Abstract
Flavor, which mainly depends on volatile compounds, is an important index for evaluating the quality of clam sauce. This study investigated the volatile compounds in clam sauce prepared using four different methods and the influence of aroma characteristics. Fermenting a mixture of soybean koji and clam meat improved the flavor of the final product. Solid-phase microextraction (SPME) combined with gas chromatography-mass spectrometry (GC-MS) identified 64 volatile compounds. Nine key flavor compounds, namely, 3-methylthio-1-propanol, 2-methoxy-4-vinylphenol, phenylethyl alcohol, 1-octen-3-ol, α-methylene phenylacetaldehyde, phenyl-oxirane, 3-phenylfuran, phenylacetaldehyde, and 3-octenone, were selected using variable importance in projection (VIP). The results of the electronic nose and tongue detection of the aroma characteristics of the samples prepared by four different fermentation methods were consistent with those of GC-MS analysis. The clam sauce prepared by mixing soybean koji with fresh clam meat possessed better flavor and quality than that prepared via other methods.
1. Introduction
Condiments could coordinate the smell and taste of food, increase flavor and remove the fishy smell [1]. In 2022, the scale of the condiment market in China reached CNY 513.3 billion, and the total annual output exceeded 13 million tons. There is a broad market for the condiment industry [2]. With people’s increasing demand for nutrition, naturalness and diversity of condiments, fermented seasonings are widely selected in cooking or food preparation because of their delicious taste, rich aroma and unique flavor [3]. The seafood flavor condiment with excellent umami taste is especially popular within the condiment market [4]. One of the most cultured seafoods is clam, the annual output of which reached about 4.28 million tons per year, with an annual output value of USD 150 million [5]. In addition, clam meat is rich in protein (9.09%~12.75%) [6], which has potential for the preparation of the fermented condiment.
At present, there are few reports about the preparation of clam fermented sauce. Nevertheless, similar to the preparation of fish sauce, clam meat could be fermented at high salt concentrations for sauce preparation [7]. The fermentation cycle of a traditional sauce production process requires at least six months [8]. The fermentation time is shortened by increasing the fermentation temperature and adding high-concentration saltwater to inhibit the growth of bacteria [9]; however, the salinity of clam sauce produced by this method is exceedingly high, which affects its taste. Flavor formation in fermentation sauce is a complex process [10] in which the proteins in raw meat are degraded into taste compounds such as amino acids or peptides through biochemical metabolic pathways, under the joint action of microorganisms and enzymes [11]. In addition to these flavor precursor substances, such as amino acids, fatty acids could further produce a variety of small-molecule flavor compounds such as aldehydes, ketones, alcohols, and pyrazines, which ultimately contribute to the flavor and quality of clam sauce products [12]. To some extent, different fermentation strategies will produce different flavor precursors and flavor compounds, resulting in a different flavor of the final product. Therefore, it is necessary to select a fermentation strategy to prepare clam sauce with acceptable flavor.
This study identified volatile compounds in clam sauce using headspace solid-phase microextraction combined with gas chromatography-mass spectrometry (HS-SPME-GC-MS), and further analyzed the volatile compounds in clam sauces produced by different fermentation methods using heatmap and partial least squares discriminant analyses (PLS-DA). In addition, the sensory values measured by an electronic nose and tongue were selected as the key evaluation indexes, along with the contents of free amino acids and amino acid nitrogen (AAN). Finally, the optimum fermentation method was selected by comparison of the observed volatile flavor substances, which provided a theoretical reference for the preparation of high-quality clam sauce products.
2. Materials and Methods
2.1. Materials
Trichloroacetic acid (TCA, 99%) was purchased from Macklin (Shanghai, China). Acetone (99.5%) was purchased from Damao Chemical Reagent Co., Ltd. (Tianjin, China). Hydrochloric acid (HCl, AR) was purchased from Xilong Chemical (Shantou, China). Ruditapes philippinarum and soybeans were purchased from the local seafood market and a local supermarket, respectively, in Dalian, China. All other reagents used in this study were of analytical grade or higher purity.
2.2. Preparation of Bean Koji
Soybeans were screened, cleaned, and soaked for 8–10 h at 25 °C, based on the standard degree of the soaking of soybeans covered with water. The soybeans were drained and cooked in a sterilized pot (100 °C) for 2 h [13]. After cooking, the soybeans were cooled to approximately 40 °C before adding Aspergillus oryzae and flour (w/w 1:60). The mixture was then covered with two layers of clean, wet gauze and cultured at 31 °C and 85% humidity for 2–3 days.
2.3. Preparation of Clam Meat Koji
Clam meat was used in this study. The clam meat was washed with water 2–3 times to remove impurities and then broken up before being mixed with flour (85% clam meat, 15% flour) and cooked in a sterile pot (100 °C) for 1 h. When the temperature dropped to approximately 40 °C, the clam meat was cut into small pieces (2.5 cm × 2.5 cm × 1.4 cm). The clam meat was then mixed with Aspergillus oryzae and flour at a ratio of 1:60 (w/w), stirred well, and covered with two layers of clean, wet gauze and incubated at 31 °C and 85% humidity for culturing [13].
2.4. Preparation of Mixed Koji
The soybeans were screened, cleaned, and soaked for 8–10 h at room temperature, based on the standard degree of the soaking of soybeans covered with water. High-quality clam meat was washed with water 2–3 times to remove impurities and then broken up. Soybeans (55%) and clam meat (30%) were then mixed with flour (15%) and cooked in a sterile pot (100 °C) for 1 h. When the temperature dropped to approximately 40 °C, the clam meat was cut into small pieces (2.5 cm × 2.5 cm × 1.4 cm) and mixed with Aspergillus oryzae and flour in a ratio of 1:60 (w/w). The mixture was stirred well, covered with two layers of clean, wet gauze and incubated at 31 °C and 85% humidity for culturing [14].
2.5. Preparation of Fermented Clam Sauce
Various fermentation methods were used to prepare the clam sauce. The precursor of sauce I was mixed koji (100%). The precursors of sauce II were meat koji (67%) and soybean koji (33%). The precursors of sauce III were clam meat (67%) and soybean koji (33%). The precursor of sauce IV was meat koji (100%).
The precursor of each sauce was mixed with salt water (12%) and fermented at 45 °C for 12 days, then at 35 ℃ for 18 days. The mixture was stirred every two days during the fermentation process to produce a delicious and fragrant clam sauce [15], which was stored at −30 °C prior to use.
2.6. E-Tongue Analysis
The taste of the clam sauce was analyzed using a TS-5000Z electronic tongue (Insent Inc., Japan), employing an established method with some modifications [16]. The sensors on the electronic tongue included bitterness (SB2C00), umami (SB2AAE), salinity (SB2CT0), acidity (SB2CA0), and astringency sensors (SB2AE1). A sample of the clam sauce (2 g) was added to deionized water (100 mL) and shaken well. After centrifugation, the supernatant (60 mL) was collected for testing. Each sample was analyzed using three parallel experiments.
2.7. Amino Acid Nitrogen Analysis
The reference analysis of the AAN content in soy sauce was conducted in accordance with the official analytical method in China (GB/T 5009.235—2016). The AAN content was determined using the titration method, in which diluted samples (20 mL) were mixed with distilled water (60 mL) and titrated to pH 8.2 with 0.05 mol/L NaOH (0.05 mol/L). Formaldehyde solution (10 mL, 40%) was then added, and the mixture was titrated to pH 9.2 with NaOH (0.05 mol/L). The AAN content was calculated from the volume of NaOH and a blank test was performed using distilled water [17].
2.8. Amino Acid Analysis
The amino acids in the clam sauces were identified using a Hitachi LA8080 amino acid analyzer (LA8080, Hitachi, Japan) with a high-performance appraisal-exchange column (Hitachi, Japan) [18]. Clam sauces (0.1 g) were diluted 10 times in distilled water before being mixed with an equal volume of TCA (10%). The mixture was maintained at 4 °C for 1 h to precipitate the protein and then centrifuged at 10,000× g for 10 min. The supernatant was collected, and the centrifugation procedure was repeated. Hydrochloric acid was added to obtain a final hydrochloric acid concentration of 0.02 M and the sample was passed through a filter (0.22 µm). The samples were eluted at 57 °C in the column oven, and the derivatization of amino acid with ninhydrin was performed at 135 °C in the reaction oven. The injection volume was 20 µL.
2.9. E-Nose Analysis
The flavor profile of the clam sauce was analyzed using the PEN3 electronic nose (WinMuster Airsense Analytics Inc., Schwerin, Germany), which contains 10 metal oxide sensors that have different sensitivities for each characteristic volatile compound [19]. The substances to which each type of sensing element is sensitive are listed in Table 1. The samples of the clam sauce (5.0 g) were shaken vigorously in an electronic nose (e-nose) sample bottle (20 mL). After cleaning, the probe with filtered air, the inlet of the electronic nose was inserted into the sample bottle and analysis was performed over 100 s. After analysis, the probe was cleaned with filtered air for 20 s [20].

Table 1.
Sensor array of the E-nose.
2.10. GC-MS Analysis
The characteristic flavor compounds of the clam sauce were identified using HS-SPME-GC-MS. The clam sauce (0.5 g) was added to a cyclohexanone internal standard (10 mg/L) in a headspace bottle (10 mL) before extraction. The volatile compounds were extracted in headspace vials and incubated in a water bath at 60 °C for 20 min. The lab-made SPME fiber with a hydrophilic–lipophilic balance (HLB) [21] was exposed to the headspace for 40 min. The fibers were then injected into the gas chromatography (GC) system, which operated at 250 °C for 10 min in the splitless mode to thermally desorb the extracted compounds [22].
GC was performed using an Agilent 7890 B chromatograph (Agilent Technologies Inc., Santa Clara, CA, USA) with a non-polar HP-5 ms ultra-inert Agilent column (30 m × 250 µm × 0.25 µm). The initial temperature of the GC oven was 35 °C, which was increased to 230 °C at a rate of 5 °C/min and maintained for 10 min. Helium was used as the carrier gas at a flow rate of 1 mL/min. MS (5977 B, Agilent Technologies Inc., Santa Clara, CA, USA) detection was performed in scan mode, within a scanning range of m/z 35–500, and used an electron impact ion source (70 eV) at 230 °C [23].
2.11. Statistical Analysis
All experiments were conducted in triplicate, and the results are presented as the mean and standard deviation of the three experiments. The SPSS software package (SPSS 10.0 for Windows) was used to determine significant differences among the experiments (p < 0.05), using analysis of variance (ANOVA).
3. Results and Discussion
3.1. Analysis of the Taste of Clam Sauces by an Electronic Tongue
The electronic tongue, using an artificial lipid membrane sensor, was used to quantify the six basic tastes of clam sauces, including taste, bitterness, astringency, richness, aftertaste astringency (aftertaste-A), and aftertaste bitterness (aftertaste-B) [24]. The saltiness response values of the clam sauces prepared by the four different processes were all high (Figure 1), which may occur because clams are mariculture species with a strong capacity to adsorb substances from the environment. In addition, the preparation of clam sauce requires extra salt, further increasing its saltiness. In contrast, sauces I, II, III, and IV were all less bitter, likely because the raw clam proteins in sauce III were hydrolyzed into small peptides and had less exposure to hydrophobic amino acid residues [25]. The umami response values of sauce III were significantly higher than those of the other three sauces. These results demonstrate that sauce III had a better taste than sauces I, II, and IV.
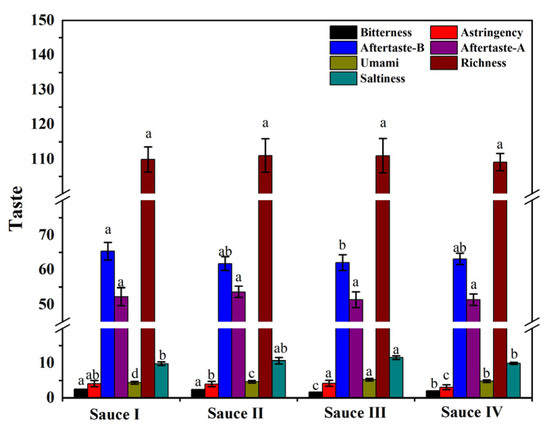
Figure 1.
Electronic tongue (E-tongue) analysis of clam sauces prepared using different fermentation methods (sauce I, sauce II, sauce III, and sauce IV). Different lowercase letters indicate significant differences in the average value within each group (p < 0.05).
3.2. Analysis of Amino Acid Nitrogen in Clam Sauces
The free amino acid content of clam sauces is mainly characterized by the content of amino acid nitrogen, which reflects the fermentation maturity and flavor characteristics of the clam sauce [26]. The amino acid content is an important component of the umami substance, that is crucial in forming the flavor of the clam sauce [27]. The amino acid nitrogen contents of the clam sauces are shown in Figure 2, in which sauce I, sauce II, sauce III, and sauce IV are all higher than the Chinese national standard of 0.5 g/100 mL, and the product therefore satisfies the quality requirements of this standard. The amino acid nitrogen content in sauce III was higher than that of the other three clam sauces (p < 0.05), indicating that sauce III had the strongest umami flavor and good product quality. This is consistent with the electronic tongue analysis of the sauces.
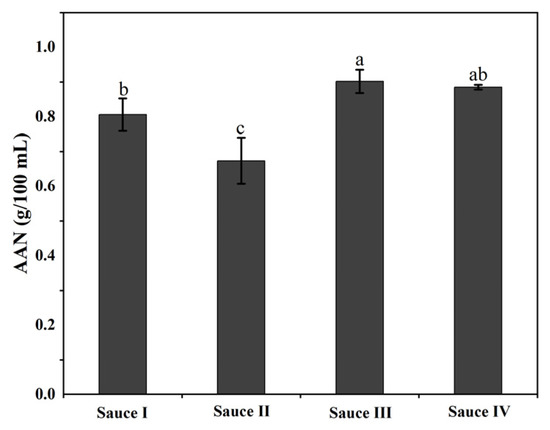
Figure 2.
Amino acid nitrogen (AAN) analysis of the clam sauces prepared using different fermentation methods (sauce I, sauce II, sauce III, and sauce IV). Different lowercase letters indicate significant differences in the average value within each group (p < 0.05).
3.3. Analysis of Free Amino Acids in Clam Sauces
The main taste substances in clam sauce are amino acids, which play an important role in the formation of flavors [28] and are released during the degradation of proteins in clam meat. A total of 14 free amino acids were detected in the sauce samples produced by the four fermentation processes (Table 2). The contents of total free amino acids (TFAA) in clam sauce I, sauce II, sauce III, and sauce IV were 2193.84 mg/100 g, 2016.22 mg/100 g, 2343.69 mg/100 g, and 2172.89 mg/100 g, respectively. The TFAA content of sauce III was significantly higher than that of the other three sauces (p < 0.05), likely owing to the more complete degradation of the protein during the fermentation process. The increased amino acid content of sauce III facilitates its digestion and absorption. The main delicious amino acids (FAAs) in clam sauce are Glu, Asp, Gly, and Ala. The total content of these umami amino acids is higher than 30%, which may give the clam sauce a strong umami flavor [29]. Therefore, sauce III had an umami amino acid content of 1150.79 mg/100 g, which was significantly higher than that of sauces I, II, and IV (996.97 mg/100 g, 900.70 mg/100 g, and 915.46 mg/100 g, respectively). This means that sauce III rendered a superior umami flavor. The bitter amino acid content of sauce III was lower than that of clam sauces I, II, and IV. Among bitter amino acids, Met has a strong bitter taste, and bitter amino acids can produce bitter substances. In summary, the amino acid content of sauce III suggests that it has a better overall flavor than the other sauces, which is also consistent with the observed levels of amino acid nitrogen.

Table 2.
Amino acid analysis of clam sauces a.
3.4. Electronic Nose Analysis of Clam Sauce Favor Profiles
Flavor is an important quality of clam sauce condiments [30]. The flavor profile diagram of the response of 10 sensors in the PEN3 electronic nose to the four different clam sauces is shown in Figure 3A. The flavor profiles of sauce I, sauce II, and sauce IV are essentially the same. The response values of W5S, W1W, and W2W of sauce III were significantly high, indicating that sauce III contained more nitrogen oxides, sulfides, organic sulfides, and aromatic components than sauces I, II, and IV [31]. To further identify the differences among the flavor profiles, the data from the electronic nose were analyzed by principal component analysis (PCA), which clearly showed an obvious distinction between sauces III and I, and between sauces II and IV in PC1. Sauces I, II, and IV could not be clearly distinguished in PC1, indicating the similarity of the flavor profiles of the clam sauces prepared using techniques I, II, and IV.
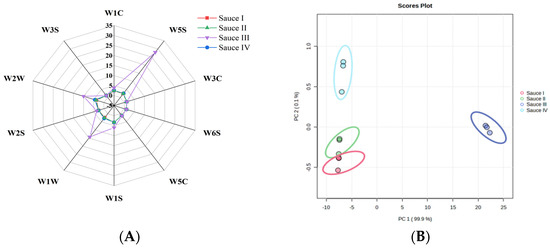
Figure 3.
Flavor intensity and principal component analysis of the sauces prepared using different fermentation methods. (A) Radar map of the electronic nose. (B) Principal component analysis map of the flavor intensity of sauce I, sauce II, sauce III, and sauce IV.
3.5. Identification of Volatile Compounds in Clam Sauces via HS-SPME/GC-MS
To further confirm the difference in volatile flavor compounds in clam sauces fermented using different methods, the clam sauces were analyzed using HS-SPME-GC-MS [32]. Figure 4A shows the chromatogram of the clam sauce samples. Sixty-four volatile compounds, including 22 aldehydes and ketones, 11 alcohols, 13 heterocyclic compounds, five esters, nine alkanes and alkenes, and four acids, were identified and classified into five groups according to their general properties and chemical structures (Table 3).
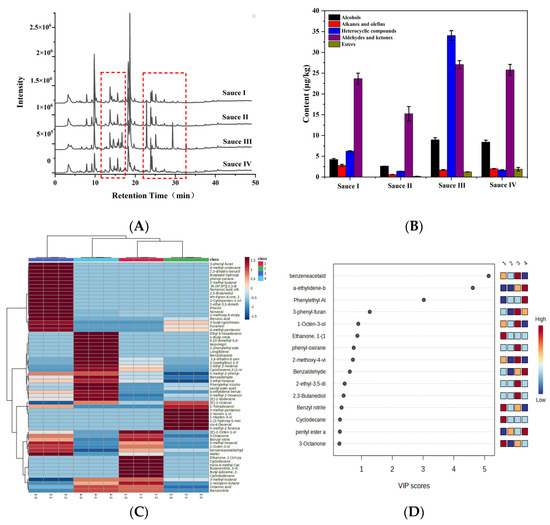
Figure 4.
GC-MS and PLS-DA analyses of clam sauces prepared using different fermentation methods. (A) GC-MS total ion chromatograms of the clam sauces. (B) The total volatile compounds in clam sauces prepared using different fermentation methods. (C) Heatmaps of volatile matter spectra in the whole samples prepared with different fermentation methods. (D) PLS-DA analysis of the volatile compound content in the whole samples prepared using different fermentation methods (sauce I, sauce II, sauce III, and sauce IV).

Table 3.
Volatile compounds in the sauce identified via HS-SPME-GC-MS.
Aldehydes are among the most important flavor volatiles in clam sauce. They have a low odor threshold and may be derived from the degradation of amino acids or oxidation of unsaturated fatty acids [33]. Aldehydes are therefore important in determining the flavor characteristics of fermented aquatic products. Further, 2-methylbutanal was detected only in sauce III, with a content of 0.83 µg/kg, because of the high fat content of its raw clam meat [34]. Relatively high amounts of phenylacetaldehyde, benzaldehyde, 5-methyl-2-phenyl-2-hexenal, and nonanal were detected in sauces I, II, III, and IV. Benzaldehyde is known to produce sweet odors in foods, including almonds and caramel, and it is one of the most important compounds in the aroma of fermented sauces [35]. Phenacetaldehyde has a strong floral odor [24] and it was found at the highest concentration in sauce III (22.56 µg/kg). These compounds are important components of the flavor of clam sauce and give the sauce a fresh aroma. Additionally, 5-methyl-2-phenyl-2-hexenal can give clam sauce toasty, sweet, and nutty aromas [36]. The contents of nonanal in sauces I, II, III, and IV were 1.54, 0.43, 0.36, and 0.71 µg/kg, respectively, with III having the lowest nonanal content. Nonanals mainly originate from the oxidative decomposition of unsaturated fatty acids, such as linoleic and linolenic acids, and they may also be formed by the Strecker degradation of amino acids [37]. Nonanal can produce an off flavor, which may be responsible for the fishy flavor of clam sauce.
The ketones in clam sauces are produced through the oxidation of unsaturated fatty acids, thermal degradation of amino acids, Maillard reaction, or microbial oxidation [38]. Ketones contribute less to the flavor of clam sauce than aldehydes, owing to their lower relative content having only a slight effect on odor [39]. The differences in the flavors of the various raw materials are mainly due to the difference in the quality and quantity of carbonyl compounds. A total of nine ketones were detected in sauces I, II, III, and IV, with 3-octanone being the most common compound, which can, in turn, give the sauce an odor of mushrooms and butter [39].
Alcohols, which have a botanical and aromatic odor, are derived mainly from the thermal oxidation of lipids and degradation of carbohydrates [38]. In clam sauce, the contribution of alcohols to the aroma is less important than that of aldehydes and ketones, but alcohols still play a key role in the formation of flavor of the clam sauce [40]. Alcohols mainly originate from the degradation of polyunsaturated fatty acids, and 1-octen-3-ol and 2,3 butanediol were the most common alcohols detected in the samples. In addition, 2,3 butanediol, which results in a burnt flavor [41], was detected only in sauce III, with a high relative content of 3.19 µg/kg. This sauce had a particularly strong odor, which demonstrates the importance of this substance for the flavor of sauce III. Another key aroma substance in clam sauce is 1-octene-3-ol, and the relative contents detected in sauces I, II, and III were 2.84, 0.2, and 3.58 µg/kg, respectively, among which sauce III had the highest relative content, giving a mushroom flavor to the overall aroma of the clam sauce [42].
Thirteen heterocyclic compounds were detected in the clam sauces prepared using sauces I, II, III, and IV. Furan is known to greatly enhance the aroma of fermented foods, and it is typically formed via the Amadori rearrangement pathway [35]. The compound 2-ethylfuran has a strong influence on the aroma of clam sauce, because it generates a rubbery, pungent smell in the sauce; therefore, the threshold is low. Moreover, 2-methoxy-4-vinylphenol was detected in sauces I and III, with sauce III having a higher relative content, and 2-methoxy-4-vinylphenol has a typical soy sauce flavor and smokiness and is one of the main components of soy sauce aroma [33]. This molecule is one of the representative compounds that gives clam sauce its characteristic flavor. Pyrazine compounds are the fat oxidation products of the Maillard reaction, which are also characteristic of clam sauce and mainly reflect the flavor of the roast and meat. Alongside that, 2-ethyl-3,5-dimethylpyrazine has a unique soy sauce flavor, and it may be a flavor substance in clam sauce; however, this substance was only detected in sauce III, at a relative content of 3.66 µg/kg. We therefore inferred that this substance could be responsible for the special flavor of sauce III, which differs from those of sauces I, II, and IV. Maltol was also detected, which enriches the clam sauce with a caramel flavor [43].
Esters, produced by the dehydration of hydroxyl fatty acids, usually exhibit a sweet, fruity taste and constitute some portion of the odor component of clam sauce. Five lipids were detected in sauces I, II, III, and IV. In particular, acetic acid, pentyl ester, and nonanoic acid ethyl ester were detected in sauce III, but these lipids may not have strong effects on the aroma of the clam sauce samples [44].
Fourteen hydrocarbons, which are generally aromatic and sweet, were detected in the clam sauce and contributed to its overall flavor to some degree. Alkanes have a weaker effect on odor than other compounds, owing to their higher odor threshold. Alkenes tend to have low odor thresholds, and have a seeming floral and fruity aroma. The clam sauce contained mainly long-chain aliphatic hydrocarbons [29]. Hydrocarbons with a range of 6–19 carbon atoms were detected in the volatiles of crustaceans and fish, but higher thresholds made little contribution to the overall flavor.
The volatile compounds detected in the clam sauce mainly consist of volatile carbonyl compounds and alcohols. Carbonyl compounds, including aldehydes and ketones, are important in the characterization of the product’s odor, owing to their low thresholds. Clam sauce typically contains more aldehydes and ketones than heterocyclic compounds and alcohols (Figure 4B). The total relative content of volatile substances in sauce III was higher than that in sauces I, II, and IV, along with a higher total relative content of alcohols and esters. This demonstrates the superior flavor of clam sauce III.
PLS-DA Analysis of Volatile Compound Content in Clam Sauces
A heatmap indicates the differences in the amount of various volatile organic compounds by plotting different shades of colors based on the average amount, which provides an intuitive visualization of the differences between the samples. The relative content of each volatile flavor compound is color-coded in the heat map. The darker the red, the greater the relative content, and the darker the blue, the lower the relative content. As shown in Figure 4C, sauce III contained significantly higher relative levels of nonanal, 2-methoxy-4-vinylphenol, maltol, 1-octene-3-ol, 2-ethyl-3,5-dimethylpyrazine, 2-methylbutaldehyde, 3-octenone, 3-phenylfuran, and phenylacetaldehyde than the other sauces, along with a higher relative content of other volatile compounds, giving it a richer aroma. PLS-DA analysis of the volatile components of clam sauce prepared using different processes was performed based on the obtained heatmaps. The variable importance in projection (VIP) > 1.0 was used to identify whether a substance was an important differential volatile compound. Figure 4D shows four key volatile compounds, phenylacetaldehyde, α-methylene phenylacetaldehyde, phenylethyl alcohol, and 3-phenylfuran (VIP > 1.0), which were found at a higher relative content in sauce III. These results demonstrate the superior flavor quality of sauce III, and they are also consistent with the observations made using the electronic nose.
4. Conclusions
The clam sauces prepared using four different fermentation methods (sauce I, sauce II, sauce III, and sauce IV) were analyzed via HS-SPME-GC-MS, which identified 64 volatile compounds. Sauce III, which was prepared through the mixed fermentation of bean koji and clam meat, had the highest free amino acid content of the four sauces, and was superior in flavor. Analysis of the sauces using an electronic nose and tongue demonstrated the superior flavor quality of sauce III and was in good agreement with the GC-MS analysis.
Author Contributions
Conceptualization, T.Z. and Y.M.; methodology, T.Z.; software, W.J.; validation, T.Z., Y.M. and W.J.; formal analysis, T.Z.; investigation, T.Z.; resources, T.Z.; data curation, T.Z.; writing—original draft preparation, T.Z.; writing—review and editing, X.X.; visualization, B.F.; supervision, X.X.; project administration, X.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Marine Economic Development Project of Liaoning Province grant number 2022-47.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to express our thanks to all the participants of the present research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spence, C. The psychology of condiments: A review. Int. J. Gastron. Food Sci. 2018, 11, 41–48. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, X. The Development Trend and Problems of Chinese Condiments. IOP Conf. Ser.: Earth Environ. Sci. 2020, 615, 012088. [Google Scholar] [CrossRef]
- Hajeb, P.; Jinap, S. Umami taste components and their sources in Asian foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Teo, J.N.; Liu, S.Q. Fermented shellfish condiments: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4447–4477. [Google Scholar] [CrossRef]
- Zhou, C.; Xu, L.; Song, H.; Feng, J.; Hu, Z.; Yang, M.-J.; Shi, P.; Li, Y.-R.; Guo, Y.-J.; Li, H.-Z. Examination of the regulation of energy metabolism, antioxidant response, and ammonia detoxification in hard clam, Mercenaria mercenaria, under hypersalinity stress. Aquaculture 2023, 563, 738916. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S.; Kishimura, H.; Tsai, Y.-H. Chemical compositions and nutritional value of Asian hard clam (Meretrix lusoria) from the coast of Andaman Sea. Food Chem. 2013, 141, 4138–4145. [Google Scholar] [CrossRef]
- Han, J.; Kong, T.; Wang, Q.; Jiang, J.; Zhou, Q.; Li, P.; Zhu, B.; Gu, Q. Regulation of microbial metabolism on the formation of characteristic flavor and quality formation in the traditional fish sauce during fermentation: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Q.; Dong, Z.; Liu, S.; Zhang, C.; Li, J. Biochemical, Nutritional, and Sensory Quality of the Low Salt Fermented Shrimp Paste. J. Aquat. Food Prod. Technol. 2017, 26, 706–718. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J.; Meng, X.; Wu, X. Characterization of volatile profile from ten different varieties of Chinese jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef]
- Sakpetch, P.; Benchama, O.; Masniyom, P.; Salaipeth, L.; Kanjan, P. Physicochemical characteristics and flavor profiles of fermented fish sauce (budu) during fermentation in commercial manufacturing plant. J. Food Sci. Technol. 2022, 59, 693–702. [Google Scholar] [CrossRef]
- Chen, Q.; Kong, B.; Han, Q.; Xia, X.; Xu, L. The role of bacterial fermentation in lipolysis and lipid oxidation in Harbin dry sausages and its flavour development. LWT 2017, 77, 389–396. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, C.; Zhao, H.; Gao, X.; Zhao, M.; Sun, W. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int. J. Food Sci. Technol. 2013, 48, 609–619. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Feng, X.; Zhang, M.; Ding, T.; Zhao, Y.; Wang, C. Comparative proteome and volatile metabolome analysis of Aspergillus oryzae 3.042 and Aspergillus sojae 3.495 during koji fermentation. Food Res. Int. 2023, 165, 112527. [Google Scholar] [CrossRef] [PubMed]
- Trang, N.H.; Shimada, K.-I.; Sekikawa, M.; Ono, T.; Mikami, M. Fermentation of meat with koji and commercial enzymes, and properties of its extract. J. Sci. Food Agric. 2005, 85, 1829–1837. [Google Scholar] [CrossRef]
- Ruan, L.; Ju, Y.; Zhan, C.; Hou, L. Improved umami flavor of soy sauce by adding enzymatic hydrolysate of low-value fish in the natural brewing process. LWT 2022, 155, 112911. [Google Scholar] [CrossRef]
- Chen, D.; Wang, S.; Li, M.; Hao, T.; Lin, S. The dynamic changes in product attributes of shiitake mushroom pilei and stipes during dehydration by hot air drying. J. Food Process. Preserv. 2021, 45, e15648. [Google Scholar] [CrossRef]
- Li, W.; Lu, H.; He, Z.; Sang, Y.; Sun, J. Quality characteristics and bacterial community of a Chinese salt-fermented shrimp paste. LWT 2021, 136, 110358. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, F.; Wu, D.; Yu, C.; Wang, Z.; Du, M. Antioxidant and ACE Inhibitory Activity of Enzymatic Hydrolysates from Ruditapes philippinarum. Molecules 2018, 23, 1189. [Google Scholar] [CrossRef]
- Liu, M.; Han, X.; Tu, K.; Pan, L.; Tu, J.; Tang, L.; Liu, P.; Zhan, G.; Zhong, Q.; Xiong, Z. Application of electronic nose in Chinese spirits quality control and flavour assessment. Food Control 2012, 26, 564–570. [Google Scholar] [CrossRef]
- Luo, X.; Hu, S.; Xu, X.; Du, M.; Wu, C.; Dong, L.; Wang, Z. Improving air-fried squid quality using high internal phase emulsion coating. J. Food Meas. Charact. 2022, 16, 3844–3854. [Google Scholar] [CrossRef]
- Yu, J.; Xu, X.B.; Murtada, K.; Pawliszyn, J. Untargeted analysis of microbial metabolites and unsaturated fatty acids in salmon via hydrophilic-lipophilic balanced solid-phase microextraction arrow. Food Chem. 2022, 380, 132219. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, F.; Zhu, B.; Yin, S.; Fu, Y.; Li, Y.; Liao, Y.; Kang, M.; Zhang, Y.; He, J.; et al. Optimization of HS-SPME-GC-MS for the Determination of Volatile Flavor Compounds in Ningxiang Pork. Foods 2023, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Zhou, T.; Jiang, W.; Zhu, B.-W.; Du, M.; Xu, X.-B. Balanced extraction of volatile and semi-volatile compounds by dynamic linked position unity solid-phase microextraction. Food Chem. 2023, 407, 135160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Feng, Y.; Hadiatullah, H.; Zheng, F.; Yao, Y. Chemical Characteristics of Three Kinds of Japanese Soy Sauce Based on Electronic Senses and GC-MS Analyses. Front. Microbiol. 2021, 11, 579808. [Google Scholar] [CrossRef] [PubMed]
- Selli, S.; Cayhan, G.G. Analysis of volatile compounds of wild gilthead sea bream (Sparus aurata) by simultaneous distillation–extraction (SDE) and GC–MS. Microchem. J. 2009, 93, 232–235. [Google Scholar] [CrossRef]
- Ouyang, Q.; Chen, Q.; Zhao, J.; Lin, H. Determination of Amino Acid Nitrogen in Soy Sauce Using Near Infrared Spectroscopy Combined with Characteristic Variables Selection and Extreme Learning Machine. Food Bioprocess Technol. 2012, 6, 2486–2493. [Google Scholar] [CrossRef]
- Wang, L.; Su, L.; Zhang, Y.; Pan, S.; Du, Y.; Zhang, J. Biochemical and Sensory Changes of Low-Salt Anchovy (Engraulis japonicus) Sauce Prepared by a Novel Technique. J. Aquat. Food Prod. Technol. 2017, 26, 695–705. [Google Scholar] [CrossRef]
- Bermúdez, R.; Franco, D.; Carballo, J.; Sentandreu, M.Á.; Lorenzo, J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014, 56, 226–235. [Google Scholar] [CrossRef]
- Zhu, W.; Luan, H.; Bu, Y.; Li, X.; Li, J.; Ji, G. Flavor characteristics of shrimp sauces with different fermentation and storage time. LWT 2019, 110, 142–151. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Liu, H.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Shi, J.; Nian, Y.; Da, D.; Xu, X.; Zhou, G.; Zhao, D.; Li, C. Characterization of flavor volatile compounds in sauce spareribs by gas chromatography–mass spectrometry and electronic nose. LWT 2020, 124, 109182. [Google Scholar] [CrossRef]
- Guan, C.; Liu, T.; Li, Q.; Wang, D.; Zhang, Y. Analyzing the Effect of Baking on the Flavor of Defatted Tiger Nut Flour by E-Tongue, E-Nose and HS-SPME-GC-MS. Foods 2022, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Cai, Y.; Sun-Waterhouse, D.; Fu, X.; Su, G.; Zhao, M. Reducing the influence of the thermally induced reactions on the determination of aroma-active compounds in soy sauce using SDE and GC-MS/O. Food Anal. Methods 2017, 10, 931–942. [Google Scholar] [CrossRef]
- Romeo, V.; Ziino, M.; Giuffrida, D.; Condurso, C.; Verzera, A. Flavour profile of capers (Capparis spinosa L.) from the Eolian Archipelago by HS-SPME/GC–MS. Food Chem. 2007, 101, 1272–1278. [Google Scholar] [CrossRef]
- Mohamed, H.N.; Man, Y.C.; Mustafa, S.; Manap, Y.A. Tentative identification of volatile flavor compounds in commercial budu, a Malaysian fish sauce, using GC-MS. Molecules 2012, 17, 5062–5080. [Google Scholar] [CrossRef]
- Li, Z.; Dong, L.; Huang, Q.; Wang, X. Bacterial communities and volatile compounds in Doubanjiang, a Chinese traditional red pepper paste. J. Appl. Microbiol. 2016, 120, 1585–1594. [Google Scholar] [CrossRef]
- Ding, A.; Zhu, M.; Qian, X.; Shi, L.; Huang, H.; Xiong, G.; Wang, J.; Wang, L. Effect of fatty acids on the flavor formation of fish sauce. LWT 2020, 134, 110259. [Google Scholar] [CrossRef]
- Wu, J.; Chen, R.; Li, X.; Fu, Z.; Xian, C.; Zhao, W.; Zhao, C.; Wu, X. Comprehensive identification of key compounds in different quality grades of soy sauce-aroma type baijiu by HS-SPME-GC-MS coupled with electronic nose. Front. Nutr. 2023, 10, 1132527. [Google Scholar] [CrossRef]
- Wen, X.; Chen, A.; Xu, Y.; Wu, Y.; Yang, Y.; Zhang, Y.; Cao, Y.; Chen, S. Comparative Evaluation of Volatile Profiles of Asian Hard Clams (Meretrix meretrix) with Different Shell Colors by Electronic Nose and GC-MS. J. Aquat. Food Prod. Technol. 2021, 30, 107–121. [Google Scholar] [CrossRef]
- Pham, A.J.; Schilling, M.W.; Yoon, Y.; Kamadia, V.V.; Marshall, D.L. Characterization of fish sauce aroma-impact compounds using GC-MS, SPME-Osme-GCO, and Stevens’ power law exponents. J. Food Sci. 2008, 73, C268–C274. [Google Scholar] [CrossRef]
- Zheng, J.; Wu, C.-D.; Huang, J.; Zhou, R.-Q.; Liao, X.-P. Analysis of volatile compounds in Chinese soy sauces moromi cultured by different fermentation processes. Food Sci. Biotechnol. 2013, 22, 605–612. [Google Scholar] [CrossRef]
- Gao, L.; Liu, T.; An, X.; Zhang, J.; Ma, X.; Cui, J. Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC-MS combined with HS-SPME and discrimination with electronic nose. J. Food Sci. Technol. 2017, 54, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, M.; Song, H.; Meng, Q.; Guan, X. Characterization of key odor-active compounds in commercial high-salt liquid-state soy sauce by switchable GC/GC× GC–olfactometry–MS and sensory evaluation. Food Chem. 2021, 342, 128224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, C.; Zhuang, H.; Liu, Y.; Feng, T.; Nie, B. Characterization of Volatile Component Changes in Peas under Different Treatments by GC-IMS and GC-MS. J. Food Qual. 2021, 2021, 6533083. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).