Recent Advances in Sources, Migration, Public Health, and Surveillance of Bisphenol A and Its Structural Analogs in Canned Foods
Abstract
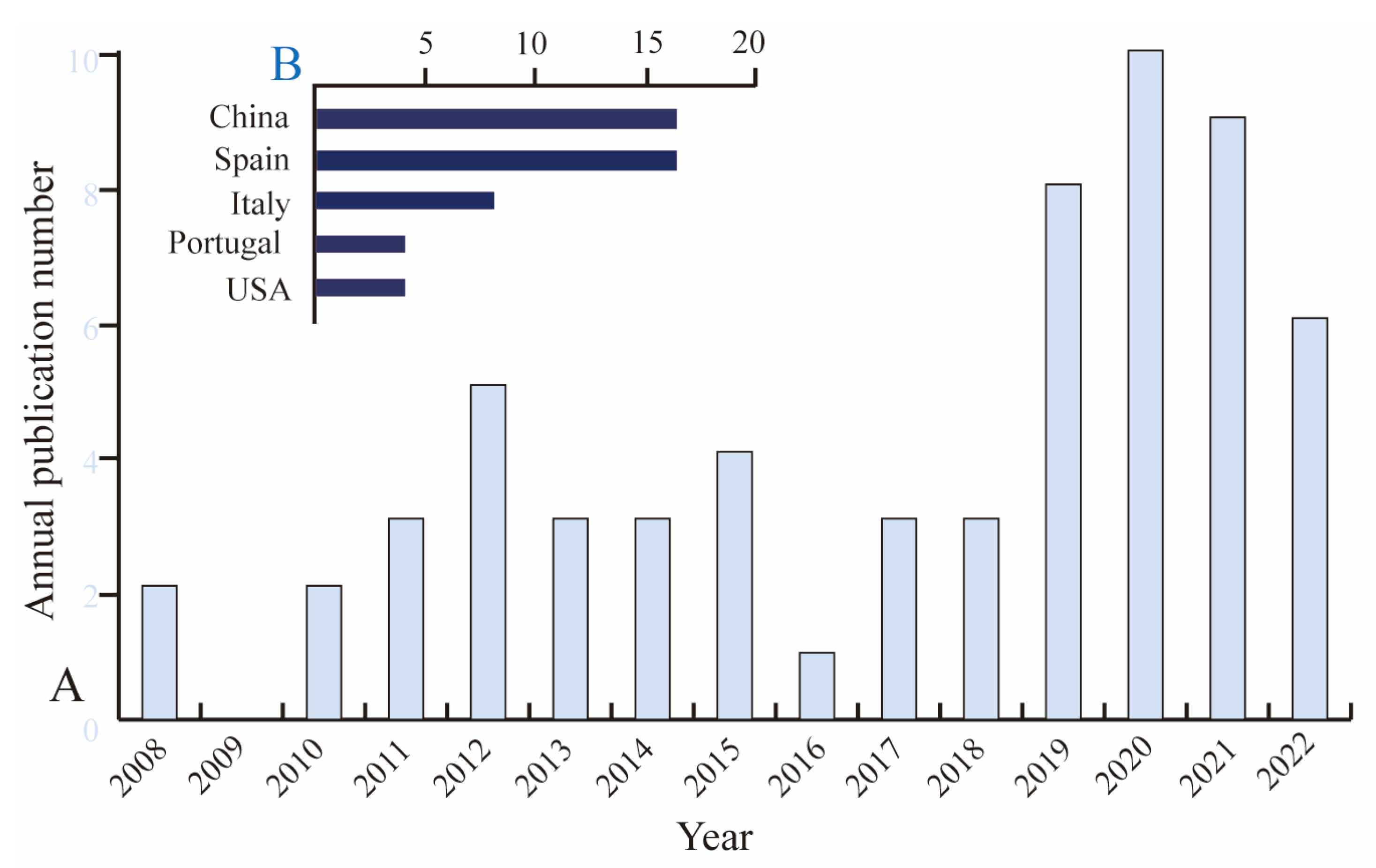
1. Introduction
2. Contamination Sources of BPA and Its Analogs in Canned Foods
2.1. BPA and Its Analogs from Packaging Materials Used in Canned Foods
2.2. BPA and Its Analogs from Raw Materials of Canned Products
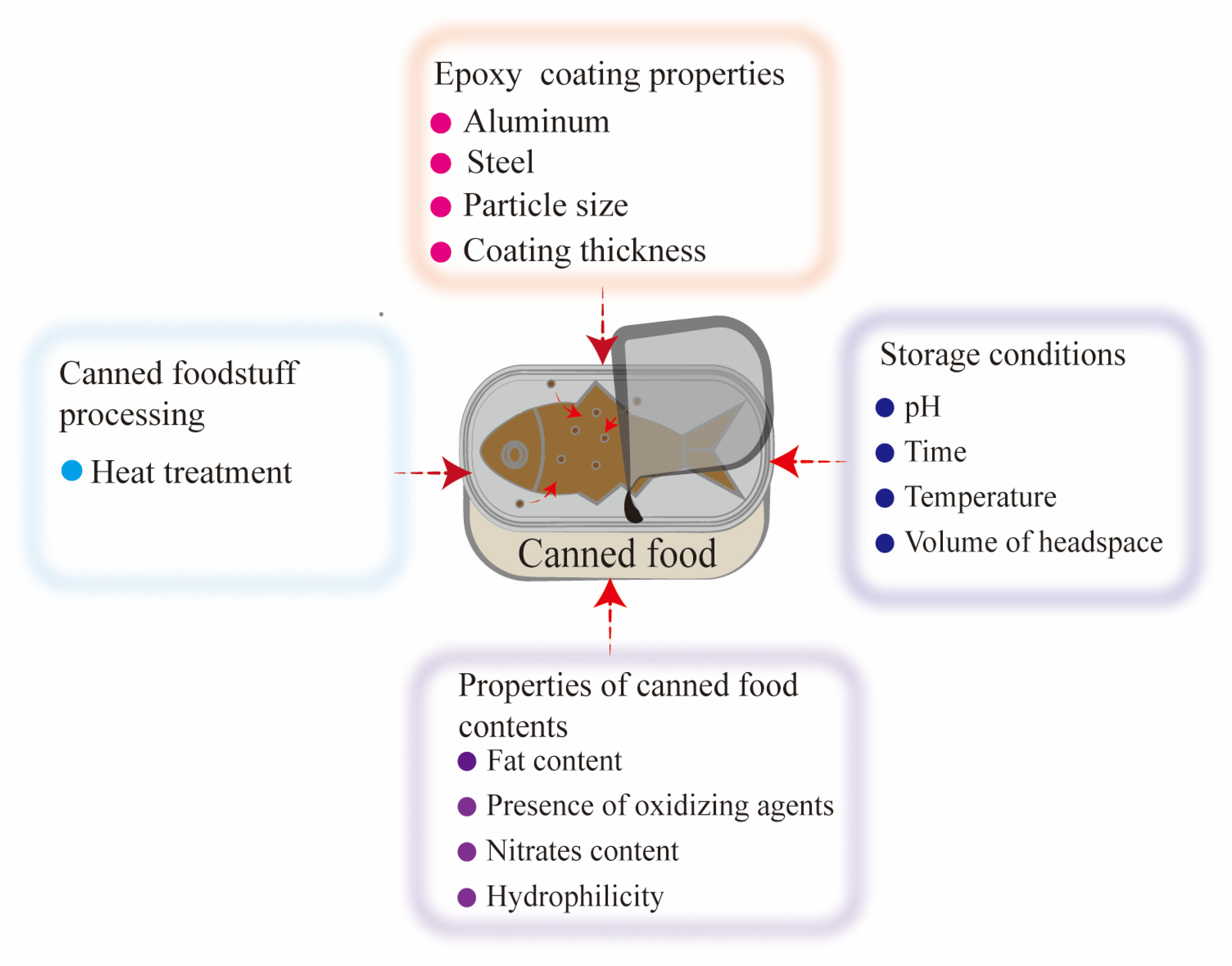
3. Factors Influencing the Migration of BPA and Its Analogs in Canned Foods
4. Surveillance of BPA and Its Analogs in Canned Foods
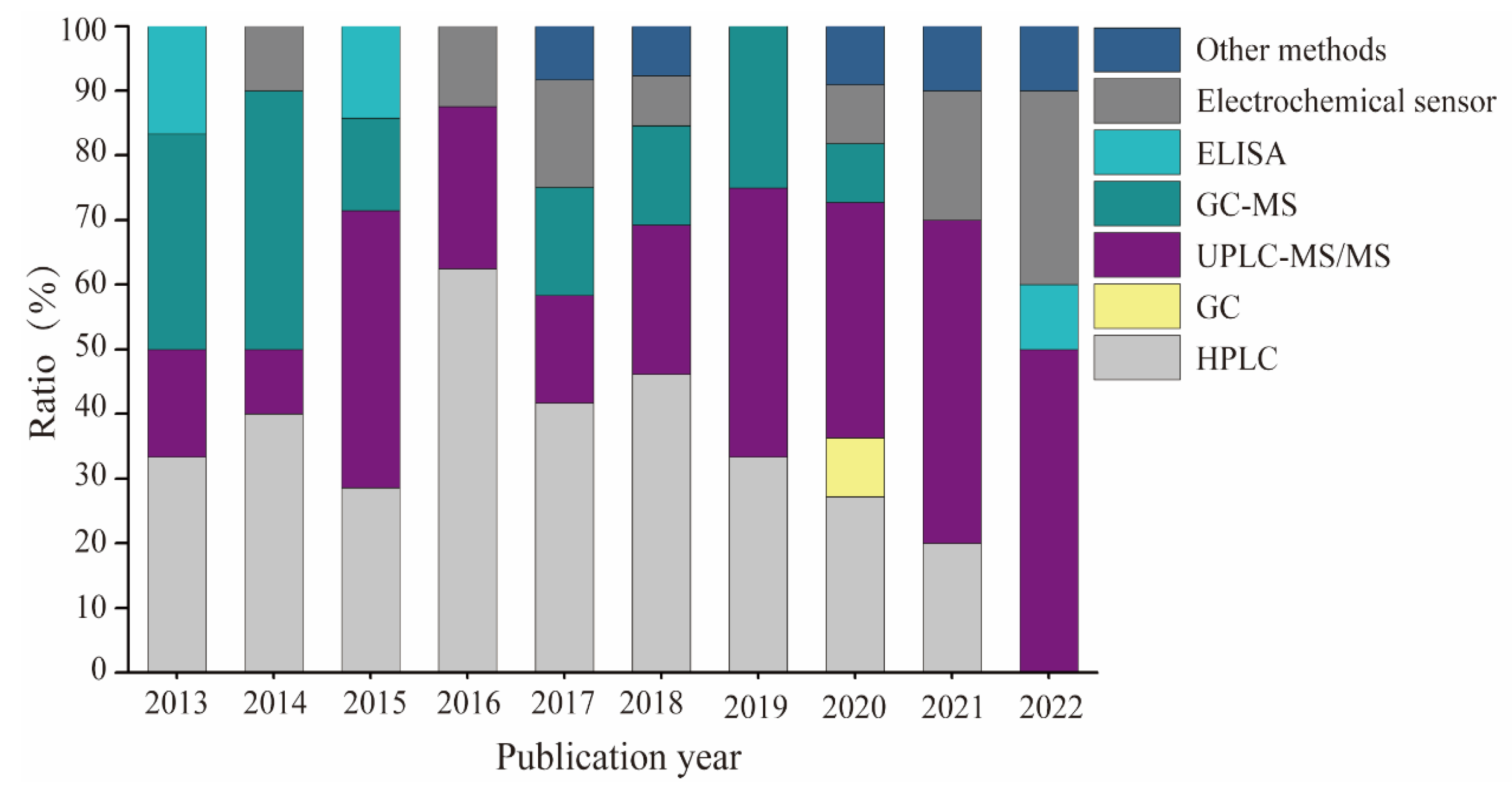
4.1. Analytical Methods for Determining BPA and Its Analogs in Canned Foods
4.2. Estimates of BPA and Its Analogs in Canned Foods
5. Impact of BPA and Its Analogs from Canned Foods on Public Health
5.1. Exposure Assessment Examples of BPA and Its Analogs by Analysing Residual Levels of These Chemicals in Canned Foods
5.2. Toxicological Mechanism of BPA and Its Analogs in Canned Products
6. Bisphenols Current Legislation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jalal, N.; Surendranath, A.R.; Pathak, J.L.; Yu, S.; Chung, C.Y. Bisphenol A (BPA) the mighty and the mutagenic. Toxicol. Rep. 2018, 5, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Majewska, J.; Berg, A.; Owczarek, K.; Zajdel, R.; Kaleta, D.; Wasik, A.; Rachon, D. Serum bisphenol A analogues in women diagnosed with the polycystic ovary syndrome—Is there an association? Environ. Pollut. 2021, 272, 115962. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, B.; Yang, R.; Wu, Y.; Zhao, Y.; Li, C.; Zhang, J.; Xing, Y.; Shao, B. Bisphenol analogues and their chlorinated derivatives in breast milk in China: Occurrence and exposure assessment. J. Agric. Food Chem. 2021, 69, 1391–1397. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef]
- Von Goetz, N.; Pirow, R.; Hart, A.; Bradley, E.; Pocas, F.; Arcella, D.; Lillegard, I.T.L.; Simoneau, C.; van Engelen, J.; Husoy, T.; et al. Including non-dietary sources into an exposure assessment of the European Food Safety Authority: The challenge of multi-sector chemicals such as Bisphenol A. Regul. Toxicol. Pharmacol. 2017, 85, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef] [PubMed]
- McDonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive; nervous; and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300. [Google Scholar] [CrossRef]
- Farrugia, F.; Aquilina, A.; Vassallo, J.; Pace, N.P. Bisphenol A and Type 2 Diabetes Mellitus: A review of epidemiologic, functional, and early life factors. Int. J. Environ. Res. Public Health 2021, 18, 716. [Google Scholar] [CrossRef]
- Perez-Bermejo, M.; Mas-Perez, I.; Murillo-Llorente, M.T. The role of the bisphenol A in diabetes and obesity. Biomedicines 2021, 9, 666. [Google Scholar] [CrossRef]
- Cai, S.F.; Rao, X.M.; Ye, J.H.; Ling, Y.X.; Mi, S.; Chen, H.Z.; Fan, C.H.; Li, Y.J. Relationship between urinary bisphenol a levels and cardiovascular diseases in the U.S. adult population, 2003–2014. Ecotoxicol. Environ. Safe 2020, 192, 110300. [Google Scholar] [CrossRef]
- Moreno-Gomez-Toledano, R.; Arenas, M.I.; Velez-Velez, E.; Coll, E.; Quiroga, B.; Bover, J.; Bosch, R.J. Bisphenol A exposure and kidney diseases: Systematic review, meta-analysis; and NHANES 03-16 Study. Biomolecules 2021, 11, 1046. [Google Scholar] [CrossRef]
- İyİgÜndoĞdu, İ.; ÜstÜndaĞ, A.; Duydu, Y. Toxicological evaluation of bisphenol A and its analogues. Turk. J. Pharm. Sci. 2020, 17, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Cai, W.; Liu, H.; Jiang, H.; Bi, Y.; Wang, H. The association of bisphenol A and phthalates with risk of breast cancer: A meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 2375. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, C.; Muscogiuri, G.; Nardone, A.; Garifalos, F.; Provvisiero, D.P.; Verde, N.; Pivonello, R. Bisphenol A: An emerging threat to female fertility. Reprod. Biol. Endocrinol. 2020, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Vilarinho, F.; Sendón, R.; van de Kellen, A.; Vaz, M.F.; Silva, A.S. Bisphenol A in food as a result of its migration from food packaging. Trends Food Sci. Technol. 2019, 91, 33–65. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) No 10/2011 as regards the use of that substance in plastic food contact materials. Off. J. Eur. Union 2018, L41, 6–12. Available online: https://data.europa.eu/eli/reg/2018/213/oj (accessed on 11 October 2022).
- France. Law No. 2012-1442 on Suspension of the Manufacture, Import, Export and Placing on the Market of Any Food Package Containing Bisphenol A. 2012. Available online: https://www.legifrance.gouv.fr/dossierlegislatif/JORFDOLE000024664321/ (accessed on 1 October 2022).
- Shamhari, A.; Hamid, Z.A.; Budin, S.B.; Shamsudin, N.J.; Taib, I.S. Bisphenol A and its analogues deteriorate the hormones physiological function of the male reproductive system: A mini-review. Biomedicines 2021, 9, 1744. [Google Scholar] [CrossRef]
- Husoy, T.; Andreassen, M.; Hjertholm, H.; Carlsen, M.H.; Norberg, N.; Sprong, C.; Papadopoulou, E.; Sakhi, A.K.; Sabaredzovic, A.; Dirven, H. The Norwegian biomonitoring study from the EU project EuroMix: Levels of phenols and phthalates in 24-hour urine samples and exposure sources from food and personal care products. Environ. Int. 2019, 132, 105103. [Google Scholar] [CrossRef]
- Andújar, N.; Glvez-Ontiveros, Y.; Zafra-Gmez, A.; Rodrigo, L.; Álvarez-Cubero, M.J.; Aguilera, M.; Monteagudo, C.; Rivas, A. Bisphenol A analogues in food and their hormonal and obesogenic effects: A review. Nutrients 2019, 11, 2136. [Google Scholar] [CrossRef]
- Oliveira, W.Q.; Azeredo, H.M.C.; Neri-Numa, I.A.; Pastore, G.M. Food packaging wastes amid the COVID-19 pandemic: Trends and challenges. Trends Food Sci. Technol. 2021, 116, 1195–1199. [Google Scholar] [CrossRef]
- González, N.; Cunha, S.C.; Ferreira, R.; Fernandes, J.O.; Domingo, J.L. Concentrations of nine bisphenol analogues in food purchased from Catalonia (Spain): Comparison of canned and non-canned foodstuffs. Food Chem. Toxicol. 2020, 136, 110992. [Google Scholar] [CrossRef]
- Prudencio, T.M.; Swift, L.M.; Guerrelli, D.; Cooper, B.; Reilly, M.; Ciccarelli, N.; Sheng, J.; Jaimes, R.; Posnack, N.G. Bisphenol S and bisphenol F are less disruptive to cardiac electrophysiology, as compared to bisphenol A. Toxicol. Sci. 2021, 183, 214–226. [Google Scholar] [CrossRef]
- Siracusa, J.S.; Yin, L.; Measel, E.; Liang, S.; Yu, X. Effects of bisphenol A and its analogs on reproductive health: A mini review. Reprod. Toxicol. 2018, 79, 96–123. [Google Scholar] [CrossRef]
- Liu, J.C.; Zhang, L.Y.; Lu, G.H.; Jiang, R.R.; Yan, Z.H.; Li, Y.P. Occurrence, toxicity and ecological risk of bisphenol A analogues in aquatic environment—A review. Ecotoxicol. Environ. Safe 2021, 208, 111481. [Google Scholar] [CrossRef] [PubMed]
- Deceuninck, Y.; Bichon, E.; Geny, T.; Veyrand, B.; Grandin, F.; Viguie, C.; Marchand, P.; Le Bizec, B. Quantitative method for conjugated metabolites of bisphenol A and bisphenol S determination in food of animal origin by Ultra High-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1601, 232–242. [Google Scholar] [CrossRef]
- El Moussawi, S.N.; Ouaini, R.; Matta, J.; Chébib, H.; Cladière, M.; Camel, V. Simultaneous migration of bisphenol compounds and trace metals in canned vegetable food. Food Chem. 2019, 288, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, A.A.; Babalola, B.A. Bisphenol-A (BPA) in Foods commonly consumed in Southwest Nigeria and its Human Health Risk. Sci. Rep. 2019, 9, 17458. [Google Scholar] [CrossRef]
- El Moussawi, S.N.; Cladière, M.; Chébib, H.; Ouaini, R.; Camel, V. Empirical models to predict the effect of sterilisation and storage on bisphenols migration from metallic can coatings into food simulants. Food Addit. Contam. A 2019, 36, 1937–1949. [Google Scholar] [CrossRef]
- Cao, X.L.; Corriveau, J.; Popovic, S. Sources of low concentrations of bisphenol A in canned beverage products. J. Food Prot. 2010, 73, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- EFSA. No Consumer Health Risk from Bisphenol A Exposure. 2015. Available online: http://www.efsa.europa.eu/en/press/news/150121 (accessed on 18 October 2022).
- Akhbarizadeh, R.; Moore, F.; Monteiro, C.; Fernandes, J.O.; Cunha, S.C. Occurrence, trophic transfer, and health risk assessment of bisphenol analogues in seafood from the Persian Gulf. Mar. Pollut. Bull. 2020, 154, 111036. [Google Scholar] [CrossRef]
- Repossi, A.; Farabegoli, F.; Gazzotti, T.; Zironi, E.; Pagliuca, G. Bisphenol A in edible part of seafood. Ital. J. Food Saf. 2016, 5, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, Y.; Dong, S.; Wang, P.; Su, X. The occurrence of bisphenol compounds in animal feed plastic packaging and migration into feed. Chemosphere 2021, 265, 129022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, X.H.; Pan, S.D.; Wang, J.L.; Zheng, Y.B.; Xu, J.J.; Zhao, Y.G.; Cai, Z.X.; Jin, M.C. Contamination status of bisphenol A and its analogues (bisphenol S, F and B) in foodstuffs and the implications for dietary exposure on adult residents in Zhejiang Province. Food Chem. 2019, 294, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.L.; Kosarac, I.; Popovic, S.; Zhou, S.; Smith, D.; Dabeka, R. LC-MS/MS analysis of bisphenol S and five other bisphenols in total diet food samples. Food Addit. Contam. A 2019, 36, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Michalowicz, J. Bisphenol A—Sources, toxicity and biotransformation. Environ. Toxicol. Pharmacol. 2014, 37, 738–758. [Google Scholar] [CrossRef]
- Zhang, N.; Scarsella, J.B.; Hartman, T.G. Identification and quantitation studies of migrants from BPA alternative food-contact metal can coatings. Polymers 2020, 12, 2846. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Castle, L.; Oldring, P.K.; Moschakis, T.; Wedzicha, B.L. Factors affecting migration kinetics from a generic epoxy-phenolic food can coating system. Food Res. Int. 2018, 106, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kito, K.; Kondo, F. Factors influencing the migration of bisphenol A from cans. J. Food Prot. 2003, 66, 1444–1447. [Google Scholar] [CrossRef]
- Choi, S.J.; Yun, E.S.; Shin, J.M.; Kim, Y.S.; Lee, J.S.; Lee, J.H.; Kim, D.G.; Oh, Y.H.; Jung, K.; Kim, G.H. Concentrations of bisphenols in canned foods and their risk assessment in Korea. J. Food Prot. 2018, 81, 903–916. [Google Scholar] [CrossRef]
- Tarafdar, A.; Sirohi, R.; Balakumaran, P.A.; Reshmy, R.; Madhavan, A.; Sindhu, R.; Binod, P.; Kumar, Y.; Kumar, D.; Sim, S.J. The hazardous threat of bisphenol A: Toxicity, detection and remediation. J. Hazard. Mater. 2022, 423, 127097. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.N.; Song, J.Y.; Wu, M.T.; Zhang, Z. Development of ic-ELISAs for the detection of bisphenol A diglycidyl ether and its derivatives in canned luncheon meats. ACS Food Sci. Technol. 2022, 2, 160–168. [Google Scholar] [CrossRef]
- Ali, H.; Mukhopadhyay, S.; Jana, N.R. Selective electrochemical detection of bisphenol A using a molecularly imprinted polymer nanocomposite. New J. Chem. 2019, 43, 1536–1543. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zeng, Q.; Wang, M.; Wang, L. An electropolymerized molecularly imprinted electrochemical sensor for the selective determination of bisphenol A diglycidyl ether. ChemistrySelect 2020, 5, 3574–3580. [Google Scholar] [CrossRef]
- Ebrahimi-Tazangi, F.; Beitollahi, H.; Hekmatara, H.; Seyed-Yazdi, J. Design of a new electrochemical sensor based on the CuO/GO nanocomposites: Simultaneous determination of Sudan I and bisphenol A. J. Iran. Chem. Soc. 2020, 18, 191–199. [Google Scholar] [CrossRef]
- Sanko, V.; Senocak, A.; Tumay, S.O.; Orooji, Y.; Demirbas, E.; Khataee, A. An electrochemical sensor for detection of trace-level endocrine disruptor bisphenol A using Mo2Ti2AlC3 MAX phase/MWCNT composite modified electrode. Environ. Res. 2022, 212, 113071. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhong, H.N.; Qiu, K.; Li, D.; Song, Y. Exposure to bisphenol A and its substitutes, bisphenol F and bisphenol S from canned foods and beverages on Chinese market. Food Control 2021, 120, 107502. [Google Scholar] [CrossRef]
- Fattore, M.; Russo, G.; Barbato, F.; Grumetto, L.; Albrizio, S. Monitoring of bisphenols in canned tuna from Italian markets. Food Chem. Toxicol. 2015, 83, 68–75. [Google Scholar] [CrossRef]
- Tzatzarakis, M.N.; Karzi, V.; Vakonaki, E.; Goumenou, M.; Kavvalakis, M.; Stivaktakis, P.; Tsitsimpikou, C.; Tsakiris, I.; Rizos, A.K.; Tsatsakis, A.M. Bisphenol A in soft drinks and canned foods and data evaluation. Food Addit. Contam. Part B 2017, 10, 85–90. [Google Scholar] [CrossRef]
- Cunha, S.C.; Inacio, T.; Almada, M.; Ferreira, R.; Fernandes, J.O. Gas chromatography-mass spectrometry analysis of nine bisphenols in canned meat products and human risk estimation. Food Res. Int. 2020, 135, 109293. [Google Scholar] [CrossRef]
- Lestido-Cardama, A.; Sendón, R.; Bustos, J.; Santillana, M.I.; Losada, P.P.; Quirós, R.B.D. Multi-analyte method for the quantification of bisphenol related compounds in canned food samples and exposure assessment of the Spanish adult population. Food Packag. Shelf Life 2021, 28, 100671. [Google Scholar] [CrossRef]
- Tian, L.; Zheng, J.Y.; Pineda, M.; Yargeau, V.; Furlong, D.; Chevrier, J.; Bornman, R.; Obida, M.; Gates Goodyer, C.; Bayen, S. Targeted screening of 11 bisphenols and 7 plasticizers in food composites from Canada and South Africa. Food Chem. 2022, 385, 132675. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, T.; Fasano, E.; Scognamiglio, G.; Pisciottano, I.D.M.; Mita, G.D.; Gallo, P. BPA, BPB, BPF, BADGE and BFDGE in canned beers from the Italian market. Food Addit. Contam. B 2019, 12, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Varriale, F.; Barbato, F.; Grumetto, L. Are canned beverages industries progressively switching to bisphenol AF? J. Food Sci. 2019, 84, 3303–3311. [Google Scholar] [CrossRef] [PubMed]
- Al Ghoul, L.; Abiad, M.G.; Jammoul, A.; Matta, J.; Darra, N.E. Zinc, aluminium, tin and Bis-phenol a in canned tuna fish commercialized in Lebanon and its human health risk assessment. Heliyon 2020, 6, e04995. [Google Scholar] [CrossRef]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. F 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Lucarini, F.; Krasniqi, T.; Rosset, G.B.; Roth, N.; Hopf, N.B.; Broillet, M.C.; Staedler, D. Exposure to new emerging bisphenols among young children in Switzerland. Int. J. Environ. Res. Public Health 2020, 17, 4793. [Google Scholar] [CrossRef]
- Provvisiero, D.P.; Pivonello, C.; Muscogiuri, G.; Negri, M.; de Angelis, C.; Simeoli, C.; Pivonello, R.; Colao, A. Influence of bisphenol A on type 2 diabetes mellitus. Int. J. Environ. Res. Public Health 2016, 13, 989. [Google Scholar] [CrossRef]
- Gauderat, G.; Picard-Hagen, N.; Toutain, P.L.; Corbel, T.; Viguié, C.; Puel, S.; Lacroix, M.Z.; Mindeguia, P.; Bousquet-Melou, A.; Véronique, G. Bisphenol A glucuronide deconjugation is a determining factor of fetal exposure to bisphenol A. Environ. Int. 2016, 86, 52–59. [Google Scholar] [CrossRef]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Yue, S.; Yu, J.; Kong, Y.; Chen, H.; Mao, M.; Ji, C.; Shao, S.; Zhu, J.; Gu, J.; Zhao, M. Metabolomic modulations of HepG2 cells exposed to bisphenol analogues. Environ. Int. 2019, 129, 59–67. [Google Scholar] [CrossRef]
- Li, C.H.; Zhang, D.H.; Jiang, L.D.; Qi, Y.H.; Guo, L.H. Binding and activity of bisphenol analogues to human peroxisome proliferator-activated receptor β/δ. Ecotoxicol. Environ. Safe 2021, 226, 112849. [Google Scholar] [CrossRef]
- Yang, F.; Qiu, W.; Li, R.; Hu, J.; Luo, S.; Zhang, T.; He, X.; Zheng, C. Genome-wide identification of the interactions between key genes and pathways provide new insights into the toxicity of bisphenol F and S during early development in zebrafish. Chemosphere 2018, 213, 559–567. [Google Scholar] [CrossRef]
- Kim, S.; Gwon, D.; Kim, J.A.; Choi, H.; Jang, C.Y. Bisphenol A disrupts mitotic progression via disturbing spindle attachment to kinetochore and centriole duplication in cancer cell lines. Toxicol. Vitro 2019, 59, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, C.; Zhong, H.; Zhang, S.; Xia, Y.; Cai, Z. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ. Pollut. 2019, 246, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Wang, H.; Wei, P.; Jiang, G.; Wang, W.; Zhang, X.; Ru, S. Impairment of bisphenol F on the glucose metabolism of zebrafish larvae. Ecotoxicol. Environ. Safe 2018, 165, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ru, S.; Wang, W.; Hao, L.; Ru, Y.; Wang, J.; Zhang, X. Long-term bisphenol S exposure aggravates non-alcoholic fatty liver by regulating lipid metabolism and inducing endoplasmic reticulum stress response with activation of unfolded protein response in male zebrafish. Environ. Pollut. 2020, 263, 114535. [Google Scholar] [CrossRef]
- Azevedo, L.F.; Hornos Carneiro, M.F.; Dechandt, C.R.P.; Cassoli, J.S.; Alberici, L.C.; Barbosa, F. Global liver proteomic analysis of Wistar rats chronically exposed to low-levels of bisphenol A and S. Environ. Res. 2020, 182, 109080. [Google Scholar] [CrossRef]
- Haq, M.E.U.; Akash, M.S.H.; Rehman, K.; Mahmood, M.H. Chronic exposure of bisphenol A impairs carbohydrate and lipid metabolism by altering corresponding enzymatic and metabolic pathways. Environ. Toxicol. Pharmacol. 2020, 78, 103387. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wang, Z.; Qin, J.; Wang, W.; Tian, H.; Ru, S. Bisphenol S induces obesogenic effects through deregulating lipid metabolism in zebrafish (Danio rerio) larvae. Chemosphere 2018, 199, 286–296. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Qin, J.; Wei, P.; Jia, Y.; Wang, J.; Ru, S. Long-term bisphenol S exposure induces fat accumulation in liver of adult male zebrafish (Danio rerio) and slows yolk lipid consumption in F1 offspring. Chemosphere 2019, 221, 500–510. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Huang, Q.; Chi, Y.; Dong, S.; Fan, J. Bisphenol-A induces neurodegeneration through disturbance of intracellular calcium homeostasis in human embryonic stem cells-derived cortical neurons. Chemosphere 2019, 229, 618–630. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Chen, H.; Luo, S.; Xiong, Y.; Wang, X.; Xu, B.; Zheng, C.; Wang, K.J. The comparative toxicities of BPA, BPB, BPS, BPF, and BPAF on the reproductive neuroendocrine system of zebrafish embryos and its mechanisms. J. Hazard. Mater. 2021, 406, 124303. [Google Scholar] [CrossRef]
- Gu, J.; Wang, H.; Zhou, L.; Fan, D.; Shi, L.; Ji, G.; Gu, A. Oxidative stress in bisphenol AF-induced cardiotoxicity in zebrafish and the protective role of N-acetyl N-cysteine. Sci. Total Environ. 2020, 731, 139190. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Leon, C.T.G.D.; Garcia-Becerra, R.; Ambrosio, J.; Nava-Castro, K.E.; Morales-Montor, J. The chemical environmental pollutants BPA and BPS induce alterations of the proteomic profile of different phenotypes of human breast cancer cells: A proposed interactome. Environ. Res. 2020, 191, 109960. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, X.; Zheng, S.; Wu, R.; Liu, C.; Wu, K. Effect of bisphenol A on craniofacial cartilage development in zebrafish (Danio rerio) embryos: A morphological study. Ecotoxicol. Environ. Safe 2021, 212, 111991. [Google Scholar] [CrossRef]
- Huang, W.L.; Zheng, S.; Xiao, J.; Liu, C.; Du, T.; Wu, K. Parental exposure to bisphenol A affects pharyngeal cartilage development and causes global transcriptomic changes in zebrafish (Danio rerio) offspring. Chemosphere 2020, 249, 126537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Wang, Q.; Liu, Y.; Liu, X.; Wu, B.; Lu, G. Intestinal toxicity and microbial community disorder induced by bisphenol F and bisphenol S in zebrafish. Chemosphere 2021, 280, 130711. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, S.; Yang, F.; Dong, P.; Yang, M.; Wong, M.; Zheng, C. Metabolism disruption analysis of zebrafish larvae in response to BPA and BPA analogs based on RNA-Seq technique. Ecotoxicol. Environ. Safe 2019, 174, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kuang, H.; Luo, Y.; Liu, S.; Meng, L.; Pang, Q.; Fan, R. Low-dose bisphenol A exposure impairs learning and memory ability with alterations of neuromorphology and neurotransmitters in rats. Sci. Total Environ. 2019, 697, 134036. [Google Scholar] [CrossRef]
- Jardim, N.S.; Sartori, G.; Sari, M.H.M.; Muller, S.G.; Nogueira, C.W. Bisphenol A impairs the memory function and glutamatergic homeostasis in a sex-dependent manner in mice: Beneficial effects of diphenyl diselenide. Toxicol. Appl. Pharmacol. 2017, 329, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, X.; Ye, Y.; Li, X. Bisphenol A affects estradiol metabolism by targeting CYP1A1 and CYP19A1 in human placental JEG-3 cells. Toxicol. Vitro 2019, 61, 104615. [Google Scholar] [CrossRef]
- Giesbrecht, G.F.; Liu, J.; Ejaredar, M.; Dewey, D.; Letourneau, N.; Campbell, T.; Martin, J.W.; Team, A.P.S. Urinary bisphenol A is associated with dysregulation of HPA-axis function in pregnant women: Findings from the APrON cohort study. Environ. Res. 2016, 151, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Harnett, K.G.; Moore, L.G.; Chin, A.; Cohen, I.C.; Lautrup, R.R.; Schuh, S.M. Teratogenicity and toxicity of the new BPA alternative TMBPF, and BPA, BPS, and BPAF in chick embryonic development. Curr. Res. Toxicol. 2021, 2, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhao, F.; Zhang, X.; Liu, W.; Jiang, G.; Wang, H.; Ru, S. Transgenerational thyroid endocrine disruption induced by bisphenol S affects the early development of zebrafish offspring. Environ. Pollut. 2018, 243, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Gu, J.; Guo, M.; Zhou, L.; Wang, Z.; Shi, L.; Gu, A. A systematic comparison of the developmental vascular toxicity of bisphenol A and its alternatives in vivo and in vitro. Chemosphere 2022, 291, 132936. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) No 10/2011 of 14 January 2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Off. J. Eur. Union 2011, L012, 1. Available online: https://data.europa.eu/eli/reg/2011/10/2019-08-29 (accessed on 29 October 2022).
- GB 9685-2016; Coatings and Coatings for Food Contact. National Health and Family Planning Commission of China (NHFPC): Beijing, China, 2016. Available online: http://down.foodmate.net/standard/yulan.php?itemid=49855 (accessed on 28 October 2022).
- Japan. Food Sanitation Law 370 in Japan. 2012. Available online: https://www.mhlw.go.jp/web/t_doc?dataId=78334000&dataType=0&pageNo=1 (accessed on 29 October 2022).
- EFSA. Have Your Say Now on EFSA’s Next BPA Re-Evaluation. 2017. Available online: https://www.efsa.europa.eu/en/press/news/170630 (accessed on 31 October 2022).
| No. | Name | Molecular Information | ||||
|---|---|---|---|---|---|---|
| Full Name | Abbreviation | Chemical Structure | Molecular Formula | Molecular Weight (g/mol) | CAS | |
| 1 | Bisphenol A | BPA | 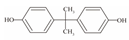 | C15H16O2 | 228.286 | 80-05-7 |
| 2 | Bisphenol AF | BPAF | 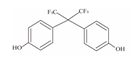 | C15H10F6O2 | 336.23 | 1478-61-1 |
| 3 | Bisphenol AP | BPAP | 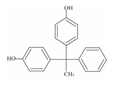 | C20H18O2 | 290.36 | 1571-75-1 |
| 4 | Bisphenol B | BPB | 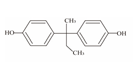 | C16H18O2 | 242.31 | 77-40-7 |
| 5 | Bisphenol C | BPC |  | C17H20O2 | 281.13 | 79-97-0 |
| 6 | Bisphenol E | BPE | 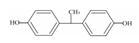 | C14H12O2 | 214.26 | 2081-08-5 |
| 7 | Bisphenol F | BPF | 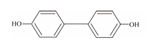 | C13H12O2 | 200.23 | 620-92-8 |
| 8 | Bisphenol G | BPG | 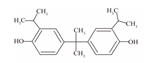 | C21H28O2 | 312.45 | 127-54-8 |
| 9 | Bisphenol M | BPM | 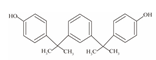 | C24H26O2 | 346.47 | 13595-25-0 |
| 10 | Bisphenol P | BPP |  | C24H26O2 | 346.47 | 2167-51-3 |
| 11 | Bisphenol PH | BPPH | 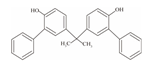 | C27H24O2 | 380.48 | 24038-68-4 |
| 12 | Bisphenol S | BPS | 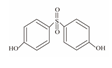 | C12H10O4S | 250.27 | 080-09-1 |
| 13 | Bisphenol TMC | BPTMC | 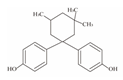 | C21H26O2 | 310.43 | 129188-99-4 |
| 14 | Bisphenol Z | BPZ | 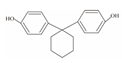 | C18H20O2 | 268.35 | 843-55-0 |
| 15 | Bisphenol A Diglycidyl Ether | BADGE | 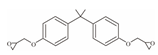 | C21H24O4 | 340.41 | 1675-54-3 |
| 16 | Bisphenol F Diglycidyl Ether | BFDGE | 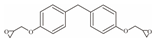 | C19H20O4 | 312.365 | 2095-03-6 |
| Sample Origin | Food Matrix | Sample Size | Sampling Time | Compound | Concentration (µg/kg) | Reference |
|---|---|---|---|---|---|---|
| Korea | Canned foods including meats, hams, fish, vegetables, and fruits | 104 | 2015 | BPA | 1.41–278.50 | [41] |
| BPF | <0.32 | |||||
| BPB | <0.14 | |||||
| BADGE·2HCl | 1.71–1525.00 | |||||
| BADGE·2H2O | 0.98–655.50 | |||||
| BADGE | 0.76–27.70 | |||||
| BADGE·H2O | 2.09–24.73 | |||||
| BADGE·HCl | 1.50–384 | |||||
| BADGEHCl·H2O | 2.42–488 | |||||
| BFDGE | <2.85 | |||||
| China | Canned foods including meats, seafood products, mushrooms, fruits, and vegetables | 151 | 2017–2018 | BPA | 0.3–837 | [48] |
| BPF | 0.3–75.4 | |||||
| BPS | 0.05–1.6 | |||||
| Italy | Canned tuna fish | 33 | / | BPA | 3.7–187.0 | [49] |
| BPB | 3.0–74.8 | |||||
| BADGE | 7.7–91.1 | |||||
| BFDGE | 6.9–38.5 | |||||
| Greek | Canned foods including juices, meats, sauces, fish, and vegetables | 14 | 2014 | BPA | 0.2–66.0 | [50] |
| Portugal | Canned meats | 30 | / | BPA | 0.15–202.3 | [51] |
| BPAF | 0.4–13.0 | |||||
| BPF | 0.4–153.0 | |||||
| BPB | 0.3–33.5 | |||||
| Spain | Canned foods including fish, seafood, vegetables, grains, and fruit | 12 | / | BADGE | <0.5 | [52] |
| BADGE·H2O | <0.15 | |||||
| BADGE·2H2O | 0.5–724 | |||||
| BADGE·HCl | <0.5 | |||||
| BADGE·2HCl | <0.5 | |||||
| BADGE·H2O·HCl | 0.5–189 | |||||
| Canada | Canned tuna | 73 | 2017–2019 | BPA | 0.01–29.38 | [53] |
| BPS | <0.01 | |||||
| Italy | Canned beers | 40 | 2015 | BPA | 0.50–0.80 | [54] |
| BPF | 1.1–2.5 | |||||
| BADGE | 1.2 | |||||
| BPB | <0.15 | |||||
| BFDGE | <0.15 | |||||
| Italy | Canned beverages | 52 | 2018 | BPA | 6.1–76 | [55] |
| BPB | 9.9–183 | |||||
| BPE | 5.2–59 | |||||
| BPF | 8.0–139 | |||||
| BPM | 23–1358 | |||||
| BPAF | <5.3 | |||||
| BADGE | 114 | |||||
| BFDGE·2H2O | 1.21–112.50 | |||||
| BFDGE·2HCl | 2.15–884.00 | |||||
| Lebanon | Canned vegetables including fava beans, red beans, chickpeas, and okra | 177 | / | BPA | 12.8–54.6 | [27] |
| BADGE·2H2O | 101–146 | |||||
| BPZ | 1.5–41.0 | |||||
| Spain | Canned foods including artichokes, asparagus, corn, fruit salad in syrup, green beans, red beans, mackerel, mushrooms, nuts, olive oil, pâté, peach in syrup, squid, and tuna | 15 | / | BPA | 3.45–88.66 | [22] |
| BPB | 0.33–3.86 | |||||
| BPE | <0.83 | |||||
| Thailand | Canned tuna fish | 137 | 2018–2019 | BPA | 0.195–0.20 | [56] |
| Area | The Average Exposure Dose (ng/kg bw/day) | Reference | |
|---|---|---|---|
| BPA | BPA Analogs a | ||
| China | 32.9 | 5.3 | [48] |
| France | 22.5 | 17.15 | [50] |
| Korea | 19.33 | 84.88 | [41] |
| Spain | 21.5 | 156.5 | [52] |
| Spain | 370 | 11 | [22] |
| Texas, USA | 12.6 | - | [6] |
| Adverse Effect | Compound | Subject | Main Discovery | Reference |
|---|---|---|---|---|
| Cardiotoxicity | BPAF | Zebrafish | Exposure to BPAF increased oxidative stress, inhibited the expression of genes that participate in cardiac development, and played a key role in the mechanism of BPAF-induced cardiac toxicity. | [75] |
| Cancer | BPA | HeLa cells | BPA increased chromosomal instability by interfering with mitotic processes such as the formation of bipolar spindles and the attachment of spindle microtubules to kinetocomes to stimulate carcinogenic effects. | [65] |
| BPS | MCF-7 cells | BPS could change methylation status in the promoter of breast-cancer-related genes. | [66] | |
| BPA and BPS | MCF-7, MDA-MB-231 breast cancer cells, and SK-BR-3 | Stem cell markers and invasion proteins were increased by BPA and BPS in estrogen receptor-positive breast cancer cells. | [76] | |
| Developmental toxicity | BPA | Zebrafish | BPA-induced pharyngeal cartilage defects via cellular pathways such as estrogen receptors, androgen receptors, and estrogen-related receptors. | [77] |
| BPA | Zebrafish | The exposure to BPA altered gene expression involved in apoptosis, defense responses, reactive oxygen species metabolism, and signaling pathways in zebrafish larvae and embryos, leading to long-term adverse morphological and functional consequences, including the observed pharyngeal cartilages and craniofacial defects. | [78] | |
| Intestinal toxicity | BPF and BPS | Zebrafish | The individual and combined exposures of BPS and BPF caused oxidative damage and inflammatory effects in the zebrafish intestine. In addition, BPF and BPS exposures changed the microbial community composition in the zebrafish intestine. | [79] |
| Metabolic disorder | BPA and BPS | Male Wistar rats | The exposure to BPA and BPS elevated the levels of serum lipid markers and upregulated the expression of enzymes involved in triglycerides synthesis. BPS treatment could also enhance liver lipogenic enzyme expressions and have more obesogenic effects compared to BPA. | [69] |
| BPS | Male zebrafish | BPS exposure stimulated the expression of autophagy and inflammatory genes, increased the levels of triacylglycerol and total cholesterol in male zebrafish liver, and induced hepatic apoptosis and fibrosis. Long-term exposure to BPS may promote the progression of simple steatosis to nonalcoholic steatohepatitis. | [68] | |
| BPA | Rats | The exposure to BPA inhibited the mRNA expression of genes encoding insulin leading to poor insulin production. In addition, it significantly reduced glucose uptake via the insulin signaling pathway. | [70] | |
| BPS | Translucent zebrafish (Danio rerio) larvae | The obesity-inducing effect of BPS is related to the disruption of triacylglycerol metabolism. | [71] | |
| BPA, BPF, and BPS | Zebrafish embryos/larvae | BPA and its analogs treatment affected several signaling pathways and physiological processes in zebrafish embryos and larvae, especially the metabolic systems. | [80] | |
| BPS | Zebrafish | BPS exposure significantly reduced the mRNA levels of lipogenic transcription factor srebp1 and increased that of fatty acid synthetase. | [72] | |
| BPF | Zebrafish larvae | BPF disrupts glucose metabolism and induces hyperglycemia by impairing insulin signaling transduction, increasing the risk of nonalcoholic fatty liver disease in male zebrafish. | [67] | |
| Neurotoxicity | BPB, BPS, BPF, and BPAF | Zebrafish embryos | BPA and its analogs increased the expression of reproductive neuroendocrine-related genes, thereby reducing the body length of zebrafish larvae and affecting their motor behavior. | [80] |
| BPA | Human cortical neurons | BPA induced the disorder of intracellular Ca2+ homeostasis, thus leading to reactive oxygen species production and anti-oxidative response defect, and then neurite outgrowth disorder, neural network degeneration, and neuron apoptosis. | [73] | |
| BPA | Rats | Low-dose exposure to BPA disrupted dendritic development and neurotransmitter homeostasis in the hippocampus, leading to impaired spatial learning and memory abilities in rats. | [81] | |
| BPA | Mice | BPA harmed different memory types and the glutamatergic parameters in a sex-dependent manner. | [82] | |
| Reproductive toxicity | BPA | JEG-3 cells | BPA altered human placental JEG-3 estradiol synthesis and catabolism and interfered with the normal placenta formation process and embryonic development during early pregnancy. | [83] |
| BPA | Pregnant women | BPA exposure led to the dysfunction of the hypothalamic pituitary adrenal axis during pregnancy. | [84] | |
| BPA, BPS, BPAF, and TMBPF | Chicken embryo | BPA and its analogs significantly impaired development, growth, and survival in a dose-dependent manner in the order of BPAF > TMBPF > BPS > BPA. The most common and severe dysmorphologies were body pigmentation abnormalities, craniofacial, eyes, and gastrointestinal. | [85] | |
| BPS | Zebrafish | BPS has significantly increased 3,5,3′-triiodothyronine plasma levels causing dysontogenesis and incubation delay, bladder inflation defects, decreased motor ability, developmental neurotoxicity, and lateral stripe hypopigmentation in unexposed embryos and larvae. | [86] | |
| Vascular developmental toxicity | BPF, BPS, and BPAF | Zebrafish embryos and human vascular | Exposure to BPA and its analogs increased oxidative stress, including a significant decrease in superoxide dismutase and catalase activity, and increased the levels of malondialdehyde and reactive oxygen species in both zebrafish and human vascular. The order of their vascular toxicity and oxidative stress potency of was as follows: BPAF > BPF > BPA > BPS. | [87] |
| Countries or Organization | Compound | t-TDI (μg/kg bw/day) | SML a (mg/kg) | Reference |
|---|---|---|---|---|
| European Union | BPA b | 0.04 | 0.05 | [16] |
| BPS | 0.05 | [88] | ||
| China | BPA | / | 0.6 | [89] |
| BPS | 0.05 | |||
| Japan | BPA | / | 2.5 | [90] |
| BPS | 0.05 | |||
| France | BPA | / | not detect | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Zhong, J.; Chi, H.; Lin, N.; Liu, Z. Recent Advances in Sources, Migration, Public Health, and Surveillance of Bisphenol A and Its Structural Analogs in Canned Foods. Foods 2023, 12, 1989. https://doi.org/10.3390/foods12101989
Ni L, Zhong J, Chi H, Lin N, Liu Z. Recent Advances in Sources, Migration, Public Health, and Surveillance of Bisphenol A and Its Structural Analogs in Canned Foods. Foods. 2023; 12(10):1989. https://doi.org/10.3390/foods12101989
Chicago/Turabian StyleNi, Ling, Jian Zhong, Hai Chi, Na Lin, and Zhidong Liu. 2023. "Recent Advances in Sources, Migration, Public Health, and Surveillance of Bisphenol A and Its Structural Analogs in Canned Foods" Foods 12, no. 10: 1989. https://doi.org/10.3390/foods12101989
APA StyleNi, L., Zhong, J., Chi, H., Lin, N., & Liu, Z. (2023). Recent Advances in Sources, Migration, Public Health, and Surveillance of Bisphenol A and Its Structural Analogs in Canned Foods. Foods, 12(10), 1989. https://doi.org/10.3390/foods12101989







