Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review
Abstract
:1. Introduction
2. NIR Spectroscopy
2.1. Analytical Framework of NIR Spectroscopy
2.2. NIR Spectrometers and Miniaturization
2.3. Image-Based Methods
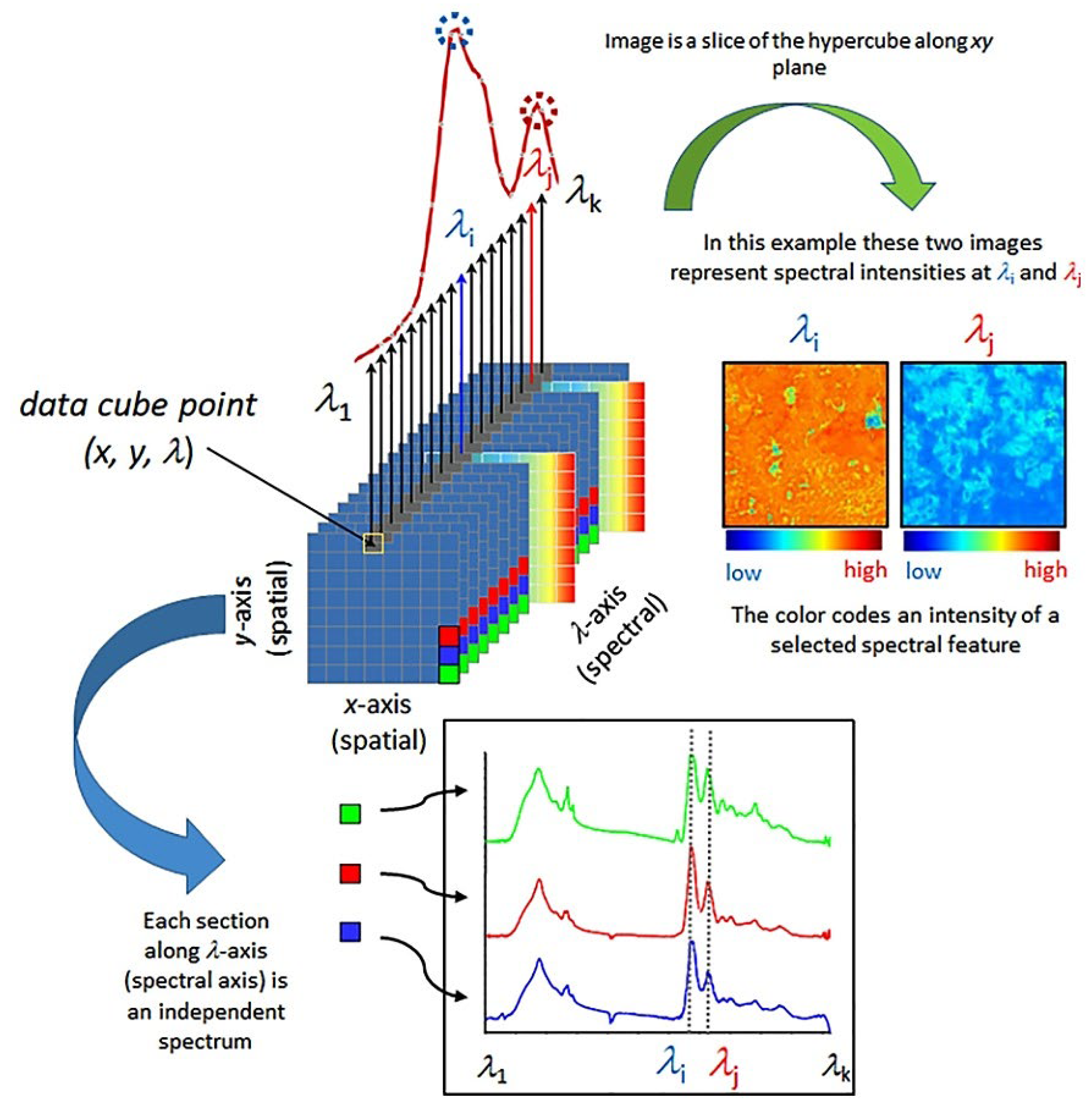
3. Analyzing the Quality Parameters of Apples; Internal and External Characteristics
3.1. Internal Quality Parameters
3.1.1. Taste
3.1.2. Aroma
3.1.3. Texture
Firmness
Mealiness
3.1.4. Nutrient Content
3.1.5. Factors That Affect Internal Quality
Phenolic Compounds
Soluble Solid Content (SSC)
Sugar Profile
Total Titratable Acidity (TA)
Starch Pattern Index (SPI) or Starch Content Index (SCI)
Total Dry Matter Concentration (DM)
3.2. External Quality Parameters
3.2.1. Color
3.2.2. Size
3.2.3. Shape
3.2.4. Surface Defects
4. Miscellaneous
4.1. Identification/Origin/Authenticity
4.2. Evaluation of Apple Maturity
4.3. Optimization of Storage Conditions
4.4. Quality Compromise Effects Related to Storage Conditions
5. Use of Portable/Handheld NIR Spectrometers
5.1. Current Analytical Potential of Miniaturized Spectrometers in Apple Quality Analysis
5.2. Assessment of Analytical Performance and Specific Applicability
5.3. Calibration Transfer
6. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Apple Production by Country 2023. Available online: https://worldpopulationreview.com/country-rankings/apple-production-by-country (accessed on 13 February 2023).
- DeEll, J.R.; Murr, D.P.; Porteous, M.D.; Vasantha Rupasinghe, H.P. Influence of temperature and duration of 1-methylcyclopropene (1-MCP) treatment on apple quality. Postharvest Biol. Technol. 2002, 24, 349–353. [Google Scholar] [CrossRef]
- Arundhati; Bhagat, R.M. Impact of climate change on apples production: World scenario. J. Pharmacogn. Phytochem. 2020, 9, 1219–1228. [Google Scholar]
- Wang, B.; Ranjan, R.; Khot, L.R.; Peters, R.T. Smartphone application-enabled apple fruit surface temperature monitoring tool for in-field and real-time sunburn susceptibility prediction. Sensors 2020, 20, 608. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K. (Eds.) Near-Infrared Technology: In the Agricultural and Food Industries, 2nd ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2001. [Google Scholar]
- Zude, M. Optical Monitoring of Fresh and Processed Agricultural Crops; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Povh, F.P.; dos Anjos, W.D.P.G. Optical Sensors applied in agricultural crops. In Optical Sensors—New Developments and Practical Applications; Yasin, M., Wadi Harun, S., Arof, H., Eds.; InTech: London, UK, 2014. [Google Scholar] [CrossRef]
- Zou, X.; Zhao, J. Nondestructive Measurement in Food and Agro-Products; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- García-Sánchez, F.; Galvez-Sola, L.; Martínez-Nicolás, J.J.; Muelas-Domingo, R.; Nieves, M. Using near-infrared spectroscopy in agricultural systems. In Developments in Near-Infrared Spectroscopy; Kyprianidis, K.G., Skvaril, J., Eds.; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Tsuchikawa, S.; Ma, T.; Inagaki, T. Application of near-infrared spectroscopy to agriculture and forestry. Anal. Sci. 2022, 38, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘point’ spectroscopy in postharvest fruit and vegetable assessment: The science behind three decades of commercial use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Nicolaı, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Pandiselvam1, R.; Prithviraj, V.; Manikantan, M.R.; Kothakota, A.; Rusu, A.V.; Trif, M.; Mousavi Khaneghah, A. Recent advancements in NIR spectroscopy for assessing the quality and safety of horticultural products: A comprehensive review. Front. Nutr. 2022, 9, 973457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, J.; Xie, C.; Bao, Y.; He, Y. Fruit quality evaluation using spectroscopy technology: A review. Sensors 2015, 15, 11889–11927. [Google Scholar] [CrossRef]
- Cortes, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring strategies for quality control of agricultural products using visible and near-infrared spectroscopy: A review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Walsh, K.B.; Golic, M.; Greensill, C.V. Sorting of fruit using near infrared spectroscopy: Application to a range of fruit and vegetables for soluble solids and dry matter content. J. Near Infrared Spectrosc. 2004, 12, 141–148. [Google Scholar] [CrossRef]
- Schmilovitch, Z.; Aharon, H.; Ignat, T.; Alchanati, V.; Haim, E.; Tamir, M. Inline application of NIR system in produce sorting machines. J. Agric. Res. 2018, 3, 000155. [Google Scholar]
- McCormick, R.; Biegert, K. Monitoring the growth and maturation of apple fruit on the tree with handheld Vis/NIR devices. NIR News 2018, 30, 12–15. [Google Scholar] [CrossRef]
- Buccheri, M.; Grassi, M.; Lovati, F.; Petriccione, M.; Rega, P.; Scalzo, R.L.; Cattaneo, T.M.P. Near infrared spectroscopy in the supply chain monitoring of Annurca apple. J. Near Infrared Spectrosc. 2019, 27, 86–92. [Google Scholar] [CrossRef]
- Norris, K.H. Early history of near infrared for agricultural applications. NIR News 1992, 3, 12–13. [Google Scholar] [CrossRef]
- Cattano, T.M.P.; Stellari, A. Review: NIR spectroscopy as a suitable tool for the investigation of the horticultural field. Agronomy 2019, 9, 503. [Google Scholar] [CrossRef]
- Saeys, W.; Do Trong, N.N.; Van Beers, R.; Nicolai, B.M. Multivariate calibration of spectroscopic sensors for postharvest quality evaluation: A review. Postharvest Biol. Technol. 2019, 158, 110981. [Google Scholar] [CrossRef]
- Arsende, E.; Fawole, O.A.; Magwaza, L.S.; Opara, U.L. Non-destructive prediction of internal and external quality attributes of fruit with thick rind: A review. J. Food Eng. 2018, 217, 11–23. [Google Scholar] [CrossRef]
- Li, L.; Peng, Y.; Yang, C.; Li, Y. Optical sensing system for detection of the internal and external quality attributes of apples. Postharvest Biol. Technol. 2020, 162, 111101. [Google Scholar] [CrossRef]
- Lin, H.; Ying, Y. Theory and application of near infrared spectroscopy in assessment of fruit quality: A review. Sens. Instrumen. Food Qual. 2009, 3, 130–141. [Google Scholar] [CrossRef]
- Roberts, J.; Power, A.; Chapman, J.; Chandra, S.; Cozzolino, D. Vibrational spectroscopy methods for agro-food product analysis. Compr. Anal. Chem. 2018, 80, 51–68. [Google Scholar] [CrossRef]
- Cozzolino, D. Near infrared spectroscopy and food authenticity. In Advances in Food Traceability Techniques and Technologies: Improving Quality throughout the Food Chain; Espiñeira, M., Santaclara, F.J., Eds.; Elsevier: Cambridge, MA, USA, 2016; pp. 119–136. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. NIR spectral analysis of natural medicines supported by novel instrumentation, methods of data analysis and interpretation. J. Pharm. Biomed. Anal. 2020, 193, 113686. [Google Scholar] [CrossRef] [PubMed]
- Beć, K.B.; Grabska, J.; Huck, C.W. Infrared and near-infrared spectroscopic techniques for the quality control of herbal medicines. In Evidence-Based Validation of Herbal Medicine, 2nd ed.; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 603–627. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Miniaturized near-infrared spectroscopy in current analytical chemistry: From natural products to forensics. In Molecular and Laser Spectroscopy—Advances and Applications; Gupta, V.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 3, pp. 141–188. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Huck, C.W. Current and future applications of IR and NIR spectroscopy in ecology, environmental studies, wildlife and plant investigations. Compr. Anal. Chem. 2022, 98, 45–76. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-infrared (NIR) sensors in environmental analysis. In Encyclopedia of Sensors and Biosensors; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 4, pp. 484–503. [Google Scholar] [CrossRef]
- Chapman, J.; Truong, V.K.; Elbourne, A.; Gangadoo, S.; Cheeseman, S.; Rajapaksha, P.; Latham, K.; Crawford, R.J.; Cozzolino, D. Combining chemometrics and sensors: Toward new applications in monitoring and environmental analysis. Chem. Rev. 2020, 120, 6048–6069. [Google Scholar] [CrossRef]
- Brereton, R.G.; Jansen, J.; Lopes, J.; Marini, F.; Pomerantsev, A.; Rodionova, O.; Roger, J.M.; Walczak, B.; Tauler, R. Chemometrics in analytical chemistry—Part I: History, experimental design and data analysis tools. Anal. Bioanal. Chem. 2017, 409, 5891–5899. [Google Scholar] [CrossRef]
- Brereton, R.G.; Jansen, J.; Lopes, J.; Marini, F.; Pomerantsev, A.; Rodionova, O.; Roger, J.M.; Walczak, B.; Tauler, R. Chemometrics in analytical chemistry—Part II: Modeling, validation, and applications. Anal. Bioanal. Chem. 2018, 410, 6691–6704. [Google Scholar] [CrossRef] [PubMed]
- Mark, H. Principles and Practice of Spectroscopic Calibration; John Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Cocchi, M. Chapter 1—Introduction: Ways and means to deal with data from multiple sources. Data Handl. Sci. Technol. 2019, 31, 1–26. [Google Scholar] [CrossRef]
- Hayes, E.; Greene, D.; O’Donnell, C.; O’Shea, N.; Fenelon, M.A. Spectroscopic technologies and data fusion: Applications for the dairy industry. Front Nutr. 2022, 9, 1074688. [Google Scholar] [CrossRef]
- Castanedo, F. A review of data fusion techniques. Sci. World J. 2013, 2013, 704504. [Google Scholar] [CrossRef]
- Mendoza, F.A.; Lu, R.; Cen, H. Data Fusion of Visible/Near-Infrared Spectroscopy and Spectral Scattering for Apple Quality Assessment; An ASABE Meeting Presentation; The American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011. [Google Scholar] [CrossRef]
- Mendoza, F.; Lu, R.; Cen, H. Comparison and fusion of four nondestructive sensors for predicting apple fruit firmness and soluble solids content. Postharvest Biol. Technol. 2012, 73, 89–98. [Google Scholar] [CrossRef]
- Fathizadeh, Z.; Aboonajmi, M.; Hassan-Beygi, S.R. Nondestructive methods for determining the firmness of apple fruit flesh. Inf. Process. Agric. 2021, 8, 515–527. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Physical principles of infrared spectroscopy. Compr. Anal. Chem. 2022, 98, 1–43. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Principles and applications of miniaturized near-infrared (NIR) spectrometers. Chem. Eur. J. 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Siesler, H.W.; Huck, C.W. Handheld near-infrared spectrometers: Where are we heading? NIR News 2020, 31, 28–35. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Miniaturized NIR spectroscopy in food analysis and quality control. Promises, challenges and perspectives. Foods 2022, 11, 1465. [Google Scholar] [CrossRef]
- Grabska, J.; Beć, K.B.; Huck, C.W. Miniaturized Near-Infrared Spectroscopy—The Ultimate Analytical Tool in Food and Agriculture, Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Huber, M.R., III; Jänkälä, K. Portable near-infrared spectroscopy for analysis of crops. Cereal Foods World 2020, 65, 6. [Google Scholar] [CrossRef]
- Gowen, A.A.; O’Donnell, C.P.; Cullen, P.J.; Downey, G.; Frias, J.M. Hyperspectral imaging—An emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, Y.; Lu, R. Innovative hyperspectral imaging-based techniques for quality evaluation of fruits and vegetables: A review. Appl. Sci. 2017, 7, 189. [Google Scholar] [CrossRef]
- Sun, D.-W. Hyperspectral Imaging for Food Quality Analysis and Control; Academic Press: London, UK, 2010. [Google Scholar]
- Zhu, H.; Gowen, A.; Feng, H.; Yu, K.; Xu, J.-L. Deep spectral-spatial features of near infrared hyperspectral images for pixel-wise classification of food products. Sensors 2020, 20, 5322. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Y.; Sun, Y.; Zhou, H.; Chen, K. Identification of apple varieties using a multichannel hyperspectral imaging system. Sensors 2020, 20, 5120. [Google Scholar] [CrossRef] [PubMed]
- Mendez, J.; Mendoza, L.; Cruz-Tirado, J.P.; Quevedo, R.; Siche, R. Trends in application of NIR and hyperspectral imaging for food authentication. Sci. Agropecu. 2019, 10, 143–161. [Google Scholar] [CrossRef]
- Ozdogan, G.; Lin, X.; Sun, D.-W. Rapid and noninvasive sensory analyses of food products by hyperspectral imaging: Recent application developments. Trends Food Sci. Technol. 2021, 111, 151–165. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Issues in hyperspectral traceability of foods. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 3, pp. 258–289. [Google Scholar] [CrossRef]
- Kakani, V.; Nguyen, V.H.; Kumar, B.P.; Kim, H.; Pasupuleti, V.R. A critical review on computer vision and artificial intelligence in food industry. J. Agric. Res. 2020, 2, 100033. [Google Scholar] [CrossRef]
- Li, Y.; Feng, X.; Liu, Y.; Han, X. Apple quality identification and classification by image processing based on convolutional neural networks. Sci. Rep. 2021, 11, 16618. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, A.; Bansal, A. Fruits and vegetables quality evaluation using computer vision: A review. J. King Saud Univ. 2021, 33, 243–257. [Google Scholar] [CrossRef]
- Paulus, I.; De Busscher, R.; Schrevens, E. Linking internal and external quality-factors of apples, measured with non-destructive and destructive methods. Acta Hortic. 1998, 464, 79–84. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Quality evaluation of apples. In Computer Vision Technology for Food Quality Evaluation, 2nd ed.; Sun, D.-W., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 273–304. [Google Scholar] [CrossRef]
- Harker, F.R.; Gunson, F.A.; Jaeger, S.R. The case for fruit quality: An interpretive review of consumer attitudes, and preferences for apples. Postharvest Biol. Technol. 2003, 28, 333–347. [Google Scholar] [CrossRef]
- Costa, G.; Fiori, G.; Noferini, M.; Ziosi, V. Internal fruit quality: How to influence it, how to define it. Acta Hortic. 2006, 712, 339–346. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Z.; Jiang, S.; Wang, Y.; Fang, H.; Zhang, Z.; Chen, X.; Wang, N. Research progress on genetic basis of fruit quality traits in apple (Malus × domestica). Front Plant Sci. 2022, 13, 918202. [Google Scholar] [CrossRef] [PubMed]
- Argenta, L.C.; Talamini do Amarante, C.V.; Tonetto de Freitas, S.; Lays Brancher, T.; Nunes Nesi, C.; Mattheis, J.P. Fruit quality of ‘Gala’ and ‘Fuji’ apples cultivated under different environmental conditions. Sci. Hortic. 2022, 303, 111195. [Google Scholar] [CrossRef]
- Shewa, A.G.; Gobena, D.A.; Ali, M.K. Review on postharvest quality and handling of apple. J. Agric. Sci. Food Technol. 2022, 8, 028. [Google Scholar] [CrossRef]
- Glanz, K.; Basil, M.; Maibach, E.; Goldberg, J.; Snyder, D. Why Americans eat what they do: Taste, nutrition, cost, convenience, and weight control concerns as influences on food consumption. J. Am. Diet. Assoc. 1998, 98, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.C.; Liu, Y. Are beliefs stronger than taste? A field experiment on organic and local apples. Food Qual. Prefer. 2017, 61, 55–62. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Charles, M.; Aprea, E.; Gasperi, F. Factors influencing sweet taste in apple. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Sugiura, T.; Ogawa, H.; Fukuda, N.; Moriguchi, T. Changes in the taste and textural attributes of apples in response to climate change. Sci. Rep. 2013, 3, 2418. [Google Scholar] [CrossRef] [PubMed]
- Dimick, P.S.; Hoskin, J.C.; Acree, T.E. Review of apple flavor—State of the art. Crit. Rev. Food Sci. Nutr. 1983, 18, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhang, W.; Zhang, B.; Fang, T.; Wang, X.-F.; Cai, Y.; Ogutu, C.; Gao, L.; Chen, G.; Nie, X.; et al. Unraveling a genetic roadmap for improved taste in the domesticated apple. Molecular Plant 2021, 14, 1454–1471. [Google Scholar] [CrossRef]
- Chapman, J.; Elbourne, A.; Truong, V.K.; Gangadoo, S.; Rajapaksha Pathirannahalage, P.; Cheeseman, S.; Cozzolino, D. Sensomics—From conventional to functional NIR spectroscopy—Shining light over the aroma and taste of foods. Trends Food Sci. Technol. 2019, 91, 274–281. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Cho, R.K.; Sohn, M.R.; Kwon, Y.K. New observation of nondestructive evaluation for sweetness in apple fruit using near infrared spectroscopy. J. Near Infrared Spectrosc. 1998, 6, A75–A78. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Y.; Li, L.; Li, L. Nondestructive detection for forecasting the level of acidity and sweetness of apple based on NIR spectroscopy. In Proceedings of the 2017 IEEE 2nd Advanced Information Technology, Electronic and Automation Control Conference (IAEAC), Chongqing, China, 25–26 March 2017; pp. 1250–1257. [Google Scholar] [CrossRef]
- Sohn, M.-R.; Kwon, Y.-K.; Cho, R.-K. Development of robust calibration for determination sweetness of Fuji apple fruit using near infrared reflectance spectroscopy. Near Infrared Anal. 2001, 2, 55–58. [Google Scholar]
- Zhang, Y.; Chen, Y.; Ciu, C. Accurate and nondestructive detection of apple brix and acidity based on visible and near-infrared spectroscopy. Appl. Opt. 2021, 60, 4021–4028. [Google Scholar] [CrossRef] [PubMed]
- Abu-Khalaf, N.; Bennedsen, B.S. Near infrared (NIR) technology and multivariate data analysis for sensing taste attributes of apples. Int. Agrophysics 2004, 18, 203–211. [Google Scholar]
- Mehinagic, E.; Royer, G.; Bertrand, D.; Symoneaux, R.; Laurens, F.; Jourjon, F. Relationship between visible-NIR spectroscopy and sensory analysis of apples. Food Qual. Prefer. 2003, 14, 473–484. [Google Scholar] [CrossRef]
- Fuhrmann, E.; Grosch, W. Character impact odorants of the apple cultivars Elstar and Cox Orange. Mol. Nutr. Food Res. 2002, 46, 187–193. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E.W. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. 2000, 28, 155–173. [Google Scholar] [CrossRef]
- Echverria, G.; Fuentes, T.; Graell, J.; Lara, I.; Lopez, M.L. Aroma volatile compounds of ‘Fuji’ apples in relation to harvest date and cold storage technology: A comparison of two seasons. Postharvest Biol. Technol. 2004, 32, 29–44. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of Apple Aroma: A Review. Food Technol. Biotechnol. 2016, 54, 375–394. [Google Scholar] [CrossRef]
- Berger, R.G. (Ed.) Flavours and Fragrances. In Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Drkenda, P.; Culah, A.; Spaho, N.; Akagic, A.; Hudina, M. How Do consumers perceive sensory attributes of apple? Foods 2021, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.; Spadafora, N.D. Analysis of apple flavours: The use of volatile organic compounds to address cultivar differences and the correlation between consumer appreciation and aroma profiling. J. Food Qual. 2020, 2020, 8497259. [Google Scholar] [CrossRef]
- Wu, X.; Bi, J.; Fauconnier, M.-L. Characteristic volatiles and cultivar classification in 35 apple varieties: A case study of two harvest years. Foods 2022, 11, 690. [Google Scholar] [CrossRef]
- Chitarrini, G.; Dordevic, N.; Guerra, W.; Robatscher, P.; Lozano, L. Aroma investigation of new and standard apple varieties grown at two altitudes using gas chromatography-mass spectrometry combined with sensory analysis. Molecules 2020, 25, 3007. [Google Scholar] [CrossRef]
- Guo, J.; Yue, T.; Yuan, Y.; Sun, N.; Liu, P. Characterization of volatile and sensory profiles of apple juices to trace fruit origins and investigation of the relationship between the aroma properties and volatile constituents. LWT 2020, 124, 109203. [Google Scholar] [CrossRef]
- Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, Comparison and classification of volatile compounds in peels of 40 apple cultivars by HS–SPME with GC–MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Hampson, C.R.; Quamme, H.A.; Hall, J.W.; MacDonald, R.A.; King, M.C.; Cliff, M.A. Sensory evaluation as a selection tool in apple breeding. Euphytica 2000, 111, 79–90. [Google Scholar] [CrossRef]
- Martinez, A.; Hermandez, A.; Moraga, C.; Tejero, P.; de Guia Cordoba, M.; Martin, A. Detection of volatile organic compounds associated with mechanical damage in apple cv. ‘Golden Delicious’ by headspace solid-phase microextraction (HS-SPME) and GC-MS analysis. LWT 2022, 172, 114213. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z. Apple quality assessment by fusion three sensors. In Sensors; IEEE: Irvine, CA, USA, 2005; pp. 389–392. [Google Scholar] [CrossRef]
- Tahir, H.E.; Xiaobo, Z.; Jianbo, X.; Komla Mahunu, G.; Jiyong, S.; Xu, J.-L.; Sun, D.-W. Recent progress in rapid analyses of vitamins, phenolic, and volatile compounds in foods using vibrational spectroscopy combined with chemometrics: A review. Food Anal. Methods 2019, 12, 2361–2382. [Google Scholar] [CrossRef]
- Ye, M.; Gao, Z.; Li, Z.; Yuan, Y.; Yue, T. Rapid detection of volatile compounds in apple wines using FT-NIR spectroscopy. Food Chem. 2016, 190, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Yu, D.; Niu, Y.; Chen, F.; Song, S.; Zhu, J.; Zhu, G. Characterization of aroma compounds of Chinese famous liquors by gas chromatography-mass spectrometry and flash GC electronic-nose. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 945, 92–100. [Google Scholar] [CrossRef]
- Wu, X.; Fauconnier, M.-L.; Bi, J. Characterization and discrimination of apples by Flash GC E-Nose: Geographical regions and botanical origins studies in China. Foods 2022, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, M.; Zhang, J.; Ma, F. Prediction of apple hybrid offspring aroma based on hyperspectral. Foods 2022, 11, 3890. [Google Scholar] [CrossRef]
- Guine, R.P.F.; Andrade, S.; Correia, A.C.; Jordao, A.M.; Lopes, A.D.; Ferreira, D. Evaluation of textural properties in apples of regional varieties. Int. J. Food Prop. 2011, 14, 331–338. [Google Scholar] [CrossRef]
- Bejaei, M.; Stanich, K.; Cliff, M.A. Modelling and classification of apple textural attributes using sensory, instrumental and compositional analyses. Foods 2021, 10, 384. [Google Scholar] [CrossRef]
- Mureșan, A.E.; Sestras, A.F.; Militaru, M.; Păucean, A.; Tanislav, A.E.; Pușcaș, A.; Mateescu, M.; Mureșan, V.; Marc, R.A.; Sestras, R.E. Chemometric comparison and classification of 22 apple genotypes based on texture analysis and physico-chemical quality attributes. Horticulturae 2022, 8, 64. [Google Scholar] [CrossRef]
- Liu, H.; Lin, S.; Zhang, M.; Lv, Y.; Ma, Y.; Rao, J.; Zhu, Q. Changes in cell wall sugar neutral composition contribute to apple texture loss during storage among cultivars. Horticulturae 2023, 9, 292. [Google Scholar] [CrossRef]
- Dheilly, E.; Le Gall, S.; Guillou, M.-C.; Renou, J.-P.; Bonnin, E.; Orsel, M.; Lahaye, M. Cell wall dynamics during apple development and storage involves hemicellulose modifications and related expressed genes. BMC Plant Biol. 2016, 16, 201. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.M.; Aaby, K.; Sivertsen, H.; Baardseth, P.; Ellekjaer, M.R. Instrumental and sensory analysis of fresh Norwegian and imported apples. Food Qual. Prefer. 1999, 10, 305–314. [Google Scholar] [CrossRef]
- Evans, K.; Brutcher, L.; Konishi, B.; Barrit, B. Correlation of sensory analysis with physical textural data from a computerized penetrometer in the washington state university apple breeding program. HortTechnology 2010, 20, 1026–1029. [Google Scholar] [CrossRef]
- Kopacka, D.; Plocharski, W.J. Effect of storage conditions on the relationship between apple firmness and texture acceptability. Postharvest Biol. Technol. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Piazza, L.; Giovenzana, V. Instrumental acoustic-mechanical measures of crispness in apples. Int. Food Res. J. 2015, 69, 209–215. [Google Scholar] [CrossRef]
- Ropelewska, E. The application of image processing for cultivar discrimination of apples based on texture features of the skin, longitudinal section and cross-section. Eur. Food Res. Technol. 2021, 247, 1319–1331. [Google Scholar] [CrossRef]
- Letal, J.; Jirak, D.; Suderlova, L.; Hajek, M. MRI ‘texture’ analysis of MR images of apples during ripening and storage. LWT—Food Sci. Technol. 2003, 36, 719–727. [Google Scholar] [CrossRef]
- Hall, M.I.; Hallett, I.C.; Johnston, J.W. X-ray micro-computed tomography analysis of apple texture and structure. Acta Hortic. 2016, 1120, 535–538. [Google Scholar] [CrossRef]
- Li, D.; Shen, M.; Li, D.; Yu, X. Green apple recognition method based on the combination of texture and shape features. In Proceedings of the 2017 IEEE International Conference on Mechatronics and Automation (ICMA), Takamatsu, Japan, 6–9 August 2017; pp. 264–269. [Google Scholar] [CrossRef]
- Malvandi, A.; Kapoor, R.; Feng, H.; Kamruzzaman, M. Non-destructive measurement and real-time monitoring of apple hardness during ultrasonic contact drying via portable NIR spectroscopy and machine learning. Infrared Phys. Technol. 2022, 122, 104077. [Google Scholar] [CrossRef]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Texture of fresh fruit. Hortic. Rev. 2010, 20, 121–224. [Google Scholar] [CrossRef]
- Molina-Delgado, D.; Alegre, S.; Puy, J.; Recasens, I. Relationship between acoustic firmness and Magness Taylor firmness in royal gala and Golden Smoothee apples. Food Sci. Tech. Int. 2009, 15, 0031–0040. [Google Scholar] [CrossRef]
- Abbott, J.A.; Affeldt, H.A.; Liljedahl, L.A. Firmness measurement of stored ‘Delicious’ apples by sensory methods, Magness-Taylor, and sonic transmission. J. Am. Soc. Hortic. Sci. 1992, 117, 590–595. [Google Scholar] [CrossRef]
- Peleg, K. Comparison of non-destructive and destructive measurement of apple firmness. J. Agric. Eng. Res. 1993, 55, 227–238. [Google Scholar] [CrossRef]
- DeEll, J.R.; Khanizadeh, S.; Saad, F.; Ferree, C. Factors affecting apple fruit firmness: A review. J. Am. Pomol. Soc. 2001, 55, 8–27. [Google Scholar]
- Lu, R.; Guyer, D.E.; Beaudry, R.M. Determination of firmness and sugar content of apples using near-infrared diffuse reflectance. J. Texture Stud. 2000, 31, 615–630. [Google Scholar] [CrossRef]
- Fan, G.; Zha, J.; Du, R.; Gao, L. Determination of soluble solids and firmness of apples by Vis/NIR transmittance. J. Food Eng. 2009, 93, 416–420. [Google Scholar] [CrossRef]
- Sun, J.; Künnemeyer, R.; McGlone, A.; Rowe, P. Multispectral scattering imaging and NIR interactance for apple firmness predictions. Postharvest Biol. Technol. 2016, 119, 58–68. [Google Scholar] [CrossRef]
- Martínez, M.; Wulfsohn, D.; Toldam-Andersen, T. Non-invasive estimation of firmness in apple fruit using VIS/NIR spectroscopy. Acta Hortic. 2012, 934, 139–144. [Google Scholar] [CrossRef]
- Tu, Z.-H.; Ji, B.-P.; Meng, C.-Y.; Hu, D.-Z.; Shi, B.-L.; Qing, Z.-S. Analysis of NIR characteristic wavelengths for apple flesh firmness based on GA and iPLS. Guang Pu Xue Yu Guang Pu Fen Xi 2009, 29, 2760–2764. [Google Scholar]
- Blanke, M.M. Non-invasive Assessment of Firmness and NIR Sugar (TSS) Measurement in apple, pear and kiwi fruit. Erwerbs-Obstbau 2013, 55, 19–24. [Google Scholar] [CrossRef]
- Mendoza, F.; Lu, R.; Cen, H. Grading of apples based on firmness and soluble solids content using Vis/SWNIR spectroscopy and spectral scattering techniques. J. Food Eng. 2014, 125, 59–68. [Google Scholar] [CrossRef]
- Mareckova, M.; Dankova, V.; Zeleny, L.; Suran, P. Non-destructive near infrared spectroscopy externally validated using large number sets for creation of robust calibration models enabling prediction of apple firmness. J. Near Spectrosc. 2022, 30, 97–104. [Google Scholar] [CrossRef]
- Osienko, P.; Biegert, K.; McCormick, R.J.; Gohrt, T.; Devadze, G.; Steif, J.; Streif, S. Application of non-destructive sensors and big data analysis to predict physiological storage disorders and fruit firmness in ‘Braeburn’ apples. Comput. Electron. Agric. 2021, 183, 106015. [Google Scholar] [CrossRef]
- Vanoli, M.; Lovati, F.; Grassi, M.; Buccheri, M.; Zanella, A.; Cattaneo, T.M.P.; Rizzolo, A. Water spectral pattern as a marker for studying apple sensory texture. Adv. Hortic. Sci. 2018, 32, 343–352. [Google Scholar] [CrossRef]
- Ma, T.; Xia, Y.; Inagaki, T.; Tsuchikawa, S. Rapid and nondestructive evaluation of soluble solids content (SSC) and firmness in apple using Vis-NIR spatially resolved spectroscopy. Postharvest Biol. Technol. 2021, 173, 111417. [Google Scholar] [CrossRef]
- Fisher, D.V. Mealiness and quality of delicious apples affected by growing conditions, maturity and storage techniques. Sci. Agric. 1943, 23, 569–588. [Google Scholar] [CrossRef]
- Harker, F.R.; Hallett, I.C. Physiological changes associated with development of mealiness of apple during storage. HortScience 1992, 27, 1291–1294. [Google Scholar] [CrossRef]
- Corollaro, M.L.; Gasperi, F.; Corelli Grappadelli, L. An overview of sensory quality of apple fruit. J. Am. Pomol. Soc. 2014, 68, 141–157. [Google Scholar]
- Harker, F.R.; Maindonald, J.; Murray, S.H.; Gunson, F.A.; Hallet, I.C.; Walker, S.B. Sensory interpretation of instrumental measurements 1: Texture of apple fruit. Postharvest Biol. Technol. 2002, 24, 225–239. [Google Scholar] [CrossRef]
- Bechar, A.; Mizrach, A.; Barreiro, P.; Landahl, S. Determination of mealiness in apples using ultrasonic measurements. Biosyst. Eng. 2005, 91, 329–334. [Google Scholar] [CrossRef]
- Mollazade, K.; Arefi, A. Optical analysis using monochromatic imaging-based spatially-resolved technique capable of detecting mealiness in apple fruit. Sci. Hortic. 2017, 225, 589–598. [Google Scholar] [CrossRef]
- Arana, I.; Jaren, C.; Arazuri, S. Apple mealiness detection by non-destructive mechanical impact. J. Food Eng. 2004, 62, 399–408. [Google Scholar] [CrossRef]
- Barreiro, P.; Ortiz, C.; Ruiz-Altisent, M.; Ruiz-Cabello, J.; Fernández-Valle, M.E.; Recasens, I.; Asensio, M. Mealiness assessment in apples and peaches using MRI techniques. Magn. Reson. Imag. 2000, 18, 1175–1181. [Google Scholar] [CrossRef]
- Barreiro, P.; Moya, A.; Correa, E.; Ruiz-Altisent, M.; Fernandez-Valle, M.; Peirs, A.; Wright, K.M.; Hills, B.P. Prospects for the rapid detection of mealiness in apples by nondestructive NMR relaxometry. Appl. Magn. Reson. 2002, 22, 387–400. [Google Scholar] [CrossRef]
- Arefi, A.; Ahmadi Moghaddam, P.; Hassanpour, A.; Mollazade, K.; Modarres Motlagh, A. Non-destructive identification of mealy apples using biospeckle imaging. Postharvest Biol. Technol. 2016, 112, 266–276. [Google Scholar] [CrossRef]
- Huang, M.; Lu, R. Apple mealiness detection using hyperspectral scattering technique. Postharvest Biol. Technol. 2010, 58, 168–175. [Google Scholar] [CrossRef]
- Mehinagic, E.; Royer, G.; Symoneaux, R.; Bertrand, D.; Jourjon, F. Prediction of the sensory quality of apples by physical measurements. Postharvest Biol. Technol. 2004, 34, 257–269. [Google Scholar] [CrossRef]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Hyson, D.A. A Comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Kistechok, A.; Wrona, D.; Krupa, T. Quality and nutritional value of ‘Chopin’ and clone ‘JB’ in relation to popular apples growing in Poland. Agriculture 2022, 12, 1876. [Google Scholar] [CrossRef]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Verma, M.K.; Kumar Sharma, S. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef]
- Duxbury, M. Determination of minerals in apples by ICP-AES. J. Chem. Educ. 2003, 80, 1180. [Google Scholar] [CrossRef]
- Nkuimi Wandjou, J.G.; Sut, S.; Giuliani, C.; Fico, G.; Papa, F.; Ferraro, S.; Caprioli, G.; Maggi, F.; Dall’Acqua, S. Characterization of nutrients, polyphenols and volatile components of theancient apple cultivar ‘Mela Rosa Dei Monti Sibillini’ from Marche region, central Italy. Int. J. Food Sci. Nutr. 2019, 70, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Pissard, A.; Baeten, V.; Romnée, J.-M.; Dupont, P.; Mouteau, A.; Lateur, M. Classical and NIR measurements of the quality and nutritional parameters of apples: A methodological study of intra-fruit variability. Biotechnol. Agron. Soc. Environ. 2012, 16, 294–306. [Google Scholar]
- Pissard, A.; Bastiaanse, H.; Baeten, V.; Sinnaeve, G.; Romnee, J.M.; Dupont, P.; Mouteau, A.; Lateur, M. Use of NIR spectroscopy in an apple breeding program for quality and nutritional parameters. Acta Horticulturae 2013, 976, 409–414. [Google Scholar] [CrossRef]
- Pissard, A.; Fernander-Pierna, J.A.; Baeten, V.; Sinnaeve, G.; Longnay, G.; Mouteau, A.; Dupont, P.; Rondia, A.; Lateur, M. Non-destructive measurement of vitamin C, total polyphenol and sugar content in apples using near-infrared spectroscopy. J. Sci. Food Agric. 2013, 93, 238–244. [Google Scholar] [CrossRef]
- Cetin, N.; Saglam, C. Rapid detection of total phenolics, antioxidant activity and ascorbic acid of dried apples by chemometric algorithms. Food Biosci. 2022, 47, 101670. [Google Scholar] [CrossRef]
- Shoffe, Y.A.; Nock, J.F.; Zhang, Y.; Zhu, L.-W.; Watkins, C.B. Comparisons of mineral and non-mineral prediction methods for bitter pit in ‘Honeycrisp’ apples. Sci. Hortic. 2019, 254, 116–123. [Google Scholar] [CrossRef]
- Mogollon, M.R.; Contreras, C.; Tornetto de Freitas, S.; Zoffoli, J.P. NIR spectral models for early detection of bitter pit in asymptomatic ‘Fuji’ apples. Sci. Hortic. 2021, 280, 109945. [Google Scholar] [CrossRef]
- Kafle, G.K.; Khot, L.R.; Jarolmasjed, S.; Yongsheng, S.; Lewis, K. Robustness of near infrared spectroscopy based spectral features for non-destructive bitter pit detection in honeycrisp apples. Postharvest Biol. Technol. 2016, 120, 188–192. [Google Scholar] [CrossRef]
- Bonomelli, C.; Mogollon, R.; Tornetto de Freitas, S.; Zoffoli, J.P.; Contreras, C. Nutritional relationships in bitter pit-affected fruit and the feasibility of Vis-NIR models to determine calcium concentration in ‘Fuji’ apples. Agronomy 2020, 10, 1476. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic compounds: Introduction. In Natural Products; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Phenolic Compounds in Apple (Malus × domestica Borkh.): Compounds Characterization and Stability during Postharvest and after Processing. Antioxidants 2013, 2, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Ledawndowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Starowicz, M.; Achrem–Achremowicz, B.; Piskuła, M.K.; Zieliński, H. Phenolic compounds from apples: Reviewing their occurrence, absorption, bioavailability, processing, and antioxidant activity—A review. Pol. J. Food Nutr. Sci. 2020, 70, 321–336. [Google Scholar] [CrossRef]
- Kumari, S.; Manohar, S.; Kumari, P.; Krishnan, V.; Maheshwari, C.; Narwal, S.; Gupta, O.P.; Gowda, V.T.; Bansal, N.; Dahuja, A. The role of major phenolics in apple to total antioxidant capacity. In Apple Cultivation—Recent Advances; Küden, A.B., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.-H.; Jeong, M.-C.; Kim, S.S.; Miitchell, A.E.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early- to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Abukauskas, V.; Janulis, V.; Kviklys, D. Phenolic content and antioxidant activity in apples of the ‘Galaval’ cultivar grown on 17 different rootstocks. Antioxidants 2022, 11, 266. [Google Scholar] [CrossRef]
- Pissard, A.; Baeten, V.; Dardenne, P.; Dupont, P.; Lateur, M. Use of NIR spectroscopy on fresh apples to determine the phenolic compounds and dry matter content in peel and flesh. Biotechnol. Agron. Soc. Environ. 2018, 22, 3–12. [Google Scholar] [CrossRef]
- Beghi, R.; Spinardi, A.; Bodria, L.; Mignani, I.; Guidetti, R. Apples nutraceutic properties evaluation through a visible and near-infrared portable system. Food Bioprocess. Technol. 2013, 6, 2547–2554. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Singh, H.; Arif, K.M. Sensors and instruments for brix measurement: A review. Sensors 2022, 22, 2290. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Pan, Y.; Liu, Y. Nondestructive measurement soluble solids content of apple by portable and online near infrared spectroscopy. In Proceedings of the SPIE 7514, Photonics and Optoelectronics Meetings (POEM) 2009: Fiber Optic Communication and Sensors, Wuhan, China, 28 October 2009. 75140p. [Google Scholar] [CrossRef]
- Biegert, K.; Stockeler, D.; McCormick, R.J.; Braun, P. Modelling soluble solids content accumulation in ‘braeburn’ apples. Plants 2021, 10, 302. [Google Scholar] [CrossRef]
- Fan, S.; Li, J.; Xia, Y.; Tian, X.; Guo, Z.; Huang, W. Long-term evaluation of soluble solids content of apples with biological variability by using near-infrared spectroscopy and calibration transfer method. Postharvest Biol. Technol. 2019, 151, 79–87. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, W.; Peng, Y.; Chen, Q.; Ouyang, Q.; Zhao, J. Color compensation and comparison of shortwave near infrared and long wave near infrared spectroscopy for determination of soluble solids content of ‘Fuji’ apple. Postharvest Biol. Technol. 2016, 115, 81–90. [Google Scholar] [CrossRef]
- Alamar, M.C.; Bobelyn, E.; Lammertyn, J.; Nicolai, B.M.; Molto, E. Calibration transfer between NIR diode array and FT-NIR spectrophotometers for measuring the soluble solids contents of apple. Postharvest Biol. Technol. 2007, 45, 38–45. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, B.; Li, J.; Huang, W.; Wang, C. Effect of spectrum measurement position variation on the robustness of NIR spectroscopy models for soluble solids content of apple. Biosyst. Eng. 2016, 143, 9–19. [Google Scholar] [CrossRef]
- Luo, X.; Ye, Z.; Xu, H.; Zhang, D.; Bai, S.; Ying, Y. Robustness improvement of NIR-based determination of soluble solids in apple fruit by local calibration. Postharvest Biol. Technol. 2018, 139, 82–90. [Google Scholar] [CrossRef]
- Tian, X.; Fan, S.; Li, J.; Huang, W.; Chen, L. An optimal zone combination model for on-line nondestructive prediction of soluble solids content of apple based on full-transmittance spectroscopy. Biosyst. Eng. 2020, 197, 64–75. [Google Scholar] [CrossRef]
- Martinez Vega, M.V.; Sharifzadeh, S.; Wulfson, D.; Skov, T.; Harder Clemensen, L.; Toldam-Andersen, T.B. A sampling approach for predicting the eating quality of apples using visible–near infrared spectroscopy. J. Sci. Food Agric. 2013, 93, 3710–3719. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Liu, Y.; Tao, Y. Nondestructive quantification of the soluble-solids content and the available acidity of apples by Fourier-transform near-infrared spectroscopy. Appl. Opt. 2005, 44, 5224–5229. [Google Scholar] [CrossRef]
- Peirs, A.; Tirry, J.; Verlinden, B.; Darius, P.; Nicolai, B.M. Effect of biological variability on the robustness of NIR models for soluble solids content of apples. Postharvest Biol. Technol. 2003, 28, 269–280. [Google Scholar] [CrossRef]
- Sharma, D.D.; D’Souza, V.F.; McConnell, M.B.; Mazza, G. Identification and quantification of sugars in winter-hardy apples by high performance liquid chromatography. Can. Inst. Food Technol. J. 1988, 21, 435–437. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, Y.; Yu, H.; Fu, X. Comparison of the HPLC method and FT-NIR analysis for quantification of glucose, fructose, and sucrose in intact apple fruits. J. Agric. Food Chem. 2006, 54, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Morvai, M.; Molnár-Perl, I. Simultaneous gas chromatographic quantitation of sugars and acids in citrus fruits, pears, bananas, grapes, apples and tomatoes. Chromatographia 1992, 34, 502–504. [Google Scholar] [CrossRef]
- Füzfai, Z.; Katona, Z.F.; Kovács, E.; Molnár-Perl, I. Simultaneous identification and quantification of the sugar, sugar alcohol, and carboxylic acid contents of sour cherry, apple, and ber fruits, as their trimethylsilyl derivatives, by gas chromatography−mass spectrometry. J. Agric. Food Chem. 2004, 52, 7444–7452. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ying, Y.B. Measurement of sugar content in Fuji apples by FT-NIR spectroscopy. J. Zheijang Univ.-Sci. 2004, 5, 651–655. [Google Scholar] [CrossRef]
- Temma, T.; Hanamatsu, K.; Shinoki, F. Measuring the sugar content of apples and apple juice by near infrared spectroscopy. Opt. Rev. 2002, 9, 40–44. [Google Scholar] [CrossRef]
- Byun, S. Design of an integrated near-infrared Spectroscopy module for sugar content estimation of apples. Micromachines 2022, 13, 519. [Google Scholar] [CrossRef]
- Ying, Y.B.; Fu, X.; Lu, H. Experiments on predicting sugar content in apples by FT-NIR Technique. J. Food Eng. 2007, 80, 986–989. [Google Scholar] [CrossRef]
- Larson, J.E.; Perkins-Veazie, P.; Ma, G.; Kon, T.M. Quantification and prediction with near infrared spectroscopy of carbohydrates throughout apple fruit development. Horticulturae 2023, 9, 279. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ohutu, C.; Li, M.; Ma, F. Determination of predominant organic acid components in malus species: Correlation with apple domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef]
- Lamani, S.; Anu-Appaiah, K.A.; Murthy, H.N.; Dewir, Y.H.; Rikisahedew, J.J. Analysis of free sugars, organic acids, and fatty acids of wood apple (Limonia acidissima L.) fruit pulp. Horticulturae 2022, 8, 67. [Google Scholar] [CrossRef]
- Liu, Y.-D.; Ying, Y.-B.; Fu, X.-P. Prediction of valid acidity in intact apples with Fourier transform near infrared spectroscopy. J. Zhejiang Univ. Sci. B 2005, 6, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Peirs, A.; Scheerlinck, N.; Touchant, K.; Nicolai, B.M. PH—Postharvest Technology: Comparison of Fourier transform and dispersive near-infrared reflectance spectroscopy for apple quality measurements. Biosyst. Eng. 2002, 81, 305–311. [Google Scholar] [CrossRef]
- Peng, B.; Ge, N.; Ciu, L.; Zhao, H. Monitoring of alcohol strength and titratable acidity of apple wine during fermentation using near-infrared spectroscopy. LWT—Food Sci. Technol. 2016, 66, 86–92. [Google Scholar] [CrossRef]
- Sharabiani, V.R.; Sabzi, S.; Pourdarbani, R.; Solis-Carmona, E.; Hernández-Hernández, M.; Hernández-Hernández, J.L. Non-destructive prediction of titratable acidity and taste index properties of gala apple using combination of different hybrids ANN and PLSR-model based spectral data. Plants 2020, 9, 1718. [Google Scholar] [CrossRef] [PubMed]
- Nturambirwe, J.F.I.; Nieuwoudt, H.H.; Perold, W.J.; Opara, U.L. Non-destructive measurement of internal quality of apple fruit by a contactless NIR spectrometer with genetic algorithm model optimization. Sci. Afr. 2019, 3, e00051. [Google Scholar] [CrossRef]
- Pourdabani, R.; Sabzi, S.; Rohban, M.H.; Garcia-Mateos, G.; Paliwal, J.; Molina-Martinez, J.M. Using metaheuristic algorithms to improve the estimation of acidity in Fuji apples using NIR spectroscopy. Ain Shams Med. J 2022, 13, 101776. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, C.; Chen, Y.; Lu, C. Design and optimization of visible and near infrared nondestructive determination model for apple acidity. Chin. J. Quantum Electron. 2022, 39, 531. [Google Scholar]
- Hasanzadeh, B.; Abbaspour-Gilandeh, Y.; Soltani-Nazarloo, A.; De La Cruz-Gámez, E.; Hernández-Hernández, J.L.; Martínez-Arroyo, M. Non-destructive measurement of quality parameters of apple fruit by using visible/near-infrared spectroscopy and multivariate regression analysis. Sustainability 2022, 14, 14918. [Google Scholar] [CrossRef]
- Doerflinger, F.C.; Miller, W.B.; Nock, J.F.; Watkins, C.B. Relationships between starch pattern indices and starch concentrations in four apple cultivars. Postharvest Biol. Technol. 2015, 110, 86–95. [Google Scholar] [CrossRef]
- Brookfield, P.; Murphy, P.; Harker, R.; MacRae, E. Starch degradation and starch pattern indices; interpretation and relationship to maturity. Postharvest Biol. Technol. 1997, 11, 23–30. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B.; Li, H.; Shen, M.; Tian, S.; Zhang, H.; Ren, X.; Xing, L.; Zhao, J. Determination of bagged ‘Fuji’ apple maturity by visible and near-infrared spectroscopy combined with a machine learning algorithm. Infrared Phys. Technol. 2020, 111, 103529. [Google Scholar] [CrossRef]
- Bertone, E.; Venturello, A.; Leardi, R.; Geobaldo, F. Prediction of the optimum harvest time of ‘Scarlet’ apples using DR-UV–Vis and NIR spectroscopy. Postharvest Biol. Technol. 2012, 69, 15–23. [Google Scholar] [CrossRef]
- Pourdarbani, R.; Sabzi, S.; Kalantari, D.; Arribas, J.I. Non-destructive visible and short-wave near-infrared spectroscopic data estimation of various physicochemical properties of Fuji apple (Malus pumila) fruits at different maturation stages. Chemometr. Intell. Lab. Syst. 2020, 206, 104147. [Google Scholar] [CrossRef]
- Peirs, A.; Lammertyn, J.; Nicolaï, B.; De Baerdemaeker, J. Non-destructive quality measurements of apples by means of NIR-spectroscopy. Acta Hortic. 2000, 517, 435–440. [Google Scholar] [CrossRef]
- Pourdabani, R.; Sabzi, S.; Arribas, J.I. Nondestructive estimation of three apple fruit properties at various ripening levels with optimal Vis-NIR spectral wavelength regression data. Heliyon 2021, 7, e07942. [Google Scholar] [CrossRef]
- Menesatti, P.; Zanella, A.; D’Andrea, S.; Costa, C.; Paglia, G.; Pallottino, F. Supervised multivariate analysis of hyper-spectral NIR images to evaluate the starch index of apples. Food Bioprocess Technol. 2009, 2, 308–314. [Google Scholar] [CrossRef]
- Peirs, A.; Scheerlinck, N.; De Baerdemaeker, J.; Nicolaï, B.M. Starch index determination of apple fruit by means of a hyperspectral near infrared reflectance imaging system. J. Near Infrared Spectrosc. 2003, 11, 379–389. [Google Scholar] [CrossRef]
- Palmer, J.W.; Harker, F.R.; Tustin, D.S.; Johnston, J. Fruit dry matter concentration: A new quality metric for apples. J. Sci. Food Agric. 2010, 90, 2586–2594. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Lannard, B. Dry matter content association with time of on-tree maturation, quality at harvest, and changes in quality after controlled atmosphere storage for ‘Royal Gala’ apples. Can. J. Plant Sci. 2021, 101, 98–106. [Google Scholar] [CrossRef]
- Vieira, M.J.; Argenta, L.C.; Mattheis, J.P.; Talamini do Amarante, C.V.; Steffens, C.A. Relationship between dry matter content at harvest and maturity index and post-harvest quality of ‘Fuji’ apples. Rev. Bras Frutic. 2018, 40, e596. [Google Scholar] [CrossRef]
- Kovač, A.; Skendrović Babojelić, M.; Pavičić, N.; Voća, S.; Voća, N.; Dobričević, N.; Jagatić, A.M.; Šindrak, Z. Influence of harvest time and storage duration on “Cripps Pink” apple cultivar (Malus × domestica Borkh) quality parameters. J. Food 2010, 8, 1–6. [Google Scholar] [CrossRef]
- Peiris, K.H.S.; Dull, G.G.; Leffler, R.G.; Kays, S.J. Spatial variability of soluble solids or dry-matter content within individual fruits, bulbs, or tubers: Implications for the development and use of NIR spectrometric techniques. HortScience 1999, 34, 114–118. [Google Scholar] [CrossRef]
- Møller, S.M.; Travers, S.; Bertram, H.C.; Bertelsen, M.G. Prediction of postharvest dry matter, soluble solids content, firmness and acidity in apples (cv. Elshof) using NMR and NIR spectroscopy: A comparative study. Eur. Food Res. Technol. 2013, 237, 1021–1024. [Google Scholar] [CrossRef]
- Travers, S.; Bertelsen, M.G.; Kucheryavskiy, S.V. Predicting apple (cv. Elshof) postharvest dry matter and soluble solids content with near infrared spectroscopy. J. Sci. Food. Agric. 2014, 94, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nock, J.F.; Shoffle, Y.A.; Watkins, C.B. Non-destructive prediction of soluble solids and dry matter contents in eight apple cultivars using near-infrared spectroscopy. Postharvest Biol. Technol. 2019, 151, 111–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Nock, J.F.; Shoffe, Y.A.; Watkins, C.B. Non-destructive prediction of soluble solids and dry matter concentrations in apples using near-infrared spectroscopy. Acta Hortic. 2020, 1275, 341–348. [Google Scholar] [CrossRef]
- Harker, F.R.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- Norman, A.; Roding, M.; Wendin, K. Sustainable fruit consumption: The influence of color, shape and damage on consumer sensory perception and liking of diferent apples. Sustainability 2019, 11, 4626. [Google Scholar] [CrossRef]
- Bowen, A.J.; Blake, A.; Turecek, J.; Amyotte, B. External preference mapping: A guide for a consumer-driven approach to apple breeding. J. Sens. Stud. 2019, 34, e12472. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Antunez, L.; Ares, G.; Swaney-Stueve, M.; Jin, D.; Harker, F.R. Quality perceptions regarding external appearance of apples: Insights from experts and consumers in four countries. Postharvest Biol. Technol. 2018, 146, 99–107. [Google Scholar] [CrossRef]
- Lysiak, G.; Kurlus, R.; Zydlik, Z.; Walkowicz-Tomczak, D. Apple skin colour changes during harvest as an indicator of maturity. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–83. [Google Scholar]
- Kader, A.A. Fruit maturity, ripening, and quality relationships. Acta Hortic. 1999, 485, 203–208. [Google Scholar] [CrossRef]
- Dobrzański, B., Jr.; Rybczyński, R. Color as a quality factor of fruits and vegetables. In Physical Methods in Agriculture; Blahovec, J., Kutílek, M., Eds.; Springer: Boston, MA, USA, 2002. [Google Scholar] [CrossRef]
- Lancaster, J.E.; Grant, J.E.; Lister, C.E.; Taylor, M.C. Skin Color in Apples—Influence of copigmentation and plastid pigments on shade and darkness of red color in five genotypes. J. Am. Soc. Hortic. Sci. 1994, 119, 63–69. [Google Scholar] [CrossRef]
- Wang, W.; Celton, J.M.; Buck-Sorlin, G.; Balzergue, S.; Bucher, E.; Laurens, F. Skin color in apple fruit (Malus × domestica): Genetic and epigenetic insights. Epigenomes 2020, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yu, L.; Zhang, J.; Wang, N.; Chen, X. Research progress of fruit color development in apple (Malus domestica Borkh.). Plant Physiol. Biochem. 2021, 162, 267–279. [Google Scholar] [CrossRef]
- Yuri, J.A.; Moggia, C.; Sepulveda, A.; Poblete-Echeverría, C.; Valdés-Gómez, H.; Torres, C.A. Effect of cultivar, rootstock, and growing conditions on fruit maturity and postharvest quality as part of a six-year apple trial in Chile. Sci. Hortic. 2019, 253, 70–79. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, D.; Jia, W.; Ji, W.; Sun, Y. A Detection method for apple fruits based on color and shape features. IEEE Access 2019, 7, 67923–67933. [Google Scholar] [CrossRef]
- Fan, P.; Lang, G.; Yan, B.; Lei, X.; Guo, P.; Liu, Z.; Yang, F. A Method of segmenting apples based on gray-centered RGB color space. Remote Sens. 2021, 13, 1211. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, M.; He, D.; Long, Y.; Song, H. Recognition of green apples in an orchard environment by combining the GrabCut model and Ncut algorithm. Biosyst. Eng. 2019, 187, 201–213. [Google Scholar] [CrossRef]
- Kasajima, I. Measuring plant colors. Plant Biotechnol. 2019, 36, 63–75. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and carotenoid pigments in the peel and flesh of commercial apple fruit varieties. Int. Food Res. J. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Matsuoka, K. Anthocyanins in Apple Fruit and Their Regulation for Health Benefits. In Flavonoids—A Coloring Model for Cheering up Life; Badria, F.A., Ed.; IntechOpen Limited: London, UK, 2020. [Google Scholar] [CrossRef]
- Dar, J.A.; Wani, A.A.; Ahmed, M.; Nazir, R.; Zargar, S.M.; Javid, K. Peel colour in apple (Malus × domestica Borkh.): An economic quality parameter in fruit market. Sci. Hortic. 2019, 224, 50–60. [Google Scholar] [CrossRef]
- Pathare, P.B.; Opara, U.L.; Al-Said, F.A.J. Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess. Technol. 2013, 6, 36–60. [Google Scholar] [CrossRef]
- Kapoor, L.; Simkin, A.J.; George Priya Doss, C.; Siva, R. Fruit ripening: Dynamics and integrated analysis of carotenoids and anthocyanins. BMC Plant Biol. 2022, 22, 27. [Google Scholar] [CrossRef]
- Nagy, A.; Riczu, P.; Tamas, J. Spectral evaluation of apple fruit ripening and pigment content alteration. Sci. Hortic. 2016, 201, 256–264. [Google Scholar] [CrossRef]
- Solovchenko, A.; Chivkunova, O.; Gitelson, A.; Merzlyak, M. Non-destructive estimation pigment content ripening quality and damage in apple fruit with spectral reflectance in the visible range. Fresh Prod. 2010, 4, 91–102. [Google Scholar]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance spectral features and non-destructive estimation of chlorophyll, carotenoid and anthocyanin content in apple fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Lammertyn, J.; Peirs, A.; De Baerdemaeker, J.; Nicolai, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Ye, X.; Ishoioka, S.; Zhang, S. Estimation of the degree of red coloration in flesh of a red-fleshed apple cultivar ‘Kurenai no Yume’ with a UV–vis-NIR interactance device. Postharvest Biol. Technol. 2017, 124, 128–136. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Sabzi, S.; Hernández-Hernández, M.; Hernández-Hernández, J.L.; Azadshahraki, F. Nondestructive estimation of the chlorophyll b of apple fruit by color and spectral features using different methods of hybrid artificial neural network. Agronomy 2019, 9, 735. [Google Scholar] [CrossRef]
- Hirst, P.M. Advances in understanding flowering and pollination in apple trees. In Achieving Sustainable Cultivation of Apples; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 31, pp. 109–126. [Google Scholar] [CrossRef]
- Batjer, L.P.; Billingsley, H.D.; Westwood, M.N.; Rogers, B.L. Predicting harvest size of apples at different times during the growing season. Proc. Amer Soc. Hort. Sci. 1957, 70, 46–57. [Google Scholar]
- Webb, R.A.; Purves, J.V.; Beech, M.G. Size factors in apple fruit. Sci. Hortic. 1980, 13, 205–212. [Google Scholar] [CrossRef]
- Paulus, I.; Schrevens, E. Evaluating and modelling the size classification of apples. J. Agric. Eng. Res. 1999, 74, 411–419. [Google Scholar] [CrossRef]
- Marini, R.P. Estimating mean fruit weight and mean fruit value for apple trees: Comparison of two sampling methods with the true mean. J. Amer. Soc. Hort. Sci. 2001, 126, 503–510. [Google Scholar] [CrossRef]
- Marini, R.P.; Schupp, J.R.; Baugher, T.A.; Crassweller, R. Estimating apple fruit size distribution from early-season fruit diameter measurements. HortScience 2019, 54, 1947–1954. [Google Scholar] [CrossRef]
- Mirbod, O.; Choic, D.; Heinemann, P.H.; Marini, R.P.; He, L. On-tree apple fruit size estimation using stereo vision with deep learning-based occlusion handling. Biosyst. Eng. 2023, 226, 27–42. [Google Scholar] [CrossRef]
- Marini, R.P.; Schupp, J.R.; Baugher, T.A.; Crassweller, R. Relationships between fruit weight and diameter at 60 days after bloom and at harvest for three apple cultivars. HortScience 2019, 54, 86–91. [Google Scholar] [CrossRef]
- Marini, R.P.; Schupp, J.R.; Baugher, T.A.; Crassweller, R. Sampling apple trees to accurately estimate mean fruit weight and fruit size distribution. HortScience 2019, 54, 1017–1022. [Google Scholar] [CrossRef]
- Keshavarzpour, F.; Rashidi, M. Classification of apple size and shape based on mass and outer dimensions. Am.-Eurasian J. Agric. Environ. Sci. 2010, 9, 618–621. [Google Scholar]
- Naor, A.; Naschitz, S.; Gal, Y. Responses of apple fruit size to tree water status and crop load. Tree Physiol. 2008, 28, 1255–1261. [Google Scholar] [CrossRef]
- Kviklys, D.; Viskelis, J.; Lanauskas, J.; Uselis, N.; Liaudanskas, M.; Janulis, V. Effects of growth control on yield and fruit quality of the apple cultivar ‘Rubin’. Agric. Food Sci. 2020, 29, 257–264. [Google Scholar] [CrossRef]
- Karim, S.K.A.; Allan, A.C.; Schaffer, R.J.; David, K.M. Cell division controls final fruit size in three apple (Malus x domestica) cultivars. Horticulturae 2022, 8, 657. [Google Scholar] [CrossRef]
- Bain, J.M.; Robertson, R.N. The physiology of growth in apple fruits I. Cell size, cell number, and fruit development. Aust. J. Biol. Sci. 1951, 4, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Toldam-Andersen, T.B.; Hansen, P. Priority of fruit quality parameters based on differences in growing conditions. Acta Hortic. 1998, 466, 71–78. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, M.; Yao, J.; Zhang, Y.; Liu, Y. Study on the effect of apple size difference on soluble solids content model based on near-infrared (NIR) spectroscopy. J. Spectrosc. 2022, 2022, 3740527. [Google Scholar] [CrossRef]
- Jiang, X.; Zhu, M.; Yao, J.; Zhang, Y.; Liu, Y. Calibration of near infrared spectroscopy of apples with different fruit sizes to improve soluble solids content model performance. Foods 2022, 11, 1923. [Google Scholar] [CrossRef]
- Vaudelle, F.; L’Huillier, J.-P. Influence of the size and skin thickness of apple varieties on the retrieval of internal optical properties using Vis/NIR spectroscopy: A Monte Carlo-based study. Comput. Electron. Agric. 2015, 116, 137–149. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, J.; Zhnag, Z.; Zhao, J.; Zhang, Z.; Zhang, H. Effective modification through transmission Vis/NIR spectra affected by fruit size to improve the prediction of moldy apple core. Infrared Phys. Technol. 2019, 100, 117–124. [Google Scholar] [CrossRef]
- Kays, S.J. Postharvest Physiology of Perishable Plant Products; Springer: New York, NY, USA, 1991. [Google Scholar]
- Kays, S.J. Preharvest factors affecting appearance. Postharvest Biol. Technol. 1999, 15, 233–247. [Google Scholar] [CrossRef]
- Currie, A.; Ganeshanandam, S.; Noiton, D.; Shelbourne, C.J.A.; Oraguzie, N. Quantitative evaluation of apple (Malus × domestica Borkh.) fruit shape by principal component analysis of Fourier descriptors. Euphytica 2000, 111, 221–227. [Google Scholar] [CrossRef]
- Cheng, X.; Tao, Y.; Chen, R.; Luo, Y. Nir/mir dual–sensor machine vision system for online apple stem–end/calyx recognition. Trans. ASAE 2003, 46, 551–558. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, L.; Tao, Y. Automated 3D surface reconstruction and analysis of apple near-infrared data for the application of apple stem-end/calyx identification. In Proceedings of the 2007 ASAE Annual Meeting, Minneapolis, MN, USA, 17–20 June 2007. ASABE, Paper No. 073074. [Google Scholar]
- Wang, J.; Huo, Y.; Wang, Y.; Zhao, H.; Li, K.; Liu, L.; Shi, Y. Grading detection of “Red Fuji” apple in Luochuan based on machine vision and near-infrared spectroscopy. PLoS ONE 2022, 17, e0271352. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Gu, W. Computer vision based system for apple surface defect detection. Comput. Electron. Agric. 2002, 36, 215–223. [Google Scholar] [CrossRef]
- Wen, Z.; Tao, Y. Dual-camera NIR/MIR imaging for stem-end/calyx identification in apple defect sorting. Trans. ASAE 2000, 43, 449–452. [Google Scholar] [CrossRef]
- Bennedsen, B.S.; Peterson, D.L. Performance of a system for apple surface defect identification in near-infrared images. Biosyst. Eng. 2005, 90, 419–431. [Google Scholar] [CrossRef]
- Mehl, P.M.; Chen, Y.-R.; Kim, M.S.; Chan, D.E. Development of hyperspectral imaging technique for the detection of apple surface defects and contaminations. J. Food Eng. 2004, 61, 67–81. [Google Scholar] [CrossRef]
- Ariana, D.; Guyer, D.E.; Shrestha, B. Integrating multispectral reflectance and fluorescence imaging for defect detection on apples. Comput. Electron. Agric. 2006, 50, 148–161. [Google Scholar] [CrossRef]
- Codrea, M.C.; Nevalainen, O.S.; Tyystjärvi, E.; Vandeven, M.; Valcke, R. Classifying apples by the means of fluorescence imaging. Intern. J. Pattern Recognit. Artif. Intell. 2004, 18, 157–174. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, R. Non-destructive defect detection of apples by spectroscopic and imaging technologies: A review. Trans. ASABE 2017, 60, 1765–1790. [Google Scholar] [CrossRef]
- Tang, Y.; Bai, H.; Sun, L.; Wang, Y.; Hou, J.; Huo, Y.; Min, R. Multi-band-image based detection of apple surface defect using machine vision and deep learning. Horticulturae 2022, 8, 666. [Google Scholar] [CrossRef]
- Fan, S.X.; Li, J.B.; Zhang, Y.H.; Tian, X.; Wang, Q.Y.; He, X.; Zhang, C.; Huang, W.Q. On line detection of defective apples using computer vision system combined with deep learning methods. J. Food Eng. 2020, 286, 110102. [Google Scholar] [CrossRef]
- Fan, S.; Liang, X.; Huang, W.; Zhang, V.J.; Pang, Q.; He, X.; Li, L.; Zhang, C. Real-time defects detection for apple sorting using NIR cameras with pruning-based YOLOV4 network. Comput. Electron. Agric. 2022, 193, 106715. [Google Scholar] [CrossRef]
- Eisenstecken, D.; Sturz, B.; Robatscher, P.; Lozano, L.; Zanella, A.; Oberhuber, M. The potential of near infrared spectroscopy (NIRS) to trace apple origin: Study on different cultivars and orchard elevations. Postharvest Biol. Technol. 2019, 147, 123–131. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Wu, Y.; Lu, M.; Yang, Y.; Li, L. Apple variety identification using near-infrared spectroscopy. J. Spectrosc. 2018, 2018, 6935197. [Google Scholar] [CrossRef]
- Cortés, V.; Cubero, S.; Blasco, J.; Aleixos, N.; Talens, P. In-line application of visible and near-infrared diffuse reflectance spectroscopy to identify apple varieties. Food Bioprocess. Technol. 2019, 12, 1021–1030. [Google Scholar] [CrossRef]
- Eisenstecken, D.; Panarese, A.; Robatscher, P.; Huck, C.W.; Zanella, A.; Oberhuber, M. A Near infrared spectroscopy (NIRS) and chemometric approach to improve apple fruit quality management: A case study on the cultivars “Cripps Pink” and “Braeburn”. Molecules 2015, 20, 13603–13619. [Google Scholar] [CrossRef]
- Luo, W.; Huan, S.; Fu, H.; Wen, G.; Cheng, H.; Zhou, J.; Wu, H.; Shen, G.; Yu, R. Preliminary study on the application of near infrared spectroscopy and pattern recognition methods to classify different types of apple samples. Food Chem. 2011, 128, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, B.; Sun, J.; Li, M.; Du, H. Discrimination of apples using near infrared spectroscopy and sorting discriminant analysis. Int. J. Food Prop. 2016, 19, 1016–1028. [Google Scholar] [CrossRef]
- Wu, X.; Wu, B.; Sun, J.; Yang, N. Classification of apple varieties using near infrared reflectance spectroscopy and fuzzy discriminant c-means clustering model. J. Food Process Eng. 2017, 40, e12355. [Google Scholar] [CrossRef]
- Song, W.; Wang, H.; Maguire, P.; Nibouche, O. Differentiation of organic and non-organic apples using near infrared reflectance spectroscopy—A pattern recognition approach. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar] [CrossRef]
- Li, M.; Cao, J.; Lu, L. Apple NIR spectral classification method. In Proceedings of the 2015 International Conference on Electrical, Computer Engineering and Electronics, Jinan, China, 29–31 May 2015. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, X.; Wu, B.; Zhou, H. Detection of apple varieties by near-infrared reflectance spectroscopy coupled with SPSO-PFCM. J. Food Process Eng. 2022, 45, e13993. [Google Scholar] [CrossRef]
- Huck-Pezzei, V.A.; Seitz, I.; Karer, R.; Schmutzler, M.; de Benedictis, L.; Wild, B.; Huck, C.W. Alps food authentication, typicality and intrinsic quality by near infrared spectroscopy. Food Res. Int. 2014, 62, 984–990. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Shao, Y. Fast discrimination of apple varieties using Vis/NIR spectroscopy. Int. J. Food Prop. 2007, 10, 9–18. [Google Scholar] [CrossRef]
- Bobelyn, E.; Serban, A.-S.; Nicu, M.; Lammertyn, J.; Nicolaï, B.M.; Saeys, W. Postharvest quality of apple predicted by NIR-spectroscopy. Study of the effect of biological variability on spectra and model performance. Postharv. Biol. Technol. 2010, 55, 133–143. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Z.; Zou, C.; Jiang, S.; El-Seedi, H.R.; Zou, X. General model of multi-quality detection for apple from different origins by Vis/NIR transmittance spectroscopy. Food Meas. 2022, 16, 2582–2595. [Google Scholar] [CrossRef]
- Tian, G.; Li, X.; Zhang, B.; Zhou, J.; Gu, B. Comparative study of two different strategies for determination of soluble solids content of apples from multiple geographical regions by using FT-NIR spectroscopy. IEEE Access 2019, 7, 179734–179745. [Google Scholar] [CrossRef]
- Schmutzler, M.; Huck, C.W. Automatic sample rotation for simultaneous determination ofgeographical origin and quality characteristics of apples based on nearinfrared spectroscopy (NIRS). Vib. Spectrosc. 2014, 72, 97–104. [Google Scholar] [CrossRef]
- Gonçalves, M.W.; Argenta, L.C.; Schlichting de Martin, M. Maturity and quality of apple fruit durinig the harvest period at apple industry. Rev. Bras. Frutic. 2017, 39, e-825. [Google Scholar] [CrossRef]
- Warrington, I.J.; Fulton, T.A.; Halligan, E.A.; de Silva, H.N. Apple fruit growth and maturity are affected by early season temperatures. J. Am. Soc. Hortic. Sci. 1999, 124, 468–477. [Google Scholar] [CrossRef]
- Knee, M.; Hatfield, S.G.S.; Smith, S.M. Evaluation of various indicators of maturity for harvest of apple fruit intended for long-term storage. J. Hortic. Sci. 1989, 64, 403–411. [Google Scholar] [CrossRef]
- Kingston, C.M. Maturity indicies for apple and pear. In Horticultural Reviews; Janik, J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1992; Volume 13. [Google Scholar]
- Beaudry, R.; Schwallir, P.; Lennington, M. Apple maturity prediction: An extension tool to aid fruit storage decisions. HortTechnology 1993, 3, 233–239. [Google Scholar] [CrossRef]
- Young, H.; Rossiter, K.; Wang, M.; Miller, M. Characterization of Royal Gala apple aroma using electronic nose technologypotential maturity indicator. J. Agric. Food Chem. 1999, 47, 5173–5177. [Google Scholar] [CrossRef]
- Mehinagic, E.; Royer, G.; Symoneaux, R.; Jourjon, F.; Prost, C. Characterization of odor-active volatiles in apples: Influence of cultivars and maturity stage. J. Agric. Food Chem. 2006, 54, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shen, M.; Pu, Y.; Li, H.; Zhang, B.; Zhang, Z.; Ren, X.; Zhao, J. Rapid identification of apple maturity based on multispectral sensor combined with spectral shape features. Horticulturae 2022, 8, 361. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Zeb, A.; Qureshi, W.S.; Arslan, M.; Malik, A.U.; Alasmary, W.; Alanazi, E. Towards fruit maturity estimation using NIR spectroscopy. Infrared Phys. Technol. 2020, 111, 103479. [Google Scholar] [CrossRef]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of near-infrared (NIR) spectroscopy and hyperspectral imaging for quality and safety assessment of fruits: An overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Peirs, A.; Touchant, K.; Schenk, A.; Nicolaï, B. FT-NIR spectroscopy to evaluate picking date of apples. Acta Hortic. 2001, 553, 477–480. [Google Scholar] [CrossRef]
- Walsh, K.B.; McGlone, V.A.; Han, D.H. The uses of near infra-red spectroscopy in postharvest decision support: A review. Postharvest Biol. Technol. 2020, 163, 111139. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, C.; Yang, H.; Jiang, H.; Li, L.; Yang, G. Non-destructive and in-site estimation of apple quality and maturity by hyperspectral imaging. Comput. Electron. Agric. 2022, 195, 106843. [Google Scholar] [CrossRef]
- Lu, Y.; Saeys, W.; Kim, M.; Peng, Y.; Lu, R. Hyperspectral imaging technology for quality and safety evaluation of horticultural products: A review and celebration of the past 20-year progress. Postharvest Biol. Technol. 2020, 170, 111318. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Sun, D.-W. Innovative nondestructive imaging techniques for ripening and maturity of fruits—A review of recent applications. Trends Food Sci. Technol. 2018, 72, 144–152. [Google Scholar] [CrossRef]
- Kevers, C.; Pincemail, J.; Tabart, J.; Defraigne, J.-O.; Dommes, J. Influence of cultivar, harvest time, storage conditions, and peeling on the antioxidant capacity and phenolic and ascorbic acid contents of apples and pears. J. Agric. Food Chem. 2011, 59, 6165–6171. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Ma, C.; Sun, K.; Liu, Q.; Zhao, N.; Sun, Y.; Tu, K.; Pan, L. Relationship between optical properties and soluble sugar contents of apple flesh during storage. Postharvest Biol. Technol. 2020, 159, 111021. [Google Scholar] [CrossRef]
- Dobrzanski, B.; Rybczynski, R. Colour change of apple as a result of storage, shelf-life, and bruising. Int. Agrophysics 2002, 16, 261–268. [Google Scholar]
- Batkan, A.; Kundakci, A.; Ergonul, B. Effect of holding period prior to storage on the chemical attributes of Starking Delicious apples during refrigerated storage. Food Sci. Technol. 2012, 32, 223–227. [Google Scholar] [CrossRef]
- Thompson, A.K.; Prange, R.K.; Bancroft, R.; Puttongsiri, T. Controlled Atmosphere Storage of Fruit and Vegetables, 3rd ed.; CABI: Wallingford, UK, 2018. [Google Scholar]
- Jan, I.; Rab, A. Influence of storage duration on physico-chemical changes in fruit of apple cultivars. J. Anim. Plant Sci. 2012, 22, 708–714. [Google Scholar]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Zanella, A.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Shen, M.; Li, H.; Zhang, B.; Zhang, M.; Pu, Y.; Chen, A.; Zhao, J. Study on quality model of apple during controlled atmosphere storage based on VIS/NIR spectroscopy. In Proceedings of the 2021 ASABE Annual International Virtual Meeting, Online, 12–16 July 2021. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.M.; Agyekum, A.A.; Wu, J.; Chen, Q.; Zuo, M.; El-Seedi, H.-R.; Tao, F.; Shi, J.; Ouyang, Q.; et al. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Yang, X.; Cai, Z.; Li, J. Online analysis of watercore apples by considering different speeds and orientations based on Vis/NIR full-transmittance spectroscopy. Infrared Phys. Technol. 2022, 122, 104090. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Cai, Z.; Fan, S.; Zhang, H.; Zhang, Q.; Li, J. Online detection of watercore apples by Vis/NIR full-transmittance spectroscopy coupled with ANOVA method. Foods 2021, 10, 2983. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, R.; Chen, K. Detection of internal defect of apples by a multichannel Vis/NIR spectroscopic system. Postharvest Biol. Technol. 2020, 161, 111065. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zhang, Q.; Duan, D.; Chen, L. Establishment of a multi-position general model for evaluation of watercore and soluble solid content in ‘Fuji’ apples using on-line full-transmittance visible and near infrared spectroscopy. J. Food Compost Anal. 2023, 117, 105150. [Google Scholar] [CrossRef]
- Giovanelli, G.; Sinelli, N.; Beghi, R.; Guidetti, R.; Casiraghi, E. NIR spectroscopy for the optimization of postharvest apple management. Postharvest Biol. Technol. 2014, 87, 13–20. [Google Scholar] [CrossRef]
- Liu, F.; Tang, X. Fuji apple storage time rapid determination method using Vis/NIR spectroscopy. Bioengineered 2015, 6, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, M.; Shen, M.; Li, H.; Zhang, Z.; Zhang, H.; Zhou, Z.; Ren, X.; Ding, Y.; Xing, L.; et al. Quality monitoring method for apples of different maturity under long-term cold storage. Infrared Phys. Technol. 2021, 12, 103580. [Google Scholar] [CrossRef]
- Ignat, T.; Lurie, S.; Nyasordzi, J.; Ostrovsky, V.; Egozi, H.; Hoffman, A.; Friedman, H.; Weksler, A.; Schmilovitch, Z. Forecast of apple internal quality indices at harvest and during storage by VIS-NIR spectroscopy. Food Bioprocess. Technol. 2014, 7, 2951–2961. [Google Scholar] [CrossRef]
- Beghi, R.; Giovanelli, G.; Malegori, C.; Giovenzana, V.; Guidetti, R. Testing of a VIS-NIR System for the Monitoring of Long-Term Apple Storage. Food Bioprocess. Technol. 2014, 7, 2134–2143. [Google Scholar] [CrossRef]
- Kumar, S.; McGlone, A.; Whitworth, C.; Volz, R. Postharvest performance of apple phenotypes predicted by near-infrared (NIR) spectral analysis. Postharvest Biol. Technol. 2015, 100, 16–22. [Google Scholar] [CrossRef]
- Camps, C.; Guillermin, P.; Mauget, J.C.; Bertrand, D. Discrimination of storage duration of apples stored in a cooled room and shelf-life by visible-near infrared spectroscopy. J. Near Infrared Spectrosc. 2007, 15, 169–177. [Google Scholar] [CrossRef]
- Khatiwada, B.P.; Subedi, P.P.; Hayes, C.; Cunha Carlos Jnr, L.C.; Walsh, K.B. Assessment of internal flesh browning in intact apple using visible-short wave near infrared spectroscopy. Postharvest Biol. Technol. 2016, 120, 103–111. [Google Scholar] [CrossRef]
- Clark, C.J.; mcGlone, V.A.; Jordan, R.B. Detection of Brownheart in ‘Braeburn’ apple by transmission NIR spectroscopy. Postharvest Biol. Technol. 2003, 28, 87–96. [Google Scholar] [CrossRef]
- Li, G.F. Nondestructive measurement model of apple internal browning based on FT-NIR spectroscopy. Adv. Mat. Res. 2011, 304, 316–321. [Google Scholar] [CrossRef]
- Mogollon, M.R.; Jara, A.F.; Contreras, C.; Zoffoli, J.P. Quantitative and qualitative VIS-NIR models for early determination of internal browning in ‘Cripps Pink’ apples during cold storage. Postharvest Biol. Technol. 2020, 161, 111060. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Shujat, A.; Wu, J.; El-Seedi, H.R.; Shi, J.; Ouyang, Q.; Chen, Q.; Zou, X. Nondestructive monitoring storage quality of apples at different temperatures by near-infrared transmittance spectroscopy. Food Sci. Nutr. 2020, 8, 3793–3805. [Google Scholar] [CrossRef]
- Lurier, S.; Watkins, C.B. Superficial scald, its etiology and control. Postharvest Biol. Technol. 2012, 65, 44–60. [Google Scholar] [CrossRef]
- Zanella, A. Control of apple superficial scald and ripening—A comparison between 1-methylcyclopropene and diphenylamine postharvest treatments, initial low oxygen stress and ultra low oxygen storage. Postharvest Biol. Technol. 2003, 27, 69–78. [Google Scholar] [CrossRef]
- Wang, Z.; Dilley, D.R. Initial low oxygen stress controls superficial scald of apples. Postharvest Biol. Technol. 2000, 18, 201–213. [Google Scholar] [CrossRef]
- Rudell, D.R.; Mattheis, J.P.; Hertog, M.L.A.T.M. Metabolomic change precedes apple superficial scald symptoms. J. Agric. Food Chem. 2009, 57, 8459–8466. [Google Scholar] [CrossRef] [PubMed]
- Rudell, D.R.; Mattheis, J.P. Superficial scald development and related metabolism is modified by postharvest light irradiation. Postharvest Biol. Technol. 2009, 51, 174–182. [Google Scholar] [CrossRef]
- Costa, F.; Zanella, A.; Beć, K.B.; Biasioli, F.; Busatto, N.; Cappellin, L.; Chitarrini, G.; Farneti, B.; Folie, I.; Grabska, J.; et al. Scald-Cold: Joint Austrian-Italian consortium in the Euregio project for the comprehensive dissection of the superficial scald in apples. NIR News 2020, 31, 5–9. [Google Scholar] [CrossRef]
- Costa, F.; Zanella, A.; Huck, C.W.; Busatto, N.; Populin, F.; Stürz, S.; Folie, I.; Biasioli, F.; Farneti, B.; Vrhovsek, U.; et al. “Scald-Cold”: Comprehensive dissection of the superficial scald in apple. Acta Hortic. 2022, 1344, 7–12. [Google Scholar] [CrossRef]
- Zanella, A.; Stürz, S.; Sadar, N.; Ebner, I. Non-destructive prediction of scald susceptibility by means of near-infrared spectroscopy. Acta Hortic. 2021, 1311, 179–184. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Dedicated non-destructive devices for food quality measurement: A review. Trends Food Sci. Technol. 2018, 78, 197–205. [Google Scholar] [CrossRef]
- Lei, Y.; Liu, C.L.; Zhou, Z.Y. Rapid analysis model of apple sugar degree using portable near-infrared spectrometer. J. Food Sci. Technol. 2018, 36, 95–100. [Google Scholar]
- Yang, F.; Li, Y.-T.; Gu, X.; Ma, J.; Fan, X.; Wang, X.-X.; Zhang, Z.-Z. Determination of acidity and vitamin C in apples using portable NIR analyzer. Guang Pu Xue Yu Guang Pu Fen Xi 2011, 31, 2386–2389. [Google Scholar] [PubMed]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D.; Khoshtaghaza, M.H. Rapid measurement of apple quality parameters using wavelet de-noising transform with Vis/NIR analysis. Sci. Hortic. 2019, 252, 7–13. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Y.; Yang, Y.; Mu, B.; Nikitina, M.A.; Xiao, X. Vis/NIR optical biosensors applications for fruit monitoring. Biosens. Bioelectron. X 2022, 11, 100197. [Google Scholar] [CrossRef]
- Liu, W.; Han, Y.; Wang, N.; Zhang, Z.; Wang, Q.; Miao, Y. Apple sugar content non-destructive detection device based on near-infrared multi-characteristic wavelength. J. Phys. Conf. Ser. 2022, 2221, 012012. [Google Scholar] [CrossRef]
- Rateni, G.; Dario, P.; Cavallo, F. Smartphone-based food diagnostic technologies: A review. Sensors 2017, 17, 1453. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ding, C.; Feng, Z.; Ji, S.; Cui, D. Recent advances in portable devices for fruit firmness assessment. Crit. Rev. Food Sci. Nutr. 2023, 63, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Guo, Z.; Zhang, B.; Hunag, W.; Zhao, C. Using Vis/NIR diffuse transmittance spectroscopy and multivariate analysis to predicate soluble solids content of apple. Food Anal. Methods 2016, 9, 1333–1343. [Google Scholar] [CrossRef]
- Xu, X.; Mo, J.; Xie, L.; Ying, Y. Influences of detection position and double detection regions on determining soluble solids content (SSC) for apples using on-line visible/near-infrared (Vis/NIR) spectroscopy. Food Anal. Methods 2019, 12, 2078–2085. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Liu, L.; Liu, Y. Nondestructive measurement of internal quality attributes of apple fruit by using NIR spectroscopy. Multimed. Tools Appl. 2019, 78, 4179–4195. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Wang, Z.; Liu, S.; He, X.; Fan, S. Calibration transfer between developed portable Vis/NIR devices for detection of soluble solids contents in apple. Postharvest Biol. Technol. 2022, 183, 111720. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, J.; Zhang, Q.; Liu, J.; Meng, Y.; Yu, Y. Nondestructive determination of SSC in an apple by using a portable near-infrared spectroscopy system. Appl. Opt. 2022, 61, 3419–3428. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Wang, Q.; Tian, X.; Yang, G.; Xia, Y.; Li, J.; Huang, W. Non-destructive evaluation of soluble solids content of apples using a developed portable Vis/NIR device. Biosyst. Eng. 2020, 193, 138–148. [Google Scholar] [CrossRef]
- Malvandi, A.; Feng, H.; Kamruzzaman, M. Application of NIR spectroscopy and multivariate analysis for Non-destructive evaluation of apple moisture content during ultrasonic drying. Spectrochim. Acta A 2022, 269, 120733. [Google Scholar] [CrossRef] [PubMed]
- Paz, P.; Sanchez, M.-T.; Perez-Marin, D.; Guerrero, J.-E.; Garrido-Varo, A. Evaluating NIR instruments for quantitative and qualitative assessment of intact apple quality. J. Sci. Food Agric. 2009, 89, 781–790. [Google Scholar] [CrossRef]
- Kaur, H.; Künnemeyer, R.; McGlone, A. Comparison of hand-held near infrared spectrophotometers for fruit dry matter assessment. J. Near Infrared Spectrosc. 2017, 25, 267–277. [Google Scholar] [CrossRef]
- Pissard, A.; Nascimento Marques, E.J.; Dardenne, P.; Lateur, M.; Pasquini, C.; Pimentel, M.F.; Fernández Pierna, J.A.; Baeten, V. Evaluation of a handheld ultra-compact NIR spectrometer for rapid and non-destructive determination of apple fruit quality. Postharvest Biol. Technol. 2021, 172, 111375. [Google Scholar] [CrossRef]
- Schmutzler, M.; Huck, C.W. Simultaneous detection of total antioxidant capacity and total soluble solids content by Fourier transform near-infrared (FT-NIR) spectroscopy: A quick and sensitive method for on-site analyses of apples. Food Control 2016, 66, 27–37. [Google Scholar] [CrossRef]
- Workmann, J.J., Jr. A review of calibration transfer practices and instrument differences in spectroscopy. Appl. Spectrosc. 2018, 72, 340–365. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Jiang, X.; Liu, Y. A Standard-free calibration transfer strategy for a discrimination model of apple origins based on near-infrared spectroscopy. Agriculture 2022, 12, 366. [Google Scholar] [CrossRef]


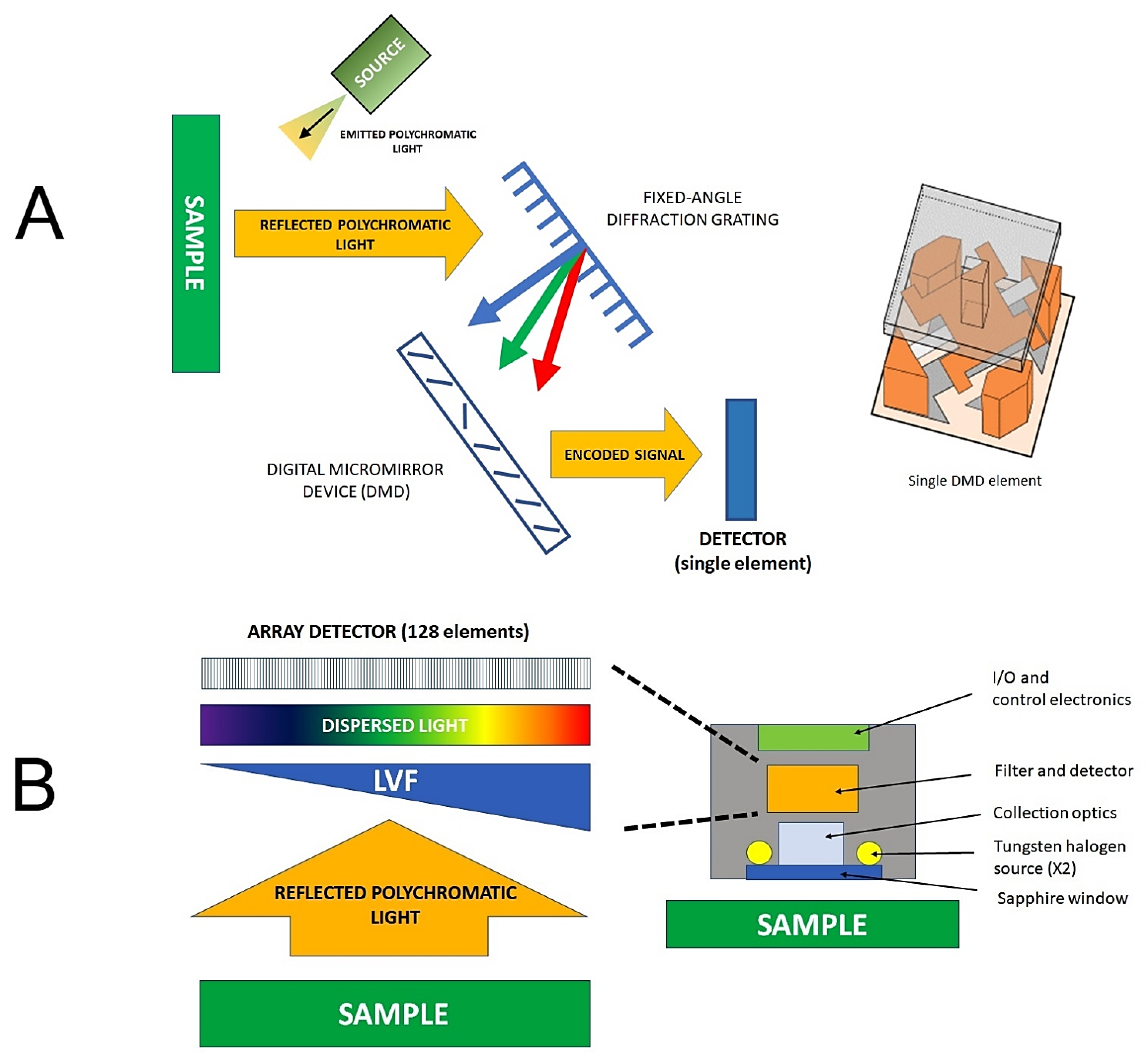
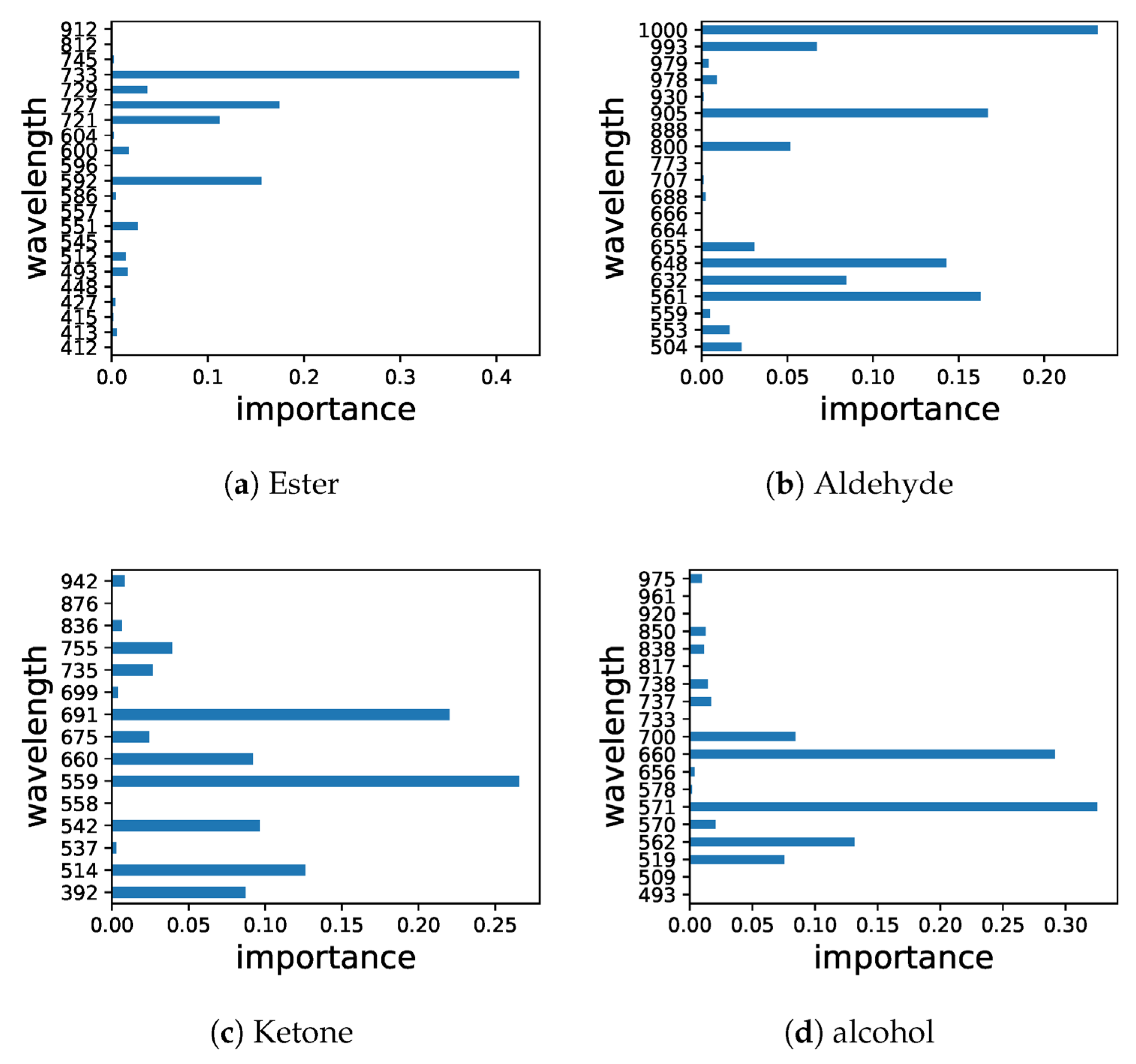
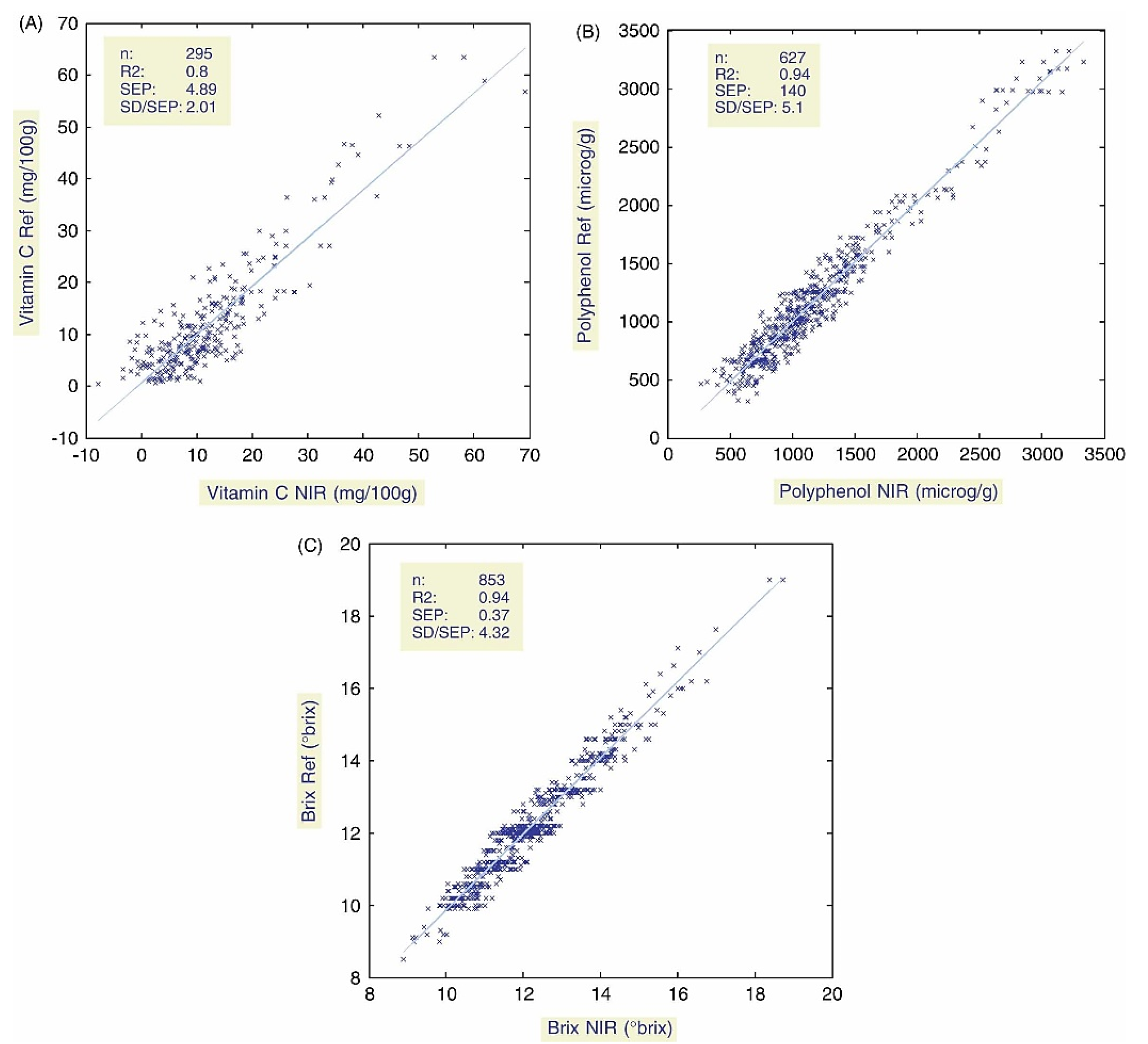
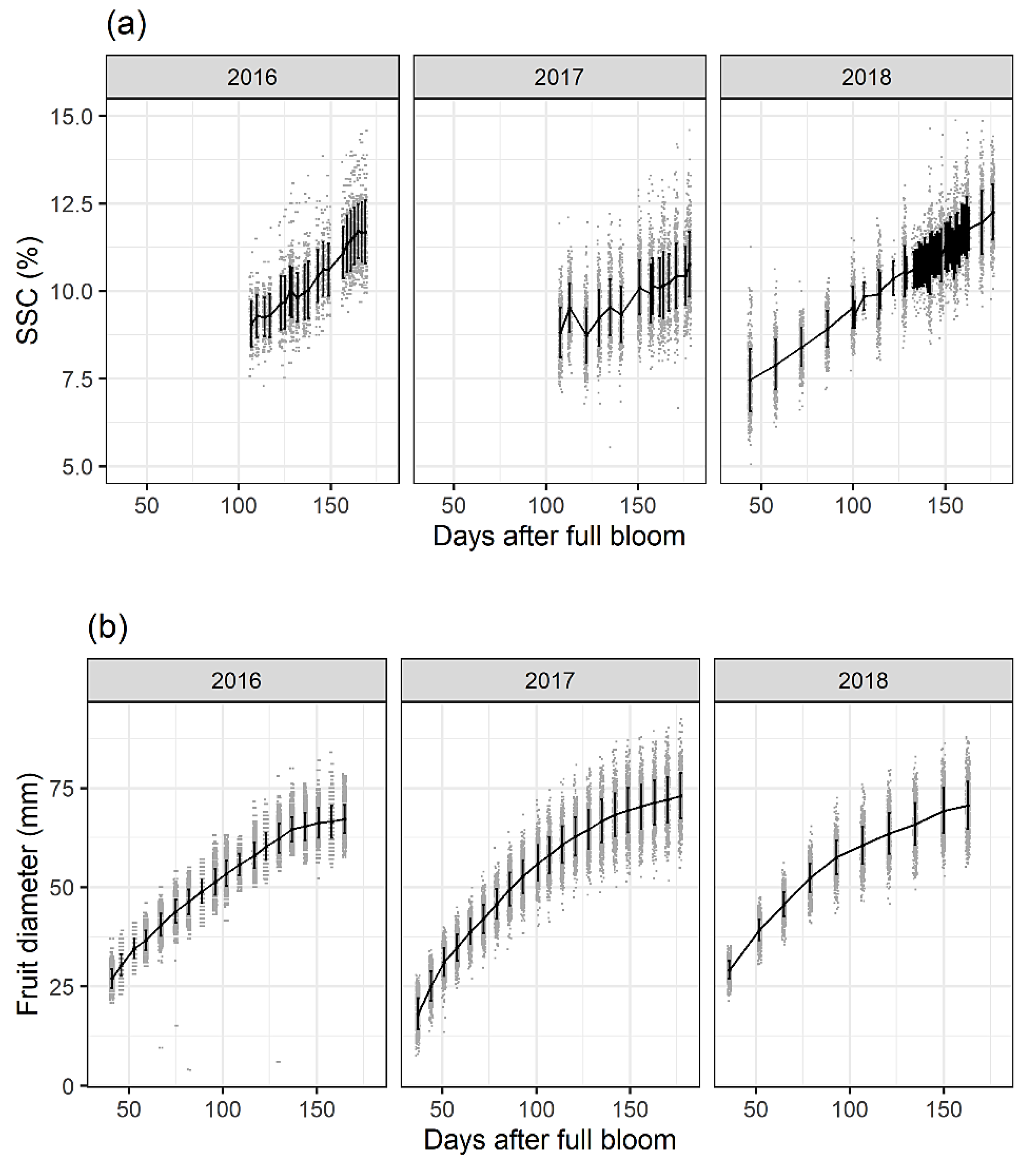
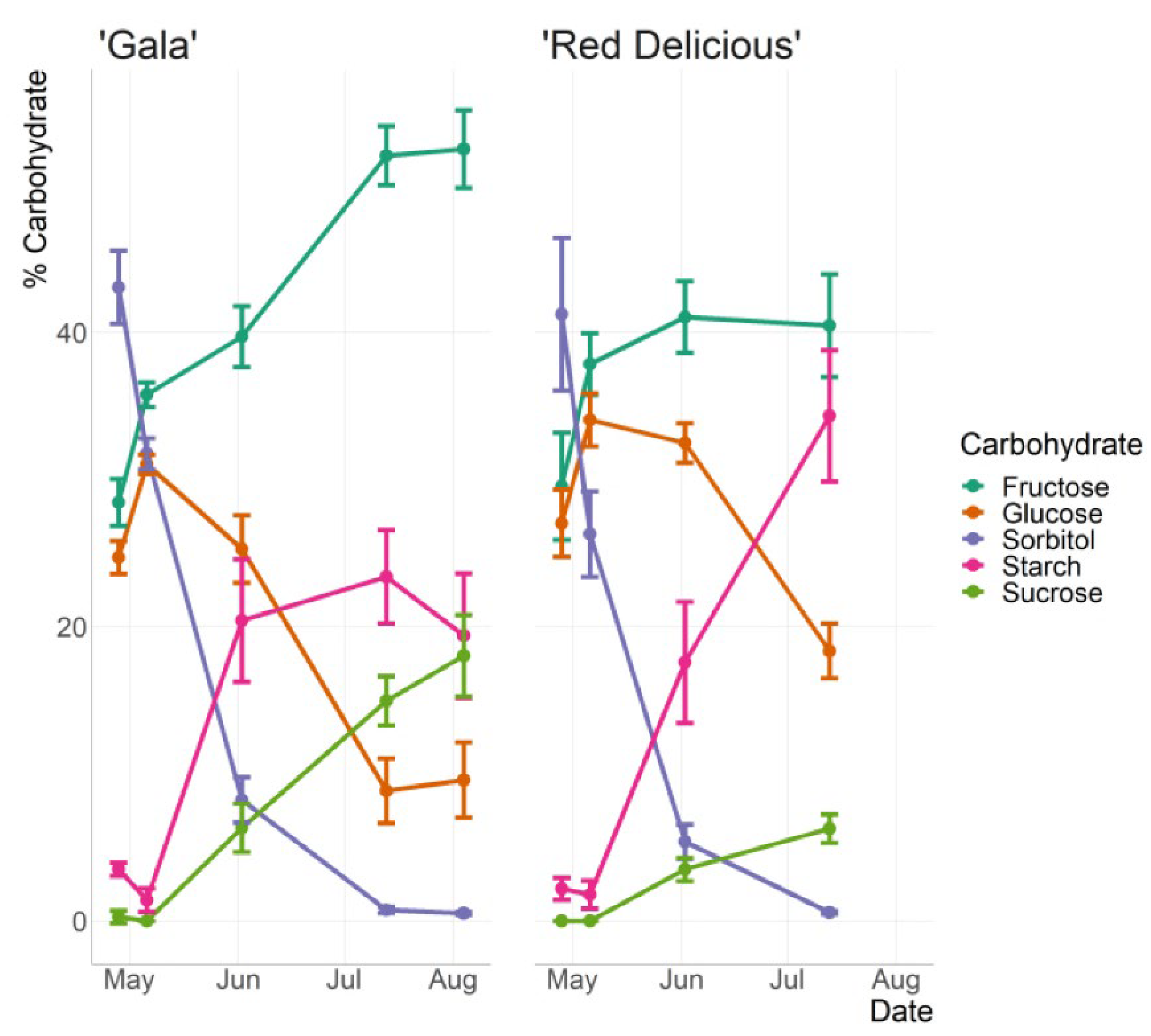
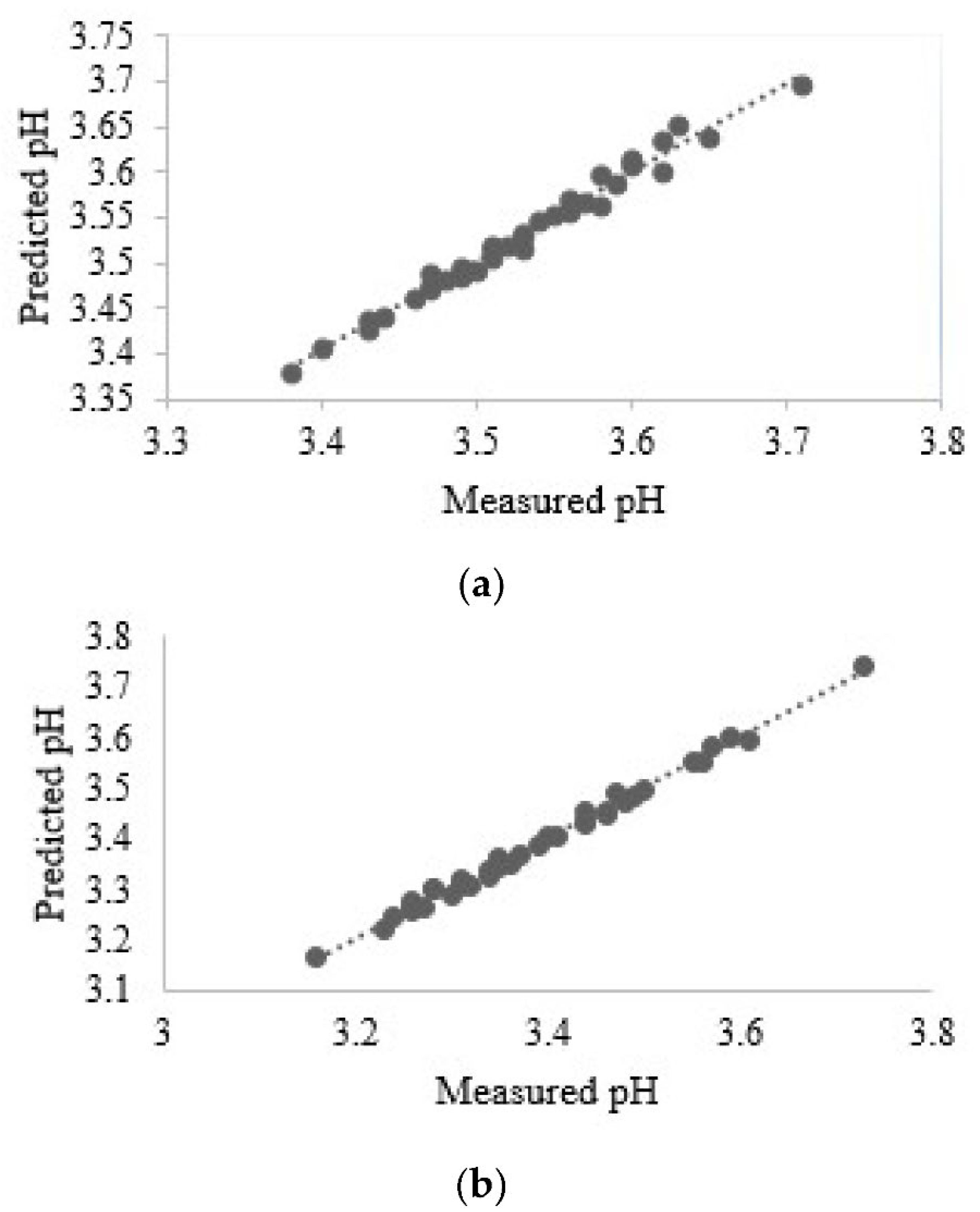
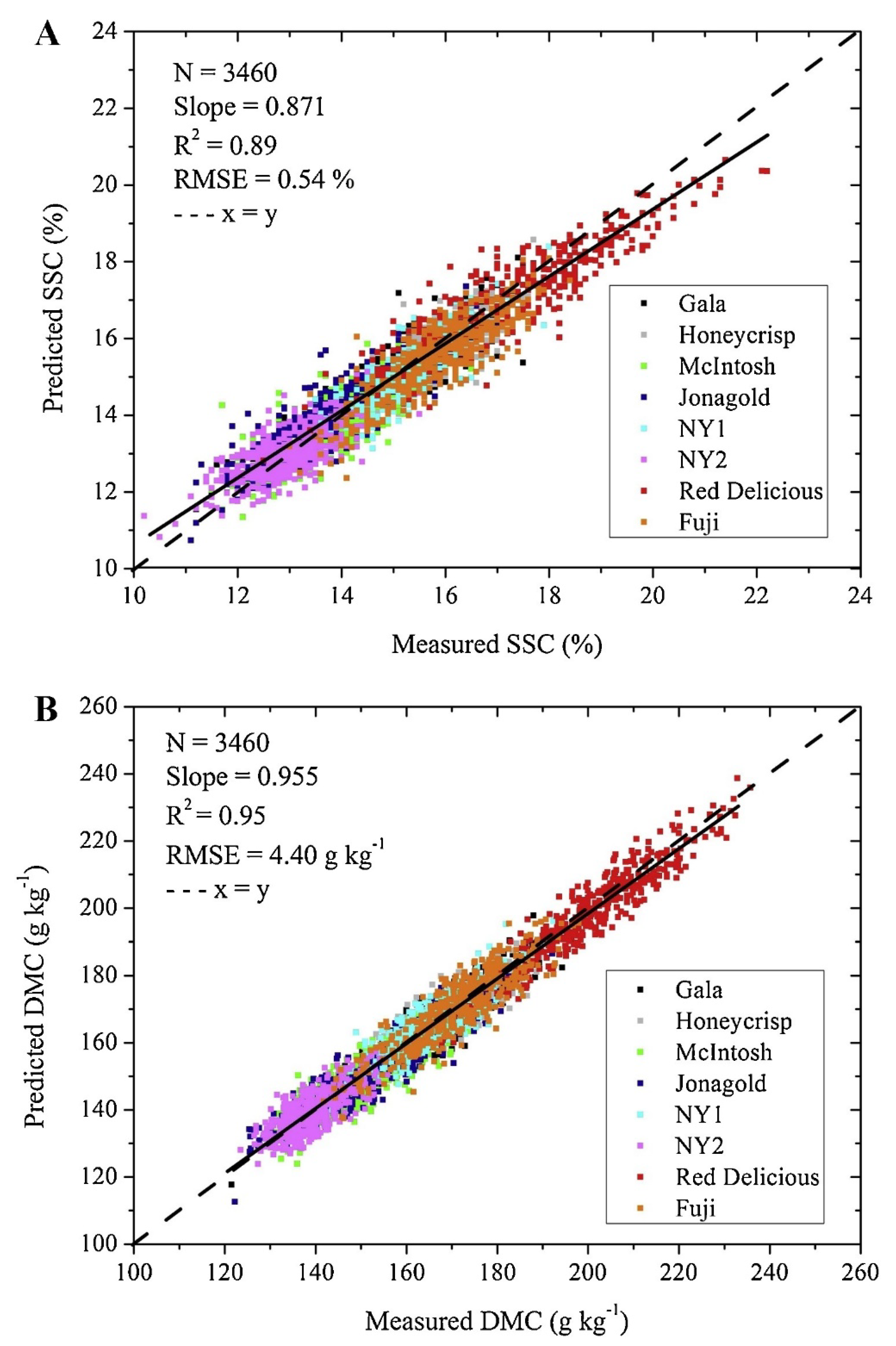
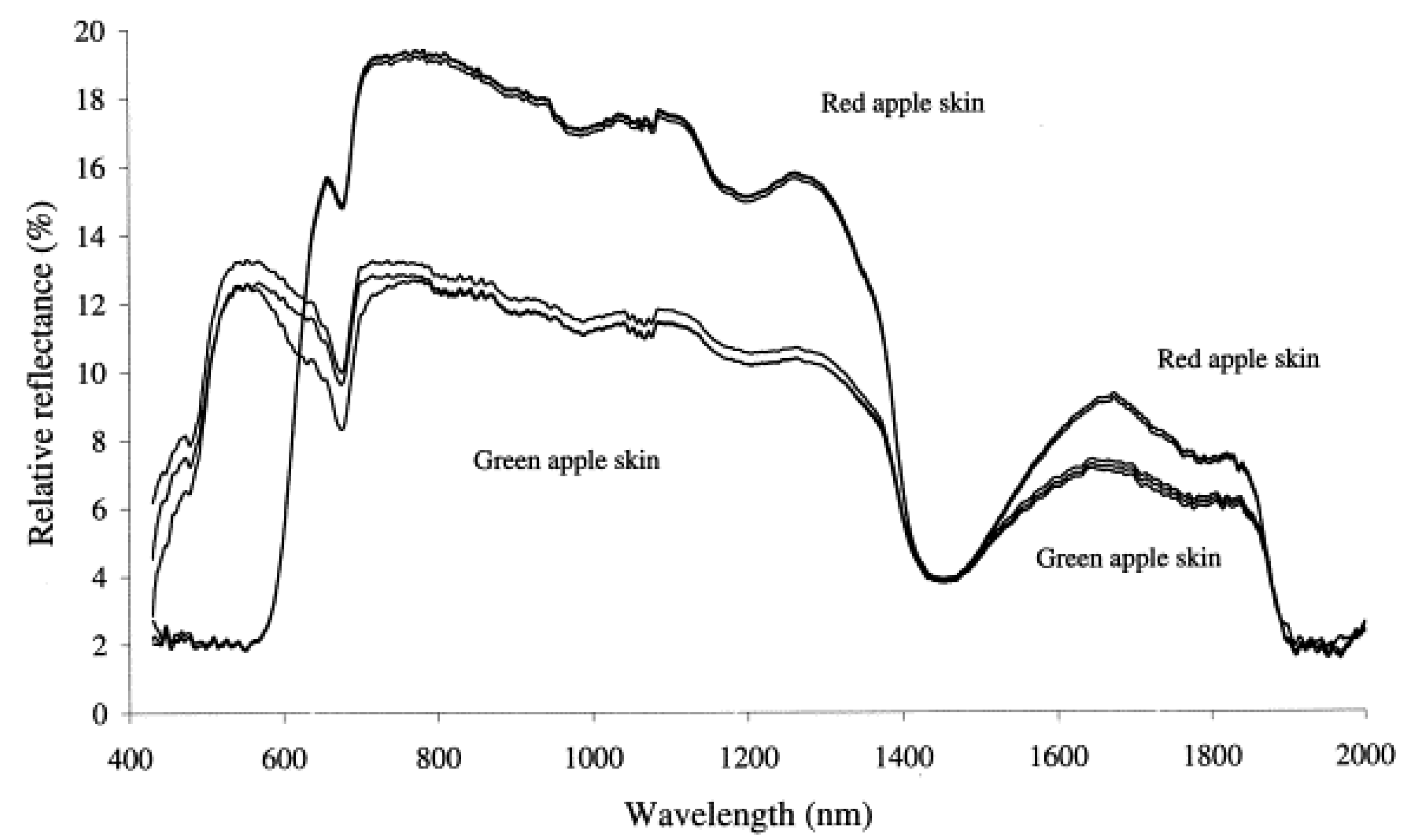
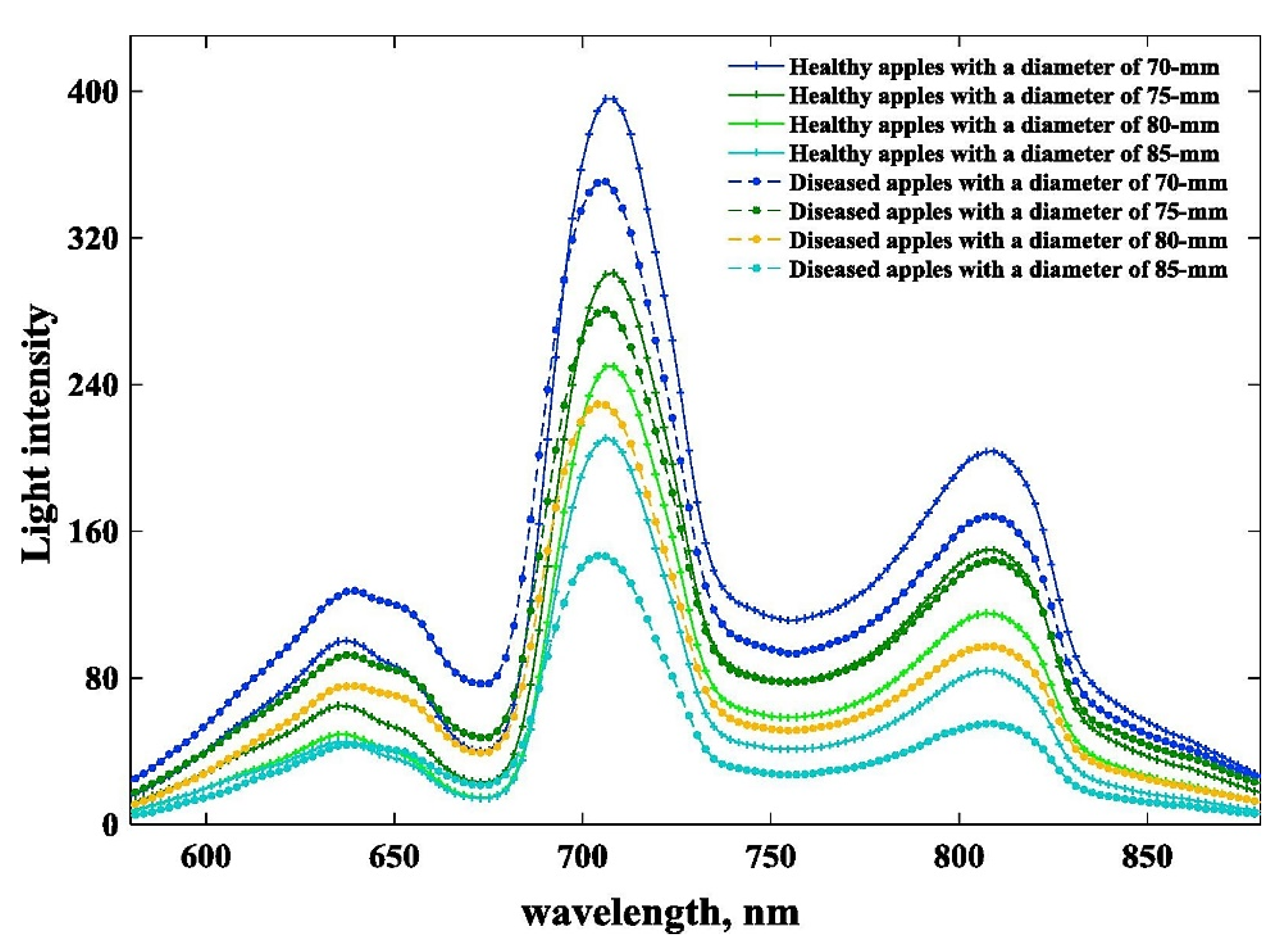
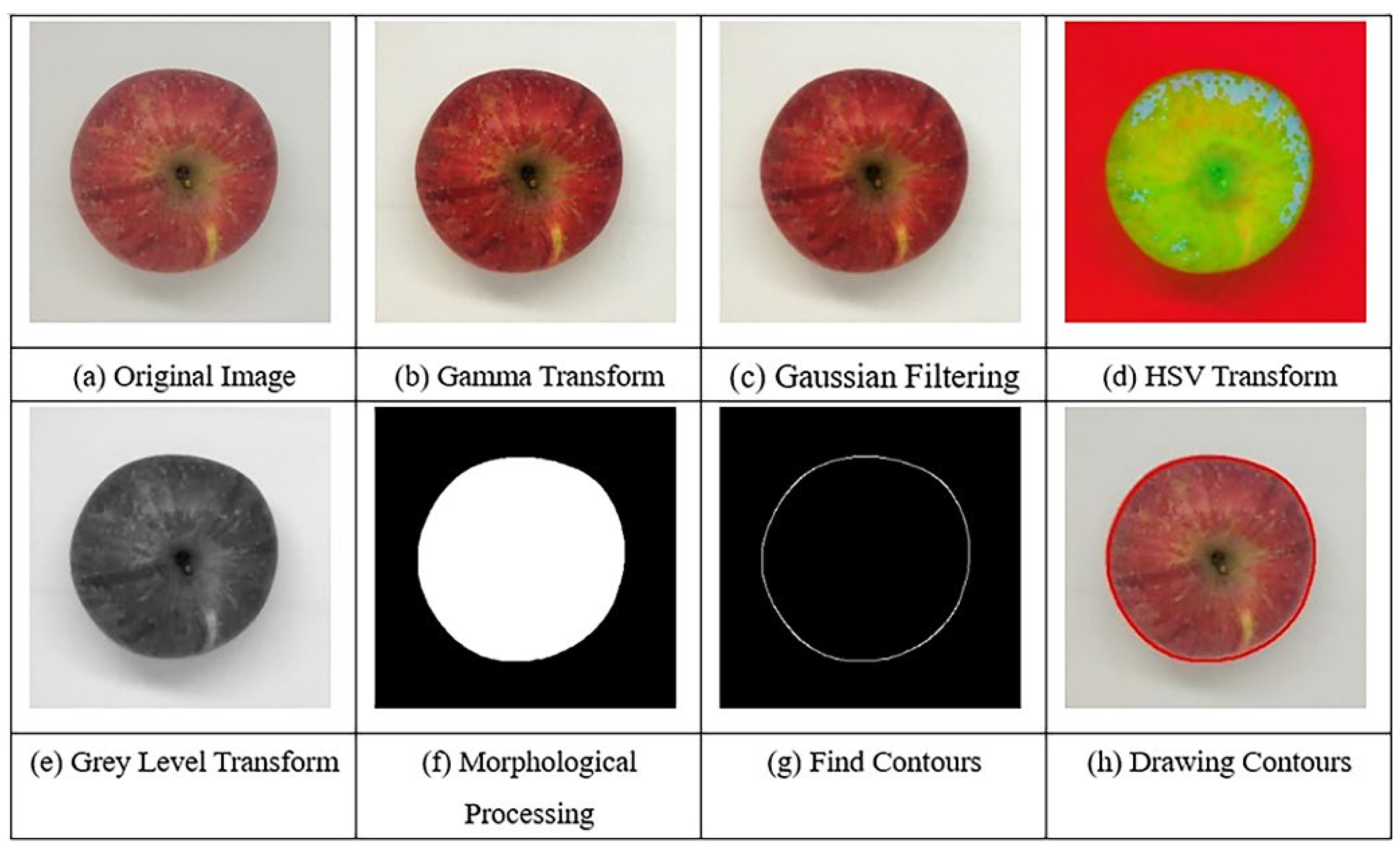
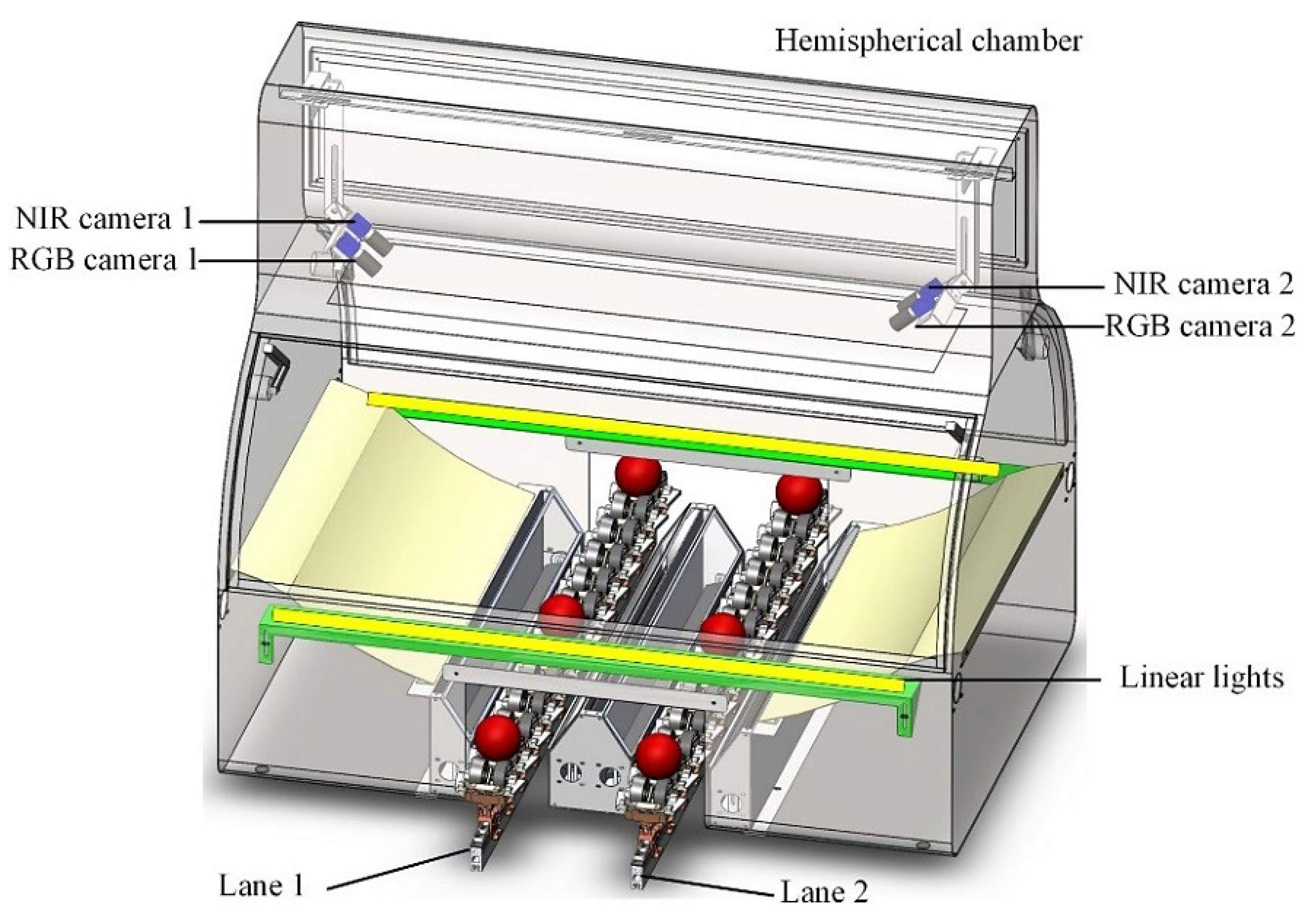

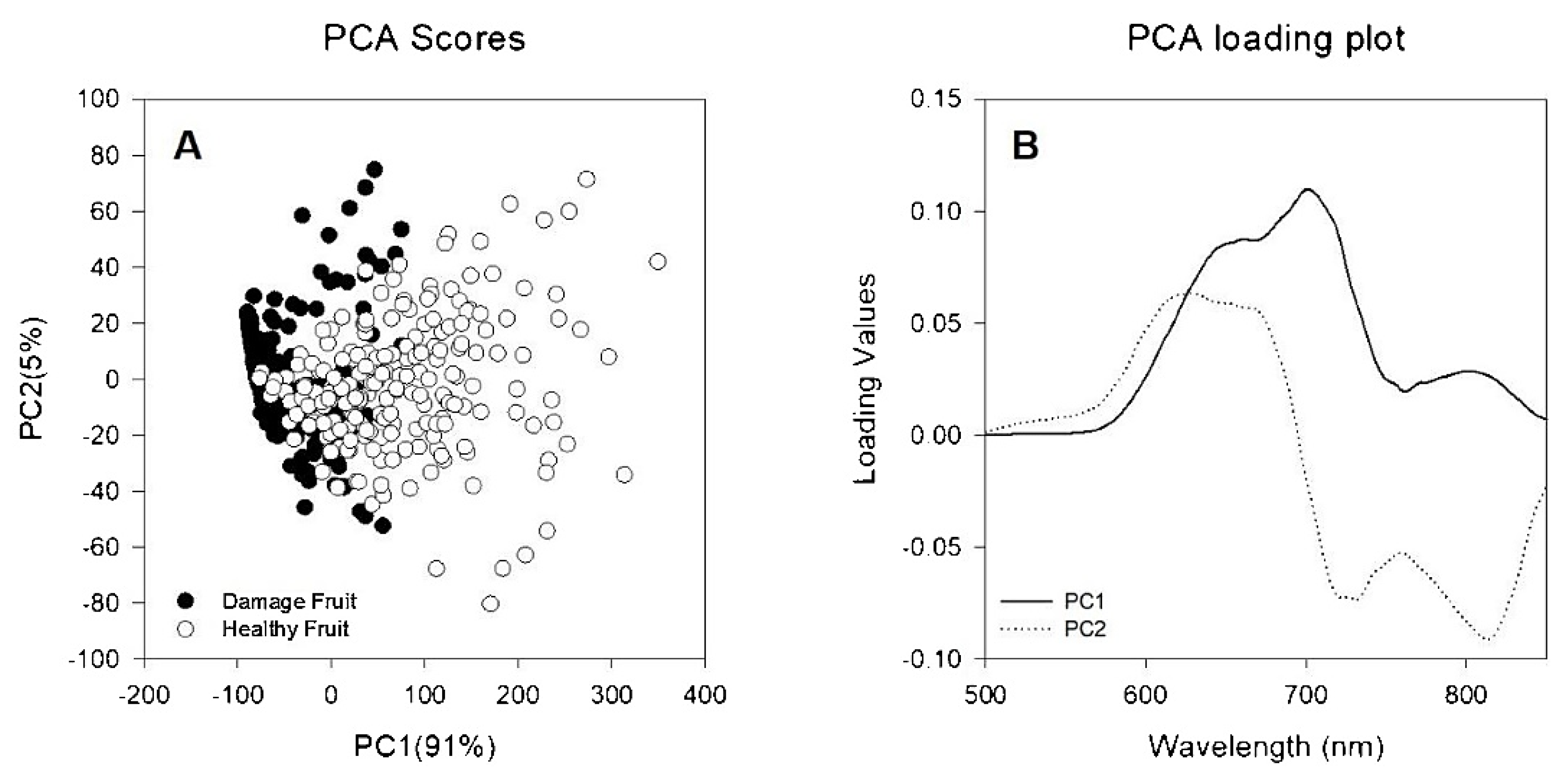
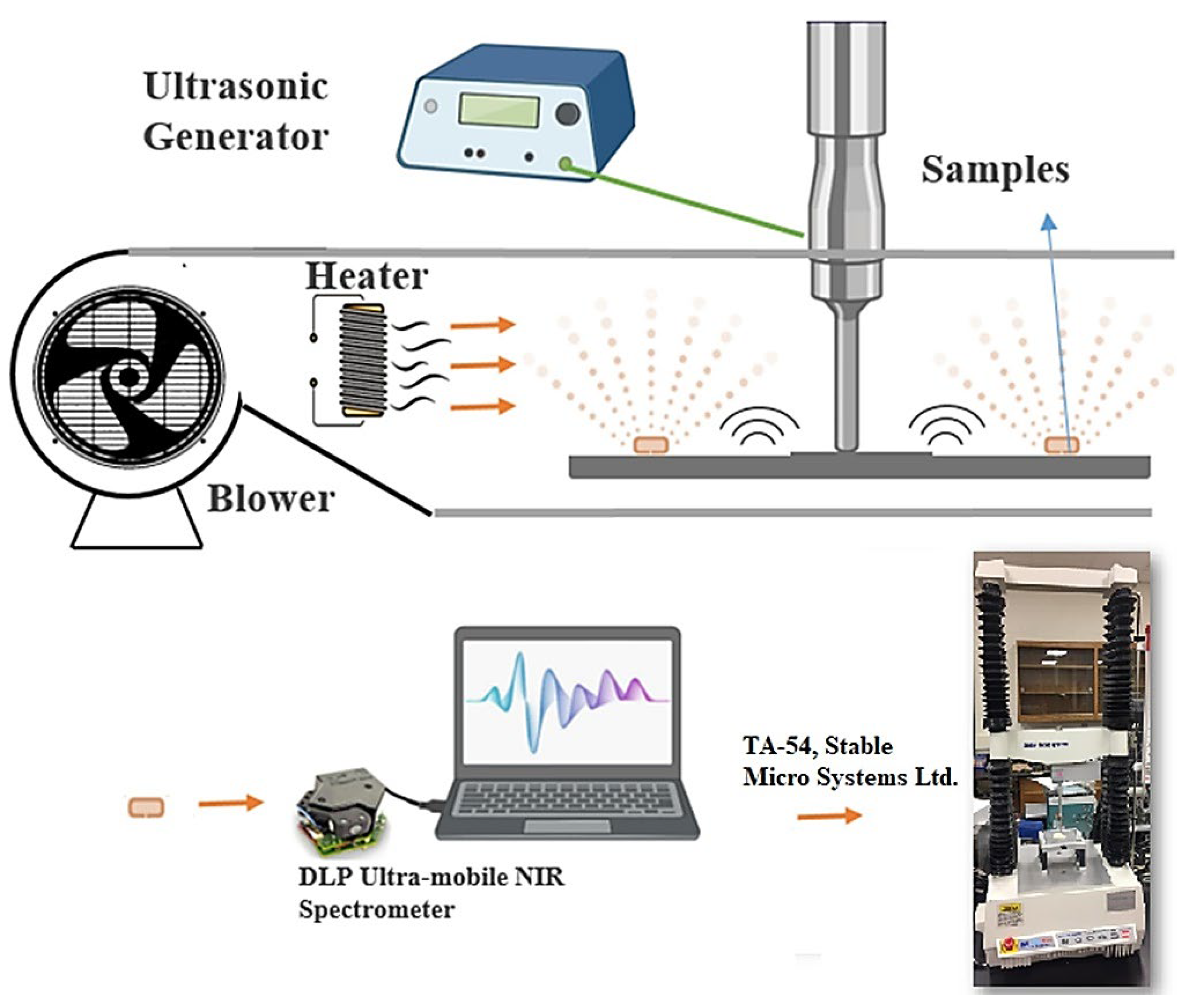
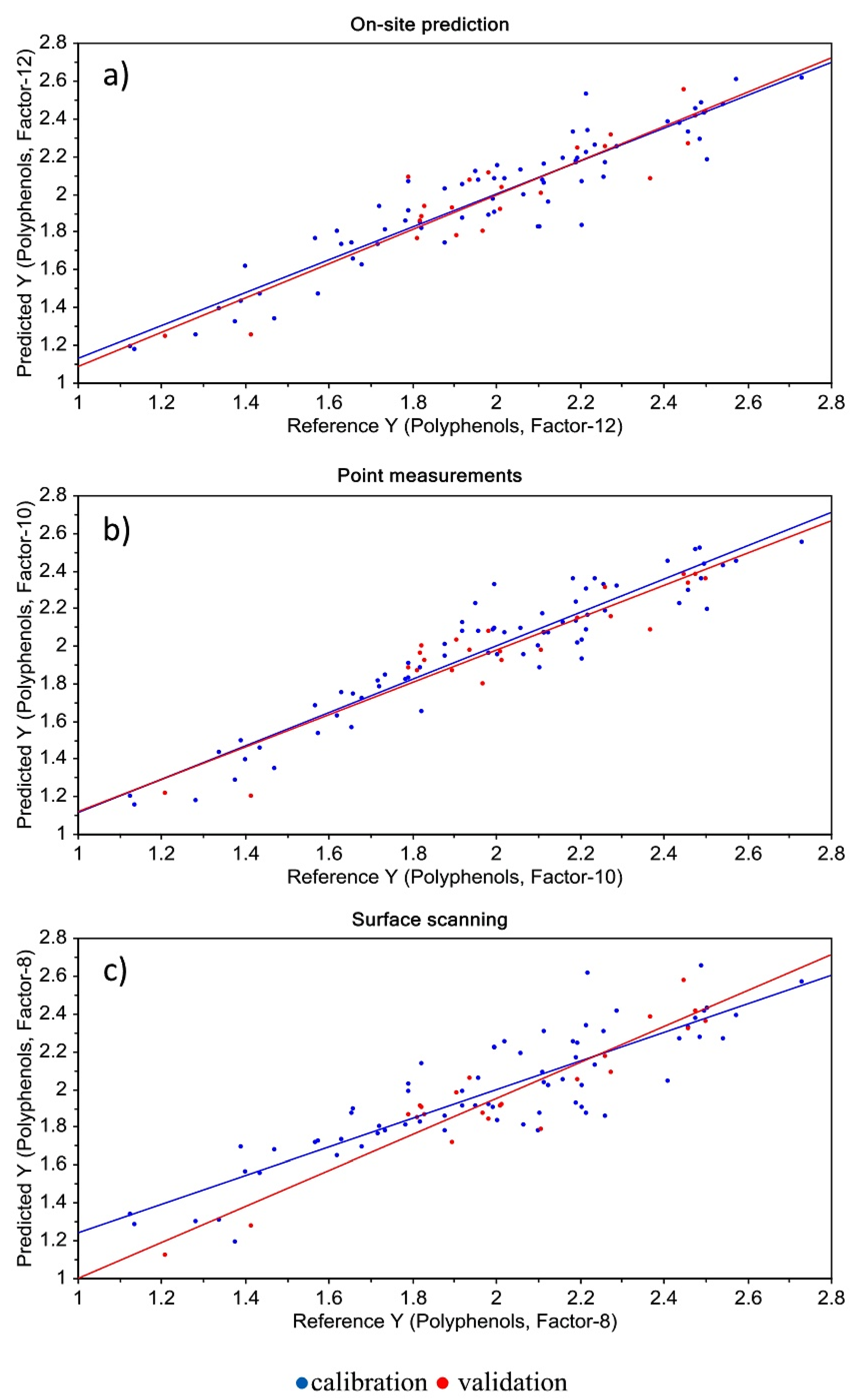
| Quality Parameters | |
|---|---|
| Internal | External |
| Color | Taste |
| Shape | Texture |
| Size | Aroma |
| Surface defect | Nutritional value |
| Internal defect | |
| Calibration Transfer | Spectral Variables | Accuracy | Fuji-1 | Fuji-2 | Fuji-3 | Fuji-4 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Recall | Precision | Recall | Precision | Recall | Precision | Recall | Precision | |||
| TCA-REA | 68 | 0.8739 | 0.9189 | 0.8095 | 0.9302 | 0.8333 | 0.5581 | 0.8571 | 0.9744 | 0.9744 |
| BDA-REA | 81 | 0.9160 | 0.9459 | 0.9091 | 0.9535 | 0.8039 | 06744 | 1 | 1 | 0.9630 |
| MEDA-REA | 77 | 0.9454 | 0.9324 | 0.9583 | 0.9302 | 0.8889 | 0.9070 | 0.8864 | 0.9872 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabska, J.; Beć, K.B.; Ueno, N.; Huck, C.W. Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review. Foods 2023, 12, 1946. https://doi.org/10.3390/foods12101946
Grabska J, Beć KB, Ueno N, Huck CW. Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review. Foods. 2023; 12(10):1946. https://doi.org/10.3390/foods12101946
Chicago/Turabian StyleGrabska, Justyna, Krzysztof B. Beć, Nami Ueno, and Christian W. Huck. 2023. "Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review" Foods 12, no. 10: 1946. https://doi.org/10.3390/foods12101946
APA StyleGrabska, J., Beć, K. B., Ueno, N., & Huck, C. W. (2023). Analyzing the Quality Parameters of Apples by Spectroscopy from Vis/NIR to NIR Region: A Comprehensive Review. Foods, 12(10), 1946. https://doi.org/10.3390/foods12101946








