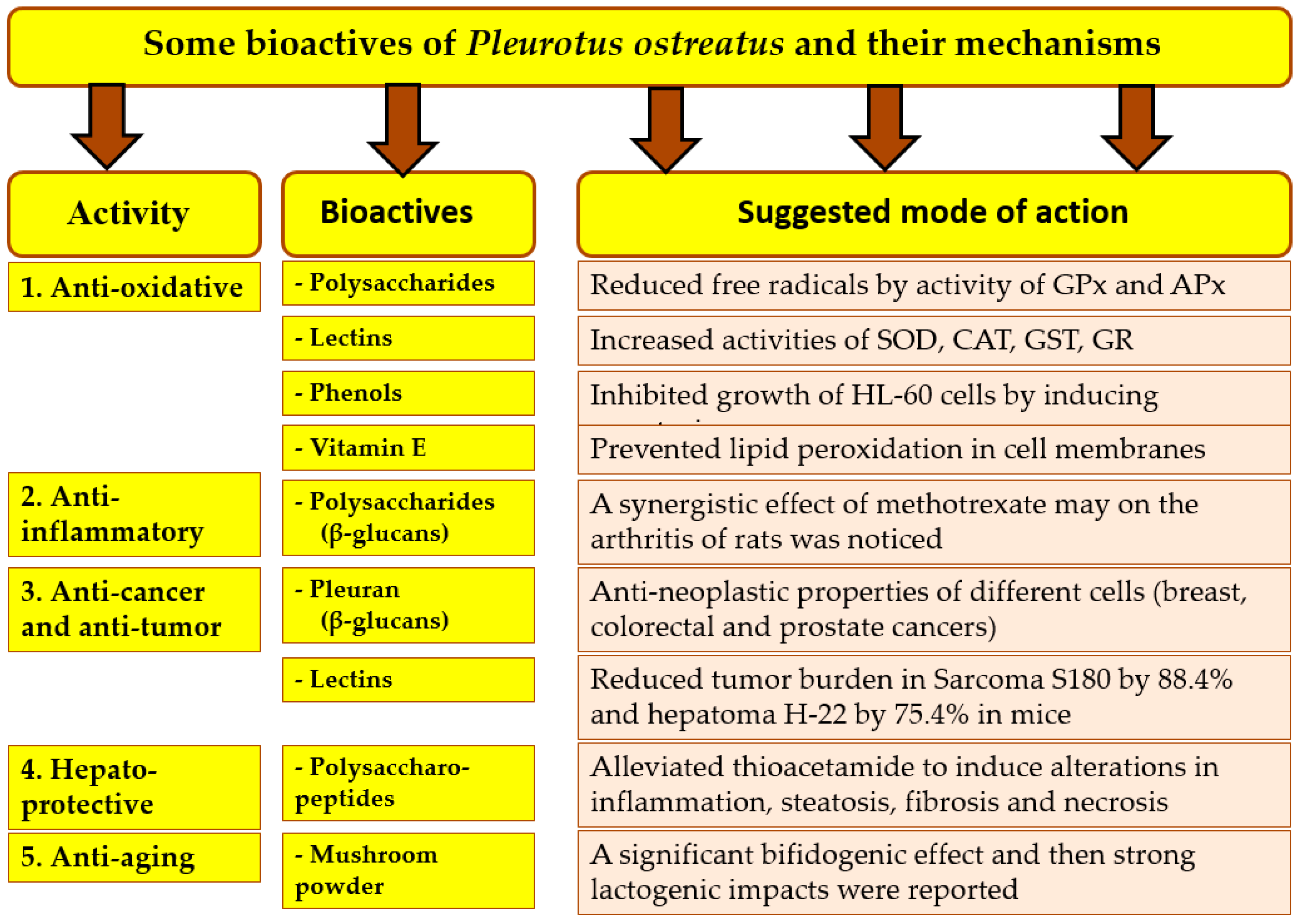
3.2. Medicinal and Pharmacological Attributes of P. ostreatus
3.3. Antimicrobial Activity of Oyster Mushroom
| Pharmacological Effect | Isolated Bioactive Compounds | Refs. |
|---|---|---|
| Antifungal | P-anisaldehyde, chitin, chitosan, 7000 Da of pleurostrin | [8,86] |
| Antibacterial | Phenolic compounds, tannins, flavonoids, terpenes, β-D Glucan (pleuran), p-anisaldehyde, chitin, chitosan | [2,9] |
| Antiviral (adjuvant in HBV vaccine) | Lectin (POL) | [10] |
| Antiviral (HCV) | Laccase | [2,10,14] |
| Antiviral (HSV-1) | Polysaccharide fraction, β-D-glucan (pleuran), insoluble β-1,3/1,6-D-glucans | [11,13,79] |
| Antiviral (HSV-2) | Polysaccharide fraction, β-glucan | [14] |
| Antiviral (COVID-19) | Terpenoids, lectins, glycoproteins, lentinan, galactomannan, and polysaccharides | [14] |
| Antiviral (HIV) | Ubiquitin-like protein, ergothioneine | [2,14] |
| Anti-helmintic (gastrointestinal parasites, larvae stage) | Secondary metabolites (alkaloids, flavonoids, phenolic compounds, quinones, peptides, terpenoids, fatty acids) | [15,16] |
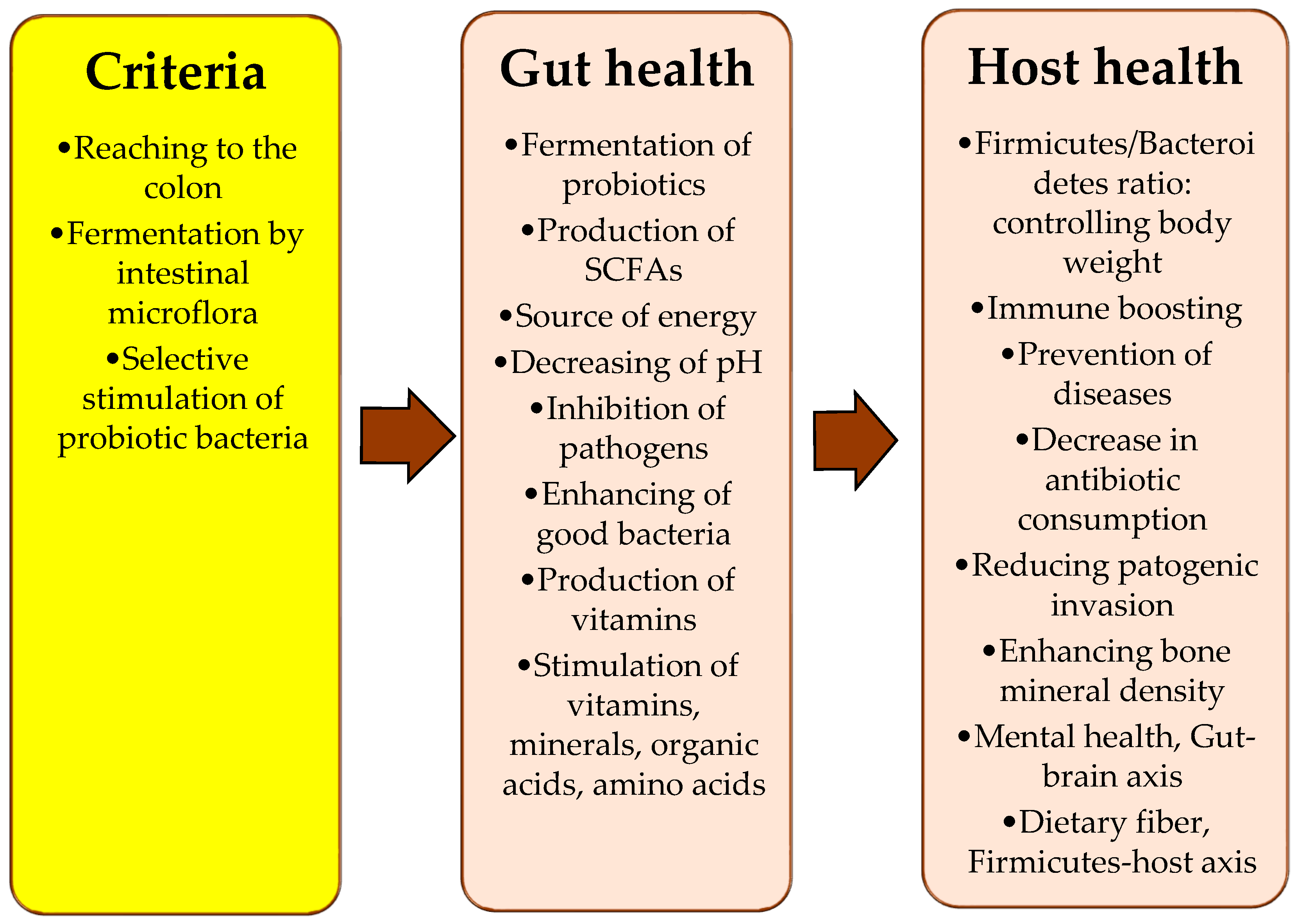
4. Prebiotics and Their Mechanism
- Reducing risk of infection by resisting pathogens and increasing natural killer activity;
- Improving mineral absorbability by lowering pH and enhancing soubility of minerals;
- Improving bowel function by increasing water binding;
- Modulation of immune cells and reducing allergies;
- Improving intestinal barrier function and lowering blood lipid levels;
- Reducing inflammation and maintaining tight junction integrity; and
5. Prebiotic Activities of Pleurotus ostreatus
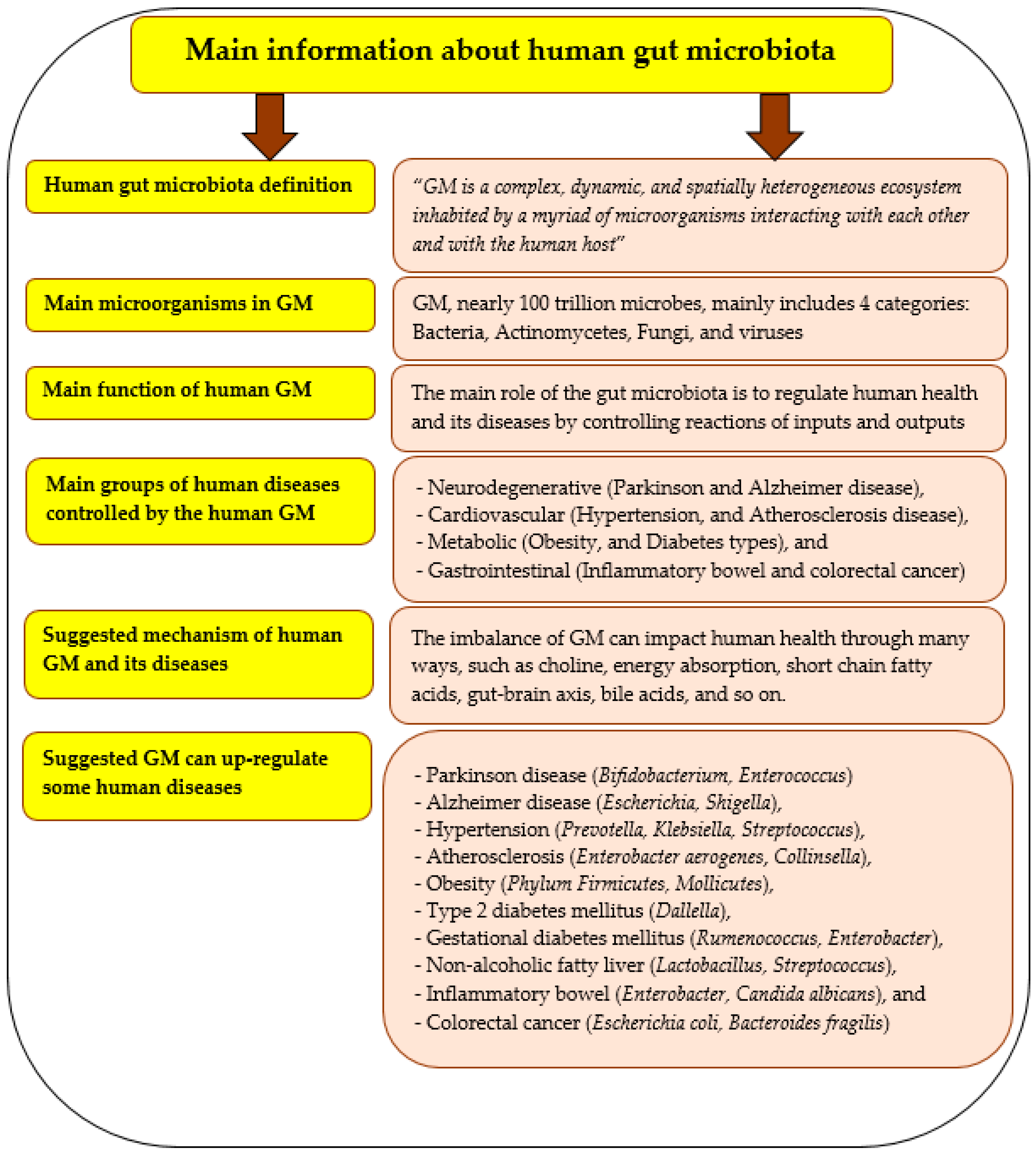
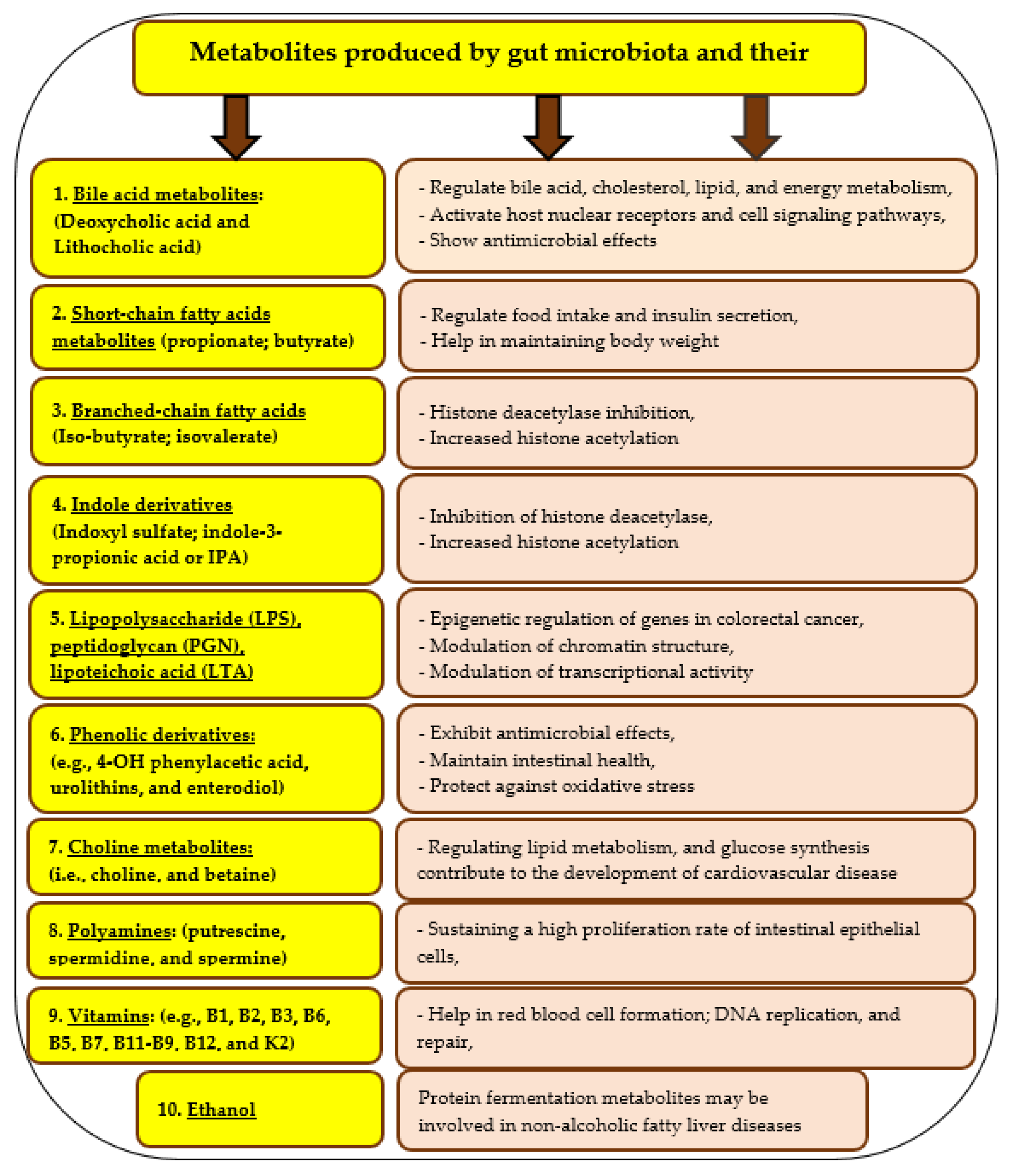
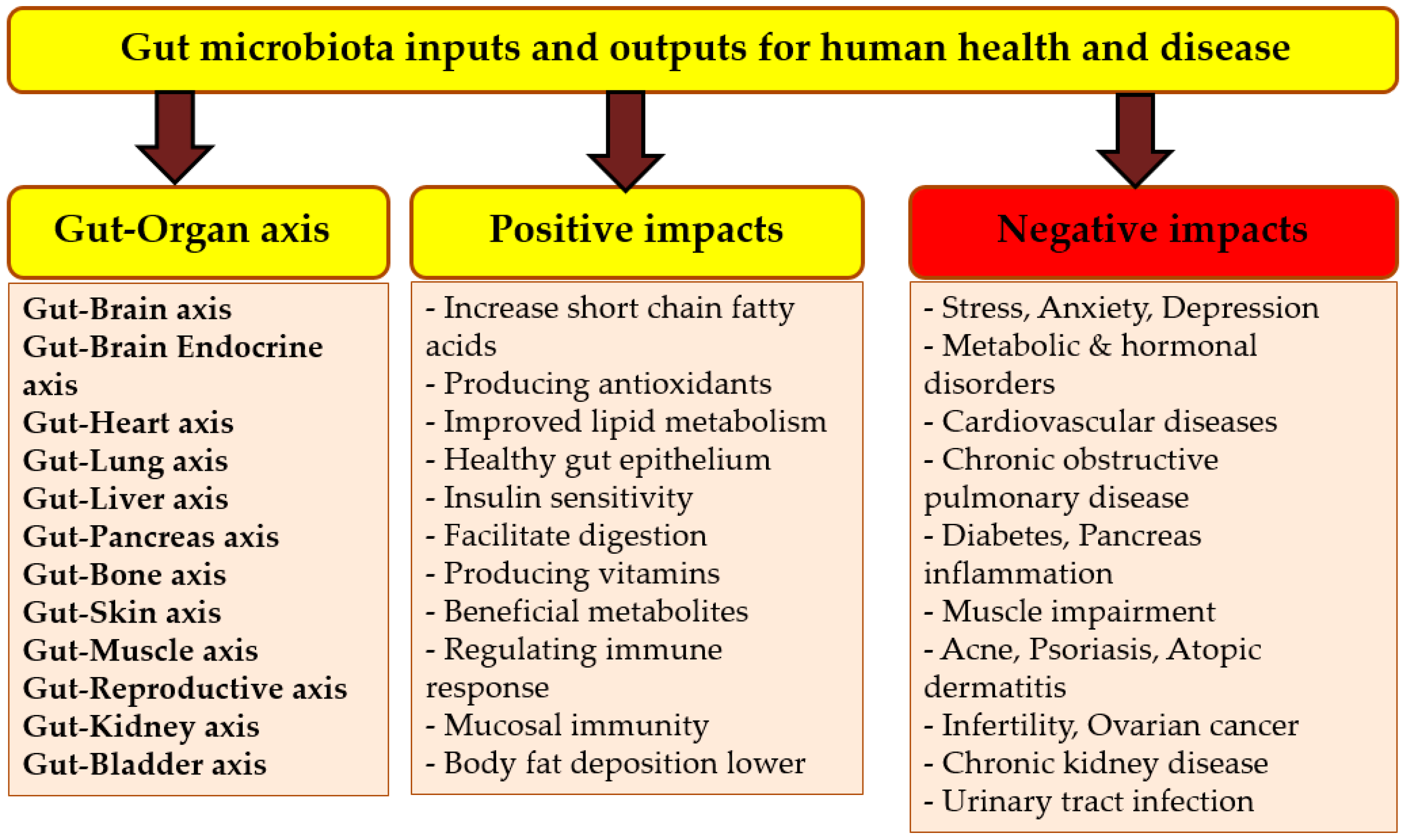
6. Gut Microbiota and Its Importance
7. Pleurotus ostreatus and Gut Microbiota
- The golden oyster mushroom (Pleurotus citrinopileatus) has distinguished pharmacological functions like modulation of hepatoprotective and human gut microbiota through the protective effects of polysaccharide peptides. These bioactives of polysaccharide peptides can extracted from this mushroom and metabolized by the gut microbiota to produce short-chain fatty acids, which promote the functions of liver [142];
- A study was conducted on the influence of isolated glucopyranose from mycelium of Pleurotus geesteranus on preventing alcoholic liver diseases and the gut microbiota. The mechanism of this bioactive compound may cause a balance in the gut–liver axis by increasing intestinal tight junction proteins, thus elevating the abundance of short-chain fatty acids producers in the intestine by regulating the composition of gut microbiota [145];
- The role of Pleurotus ostreatus in ameliorating obesity in obese mice and modulating the gut microbiota was reported. This mushroom can induce the gut microbiota functions by upregulating the metabolism of lipids and carbohydrates and the biosynthesis of bile acids, as well as downregulating the signaling pathway of adipocytokine and the biosynthesis of steroid hormones [108];
- The role of oyster mushroom (Pleurotus sajor-caju) in modulating gut microbiota in Zucker rats as a prebiotic agent was studied by enhancing the growth of SCFA-producing bacterial genera (e.g., Blautia, Bifidobacterium, Faecalibaculum, and Roseburia), while decreasing the abundance of Escherichia–Shigella [146]; and
- The role of Pleurotus eryngii was studied as a prebiotic agent in promoting the gut microbiota via in vitro fermentation in presence of selenium, which increases lead adsorption by bacteria of Desulfovibrio, leading to a reduction of lead toxicity in humans [147].
8. General Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Lesa, K.N.; Khandaker, M.U.; Mohammad Rashed Iqbal, F.; Sharma, R.; Islam, F.; Mitra, S.; Emran, T.B. Nutritional Value, Medicinal Importance, and Health-Promoting Effects of Dietary Mushroom (Pleurotus ostreatus). J. Food Qual. 2022, 2022, 2454180. [Google Scholar] [CrossRef]
- Hamad, D.; El-Sayed, H.; Ahmed, W.; Sonbol, H.; Ramadan, M.A.H. GC-MS Analysis of Potentially Volatile Compounds of Pleurotus ostreatus Polar Extract: In Vitro Antimicrobial, Cytotoxic, Immunomodulatory, and Antioxidant Activities. Front. Microbiol. 2022, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Törős, G.H.; El-Ramady, H.; Prokisch, J. Edible Mushroom of Pleurotus spp.: A Case Study of Oyster Mushroom (Pleurotus ostreatus L.). Environ. Biodiv. Soil Secur. 2022, 6, 51–59. [Google Scholar]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Töros, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- Zakil, F.A.; Xuan, L.H.; Zaman, N.; Alan, N.I.; Salahutheen, N.A.A.; Sueb, M.S.M.; Isha, R. Growth Performance and Mineral Analysis of Pleurotus ostreatus from Various Agricultural Wastes Mixed with Rubber Tree Sawdust in Malaysia. Bioresour. Technol. Rep. 2022, 17, 100873. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, L.E.I.; Yun, Z.; Ye, F.; Zhao, G. Roles of Mushroom Polysaccharides in Chronic Disease Management. J. Integr. Agric. 2022, 21, 1839–1866. [Google Scholar] [CrossRef]
- Waktola, G.; Temesgen, T. Pharmacological Activities of Oyster Mushroom (Pleurotus ostreatus). Nov. Res. Microbiol. J. 2020, 4, 688–695. [Google Scholar]
- Suliaman, S.Q.; AL-Abbasi, S.H.; Mahmood, Y.H.; AL-Azzawi, H.A. Antimicrobial Activity of Four Selected Wild Mushrooms in Iraq. Biochem. Cell. Arch. 2021, 21, 4533–4537. [Google Scholar]
- El-Maradny, Y.A.; El-Fakharany, E.M.; Abu-Serie, M.M.; Hashish, M.H.; Selim, H.S. Lectins Purified from Medicinal and Edible Mushrooms: Insights into Their Antiviral Activity against Pathogenic Viruses. Int. J. Biol. Macromol. 2021, 179, 239–258. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.; Aboshanab, K.M.; Yassien, M.A. Antiviral, Cytotoxic, and Antioxidant Activities of Three Edible Agaricomycetes Mushrooms: Pleurotus columbinus, Pleurotus sajor-caju, and Agaricus bisporus. J. Fungi 2021, 7, 645. [Google Scholar] [CrossRef]
- Elhusseiny, S.M.; El-Mahdy, T.S.; Awad, M.F.; Elleboudy, N.S.; Farag, M.M.; Yassein, M.A.; Aboshanab, K.M. Proteome Analysis and In Vitro Antiviral, Anticancer and Antioxidant Capacities of the Aqueous Extracts of Lentinula edodes and Pleurotus ostreatus Edible Mushrooms. Molecules 2021, 26, 4623. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral Bioactive Compounds of Mushrooms and Their Antiviral Mechanisms: A Review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sasidharan, S.P.; Yang, X. A Concise Review of Mushrooms Antiviral and Immunomodulatory Properties That May Combat against COVID-19. Food Chem. Adv. 2022, 1, 100023. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barrera, T.M.; Téllez-Téllez, M.; Sánchez, J.E.; Castañeda-Ramirez, G.S.; Acosta-Urdapilleta, M.; Bautista-Garfias, C.R.; Aguilar-Marcelino, L. Edible Mushrooms of the Genus Pleurotus as Biocontrol Agents of Parasites of Importance for Livestock. Sci. Fungorum 2021, 52, 1375. [Google Scholar] [CrossRef]
- Adak, M.; Kumar, P. Herbal Anthelmintic Agents: A Narrative Review. J. Tradit. Chin. Med. 2022, 42, 641. [Google Scholar]
- Guan, Z.; Feng, Q. Chitosan and Chitooligosaccharide: The Promising Non-Plant-Derived Prebiotics with Multiple Biological Activities. Int. J. Mol. Sci. 2022, 23, 6761. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulou, M.; Paschalidis, N.; Savvides, A.L.; Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Koutrotsios, G.; Zervakis, G.I.; Georgiadis, P.; Kyriacou, A. Immunomodulating Activity of Pleurotus eryngii Mushrooms Following Their In Vitro Fermentation by Human Fecal Microbiota. J. Fungi 2022, 8, 329. [Google Scholar] [CrossRef]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the Key Aroma and Phenolic Compounds of Champignon (Agaricus bisporus) and Oyster (Pleurotus ostreatus) Mushrooms after Two Cooking Treatments as Elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef] [PubMed]
- Adeoyo, O.R.; Oluborode, O.O. Antimicrobial Properties of Some Nigerian Edible Mushrooms. J. Phytopharm. 2020, 9, 110–114. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Oguoma, L.M.O.; Adigwe, P.C.; Anthony, B.B. Phytochemical Properties and In-Vitro Antimicrobial Potency of Wild Edible Mushrooms (Pleurotus ostreatus) Obtained from Yenagoa, Nigeri. J. Phytopharm. 2021, 10, 180–184. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50 Pt A, 101946. [Google Scholar] [CrossRef]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Jardon, K.M.; Canfora, E.E.; Goossens, G.H.; Blaak, E.E. Dietary Macronutrients and the Gut Microbiome: A Precision Nutrition Approach to Improve Cardiometabolic Health. Gut 2022, 71, 1214–1226. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Sheng, Y.; Liu, J.; Li, H.; Guo, M.; Xu, W.; Luo, Y.; Huang, K.; He, X. Pleurotus ostreatus Ameliorates Obesity by Modulating the Gut Microbiota in Obese Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1868. [Google Scholar] [CrossRef]
- Andrioaie, I.-M.; Duhaniuc, A.; Nastase, E.V.; Iancu, L.S.; Luncă, C.; Trofin, F.; Anton-Păduraru, D.-T.; Dorneanu, O.-S. The Role of the Gut Microbiome in Psychiatric Disorders. Microorganisms 2022, 10, 2436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Ke, S.; Weiss, S.T.; Liu, Y.-Y. Rejuvenating the Human Gut Microbiome. Trends Mol. Med. 2022, 28, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Raman, J.; Jang, K.-Y.; Oh, Y.-L.; Oh, M.; Im, J.-H.; Lakshmanan, H.; Sabaratnam, V. Cultivation and Nutritional Value of Prominent Pleurotus spp.: An Overview. Mycobiology 2021, 49, 1–14. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Dedousi, M.; Diamantopoulou, P. Cultivating Pleurotus ostreatus and Pleurotus eryngii Mushroom Strains on Agro-Industrial Residues in Solid-State Fermentation. Part I: Screening for Growth, Endoglucanase, Laccase and Biomass Production in the Colonization Phase. Carbon Resour. Convers. 2022, 5, 61–70. [Google Scholar] [CrossRef]
- Golovko, O.; Kaczmarek, M.; Asp, H.; Bergstrand, K.-J.; Ahrens, L.; Hultberg, M. Uptake of Perfluoroalkyl Substances, Pharmaceuticals, and Parabens by Oyster Mushrooms (Pleurotus ostreatus) and Exposure Risk in Human Consumption. Chemosphere 2022, 291, 132898. [Google Scholar] [CrossRef] [PubMed]
- Mayans, B.; Camacho-Arévalo, R.; García-Delgado, C.; Antón-Herrero, R.; Escolástico, C.; Segura, M.L.; Eymar, E. An Assessment of Pleurotus ostreatus to Remove Sulfonamides, and Its Role as a Biofilter Based on Its Own Spent Mushroom Substrate. Environ. Sci. Pollut. Res. 2021, 28, 7032–7042. [Google Scholar] [CrossRef]
- Kaur Gill, M.; Kocher, G.S.; Singh Panesar, A. Evaluation of Fungal Consortium Lignozyme for Biodelignification of Agricultural Residues. Biofuels Bioprod. Bioref. 2022, 16, 1772–1780. [Google Scholar] [CrossRef]
- Li, G.; Wang, Y.; Yu, D.; Zhu, P.; Zhao, G.; Liu, C.; Zhao, H. Ligninolytic Characteristics of Pleurotus ostreatus Cultivated in Cotton Stalk Media. Front. Microbiol. 2022, 13, 1035040. [Google Scholar] [CrossRef]
- Akyüz, M.; İnci, Ş.; Kırbağ, S. Evaluation of Antimicrobial, Antioxidant, Cytotoxic and DNA Protective Effects of Oyster Mushroom: Pleurotus pulmonarius (Fr.) Quel. Arab. J. Sci. Eng. 2022. [Google Scholar] [CrossRef]
- Peter, O.E.; Peter, G.R.; Obele, I.I.; Owuna, G.; Danladi, M.M.; Obiekieze, S.; Akwashiki, O. Utilization of Some Agro-Wastes for Cultivation of Pleurotus ostreatus (Oyster Mushroom) in Keffi Nigeria. Environ. Microbiol. 2019, 5, 60–69. [Google Scholar] [CrossRef]
- Akter, M.; Halawani, R.F.; Aloufi, F.A.; Taleb, M.A.; Akter, S.; Mahmood, S. Utilization of Agro-Industrial Wastes for the Production of Quality Oyster Mushrooms. Sustainability 2022, 14, 994. [Google Scholar] [CrossRef]
- Raza, M.H.; Abid, M.; Faisal, M.; Yan, T.; Akhtar, S.; Adnan, K.M.M. Environmental and Health Impacts of Crop Residue Burning: Scope of Sustainable Crop Residue Management Practices. Int. J. Environ. Res. Public Health 2022, 19, 4753. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
- Singh, C.; Pathak, P.; Chaudhary, N.; Rathi, A.; Dehariya, P.; Vyas, D. Mushrooms and Mushroom Composts in Integrated Farm Management. Res. J. Agric. Sci. 2020, 11, 1436–1443. [Google Scholar]
- Bahrololoum, S.; Kermani, M.M.M.; Koohzadi, F. Ecopreneurs and Agricultural Waste Management. J. Glob. Entrep. Res. 2022, 12, 47–51. [Google Scholar] [CrossRef]
- Calderon Lopez, J.C.; Thepanondh, S.; Sachdev, H.; Palencia Avelar, A.M.; del Carmen Leon, M.C. Sustainability and Economic Feasibility Through the Production of Oyster Mushroom (Pleurotus ostreatus (Jacq.) P. Kumm.) Derived Fromthe Waste of Coffee-Industry: A Case Studyin the Western Area of San Salvador, El Salvador. Pol. J. Environ. Stud. 2021, 30, 5617–5628. [Google Scholar] [CrossRef]
- Moshtaghian, H.; Parchami, M.; Rousta, K.; Lennartsson, P.R. Application of Oyster Mushroom Cultivation Residue as an Upcycled Ingredient for Developing Bread. Appl. Sci. 2022, 12, 11067. [Google Scholar] [CrossRef]
- Santos, F.P.d.; Magalhães, D.C.M.M.d.; Nascimento, J.d.S.; Ramos, G.L.d.P.A. Use of Products of Vegetable Origin and Waste from Hortofruticulture for Alternative Culture Media. Food Sci. Technol. 2022, 42, e00621. [Google Scholar] [CrossRef]
- Oliveira Vieira, V.; Almeida Conceição, A.; Raisa Barbosa Cunha, J.; Enis Virginio Machado, A.; Gonzaga de Almeida, E.; Souza Dias, E.; Magalhães Alcantara, L.; Neil Gerard Miller, R.; Gonçalves de Siqueira, F. A New Circular Economy Approach for Integrated Production of Tomatoes and Mushrooms. Saudi J. Biol. Sci. 2022, 29, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Berhe Sbhatu, D.; Abraha, H.B.; Fisseha, H.T. Grey Oyster Mushroom Biofarm for Small-Scale Entrepreneurship. Adv. Agric. 2019, 2019, 6853627. [Google Scholar] [CrossRef]
- Kumar, P. Effect of Different Agro-Waste Substrates on Yield Performance of Oyster Mushroom (Pleurotus sajor-caju). J. Krishi Vigyan 2020, 8, 70. [Google Scholar] [CrossRef]
- Risnawati, R.; Meitiyani; Susilo. The Effect of Adding Kepok Banana Peels (Musa paradisiaca) to Powder Media on the Growth of White Oyster Mushrooms (Pleurotus ostreatus). IOP Conf. Ser. Earth Environ. Sci. 2021, 755, 012066. [Google Scholar] [CrossRef]
- Otieno, O.D.; Mulaa, F.J.; Obiero, G.; Midiwo, J. Utilization of Fruit Waste Substrates in Mushroom Production and Manipulation of Chemical Composition. Biocatal. Agric. Biotechnol. 2022, 39, 102250. [Google Scholar] [CrossRef]
- Zhou, G.; Parawira, W. The Effect of Different Substrates Found in Zimbabwe on the Growth and Yield of Oyster Mushroom Pleurotus ostreatus. S. Afr. J. Educ. Sci. Technol. 2022, 5, 73–86. [Google Scholar] [CrossRef]
- Wachira, J.; Nguluu, S.; Kimatu, J. Differential Growth and Productivity of Oyster Mushroom (Pleurotus pulmonarius) on Agro-Waste Substrates in Semi-Arid Regions of Kenya. Int. J. Recycl. Org. Waste Agric. 2022, 11, 375–383. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Lyu, X.; Chen, T.; Chen, J.; Chen, X.; Guo, S. Utilization of Functional Agro-Waste Residues for Oyster Mushroom Production: Nutritions and Active Ingredients in Healthcare. Front. Plant Sci. 2023, 13, 1085022. [Google Scholar] [CrossRef] [PubMed]
- Elkanah, F.A.; Oke, M.A.; Adebayo, E.A. Substrate Composition Effect on the Nutritional Quality of Pleurotus ostreatus (MK751847) Fruiting Body. Heliyon 2022, 8, e11841. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Rana, S.; Kapoor, S.; Rana, A.; Dhaliwal, Y.S.; Bhushan, S. Industrial Apple Pomace as a Bioresource for Food and Agro Industries. In Sustainable Agriculture Reviews; Rana, A., Saneja, A., Kumar, S., Lichtfouse, E., Eds.; Springer International Publishing: Cham, Switzerland, 2021; Volume 56, pp. 39–65. ISBN 978-3-030-84404-2. [Google Scholar]
- Ritota, M.; Manzi, P. Pleurotus spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef]
- Díaz, R.; Díaz-Godínez, G. Substrates for Mushroom, Enzyme and Metabolites Production: A Review. J. Environ. Biol. 2022, 43, 350–359. [Google Scholar] [CrossRef]
- Doroški, A.; Klaus, A.; Režek Jambrak, A.; Djekic, I. Food Waste Originated Material as an Alternative Substrate Used for the Cultivation of Oyster Mushroom (Pleurotus ostreatus): A Review. Sustainability 2022, 14, 12509. [Google Scholar] [CrossRef]
- Arya, S.S.; Venkatram, R.; More, P.R.; Vijayan, P. The Wastes of Coffee Bean Processing for Utilization in Food: A Review. J. Food Sci. Technol. 2022, 59, 429–444. [Google Scholar] [CrossRef]
- Ivarsson, E.; Grudén, M.; Södergren, J.; Hultberg, M. Use of Faba Bean (Vicia faba L.) Hulls as Substrate for Pleurotus ostreatus—Potential for Combined Mushroom and Feed Production. J. Clean. Prod. 2021, 313, 127969. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H.; El Sebaaly, Z.; Najjar, R.; Sassine, Y.N. Effect of Olive Pruning Residues on Substrate Temperature and Production of Oyster Mushroom (Pleurotus ostreatus). Acta Hortic. 2021, 1327, 245–252. [Google Scholar] [CrossRef]
- Saha, T.; Das, S.; Sau, S.; Datta, D.; Kundu, S.; Saha, S.; Chakraborty, S.; Mitra, A.K. Toxic Metal Uptake by Oyster Mushrooms Grown in Sugarcane Bagasse. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tesfay, T.; Godifey, T.; Mesfin, R.; Kalayu, G. Evaluation of Waste Paper for Cultivation of Oyster Mushroom (Pleurotus ostreatus) with Some Added Supplementary Materials. AMB Express 2020, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Mihai, R.A.; Melo Heras, E.J.; Florescu, L.I.; Catana, R.D. The Edible Gray Oyster Fungi Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm a Potent Waste Consumer, a Biofriendly Species with Antioxidant Activity Depending on the Growth Substrate. J. Fungi 2022, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Abidin, M.H.Z.; Abdullah, N.; Abidin, N.Z. Therapeutic Properties of Pleurotus Species (Oyster Mushrooms) for Atherosclerosis: A Review. Int. J. Food Prop. 2017, 20, 1251–1261. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G. Blood Glucose Lowering and Effect of Oyster (Pleurotus ostreatus)- and Shiitake (Lentinus subnudus)-supplemented Diet on Key Enzymes Linked Diabetes and Hypertension in Streptozotocin-induced Diabetic in Rats. Food Front. 2022, 3, 161–171. [Google Scholar] [CrossRef]
- Soodpakdee, K.; Nacha, J.; Rattanachart, N.; Owatworakit, A.; Chamyuang, S. Fermentation with Pleurotus ostreatus Enhances the Prebiotic Properties of Germinated Riceberry Rice. Front. Nutr. 2022, 9, 839145. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Patsou, M.; Mitsou, E.K.; Bekiaris, G.; Kotsou, M.; Tarantilis, P.A.; Pletsa, V.; Kyriacou, A.; Zervakis, G.I. Valorization of Olive By-Products as Substrates for the Cultivation of Ganoderma Lucidum and Pleurotus ostreatus Mushrooms with Enhanced Functional and Prebiotic Properties. Catalysts 2019, 9, 537. [Google Scholar] [CrossRef]
- Sołowiej, B.G.; Nastaj, M.; Waraczewski, R.; Szafrańska, J.O.; Muszyński, S.; Radzki, W.; Mleko, S. Effect of Polysaccharide Fraction from Oyster Mushroom (Pleurotus ostreatus) on Physicochemical and Antioxidative Properties of Acid Casein Model Processed Cheese. Int. Dairy J. 2023, 137, 105516. [Google Scholar] [CrossRef]
- Inyod, T.; Ayimbila, F.; Payapanon, A.; Keawsompong, S. Antioxidant Activities and Prebiotic Properties of the Tropical Mushroom Macrocybe Crassa. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100298. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Hussein, D.E.; Algammal, A.M.; George, T.T.; Jeandet, P.; Al-Snafi, A.E.; Tiwari, A.; Pagnossa, J.P.; Lima, C.M.; Thorat, N.D.; et al. Application of Natural Antimicrobials in Food Preservation: Recent Views. Food Control 2021, 126, 108066. [Google Scholar] [CrossRef]
- Rangel-Vargas, E.; Rodriguez, J.A.; Domínguez, R.; Lorenzo, J.M.; Sosa, M.E.; Andrés, S.C.; Rosmini, M.; Pérez-Alvarez, J.A.; Teixeira, A.; Santos, E.M. Edible Mushrooms as a Natural Source of Food Ingredient/Additive Replacer. Foods 2021, 10, 2687. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Fayez, S.; Xiao, H.; Xu, B. New Insights into Antimicrobial and Antibiofilm Effects of Edible Mushrooms. Food Res. Int. 2022, 162, 111982. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Gullón, P.; Sivaraman, G.K.; McClements, D.J.; Gullón, B.; Lorenzo, J.M. Edible Mushrooms as Functional Ingredients for Development of Healthier and More Sustainable Muscle Foods: A Flexitarian Approach. Molecules 2021, 26, 2463. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Z.; Song, K.; Li, L.; Chen, M. Medicinal Value of Edible Mushroom Polysaccharides: A Review. J. Future Foods 2023, 3, 16–23. [Google Scholar] [CrossRef]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A Comprehensive Review on Phenolic Compounds from Edible Mushrooms: Occurrence, Biological Activity, Application and Future Prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef] [PubMed]
- Babota, M.; Frumuzachi, O.; Nicolescu, A.; Ielciu, I.; Paltinean, R.; Cris-An, G.; Mocan, A. Bioactive Phenolic Compounds from Mushrooms. In Edible Fungi: Chemical Composition, Nutrition and Health Effects; Royal Society of Chemistry: London, UK, 2022; p. 139. [Google Scholar]
- Minov, J.; Stoleski, S.; Karadzinska-Bislimovska, J.; Petrova, T.; Vasilevska, K.; Mijakoski, D.; Jesenak, M. Effects of Pleuran (β-Glucan from Pleurotus ostreatus) Supplementation on Incidence and Duration of Bronchiectasis Exacerbations. Open Access Maced. J. Med. Sci. 2020, 8, 906–912. [Google Scholar] [CrossRef]
- Urbancikova, I.; Hudackova, D.; Majtan, J.; Rennerova, Z.; Banovcin, P.; Jesenak, M. Efficacy of Pleuran (β -Glucan from Pleurotus ostreatus) in the Management of Herpes Simplex Virus Type 1 Infection. Evid.-Based Complement. Altern. Med. 2020, 2020, 8562309. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Machado, M.; Morais, R.M.; Calhau, C.; Pintado, M. Selective Activity of an Anthocyanin-Rich, Purified Blueberry Extract upon Pathogenic and Probiotic Bacteria. Foods 2023, 12, 734. [Google Scholar] [CrossRef]
- Pérez-Bassart, Z.; Fabra, M.J.; Martínez-Abad, A.; López-Rubio, A. Compositional Differences of β-Glucan-Rich Extracts from Three Relevant Mushrooms Obtained through a Sequential Extraction Protocol. Food Chem. 2023, 402, 134207. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, Production and Commercial Applications of Fungal Chitosan: A Review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and Eco-Friendly Approaches for the Extraction of Chitin and Chitosan: A Review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef]
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Landi, N.; Clemente, A.; Pedone, P.V.; Ragucci, S.; Di Maro, A. An Updated Review of Bioactive Peptides from Mushrooms in a Well-Defined Molecular Weight Range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Abdeltwab, W.M.; Abdelaliem, Y.F.; Metry, W.A.; Eldeghedy, M. Antimicrobial Effect of Chitosan and Nano-Chitosan against Some Pathogens and Spoilage Microorganisms. J. Adv. Lab. Res. Biol. 2019, 10, 8–15. [Google Scholar]
- Bawadekji, A.; Mridha, M.A.U.; Al Ali, M.; Jamith Basha, W.J. Antimicrobial Activities of Oyster Mushroom Pleurotus ostreatus (Jacq. Ex. Fr.) Kummer. J. Appl. Environ. Biol. Sci. 2017, 7, 227–231. [Google Scholar]
- Sitara, U.; Baloch, P.A.; Pathan, A.U.K.; Bhutto, M.A.; Ali, Q.M.; Ali, A.; Iqbal, M. In Vitro Studies to Determine Antibacterial and Antifungal Properties of Three Pleurotus Species (Oyster Mushroom). Pak. J. Bot. 2023, 55, 387–392. [Google Scholar] [CrossRef]
- Shanmugavelu, M.; Sevugaperumal, G. Screening and Potential Uses of Contaminated Spent Mushroom (Pleurotus spp.). In Emerging Contaminants; IntechOpen: London, UK, 2021. [Google Scholar]
- Gashaw, G.; Fassil, A.; Redi, F. Evaluation of the Antibacterial Activity of Pleurotus spp. Cultivated on Different Agricultural Wastes in Chiro, Ethiopia. Int. J. Microbiol. 2020, 2020, 9312489. [Google Scholar] [CrossRef]
- Garber, A.; Barnard, L.; Pickrell, C. Review of Whole Plant Extracts with Activity against Herpes Simplex Viruses In Vitro and In Vivo. J. Evid.-Based Integr. Med. 2021, 26, 2515690X20978394. [Google Scholar] [CrossRef]
- Youssef, M.M.A.; El-Nagdi, W.M.A. New Approach for Biocontrolling Root-Knot Nematode, Meloidogyne Incognita on Cowpea by Commercial Fresh Oyster Mushroom (Pleurotus ostreatus). Jordan J. Biol. Sci. 2021, 14, 173–177. [Google Scholar] [CrossRef]
- Valdez-Uriostegui, L.A.; Sánchez-García, A.D.; Zamilpa, A.; Sánchez, J.E.; González-Garduño, R.; Mendoza-de-Gives, P.; Castañeda-Ramirez, G.S.; González-Cortázar, M.; Aguilar-Marcelino, L. In Vitro Evaluation of hydroalcoholic extracts of mycelium, basidiomata and spent substrate of Pleurotus ostreatus against Haemonchus contortus. Trop. Subtrop. Agroecosyst 2021, 24, 62. [Google Scholar] [CrossRef]
- Bell, V.; Silva, C.R.P.G.; Guina, J.; Fernandes, T.H. Mushrooms as Future Generation Healthy Foods. Front. Nutr. 2022, 9, 1050099. [Google Scholar] [CrossRef]
- Roy, D.; Ansari, S.; Chatterjee, A.; Luganini, A.; Ghosh, S.K.; Chakraborty, N. In Vitro Search for Antiviral Activity against Human Cytomegalovirus from Medicinal Mushrooms Pleurotus sp. and Lentinus sp. J. Antivir. Antiretrovir. 2020, 12, 201. [Google Scholar]
- Ashaolu, T.J. Immune Boosting Functional Foods and Their Mechanisms: A Critical Evaluation of Probiotics and Prebiotics. Biomed. Pharmacother. 2020, 130, 110625. [Google Scholar] [CrossRef] [PubMed]
- Spacova, I.; Dodiya, H.B.; Happel, A.-U.; Strain, C.; Vandenheuvel, D.; Wang, X.; Reid, G. Future of Probiotics and Prebiotics and the Implications for Early Career Researchers. Front. Microbiol. 2020, 11, 1400. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Xu, L.; Yang, C.S.; Liu, Y.; Zhang, X. Effective Regulation of Gut Microbiota with Probiotics and Prebiotics May Prevent or Alleviate COVID-19 Through the Gut-Lung Axis. Front. Pharmacol. 2022, 13, 895193. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Reynés, B.; Palou, M.; Rodríguez, A.M.; Palou, A. Regulation of Adaptive Thermogenesis and Browning by Prebiotics and Postbiotics. Front. Physiol. 2019, 9, 1908. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Nair, A.; Kulkarni, N.; Todewale, N.; Jobby, R. Exploring Mushroom Polysaccharides for the Development of Novel Prebiotics: A Review. Int. J. Med. Mushrooms 2023, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ayimbila, F.; Prayoonthien, P.; Inyod, T.; Haltrich, D.; Keawsompong, S. Bioactive Composition and Modulatory Effects of Hed-Tean-Rad Mushroom, Macrocybe Crassa on Gut Microbiota. 3 Biotech 2022, 12, 314. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the Human Gut Microbiota by Phenolics and Phenolic Fiber-Rich Foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef] [PubMed]
- Golian, M.; Chlebová, Z.; Žiarovská, J.; Benzová, L.; Urbanová, L.; Hovaňáková, L.; Chlebo, P.; Urminská, D. Analysis of Biochemical and Genetic Variability of Pleurotus ostreatus Based on the β-Glucans and CDDP Markers. J. Fungi 2022, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.R.; Mahadevakumar, S. Fungal Probiotics and Prebiotics. In Fungal Biotechnology Prospects and Avenues; CRC Press: Boca Raton, FL, USA, 2022; pp. 260–279. [Google Scholar]
- Uthan, E.T.; Yamaç, M.; Yildiz, Z. In Vitro Prebiotic Activity of Polysaccharides Extracted from Edible/Medicinal Macrofungi Species. Mantar Derg. 2022, 13, 15–29. [Google Scholar]
- Kerezoudi, E.N.; Mitsou, E.K.; Gioti, K.; Terzi, E.; Avgousti, I.; Panagiotou, A.; Koutrotsios, G.; Zervakis, G.I.; Mountzouris, K.C.; Tenta, R.; et al. Fermentation of Pleurotus ostreatus and Ganoderma Lucidum Mushrooms and Their Extracts by the Gut Microbiota of Healthy and Osteopenic Women: Potential Prebiotic Effect and Impact of Mushroom Fermentation Products on Human Osteoblasts. Food Funct. 2021, 12, 1529–1546. [Google Scholar] [CrossRef]
- Boulaka, A.; Christodoulou, P.; Vlassopoulou, M.; Koutrotsios, G.; Bekiaris, G.; Zervakis, G.I.; Mitsou, E.K.; Saxami, G.; Kyriacou, A.; Zervou, M.; et al. Genoprotective Properties and Metabolites of β-Glucan-Rich Edible Mushrooms Following Their In Vitro Fermentation by Human Faecal Microbiota. Molecules 2020, 25, 3554. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, M.; Wang, X.; Ren, Y.; Yue, T.; Wang, Z.; Gao, Z. Edible Fungal Polysaccharides, the Gut Microbiota, and Host Health. Carbohydr. Polym. 2021, 273, 118558. [Google Scholar] [CrossRef]
- Stojković, D.; Barros, L. Edible Fungi: Chemical Composition, Nutrition and Health Effects; Royal Society of Chemistry: London, UK, 2022. [Google Scholar]
- Vamanu, E.; Dinu, L.D.; Pelinescu, D.R.; Gatea, F. Therapeutic Properties of Edible Mushrooms and Herbal Teas in Gut Microbiota Modulation. Microorganisms 2021, 9, 1262. [Google Scholar] [CrossRef]
- Sawangwan, T.; Wansanit, W.; Pattani, L.; Noysang, C. Study of Prebiotic Properties from Edible Mushroom Extraction. Agric. Nat. Resour. 2018, 52, 519–524. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Fang, H.; Aoki, K.; Tokinoya, K.; Yonamine, M.; Sugasawa, T.; Kawakami, Y.; Takekoshi, K. Effects of High-Fat Diet on the Gut Microbiota of Renalase Gene Knockout Mice. Obesities 2022, 2, 303–316. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, S.; Nie, Q.; He, H.; Tan, H.; Geng, F.; Ji, H.; Hu, J.; Nie, S. Gut Firmicutes: Relationship with Dietary Fiber and Role in Host Homeostasis. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, W.; Siwicka-Gieroba, D.; Kotfis, K.; Zaid, S.; Terpilowska, S.; Robba, C.; Siwicki, A.K. The Brain-Gut Axis-Where Are We Now and How Can We Modulate These Connections? Curr. Neuropharmacol. 2021, 19, 1164–1177. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, S.; Wani, S.M.; Ahmad Mir, S.; Rizwan, D. Role of Probiotics and Prebiotics in Mitigation of Different Diseases. Nutrition 2022, 96, 111602. [Google Scholar] [CrossRef] [PubMed]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary Fibers as Beneficial Microbiota Modulators: A Proposed Classification by Prebiotic Categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Bedu-Ferrari, C.; Biscarrat, P.; Langella, P.; Cherbuy, C. Prebiotics and the Human Gut Microbiota: From Breakdown Mechanisms to the Impact on Metabolic Health. Nutrients 2022, 14, 2096. [Google Scholar] [CrossRef]
- Fanfaret, I.; Boda, D.; Ion, L.; Hosseyni, D.; Leru, P.; Ali, S.; Corcea, S.; Bumbacea, R. Probiotics and Prebiotics in Atopic Dermatitis: Pros and Cons (Review). Exp. Ther. Med. 2021, 22, 1376. [Google Scholar] [CrossRef]
- Cars, O.; Chandy, S.J.; Mpundu, M.; Peralta, A.Q.; Zorzet, A.; So, A.D. Resetting the Agenda for Antibiotic Resistance through a Health Systems Perspective. Lancet Glob. Health 2021, 9, e1022–e1027. [Google Scholar] [CrossRef]
- Pilmis, B.; Le Monnier, A.; Zahar, J.-R. Gut Microbiota, Antibiotic Therapy and Antimicrobial Resistance: A Narrative Review. Microorganisms 2020, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Florowska, A.; Hilal, A.; Florowski, T. Prebiotics and Synbiotics. In Probiotics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 19–37. ISBN 978-0-323-85170-1. [Google Scholar]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Hargis, B.M.; Tellez, G. Use of Prebiotics as an Alternative to Antibiotic Growth Promoters in the Poultry Industry. In Prebiotics and Probiotics-Potential Benefits in Nutrition and Health; IntechOpen: London, UK, 2019. [Google Scholar]
- Cheung, M.K.; Yue, G.G.L.; Chiu, P.W.Y.; Lau, C.B.S. A Review of the Effects of Natural Compounds, Medicinal Plants, and Mushrooms on the Gut Microbiota in Colitis and Cancer. Front. Pharmacol. 2020, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Kawai, J.; Mori, K.; Hartanto, T.; Komatsu, K.; Kudo, T.; Fukuda, S. Dietary Supplement of Mushrooms Promotes SCFA Production and Moderately Associates with IgA Production: A Pilot Clinical Study. Front. Nutr. 2023, 9, 1078060. [Google Scholar] [CrossRef]
- Boureghda, Y.; Satha, H.; Bendebane, F. Chitin–Glucan Complex from Pleurotus ostreatus Mushroom: Physicochemical Characterization and Comparison of Extraction Methods. Waste Biomass Valor. 2021, 12, 6139–6153. [Google Scholar] [CrossRef]
- Moumita, S.; Das, B. Assessment of the Prebiotic Potential and Bioactive Components of Common Edible Mushrooms in India and Formulation of Synbiotic Microcapsules. LWT 2022, 156, 113050. [Google Scholar] [CrossRef]
- Permana, S.; Wibowo, N.R.K.; Adani, S.F.; Ayulanda, M.; Endharti, A. The insight of in silico and in vitro evaluation of oyster mushroom (Pleurotus ostreatus) against colorectal cancer targeting timp-1. J. Southwest Jiaotong Univ. 2022, 57, 186–199. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Shaheen, S.; Shorbagi, M.; Lorenzo, J.M.; Farag, M.A. Dissecting Dietary Melanoidins: Formation Mechanisms, Gut Interactions and Functional Properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 8954–8971. [Google Scholar] [CrossRef]
- Fotschki, B.; Wiczkowski, W.; Sawicki, T.; Sójka, M.; Myszczyński, K.; Ognik, K.; Juśkiewicz, J. Stimulation of the Intestinal Microbiota with Prebiotics Enhances Hepatic Levels of Dietary Polyphenolic Compounds, Lipid Metabolism and Antioxidant Status in Healthy Rats. Food Res. Int. 2022, 160, 111754. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, Y.; Qian, C.; Hussain, M.; Liu, S.; Zhang, A.; He, R.; Sun, P. The Interaction between Mushroom Polysaccharides and Gut Microbiota and Their Effect on Human Health: A Review. Biology 2023, 12, 122. [Google Scholar] [CrossRef]
- Ye, S.; Shah, B.R.; Li, J.; Liang, H.; Zhan, F.; Geng, F.; Li, B. A Critical Review on Interplay between Dietary Fibers and Gut Microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Zheng, Z.; Deng, W.; Li, Y.; Song, H.; Chen, S. Extraction, Physiological Function and Application of Soluble Dietary Fiber from Edible Fungi: A Review. Food Sci. Technol. 2022, 42, e35422. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Role of Mushroom Polysaccharides in Improving Gut Health and Associated Diseases. In Microbiome, Immunity, Digestive Health and Nutrition; Elsevier: Amsterdam, The Netherlands, 2022; pp. 431–448. ISBN 978-0-12-822238-6. [Google Scholar]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gao, Y.; Pi, X.; Zhao, S.; Liu, W. In Vitro Hepatoprotective and Human Gut Microbiota Modulation of Polysaccharide-Peptides in Pleurotus citrinopileatus. Front. Cell. Infect. Microbiol. 2022, 12, 892049. [Google Scholar] [CrossRef]
- Wang, X.; Qu, Y.; Wang, Y.; Wang, X.; Xu, J.; Zhao, H.; Zheng, D.; Sun, L.; Tai, G.; Zhou, Y.; et al. β-1,6-Glucan from Pleurotus eryngii Modulates the Immunity and Gut Microbiota. Front. Immunol. 2022, 13, 859923. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Song, X.; Cui, W.; Meng, F.; Xia, Q.; Li, X.; Hou, M.; Jia, L.; Zhang, J. Glucopyranose from Pleurotus geesteranus Prevent Alcoholic Liver Diseases by Regulating Nrf2/HO-1-TLR4/NF-ΚB Signalling Pathways and Gut Microbiota. Food Funct. 2022, 13, 2441–2455. [Google Scholar] [CrossRef]
- Maheshwari, G.; Gessner, D.K.; Neuhaus, K.; Most, E.; Zorn, H.; Eder, K.; Ringseis, R. Influence of a Biotechnologically Produced Oyster Mushroom (Pleurotus sajor-caju) on the Gut Microbiota and Microbial Metabolites in Obese Zucker Rats. J. Agric. Food Chem. 2021, 69, 1524–1535. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, Q.; Ma, G.; Yu, A.; Zhao, L.; Zhang, X.; Zhao, R. Selenium Biofortification in Pleurotus eryngii and Its Effect on Lead Adsorption of Gut Microbiota via In Vitro Fermentation. Food Chem. 2022, 396, 133664. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current Trends in Marine Algae Polysaccharides: The Digestive Tract, Microbial Catabolism, and Prebiotic Potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Kour, H.; Kour, D.; Kour, S.; Singh, S.; Jawad Hashmi, S.A.; Yadav, A.N.; Kumar, K.; Sharma, Y.P.; Ahluwalia, A.S. Bioactive Compounds from Mushrooms: Emerging Bioresources of Food and Nutraceuticals. Food Biosci. 2022, 50, 102124. [Google Scholar] [CrossRef]
- Saggu, A.K.; Tomer, V.; Kumar, A.; Pandey, P. Consideration of Phytonutrients, Probiotics and Prebiotics for Enhanced Immunity during Disaster Relief Situation—A Review. Clin. Nutr. Open Sci. 2023, 47, 131–146. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, R. A Review on Nutritional Advantages of Edible Mushrooms and Its Industrialization Development Situation in Protein Meat Analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- De Cianni, R.; Pippinato, L.; Mancuso, T. A Systematic Review on Drivers Influencing Consumption of Edible Mushrooms and Innovative Mushroom-Containing Products. Appetite 2023, 182, 106454. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.; Niamah, A.K. Bacterial viability, antioxidant stability, antimutagenicity and sensory properties of onion types fermentation by using probiotic starter during storage. Nutr. Food Sci. 2022, 52, 901–916. [Google Scholar] [CrossRef]
- Cunningham, M.; Azcarate-Peril, M.A.; Barnard, A.; Benoit, V.; Grimaldi, R.; Guyonnet, D.; Holscher, H.D.; Hunter, K.; Manurung, S.; Obis, D.; et al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021, 29, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Kambale, R.M.; Ntagazibwa, J.N.; Kasengi, J.B.; Zigashane, A.B.; Francisca, I.N.; Mashukano, B.N.; Ngaboyeka, G.A.; Bahizire, E.; Zech, F.; Bindels, L.B.; et al. Probiotics for children with uncomplicated severe acute malnutrition (PruSAM study): A randomized controlled trial in the Democratic Republic of Congo. Am. J. Clin. Nutr. 2023, 117, 976–984. [Google Scholar] [CrossRef] [PubMed]
- D’Accolti, M.; Soffritti, I.; Bini, F.; Mazziga, E.; Cason, C.; Comar, M.; Volta, A.; Bisi, M.; Fumagalli, D.; Mazzacane, S.; et al. Shaping the subway microbiome through probiotic-based sanitation during the COVID-19 emergency: A pre-post case-control study. Microbiome 2023, 11, 64. [Google Scholar] [CrossRef]
- Yasmin, A.; Butt, M.S.; Afzaal, M.; Baak, M.; Nadeem, M.T.; Shahid, M.Z. Prebiotics, gut microbiota and metabolic risks: Unveiling the relationship. J. Funct. Foods 2015, 17, 189–201. [Google Scholar] [CrossRef]
- Yan, T.; Shi, L.; Liu, T.; Zhang, X.; Yang, M.; Peng, W.; Sun, X.; Yan, L.; Dai, X.; Yang, X. Diet-rich in wheat bran modulates tryptophan metabolism and AhR/IL-22 signalling mediated metabolic health and gut dysbacteriosis: A novel prebiotic-like activity of wheat bran. Food Res. Int. 2023, 163, 112179. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, A.; Covino, M.; Candelli, M.; Ojetti, V.; Capacci, A.; Gasbarrini, A.; Franceschi, F.; Merra, G. How Do Diet Patterns, Single Foods, Prebiotics and Probiotics Impact Gut Microbiota? Microbiol. Res. 2023, 14, 390–408. [Google Scholar] [CrossRef]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.; Saeed, F.; Afzaal, M.; Shah, Y.A.; Qamar, A.; Faisal, Z.; Ghani, S.; Ateeq, H.; Akhtar, M.N.; Tufail, T.; et al. Gut microbiota and synbiotic foods: Unveiling the relationship in COVID-19 perspective. Food Sci. Nutr. 2023, 11, 1166–1177. [Google Scholar] [CrossRef]
- Chakaroun, R.M.; Olsson, L.M.; Bäckhed, F. The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat. Rev. Cardiol. 2023, 20, 217–235. [Google Scholar] [CrossRef]
- Yang, Y.; Du, H.; Zou, G.; Song, Z.; Zhou, Y.; Li, H.; Tan, C.; Chen, H.; Fischetti, V.A.; Li, J. Encapsulation and delivery of phage as a novel method for gut flora manipulation in situ: A review. J. Control. Release 2023, 353, 634–649. [Google Scholar] [CrossRef]
- Alverdy, J.C. Rationale for Colonic Pre-Habilitation Prior to Restoration of Gastrointestinal Continuity. Surg. Infect. 2023, 24, 265–270. [Google Scholar] [CrossRef]



| Used Solvent(s) | Anti-Microbes (Bacteria) | Refs. |
|---|---|---|
| Chitosan | Bacillus cereus | [87] |
| Methanol | Bacillus pumilus | [9] |
| Hexane-dichloromethane | Bacillus subtilis | [8] |
| Ethyl acetate | Burkhoderia pseudomallei, Enterobacter aerogenes | [20] |
| Methanol | Enterococcus faecalis | [88] |
| Ethyl acetate, methanol, water extract, ethanol: chloroform: distilled water (2:2:3), plus (75%) of chloroform, ethanol, and acetone | Escherichia coli | [3,21,89,90] |
| Ethyl acetate | Klebsiella oxytoca | [20] |
| Methanol | Klebsiella pneumonia | [88] |
| Ethanol, chloroform, and distilled water, in a constant ratio of 2:2:4 | Micrococcus luteus | [3] |
| Ethyl acetate | Moraxella sp. | [20] |
| Ethyl acetate, methanol, and Hexane-dichloromethane | Pseudomonas aeruginosa | [8,20] |
| Ethyl acetate | Salmonella pullorum | [20] |
| Methanol | Salmonella typhi | [9] |
| Water and methanol | Shigella sp. | [9,21] |
| Ethyl acetate, methanol, ethanol: chloroform: distilled water (2:2:1), water plus (75%) of chloroform, ethanol, and acetone | Staphylococcus aureus | [3,21,89,90] |
| Water | Vibrio sp. | [21] |
| Used Solvent(s) | Anti-Microbes (Fungi) | Refs. |
|---|---|---|
| Hexane-dichloromethane | Aspergillus niger | [8] |
| Ethyl acetate, methanol, ethanol: chloroform: water (2:2:2), and water | Candida albicans | [3,9,90] |
| Methanol | Candida glabrata, C. krusei, C. parapsilosis, C. tropicalis | [9] |
| Water | Cryptococcus humicola, Trichosporon cutaneum | [92] |
| Methanol | Epidermophyton floccosum, Microsporum gypseum, Trichophyton rubrum | [91] |
| Hexane-dichloromethane | Fusarium oxysporum | [8] |
| Water | Penicillium sp. | [21] |
| Activity | Used Solvent(s)/Bioactives | Microbe/Disease | Refs. |
|---|---|---|---|
| Antihelmintic | Hydroalcoholic extract | Gastrointestinal nematodes | [94] |
| Antihelmintic | Aqueous extract | M. incognita parasitic nematode | [93] |
| Antiviral | Laccase purified from P. ostreatus mushroom fruiting | HCV | [10,14] |
| Antiviral | Ubiquitin-like protein purified from P. ostreatus | HIV | [2,14] |
| Antiviral | Water extract | HSV-1 | [92] |
| Antiviral | Extracted β-glucan | HSV-1 | [79] |
| Antiviral | Aqueous and methanol extracts of P. ostreatus | HSV-1 | [95] |
| Antiviral | Sodium-chloride extract of P. ostreatus mycelia | HSV-2 | [92] |
| Antiviral | Aqueous extract of P. ostreatus mushroom | SARS-CoV-2/SARS | [11] |
| Antiviral | Aqueous and methanol extracts of P. ostreatus | Human Cytomegalovirus | [96] |
| Prebiotic Activity | Probiotic Microbes | Refs. |
|---|---|---|
| High content of total carbohydrates and total reducing sugar as indicator for prebiotic yield | Lactobacillus acidophilus, Lactobacillus plantarum | [116] |
| β-glucans of P. ostreatus enhanced the gut microbiota of 65-year-old humans | Bifidobacterium spp., Faecalibacterium prausnitzii | [112] |
| Polysaccharides of P. ostreatus mushroom enhanced the gut microbiota in humans | Bifidobacterium spp. | [113] |
| Polysaccharides from P. ostreatus mushroom promoted the activity of studied microbes | Lactobacillus spp., Enterococcus spp. | [113,114] |
| Polysaccharides from P. ostreatus promoted the activity of studied microbes | Bifidobacterium spp. | [116] |
| β-glucans in the P. ostreatus mushroom promoted the activity of studied microbes | Lactobacillus spp. | [115] |
| β-glucans in P. ostreatus mushroom enhanced the gut microbiota in humans | Bacteroides spp. | [111] |
| γ-aminobutyric acid and β-glucan in P. ostreatus improved digestive system health | Pediococcus spp., Streptococcus lactis | [67] |
| Polysacharrides extracted from P. ostreatus promoted the activity of studied microbes | Lactobacillus plantarum, Lactobacillus acidophilus | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Törős, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom. Foods 2023, 12, 2010. https://doi.org/10.3390/foods12102010
Törős G, El-Ramady H, Prokisch J, Velasco F, Llanaj X, Nguyen DHH, Peles F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom. Foods. 2023; 12(10):2010. https://doi.org/10.3390/foods12102010
Chicago/Turabian StyleTörős, Gréta, Hassan El-Ramady, József Prokisch, Fernando Velasco, Xhensila Llanaj, Duyen H. H. Nguyen, and Ferenc Peles. 2023. "Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom" Foods 12, no. 10: 2010. https://doi.org/10.3390/foods12102010
APA StyleTörős, G., El-Ramady, H., Prokisch, J., Velasco, F., Llanaj, X., Nguyen, D. H. H., & Peles, F. (2023). Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus ostreatus Mushroom. Foods, 12(10), 2010. https://doi.org/10.3390/foods12102010















