Towards a Better Understanding of Texturization during High-Moisture Extrusion (HME)—Part I: Modeling the Texturability of Plant-Based Proteins
Abstract
1. Introduction
2. Material and Methods:
2.1. Materials
2.2. Sample Preparation of SPC Alpha 8
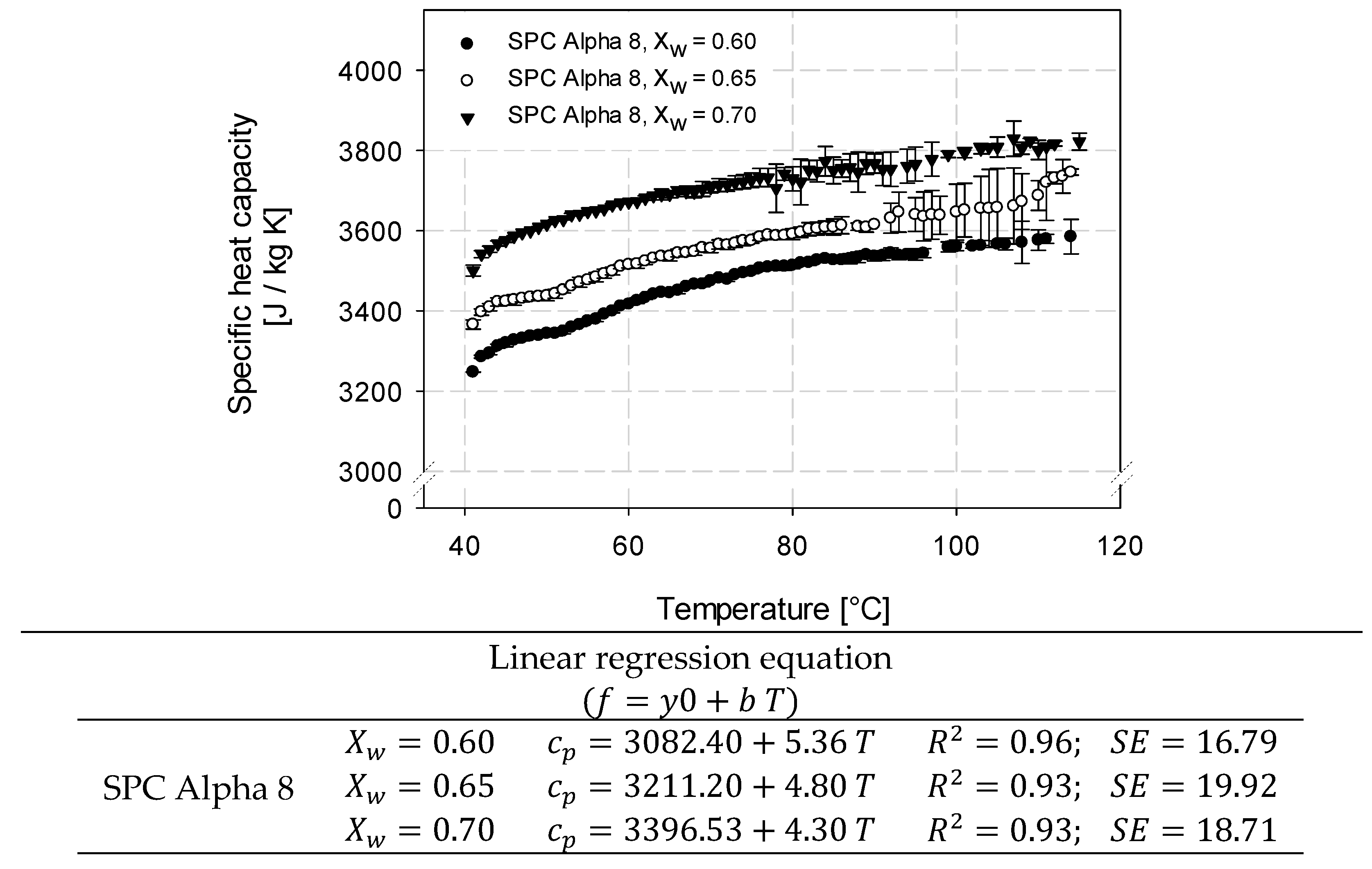
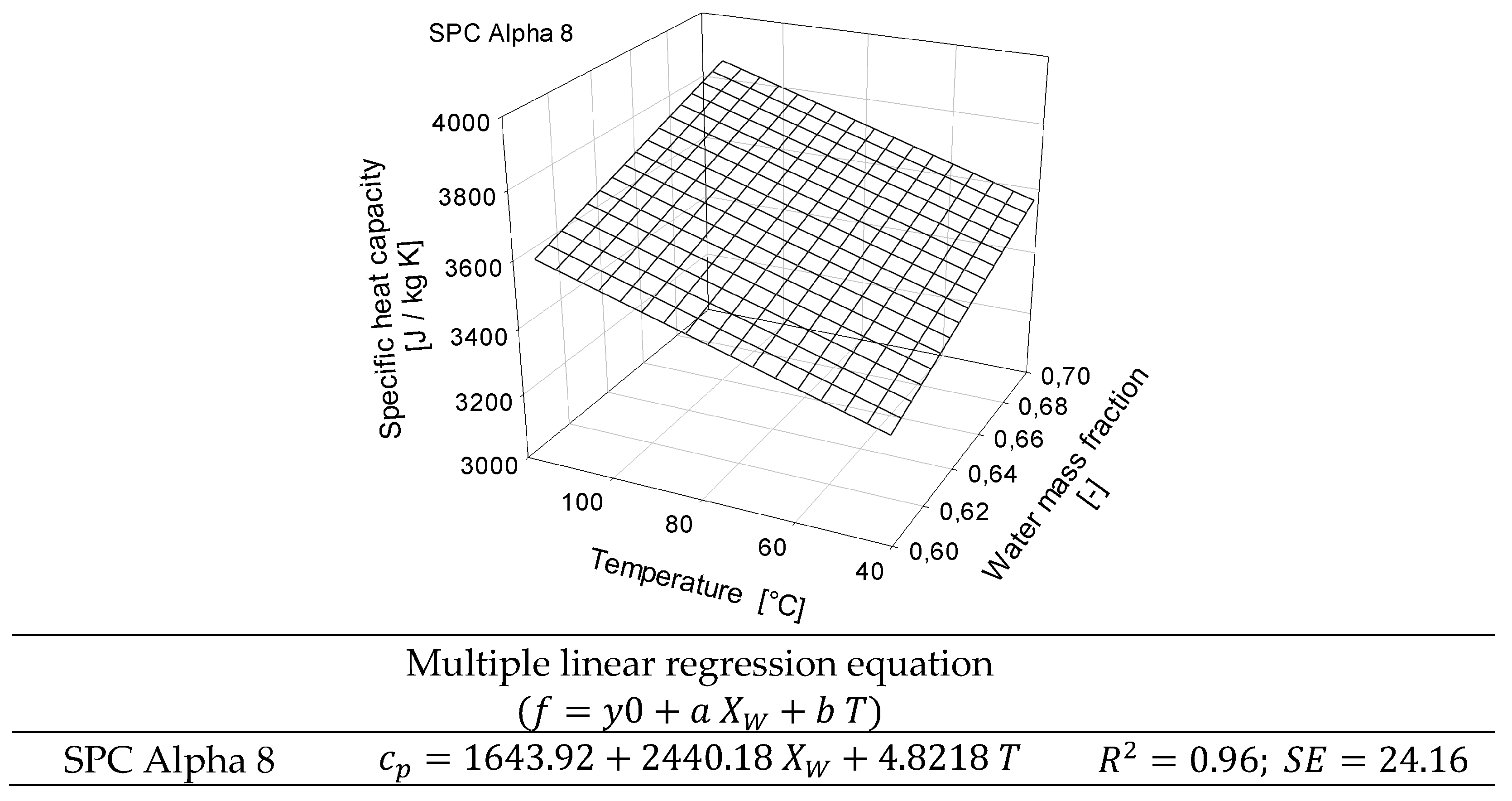
2.3. Determination of Specific Heat Capacity of SPC Alpha 8 by µDSC
2.4. Determination of Phase Transition of SPC Alpha 8 by DSC
2.5. High-Moisture Texturization of SPC Alpha 8
2.6. Sensory Evaluation of High-Moisture Texturized SPC Alpha 8
- -
- Well-textured (=HMMA):
- -
- Textured:
- -
- Poorly-textured:
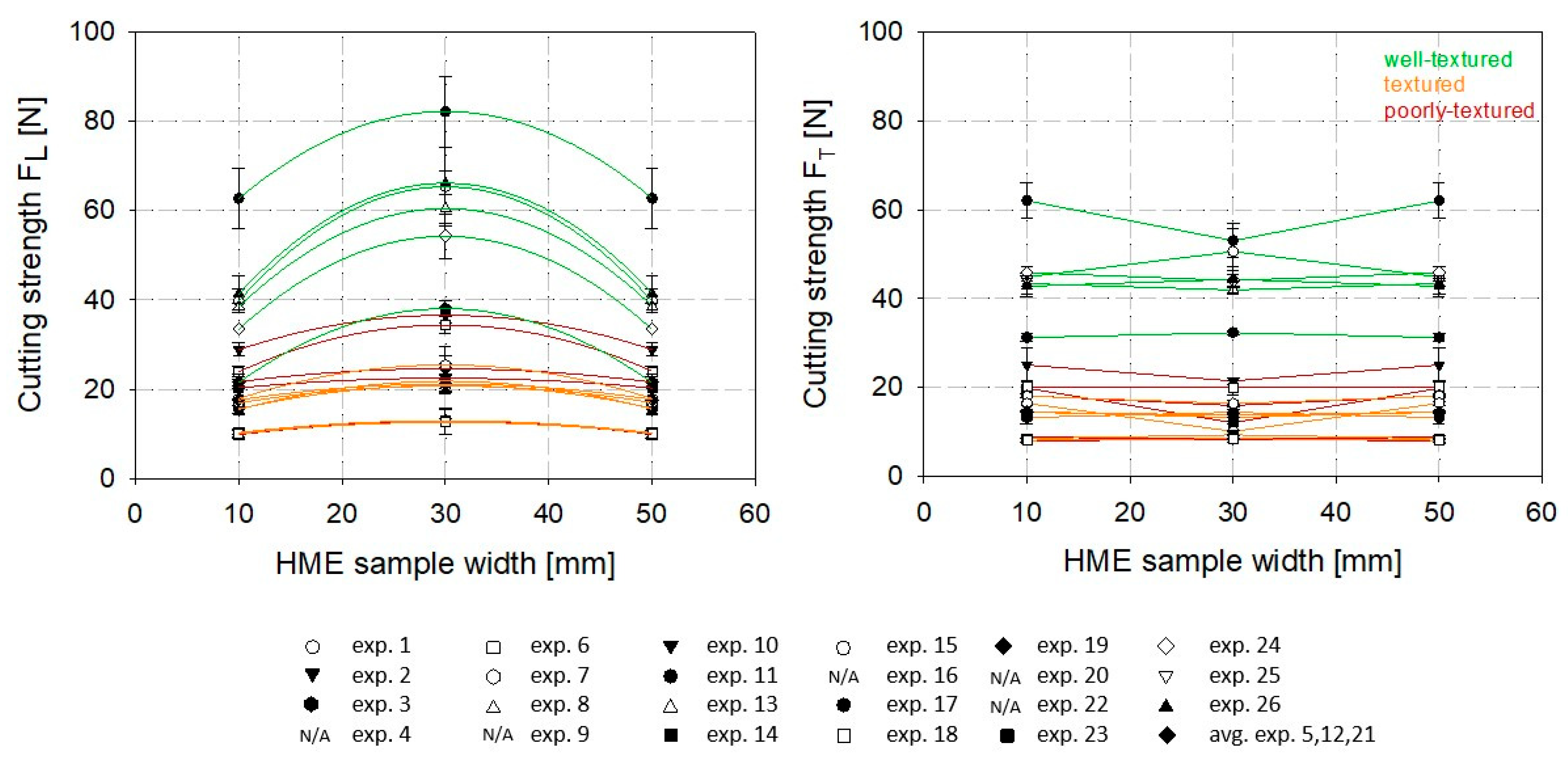
2.7. Determination of Slice Shear Force of HME Samples Made out of SPC Alpha 8
2.8. Modeling of High-Moisture Texturization of SPC Alpha 8
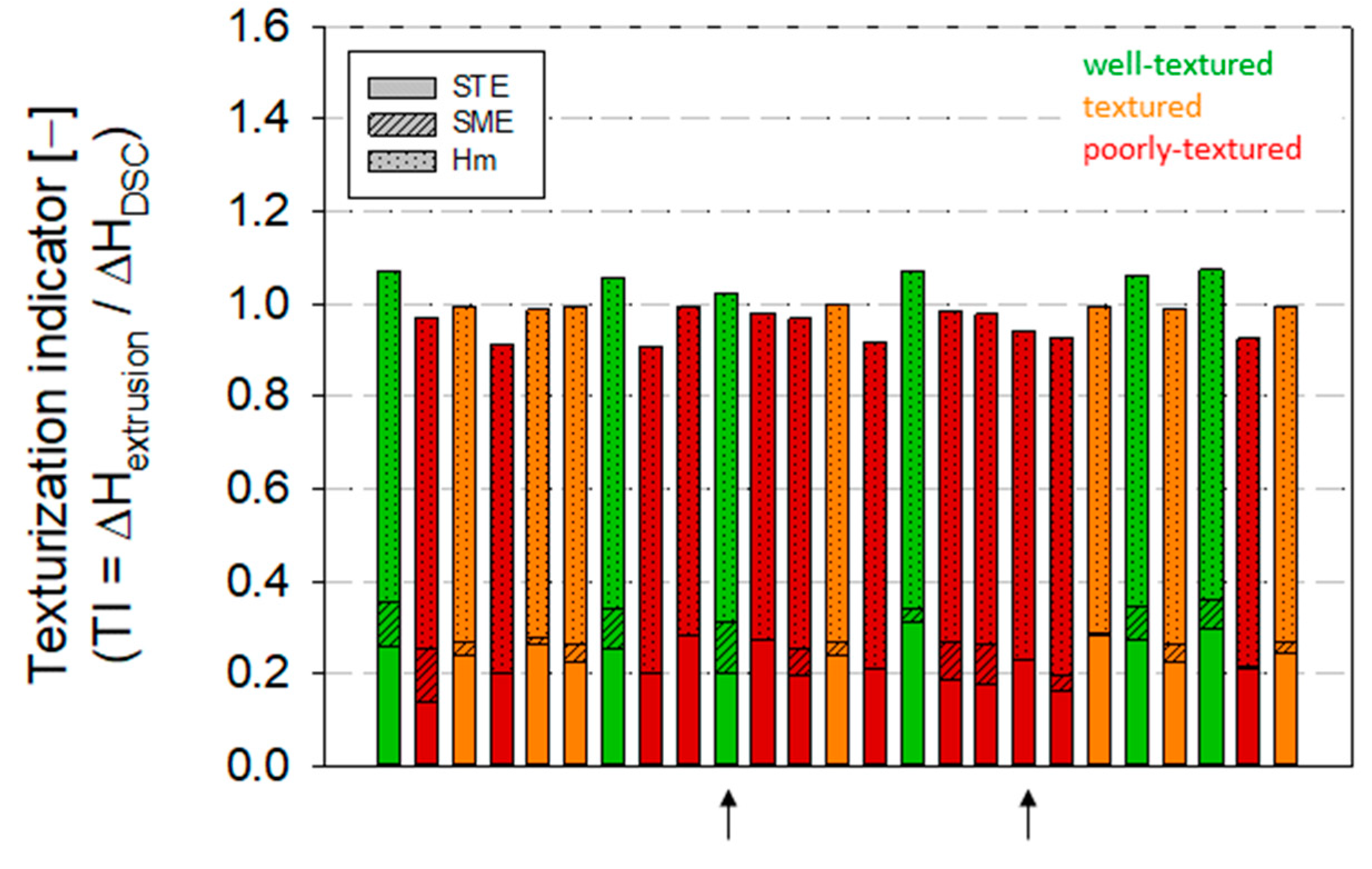
2.9. Implementation of a Texturization Indicator to Predict High-Moisture Texturization of SPC Alpha 8
2.10. Validation to Predict High-Moisture Texturization of Plant-Based Proteins
2.11. Statistical Analysis
3. Results and Discussion
3.1. High-Moisture Texturization of SPC Alpha 8
3.2. Determination of Specific Heat Capacity of SPC Alpha 8
3.3. Determination of Phase Transition of SPC Alpha 8
3.4. Modeling of High-Moisture Texturization of SPC Alpha 8
3.5. Validation to Predict High-Moisture Texturization of Plant-Based Proteins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Weele, C.; Feindt, P.; van der Jan Goot, A.; van Mierlo, B.; van Boekel, M. Meat alternatives: An integrative comparison. Trends Food Sci. Technol. 2019, 88, 505–512. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Joo, S.-T. Meat analog as future food: A review. J. Anim. Sci. Technol. 2020, 62, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bakhsh, A.; Lee, S.-J.; Lee, E.-Y.; Hwang, Y.-H.; Joo, S.-T. Traditional Plant-based Meat Alternatives, Current, and Future Perspective: A Review. J. Agirc. Life Sci. 2021, 55, 1–10. [Google Scholar] [CrossRef]
- Beniwal, A.S.; Singh, J.; Kaur, L.; Hardacre, A.; Singh, H. Meat analogs: Protein restructuring during thermomechanical processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1221–1249. [Google Scholar] [CrossRef] [PubMed]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of high moisture extrusion cooking on protein-protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food. Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Brugger, C.; Dellemann, J.-P.; Petry, C.; Laporte, M.; Müller, N.; Windhab, E. Next Generation Texturized Vegetable Proteins. Food Mark. Technol. 2017, 1, 20–25. [Google Scholar]
- Högg, E.; Horneber, T.; Rauh, C.; Emin, M.A.; Pietsch, V.L.; Schuchmann, H.P.; Herpich, J.; Pernutz, C.; Töpfl, S. Aufklärung der Texturierungsmechanismen bei der Nassextrusion von Soja- und Erbsenprotein unter besonderer Berücksichtigung biothermofluiddynamischer und proteinchemischer Aspekte auf Basis experimenteller und numerischer Untersuchungen; Schlussbericht zu IGV-Vorhaben Nr. AiF 18727 N; Das o.g. IGF-Vorhaben der Forschungsvereinigung Forschungskreis der Ernährungsindustrie e.V.: Bonn, Germany, 2018. [Google Scholar]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J.K. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Ferawati, F.; Zahari, I.; Barman, M.; Hefni, M.; Ahlström, C.; Witthöft, C.; Östbring, K. High-Moisture Meat Analogues Produced from Yellow Pea and Faba Bean Protein Isolates/Concentrate: Effect of Raw Material Composition and Extrusion Parameters on Texture Properties. Foods 2021, 10, 843. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Thermoplastic Extrusion—The Mechanism of the Formation of Extrudate Structure and Properties. J. Am. Oil Chem. Soc. 1993, 70, 417–424. [Google Scholar] [CrossRef]
- Roos, Y.; Drusch, S. Phase Transitions in Foods; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Osen, R. Texturization of Pea Protein Isolates Using High Moisture Extrusion Cooking. Ph.D. Thesis, Technische Universität Muenchen, Munich, Germany, 2017. Available online: https://mediatum.ub.tum.de/?id=1356359 (accessed on 5 January 2023).
- Harper, J. Food extruders and their applications. In Extrusion Cooking; American Association of Cereal Chemists: St. Paul, MN, USA, 1989. [Google Scholar]
- Riaz, M.N. Texturized soy protein as an ingredient. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing in Food Science and Technology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Akdogan, H. High moisture food extrusion. Int. J. Food Sci. Technol. 1999, 34, 195–207. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein-protein interactions during high-moisture extrusion for fibrous meat analogues and comparison of protein solubility methods using different solvent systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Wild, F. Manufacture of Meat Analogues through High Moisture Extrusion. In Reference Module in Food Science; Smithers, G.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.H.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef] [PubMed]
- Guyony, V.; Fayolle, F.; Jury, V. High moisture extrusion of vegetable proteins for making fibrous meat analogs: A review. Food Rev. Int. 2022, 18, 1–26. [Google Scholar] [CrossRef]
- Bouvier, J.-M.; Campanella, O. Extrusion Processing Technology. Food and Non-Food Biomaterials; John Wiley & Sons Inc.: Chichester, UK, 2014. [Google Scholar]
- Vatansever, S.; Tulbek, M.C.; Riaz, M.N. Low- and High-Moisture Extrusion of Pulse Proteins as Plant-Based Meat Ingredients: A Review. Cereal Foods World 2020, 65, 1. [Google Scholar] [CrossRef]
- Durowoju, I.B.; Bhandal, K.S.; Hu, J.; Carpick, B.; Kirkitadze, M. Differential Scanning Calorimetry—A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J. Vis. Exp. 2017, 121, 55262. [Google Scholar] [CrossRef]
- Miotto, M.; Olimpieri, P.P.; Di Rienzo, L.; Ambrosetti, F.; Corsi, P.; Lepore, R.; Tartaglia, G.G.; Milanetti, E. Insights on protein thermal stability: A graph representation of molecular interactions. Bioinformatics 2018, 35, 2569–2577. [Google Scholar] [CrossRef]
- Kotov, V.; Mlynek, G.; Vesper, O.; Pletzer, M.; Wald, J.; Teixeira-Duarte, C.M.; Celia, H.; Garcia-Alai, M.; Nussberger, S.; Buchanan, S.K.; et al. In-depth interrogation of protein thermal unfolding data with MoltenProt. Protein Sci. 2021, 30, 201–217. [Google Scholar] [CrossRef]
- Rees, D.C.; Robertson, A.D. Some thermodynamic implications for the thermostability of proteins. Protein Sci. 2001, 10, 1187–1194. [Google Scholar] [CrossRef]
- Dehghan-Nayeri, N.; Rezaei-Tavirani, M. The Interpretation of Protein Structure through Relationship Ofmelting Point (Tm) and Enthalpy of Unfolding (ΔHu). Int. J. Anakytical Pharm. Biomed. Sci. 2015, 4, 47–50. [Google Scholar]
- Sandoval Murillo, J.L.; Osen, R.; Hiermaier, S.; Ganzenmüller, G. Towards understanding the mechanism of fibrous texture formation during high-moisture extrusion of meat substitutes. J. Food Eng. 2019, 242, 8–20. [Google Scholar] [CrossRef]
- Cornet, S.H.; Snel, S.J.; Lesschen, J.; van der Goot, A.J.; van der Sman, R.G. Enhancing the water holding capacity of model meat analogues through marinade composition. J. Food Eng. 2021, 290, 110283. [Google Scholar] [CrossRef]
- Yu, L.; Christie, G. Measurement of starch thermal transitions using differential scanning calorimetry. Carbohydr. Polym. 2001, 46, 179–184. [Google Scholar] [CrossRef]
- Zhang, J.; Farkas, B.E.; Hale, S.A. Thermal properties of skipjack tuna. Int. J. Food Prop. 2001, 4, 81–90. [Google Scholar] [CrossRef]
- Högg, E.; Rauh, C. Towards a better understanding of texturization during high-moisture extrusion (HME)—Part II: Characterization of thermophysical properties of high-moisture meat analogues. Foods 2023, submitted.
- Meuser, F.; van Lengerich, B.H. Systems analytical model for the extrusion of starches. Therm. Process. Qual. Foods 1984, 10, 175–179. [Google Scholar]
- American Meat Science Association. Research Guidelines for Cookery, Sensory Evaluation, and Instrumental Tenderness Measurements of Meat; American Meat Science Association: Savoy, IL, USA, 2015; Volume 1, pp. 1–105. [Google Scholar]
- Chang, K.; Liang, B.; Halek, G.W. Analysis of Shear and Thermal History during Co-Rotating Twin-Screw Extrusion. J. Food Sci. 1991, 56, 518–531. [Google Scholar] [CrossRef]
- Olimat, A.N.; Al-Salaymeh, A.; Al-Maaitah, A. Studying the Stability of Melting and Solidification Behavior of Phase Change Material. J. Appl. Res. Ind. Eng. 2017, 4, 54731. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, D.-Y.; Shin, W.-S. Fucoidan improves the structural integrity and the molecular stability of β-lactoglobulin. Food Sci. Biotechnol. 2018, 27, 1247–1255. [Google Scholar] [CrossRef]
- Seniorita, L.; Minami, E.; Yazawa, Y.; Hayashi, H.; Saka, S. Differential Scanning Calorimetric Study of Solidification Behavior of Monoacylglycerols to Investigate the Cold-Flow Properties of Biodiesel. J. Am. Oil Chem. Soc. 2019, 96, 979–987. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion Process Parameters, Sensory Characteristics, and Structural Properties of a High Moisture Soy Protein Meat Analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Chen, F.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Leister, D. Continous Manufacturing of Pharmaceuticals: Scale-up of a hot melt extrusion. Pharam. Technol. 2018, 1, 1. [Google Scholar]
- Thiébaud, M.; Dumay, E.; Cheftel, J.C. Influence of Process Variables on the Characteristics of a High Moisture Fish Soy Protein Mix Texturized by Extrusion Cooking. LWT Food Sci. Technol. 1996, 29, 526–535. [Google Scholar] [CrossRef]
- Noguchi, A. Extrusion cooking of high-moisture protein foods. In Extrusion Cooking; Mercier, C., Linko, P., Harper, J.M., Eds.; American Association of Cereal Chemists: St. Paul, MI, USA, 1989. [Google Scholar]
- Baird, D.G.; Reed, C.M. Transport Properties of Food Doughs. In Extrusion Cooking; Mercier, C., Linko, P., Harper, J.M., Eds.; American Association of Cereal Chemists: St. Paul, MI, USA, 1989; pp. 205–234. [Google Scholar]
- Chen, R.-H.; Ker, Y.-C.; Wu, C.-S. Temperature and Shear Rate Affecting the Viscosity and Secondary Structural Changes of Soy 11S Globulin Measured by a Cone-Plate Viscometer and Fourier Transform Infrared Spectroscopy. Agric. Biol. Chem. 1990, 54, 1165–1176. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M.A. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Texture and Chemical Characteristics of Soy Protein Meat Analog Extruded at High Moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Ryu, G.-H. Extrusion cooking of high-moisture meat analogues. Chapter 7. In Extrusion Cooking Cereal Grains Processing; Woodhead Publishing: Duxford, UK, 2020; pp. 205–224. [Google Scholar]
- Kitabatake, N.; Megard, D.; Cheftel, J.C. Continuous Gel Formation by HTST Extrusion-Cooking: Soy Proteins. J. Food Sci. 1985, 50, 1260–1265. [Google Scholar] [CrossRef]
- Wagner, L. Numerical Modeling of the Cooking Extrusion of a Bio-Polymer. Ph.D. Thesis, Virginia Polytechnic Institute and State Universit, Blacksburg, VA, USA, 1987. Available online: https://vtechworks.lib.vt.edu/handle/10919/53662 (accessed on 5 January 2023).
- Carsote, C.; Badea, E. Micro differential scanning calorimetry and micro hot table method for quantifying deterioration of historical leather. Herit. Sci. 2019, 7, 1. [Google Scholar] [CrossRef]
- Sousa, I.M.; Mitchell, J.R.; Ledward, D.A.; Hill, S.E.; da Costa, M.L. Differential scanning calorimetry of lupin and soy proteins. Z. Fur Lebensm.-Unters. Und-Forsch. 1995, 201, 566–569. [Google Scholar] [CrossRef]
- Liu, K.S.; Hsieh, F.-H. Protein–Protein Interactions in High Moisture-Extruded Meat Analogs and Heat-Induced Soy Protein Gels. J. Amer. Oil Chem. Soc. 2007, 84, 741–748. [Google Scholar] [CrossRef]
- Sablani, S.S.; Syamaladevi, R.M.; Swanson, B.G. A Review of Methods, Data and Applications of State Diagrams of Food Systems. Food Eng. Rev. 2010, 2, 168–203. [Google Scholar] [CrossRef]




| Experiment Number | Experimental Plan | |||
|---|---|---|---|---|
| X1 | X2 | X3 | X4 | |
| 1 | 400 | 60 | 13 | 160 |
| 2 | 400 | 60 | 6 | 120 |
| 3 | 175 | 65 | 9.5 | 140 |
| 4 | 220 | 70 | 6 | 120 |
| 5 | 310 | 65 | 9.5 | 140 |
| 6 | 400 | 70 | 13 | 160 |
| 7 | 445 | 65 | 9.5 | 140 |
| 8 | 400 | 60 | 6 | 160 |
| 9 | 400 | 70 | 6 | 120 |
| 10 | 220 | 70 | 6 | 160 |
| 11 | 310 | 57.5 | 9.5 | 140 |
| 12 | 310 | 65 | 9.5 | 140 |
| 13 | 400 | 70 | 6 | 160 |
| 14 | 220 | 60 | 13 | 120 |
| 15 | 310 | 65 | 4.25 | 140 |
| 16 | 220 | 70 | 13 | 120 |
| 17 | 310 | 65 | 9.5 | 170 |
| 18 | 220 | 60 | 6 | 120 |
| 19 | 400 | 60 | 13 | 120 |
| 20 | 310 | 72.5 | 9.5 | 140 |
| 21 | 310 | 65 | 9.5 | 140 |
| 22 | 310 | 65 | 9.5 | 110 |
| 23 | 220 | 70 | 13 | 160 |
| 24 | 220 | 60 | 13 | 160 |
| 25 | 310 | 65 | 14.25 | 140 |
| 26 | 220 | 60 | 6 | 160 |
| 27 | 400 | 70 | 13 | 120 |
| Experiment Number | Experimental Plan | |||
|---|---|---|---|---|
| X1 | X2 | X3 | X4 | |
| SPC Procon No.1 | 400 | 60 | 13 | 130 |
| SPC Procon No.2 | 400 | 60 | 13 | 140 |
| SPI Wilpro No.1 | 400 | 60 | 13 | 120 |
| SPI Wilpro No.2 | 400 | 60 | 13 | 130 |
| PPI Pisane No.1 | 400 | 60 | 9 | 130 |
| PPI Pisane No.2 | 400 | 60 | 9 | 155 |
| Experiment Number | Experimental Plan | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Process Response | Product Response | ||||||||
| X1 | X2 | X3 | X4 | Y1 | Y2 | Y3 | Texture | ||
| Quantitative Structuring index | Qualitative Sensory | ||||||||
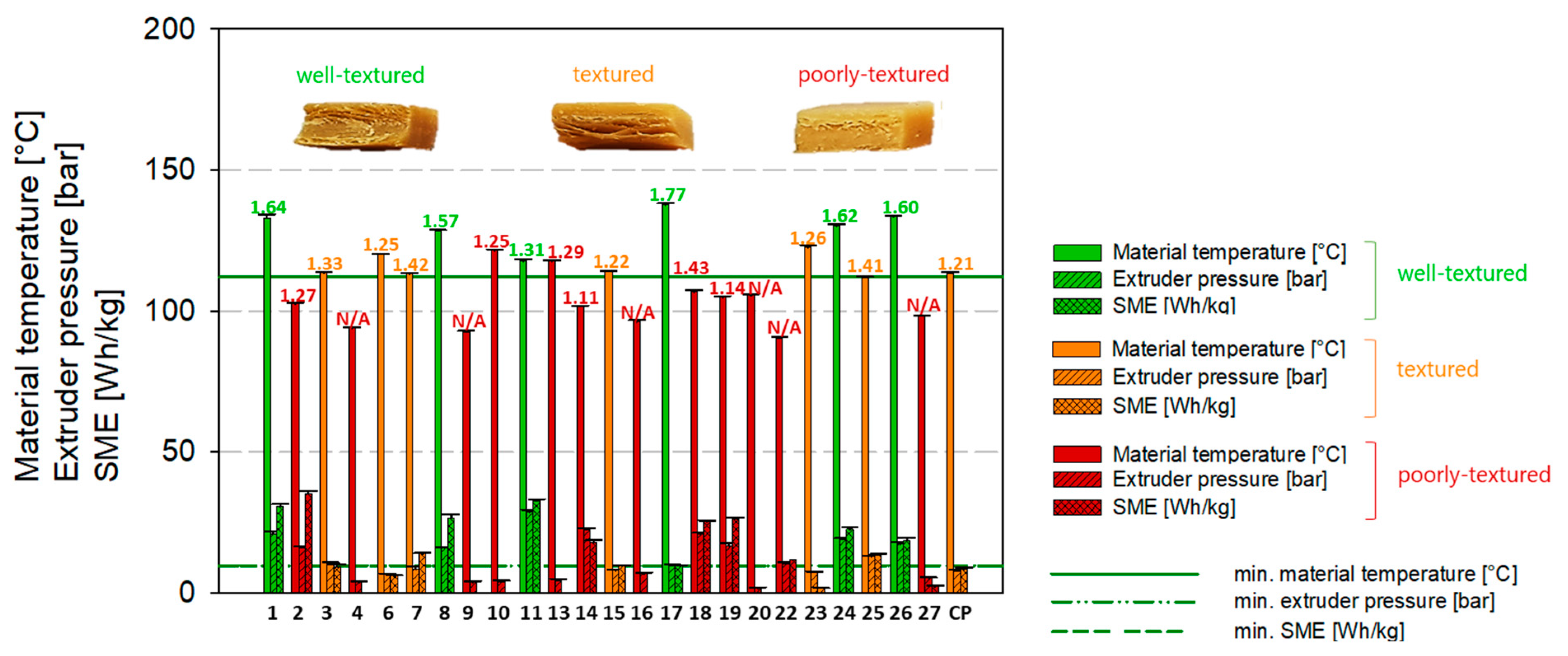
| 1 | 400 | 60 | 13 | 160 | 133.10 | 21.06 | 30.68 | 1.64 | well-textured |
| 2 | 400 | 60 | 6 | 120 | 102.57 | 16.20 | 35.34 | 1.27 | poorly-textured |
| 3 | 175 | 65 | 9.5 | 140 | 113.49 | 10.54 | 9.28 | 1.33 | textured |
| 4 | 220 | 70 | 6 | 120 | 93.90 | 3.84 | 0 | N/A | poorly-textured |
| 5 (CP) | 310 | 65 | 9.5 | 140 | 116.84 | 7.96 | 9.78 | 1.14 | textured |
| 6 | 400 | 70 | 13 | 160 | 120.11 | 6.63 | 6.09 | 1.25 | textured |
| 7 | 445 | 65 | 9.5 | 140 | 113.15 | 8.40 | 13.98 | 1.42 | textured |
| 8 | 400 | 60 | 6 | 160 | 128.48 | 15.95 | 26.74 | 1.57 | well-textured |
| 9 | 400 | 70 | 6 | 120 | 92.62 | 3.80 | 0 | N/A | poorly-textured |
| 10 | 220 | 70 | 6 | 160 | 121.53 | 4.03 | 0 | 1.25 | poorly-textured |
| 11 | 310 | 57.5 | 9.5 | 140 | 117.76 | 28.80 | 32.75 | 1.31 | well-textured |
| 12 (CP) | 310 | 65 | 9.5 | 140 | 114.44 | 8.94 | 8.62 | 1.23 | textured |
| 13 | 400 | 70 | 6 | 160 | 117.73 | 4.47 | 0 | 1.29 | poorly-textured |
| 14 | 220 | 60 | 13 | 120 | 101.80 | 22.47 | 17.86 | 1.11 | poorly-textured |
| 15 | 310 | 65 | 4.25 | 140 | 114.22 | 7.98 | 9.81 | 1.22 | textured |
| 16 | 220 | 70 | 13 | 120 | 96.28 | 6.84 | 0 | N/A | poorly-textured |
| 17 | 310 | 65 | 9.5 | 170 | 137.76 | 9.69 | 9.71 | 1.77 | well-textured |
| 18 | 220 | 60 | 6 | 120 | 106.82 | 20.96 | 25.34 | 1.43 | poorly-textured |
| 19 | 400 | 60 | 13 | 120 | 104.85 | 16.73 | 26.20 | 1.14 | poorly-textured |
| 20 | 310 | 72.5 | 9.5 | 140 | 105.67 | 1.61 | 0 | N/A | poorly-textured |
| 21 (CP) | 310 | 65 | 9.5 | 140 | 110.95 | 6.67 | 6.72 | 1.26 | textured |
| 22 | 310 | 65 | 9.5 | 110 | 90.42 | 10.49 | 11.85 | N/A | poorly-textured |
| 23 | 220 | 70 | 13 | 160 | 122.68 | 7.51 | 1.73 | 1.26 | textured |
| 24 | 220 | 60 | 13 | 160 | 130.22 | 19.08 | 22.70 | 1.62 | well-textured |
| 25 | 310 | 65 | 14.25 | 140 | 111.89 | 12.91 | 13.51 | 1.41 | textured |
| 26 | 220 | 60 | 6 | 160 | 133.58 | 17.45 | 18.70 | 1.60 | well-textured |
| 27 | 400 | 70 | 13 | 120 | 98.18 | 5.44 | 2.38 | N/A | poorly-textured |
| Water Content | [%] | 57.5 | 60 | 65 | 70 | 72.5 |
|---|---|---|---|---|---|---|
| Minimum texturization temperature | [°C] | 112.70 | 117.68 | 121.47 | 131.50 | 133.59 |
| Specific Heat Capacity cp | Phase Transition at Xw = 0.6 | ||||||
|---|---|---|---|---|---|---|---|
| Multiple linear regression equation | Tm [°C] | ΔHm [J/g] | Tonset [°C] | Tend [°C] | ΔHDSC [J/g] | ||
| SPC Procon | 115.9 | 834.1 | 86.0 | 129.9 | 1205 | ||
| ±4.7 | ±13.5 | ±3.4 | ±3.5 | ±29.6 | |||
| SPI Wilpro | 114.6 | 673.2 | 83.4 | 126.5 | 1036 | ||
| ±0.4 | ±31.7 | ±1.7 | ±2.3 | ±8.7 | |||
| PPI Pisane | 117.9 | 781.4 | 90.0 | 133.6 | 1125 | ||
| ±0.2 | ±1.2 | ±2.8 | ±0.6 | ±41.4 | |||
| Protein | Moisture Content | Calculated Minimum Texturization Temperature Tin, calculated |
|---|---|---|
| SPC Procon | 60% | 128.00 °C |
| SPI Wilpro | 60% | 124.15 °C |
| PPI Pisane | 60% | 120.40 °C |
| Protein | Experiment No. | Experimental Plan | Extrusion Response | Calculated min. Texturization Temperature | |||||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Pressure [bar] | SME [Wh/kg] | Tin [°C] | Tin [°C] | ||
| SPC Procon | No.1 | 400 | 60 | 13 | 130 | 40.00 | 42.47 | 125.17 | 128.00 |
| No.2 | 400 | 60 | 13 | 140 | 38.50 | 40.00 | 137.20 | 128.00 | |
| SPI Wilpro | No.1 | 400 | 60 | 13 | 120 | 30.18 | 33.72 | 109.59 | 124.15 |
| No.2 | 400 | 60 | 13 | 130 | 41.10 | 48.90 | 129.70 | 124.15 | |
| PPI Pisane | No.1 | 400 | 60 | 9 † | 130 | 16.60 | 16.20 | 120.10 | 120.40 |
| No.2 | 400 | 60 | 9 † | 155 | 12.40 | 11.77 | 134.15 | 120.40 | |
| Protein | Experiment No. | Product response | Texturization indicator based on Equation (8) | ||||||
| Qualitative Sensory | Product Images | TI ‡ [−] | |||||||
| SPC Procon | No.1 | poorly-textured |  | 0.99 | |||||
| No.2 | well-textured | 1.03 | |||||||
| SPI Wilpro | No.1 | poorly-textured | 0.95 | ||||||
| No.2 | well-textured | 1.02 | |||||||
| PPI Pisane | No.1 | textured | 1.00 | ||||||
| No.2 | well-textured | 1.05 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Högg, E.; Rauh, C. Towards a Better Understanding of Texturization during High-Moisture Extrusion (HME)—Part I: Modeling the Texturability of Plant-Based Proteins. Foods 2023, 12, 1955. https://doi.org/10.3390/foods12101955
Högg E, Rauh C. Towards a Better Understanding of Texturization during High-Moisture Extrusion (HME)—Part I: Modeling the Texturability of Plant-Based Proteins. Foods. 2023; 12(10):1955. https://doi.org/10.3390/foods12101955
Chicago/Turabian StyleHögg, Elisabeth, and Cornelia Rauh. 2023. "Towards a Better Understanding of Texturization during High-Moisture Extrusion (HME)—Part I: Modeling the Texturability of Plant-Based Proteins" Foods 12, no. 10: 1955. https://doi.org/10.3390/foods12101955
APA StyleHögg, E., & Rauh, C. (2023). Towards a Better Understanding of Texturization during High-Moisture Extrusion (HME)—Part I: Modeling the Texturability of Plant-Based Proteins. Foods, 12(10), 1955. https://doi.org/10.3390/foods12101955






