Abstract
Weizmannia coagulans is an important potential probiotic with dual characteristics of Bacillus and Lactobacillus. This study describes a novel Weizmannia coagulans PL-W with excellent antibacterial activity isolated from Mongolian traditional cheese, in which safety and probiotic potential were evaluated by complete genome sequencing. The crude bacteriocins of W. coagulans PL-W showed antibacterial activity against various foodborne pathogens, including Listeria monocytogenes CMCC 54,004, Bacillus cereus ATCC 14,579, and Staphylococcus aureus ATCC 25,923. Moreover, the crude bacteriocins have outstanding stability against pH, temperature, surfactants, and are sensitive to protease. The complete genome sequencing revealed W. coagulans PL-W consists of 3,666,052-base pair (bp) circular chromosomes with a GC content of 46.24% and 3485 protein-coding genes. It contains 84 tRNA, 10 23S rRNA, 10 16S rRNA, and 10 5S rRNA. In addition, no risk-related genes such as acquired antibiotic resistance genes, virulence, and pathogenic factors were identified, demonstrating that W. coagulans PL-W is safe to use. Furthermore, the presence of gene clusters involved in bacteriocin synthesis, adhesion-related genes, and genes contributing to acid and bile tolerance indicate that W. coagulans PL-W is a potential candidate probiotic. Thus, antimicrobial activity and genome characterization of W. coagulans PL-W demonstrate that it has extensive potential applications as a food protective culture.
1. Introduction
Foodborne diseases are a significant health problem for all of humanity [1]. Various antibiotics have been used for a long time to inhibit the growth of pathogenic bacteria and prevent their threat to human health. However, the overuse of antibiotics has led to increased multidrug resistance in bacteria, which is a global public health crisis that threatens our ability to treat bacterial infections [2,3]. On the other hand, several studies have shown that the human commensal microbiota can be affected by the continuous use of antibiotics [4,5]. Therefore, it is urgent to search for new antimicrobial substances, such as bacteriocin, to prevent the adverse effects of traditional antimicrobial substances. In this regard, several studies have shown that bacteriocins from probiotics which are generally recognized as safe (GRAS), could be used as a substitute for traditional antibiotics in the future [6,7].
Bacteriocins are antimicrobial peptides synthesized from ribosomes produced by bacteria, which inhibit the growth of related (narrow spectrum) or nonrelated (broad spectrum) microorganisms [8]. At present, the bacteriocins from microbials approved for application in food additives are mainly from Nisin and Pediocin PA-1 produced by lactic acid bacteria (LAB) [9,10]. Bacteriocins predominantly exert their antibacterial activity by influencing gene and protein replication, pore formation, and membrane permeabilization [11,12], which could prevent target bacteria from evolving into corresponding drug-resistant strains, making them a potential alternative to antibiotics. It is widely known that the genus Bacillus is a rich source of bacteriocins or bacteriocin-like inhibitory substances (BLIS) [13,14,15]. Compared with most LAB, bacteriocins produced by Bacillus have broader inhibition spectra and may include Gram-positive bacteria, Gram-negative bacteria, or fungi, some of which are pathogenic for humans [16]. Consequently, bacteriocins from Bacillus are increasingly becoming more critical and have attracted more and more interest from researchers.
W. coagulans is a high-temperature-resistant spore-forming bacterium with probiotic activity. It has received extensive attention since it contains the properties both of Bacillus and Lactobacillus [17]. In 2012, the Food and Drug Administration approved W. coagulans as safe to use in food [18]. Several studies have found that some W. coagulans possess good antibacterial activity by producing bacteriocin [19]. For instance, lactosporin produced by W. coagulans ATCC 7050 has been reported to inhibit pathogens [20]. The bacteriocin produced by W. coagulans can inhibit the growth of spoilage bacteria and improve the preservation of large yellow croaker [21]. W. coagulans BDU3 produces a new 1.4 kDa bacteriocin with antimicrobial activity against foodborne pathogens [22]. Therefore, W. coagulans are expected to become a new star bacterium for food preservatives. There are three conventional strategies for using bacteriocins in the food industry: pure bacteriocin, bacteriocin-containing fermentates, and bacteriocin-producing live cells. It is noteworthy that the strains used to produce bacteriocin must be safe. Thus, assessing the safety and probiotic properties of the strains is an essential step for use in food products [23,24]. For instance, Sreenadh et al. evaluated the probiotics, safety, and technology of W. coagulans S-31,876 through a number of in vitro experiments to explore its potential applications [25]. High-throughput sequencing enables the evaluation of the properties of strains at the genomic level, including their genetic, safety, and metabolic profiles. Previously, Aulitto et al. used comparative genomics to focus on the biotransformation and defense ability of W. coagulans against the external environment [26]. In this research, the W. coagulans PL-W with antibacterial activity was identified from Mongolian traditional cheese, and its complete genome was confirmed. Assessment of the genome indicates that W. coagulans PL-W may be a safe strain with probiotic properties to use and promote future research and development of the organism in food preservation. In addition, the crude bacteriocin characteristics produced by W. coagulans PL-W were evaluated to provide the theoretical basis for its potential application as a food preservative.
2. Materials and Methods
2.1. Samples and Bacterial Culture Conditions
W. coagulans PL-W were cultured in Man Rogosa and Sharpe (MRS) medium at 45 °C. The indicator strain, L. monocytogenes CMCC 54,004, was cultured in TSYEB medium at 37 °C. The medium used for the culture of other bacteria is shown in Table 1. All bacteria used in this research were stored at −80 °C in a suitable culture medium containing 25% (v/v) glycerol.

Table 1.
Antibacterial spectrum of the crude bacteriocin of W. coagulans PL-W.
2.2. Isolation of Antimicrobial Substance-Producing W. coagulans and Crude Antimicrobial Substance Preparation
The method of isolation of W. coagulans was based on the previous method with some modifications [27]. Firstly, Mongolian traditional cheese was homogenized in sterilized water and then heated to 80 °C for 10 min. Subsequently, samples were diluted in a gradient and then spread individually on MRS agar with 2 g/L CaCO3 and incubated at 45 °C for 48 h. Bacterial colonies that showed clear circles on the plates containing CaCO3 were individually picked and then inoculated on MRS broth medium and incubated at 45 °C under the shaking condition (200 rpm) for 48 h.
The cell-free supernatant (CFS) was obtained by centrifugation at 10,000× g for 10 min, and then ammonium sulfate was slowly added to the CFS to 80% saturation by stirring and at 4 °C overnight. To collect the crude antimicrobial substance, the mixture was centrifuged at 10,000× g for 20 min at 4 °C, and then the precipitate was resuspended in 1 mL of PBS (pH 6.8). The activity of crude antimicrobial substance against L. monocytogenes CMCC 54,004 was determined by the agar well diffusion method. Strains with strong antimicrobial activity against the L. monocytogenes CMCC 54,004 were selected and identified by Gram staining, lactate production capacity, catalase and oxidase activities, ability to grow at different temperatures (45–60 °C), NaCl concentrations (1–5%), and various sugars. The 16S rDNA sequencing was performed for genotype identification as described previously [28]. Subsequently, the MEGA 7.0 software was used to analyze the phylogenetics of strains.
2.3. Characteristics of Antimicrobial Substance Production in W. coagulans PL-W
2.3.1. Kinetics of Growth and Crude Antimicrobial Substance Production in W. coagulans PL-W
W. coagulans PL-W was grown in 30 mL of MRS medium at 45 °C for 48 h and then inoculated into MRS medium at a 2% inoculum (v/v). The cell density at 600 nm was determined every 4 h while CFS was collected to obtain the crude antimicrobial substance and assess antibacterial activity by the method described in Section 2.2 [28].
2.3.2. Physicochemical Properties of Crude Antimicrobial Substance
The stability of the crude antimicrobial substance was examined by detecting the changes in antibacterial activity against L. monocytogenes CMCC 54,004 under different conditions [29]. The enzyme stability of the crude antimicrobial substance was evaluated by incubation with lipase, α-amylase, proteinase K, neutral protease, flavor enzyme, trypsin, and pepsin for 2 h under the optimal reaction conditions for each enzyme, while the final concentration of each enzyme was 1 mg/mL. The pH of the crude antimicrobial substance was adjusted from 2 to 10 with 2 M HCL and 2 M NaOH to detect the change in its antibacterial activity. The thermal stability of the crude antimicrobial substance was tested by incubation at 4–121 °C for 10 and 30 min, respectively. To test the crude antimicrobial substance stability with the surfactant, the crude antimicrobial substance was incubated with various chemical reagents 1% (v/v), including Tween 80, EDTA, urea, and SDS at 37 °C for 2 h. The antimicrobial activity of the untreated antimicrobial substance was measured and used as the positive control.
2.3.3. Antimicrobial Spectrum Assay of Crude Bacteriocins
To investigate the antimicrobial spectrum of W. coagulans PL-W, the antimicrobial activity of the crude bacteriocins of W. coagulans PL-W against a range of indicator strains, including food spoilage bacteria and food-borne pathogens (Table 1), was determined by using the pour plate method described by An et al. [30]. The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) of the crude bacteriocin were calculated by observing the growth of L. monocytogenes CMCC 54004 mixed with various concentrations of the crude bacteriocins [31].
2.3.4. Purification of Bacteriocin
To identify the bacteriocin, firstly, ultrafiltration tubes of 10 KD and 3 KD were used to isolate the crude bacteriocin. The fractions containing proteins larger than 10 KD, between 3–10 KD, and smaller than 3 KD were tested for antibacterial activity. Then, the active fractions were loaded onto a C18 reverse-phase column (5 µm, 4.6 × 250 mm, Agilent, Santa Clara, CA, USA), connected to a reverse-phase high-performance liquid chromatography (RP-HPLC) system, and eluted at 0.5 mL/min flow rate by a linear gradient elution with 95% water–acetonitrile (5–95%) containing 0.1% trifluoroacetic acid (TFA) for 30 min. The different peaks were collected at an absorbance of 280 nm, and then concentrated using 1 KD ultrafiltration tubes for antibacterial activity evaluation. Using Tricine-SDS-PAGE (16.5% separated and 4% concentrated gel), the range of molecular mass of the collected active fractions was analyzed.
2.4. Genome Sequencing, Assembly, Annotation, and Classification
W. coagulans PL-W grown to mid-logarithmic phase were collected and genomic DNA were extracted using the Bacterial Genomic DNA Isolation kit (TianGen, Beijing, China) according to the kit instructions. The PromethION platform was used to sequence the W. coagulans PL-W genome. Subsequently, the sequences were mix assembled with Unicycler and corrected with Pilon (parameter: OFF). After removing the redundant part, the Circlator (parameter: fixStart) was used to move the origin of the sequence to the replication start site of the genome to obtain the final genome sequence. The encoding gene was predicted with prodigal, tRNA, rRNA, and other ncRNAs were predicted using trnascan-SE. Interproscan was used for annotation of genome-encoded proteins. BlastP was used to align the encoded proteins to KEGG, RefSeq, and COG databases, and the best results with alignment coverage greater than 30% were retained as annotation results. The circos was used to draw the nuclear genome circle map.
Using W. coagulans PL-W as a reference strain, digital DNA-DNA hybridization was performed using an online tool (http://ggdc.dsmz.de/, (accessed on 5 September 2022)). The W. coagulans PL-W genome in the form of FASTA was submitted to the Type (Strain) Genome Server (TYGS) and the genome was compared with the TYGS database.
2.5. Prediction of the Safety of W. coagulans PL-W
The Comprehensive Antibiotic Research Database (CARD) and Resistance Gene Identification Tool (RGI) were used to analyze the presence of drug-resistance genes in W. coagulans PL-W. The VFDB webserver was used to predict putative virulence factors and the pathogen finder webserver was used to predict bacterial pathogenicity.
2.6. Prediction of the Probiotic Characteristics of W. coagulans PL-W
The Hidden Markov model (HMM) was used to detect genes related to acid and bile tolerance in the genome of W. coagulans PL-W and various proteins related to adhesion and aggregation were searched in the genome annotation data. AntiSMASH5 and BAGEL4 were used to predict non-ribosomal synthetic secondary metabolites (NRPS) and bacteriocin synthesis gene clusters in the W. coagulans PL-W genome, respectively.
2.7. Statistical Analysis
Three parallel groups were set up for each group of experiments. GraphPad Prism software was used for statistical analysis of the experimental data by a one-way ANOVA, and values of p < 0.05 were considered statistically significant.
3. Results and Discussion
3.1. Screening and Identification of Antimicrobial Substance-Producing Strains
Bacteriocins are a potential alternative to antibiotics since they are safe and it is not easy to produce antibiotic resistance to indicator strains [32]. Bacteriocins produced by Bacillus have a broad inhibitory spectrum, which makes them of excellent research significance [16]. The present study isolated 243 potential lactate-producing strains from Mongolian traditional cheese. Among these strains, strain PL-W possessed the highest antibacterial activity to indicator strains (L. monocytogenes CMCC 54,004) and was selected as the target strain. It was characterized as Gram-positive, spore-forming, rod-shaped, lactate produced, and positive for catalase, indole, and the Voges–Proskauer test, and grew in medium containing 5% sodium chloride and fermented sucrose, glucose, lactose, and arabinose. The phylogenetic analysis according to the strain 16S rRNA sequence showed that the selected strain belonged to the same evolutionary branch as W. coagulans and shared 100% support degree with W. coagulans 683, W. coagulans DSM1 ATCC 7050, and W. coagulans NBRC 12,583 (Supplementary Materials, Figure S1). Thus, we classified the strain PL-W as W. coagulans PL-W.
3.2. Crude Antimicrobial Substance Production Properties of W. coagulans PL-W
3.2.1. Kinetics of Growth and Crude Antimicrobial Substance Production in W. coagulans PL-W
Kinetics of crude antimicrobial substance production of W. coagulans PL-W cultured in MRS broth at 45 °C with shaking are presented in Figure 1. The inhibition zone size of crude antimicrobial substance against L. monocytogenes 54,004 was used to detect its production. The result showed that W. coagulans PL-W entered the stable phase after 24 h of culture. Meanwhile, the inhibition zone against L. monocytogenes 54,004 was detected and reached the largest at 32 h, indicating the production of the crude antimicrobial substance may reach the maximum mass. The antibacterial activity of the crude antimicrobial starts to decrease slightly after 32 h, probably due to degradation by other substances secreted by W. coagulans PL-W. Thus, the crude antimicrobial substance obtained from W. coagulans PL-W was cultured at 32 h for further characterization.
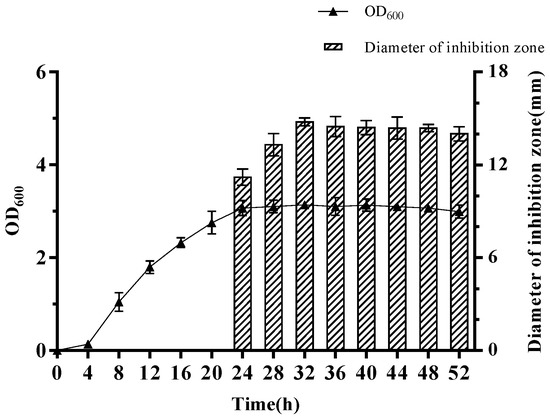
Figure 1.
Growth and dynamics of antimicrobial substance production by W. coagulans PL-W.
3.2.2. Characterization of Crude Antimicrobial Substance
Bacteriocins are antimicrobial peptides synthesized by ribosomes, produced by LAB, which have drawn wide attention owing to their characterization as food antiseptics. For example, Nisin, produced by Lactococcus lactis, has been approved by the Food and Drug Administration (FDA) and used for food preservation since 1950 [33]. Pediocin, bavaricin, leucocin, and sakacin are other bacteriocins produced by LAB that have been approved for use in food in some countries [34,35]. Although some Bacillus species are involved in various food fermentation processes, none of the Bacillus bacteriocins have been approved as food preservatives [36,37]. W. coagulans has been reported as safe by the FDA and the European Union Food Safety Authority (EFSA), which was classified as Bacillus coagulans due to its Bacillus characteristics. Therefore, there are further opportunities for W. coagulans bacteriocins to prove useful for food preservation. In this study, the antimicrobial substance of W. coagulans PL-W was treated with different enzymes to determine its nature. The antimicrobial activity against L. monocytogenes CMCC 54,004 indicated that the antimicrobial substance remained stable after lipase and α-amylase treatment. On the contrary, the antimicrobial substance was sensitive to proteinase K, indicating that the antimicrobial substance is a bacteriocin (Figure 2A). Furthermore, we examined the stability of the crude bacteriocin of W. coagulans PL-W under different conditions, including pH, temperature, and surfactants, to predict its application in the food industry. In the test of pH stability, the antibacterial activity of crude bacteriocins remained above 90% at pH 2–8, which indicates that the crude bacteriocins still have specific activity under acidic, neutral, and weakly alkaline conditions (Figure 2B). Therefore, bacteriocins produced by W. coagulans PL-W have the potential to be applied to acidic, neutral, and weakly alkaline foods. The antibacterial activities of the bacteriocins were nearly not altered under 4 °C to 40 °C, indicating that bacteriocins may have storage stability. Although the activity decreased after treatment at 60–100 °C, the crude bacteriocins show more than 70% activity making them valuable for heat-processed foods (Figure 2C). The stability of bacteriocins against surfactants facilitates their use in emulsifying food. Our study showed that the crude bacteriocins had little influence on antibacterial activity after treatment with different surfactants, suggesting that bacteriocins could be used in emulsified food (Figure 2D).
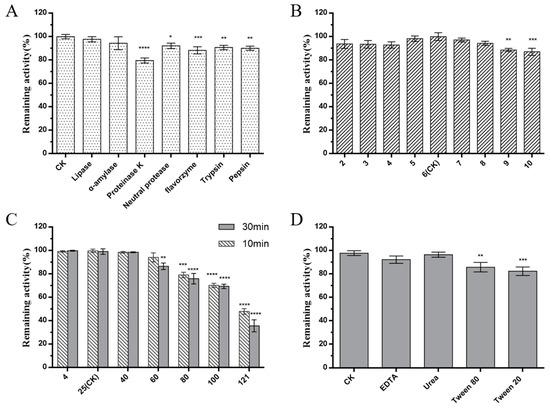
Figure 2.
Stability of crude bacteriocins. (A) enzyme; (B) pH; (C) temperature; and (D) surfactant. The experiment was repeated three times. (n = 3, * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001).
3.2.3. Antibacterial Spectrum of Crude Bacteriocins
The antibacterial spectrum of crude bacteriocins was determined by assaying for the antimicrobial activity against several indicator strains. The crude bacteriocins displayed a wide range of the antibacterial spectrum, including Gram-positive and Gram-negative bacteria (Table 1). Significantly, some of them are common spoilage bacteria in the food industry, such as L. monocytogenes CMCC 54,004, S. aureus ATCC 25,923, and B. cereus ATCC 14,579. The result of this study suggests that W. coagulans PL-W and its bacteriocin products have great utilization potential in food preservation.
3.2.4. Purification of Bacteriocin
The crude antimicrobial substances were separated into different fractions using 10 KD and 3 KD ultrafiltration tubes. The result showed that only fractions with molecular mass between 3–10 kDa exhibited antibacterial activity (Figure 3A). Therefore, it was presumed that the molecular mass of the bacteriocin was among 3–10 kDa. Further purification of the active fraction using a C18 column showed that only the third peak was able to inhibit the growth of the indicator strain (Figure 3B). Tricine-SDS-PAGE analysis of the molecular mass of peak 3 displayed a single band around 7 kDa (Figure 3C). Consequently, we conclude that W. coagulans PL-W expressed a bacteriocin with a molecular mass near 7 kDa.
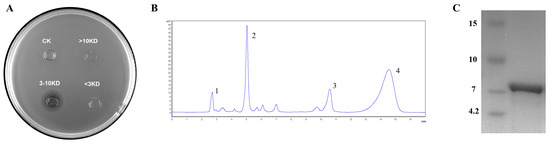
Figure 3.
Purification of Bacteriocin. (A) Ultrafiltration tubes separate the active fractions. (B) RP-HPLC process of purification. (C) Tricine–SDS–PAGE of activity fraction.
3.3. General Genome Features of W. coagulans PL-W
W. coagulans are widely used as probiotics in the food industry, medicine, and animal breeding [17]. Genetic analyses assist humans in finding potential probiotic strains with genetically encoded properties such as bile acid resistance, epithelial adhesion, and bacteriocins, but also determine whether a strain is safe to use from a genetic perspective [26,38]. There is currently insufficient information on the probiotic effects of W. coagulans on the genetic basis. Therefore, the present study aims to elucidate the safety and probiotic properties of W. coagulans PL-W and explore its potential application in food preservation through genomic analysis. Whole genome sequencing was performed using the PromethION platform. A total of 3,554,753,206 raw reads were used for genome assembly with the unicycler assembler, version 0.4.8 (Ryan R. Wick, Victoria, Australia). The complete genome of W. coagulans PL-W consists of 2 contigs of 3,666,052 bp with a GC content of 46.24%. None of the plasmid sequences were validated with the Plasmid Finder 2 tool. The protein-coding genes, ribosomal RNA, and transfer RNA of the genome are shown in Table 2. The functional classification of protein was analyzed in Table 3 through the COG database. KEGG and GO databases were also used functionally to annotate protein-coding genes (Supplementary Materials, Figures S2 and S3). The complete genome information of W. coagulans PL-W is shown in Figure 4.

Table 2.
General genome attributes of W. coagulans PL-W.

Table 3.
COG categories of coding proteins in W. coagulans PL-W.
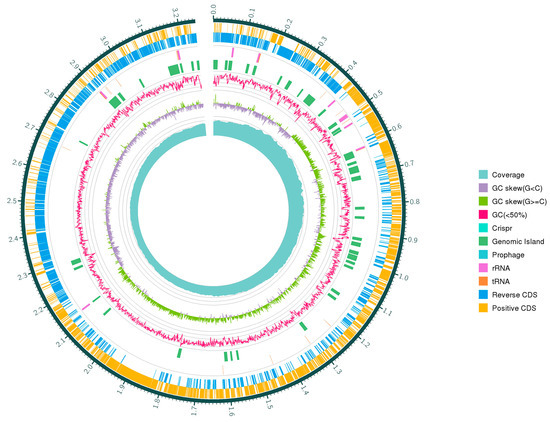
Figure 4.
Circular genome map of W. coagulans PL-W. From outside to center, ring 1: encoding gene (positive CDS); ring 2: encoding gene (reverse CDS); ring 3: tRNA and rRNA; ring 4: CRISPR, prophage and genomic island; ring 5: GC content; ring 6: GC-skew; and ring 7: coverage.
3.4. Taxonomic Classification and Phylogeny
Phylogenetic analysis at the genome level has contributed to evaluating the diversity of W. coagulans species and the taxonomic status of the species. Type (Strain) Genome Server (TYGS) is a high-throughput platform that is the most advanced genome-based classification platform [39]. The results of TYGS analysis demonstrated that W. coagulans PL-W is similar to W. coagulans ATCC7050 and W. coagulans DSM1 (Figure 5). Digital DNA–DNA hybridization (DDH) of the W. coagulans PL-W genome was performed using the publicly available genome sequences of three W. coagulans (DSM1, 2–6, ATCC7050, and XZL9) using the Genome–Genome Distance Calculator (GGDC) 3.0 server [40]. The results indicated that W. coagulans PL-W shares 94.21% and 85.32% similarity with W. coagulans XZL9 and W. coagulans 2–6, proving that W. coagulans PL-W is a new strain of Weizmannia. Using JSpeciesWS to calculate average nucleotide identity (ANI) using the method described previously revealed a 98.23% similarity of W. coagulans PL-W with W. coagulans XZL9 [41]. Thus, W. coagulans PL-W was identified as a member of the W. coagulans species based on >70% similarity in DDH and ~95% or higher ANI with a similar reference strain.
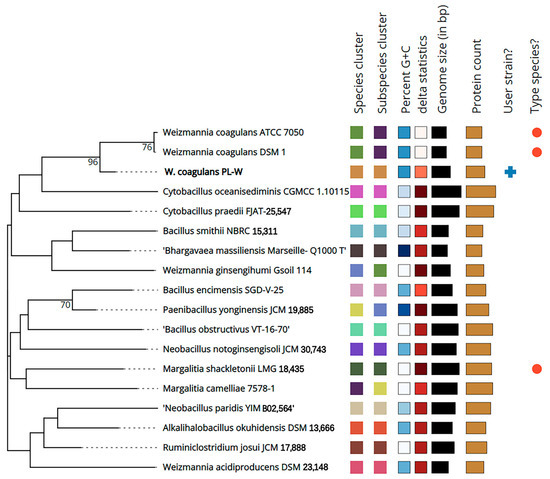
Figure 5.
Phylogenetic analysis of W. coagulans PL-W whole genome by TYGS.
3.5. Safety Assessment of W. coagulans PL-W
Safety assessment is an essential process for selecting probiotics. According to the recommendations of Qualified Safety Presumption (QPS) approved by the European Food Safety Authority (EFSA), the presence and potential mobility of antibiotic resistance genes should be considered when selecting new probiotics since the increasing resistance of bacteria to antibiotics poses a great threat to human health [42]. The CARD database contains bacterial drug resistance genes from different environmental sources (such as the gut, domestic wastewater, rivers, etc.) and their annotated information such as their resistance spectrum, mechanism of action, ontology, COG, and CDD [43]. Analysis of the W. coagulans PL-W genome from the CARD database showed that W. coagulans PL-W does not contain any antibiotic resistance genes or acquired antimicrobial genes detected. Similarly, phenotypic studies of W. coagulans PL-W sensitivity to the antibiotics were all below the cut-off values mentioned in the EFSA prescribed standards (Table 4).

Table 4.
W. coagulans PL-W sensitivity to antibiotics.
The Virulence Factor Database (VFDB) collects information on the virulence factors of bacterial pathogens [44]. The existence of any virulence genes was not detected on the W. coagulans PL-W genome using the VFDB service. At the same time, we found that W. coagulans PL-W did not exhibit hemolytic zones on the Columbia Agar plates with sheep blood (Supplementary Materials, Figure S4). Based on the above analysis, we promoted that W. coagulans PL-W was presumed to be a safe strain.
3.6. Assessment of Probiotic Properties
The ability to endure harsh conditions in the gastrointestinal tract is one of the criteria for selecting probiotics [45]. A large number of genes involved in acid and bile salt tolerance have been identified in the W. coagulans PL-W genome. ATP synthase (F1–F0-ATPase), a group of proteins mainly involved in acid resistance, are known to effectively pump protons out of the cell by hydrolyzing ATP, maintaining a low proton concentration inside the cell, thereby improving acid tolerance and thus maintaining pH homeostasis. There are eight coding F0F1 ATP synthase genes (atpC, atpD, atpG, atpA, atpH, atpF, atpE, and atpB) that were identified in the W. coagulans PL-W genome. In addition, two (Na+/H+) transport protein genes, one (H+/Cl−) antitransporter gene, ClcA, and one (Ca2+/H+) antitransporter gene, chaA, were detected in the W. coagulans PL-W genome, which has been shown to play critical roles in pH and homeostasis of cells [46,47]. Bile salt hydrolases belong to the family of glycine hydrolase, which act by binding bile salts to counteract the harmful effects of bile. A gene-encoding cholylglycine hydrolase was identified in the W. coagulans PL-W genome. Two sodium bile acid symporter family genes were also identified, and the presence of these genes was beneficial for the bile tolerance of W. coagulans PL-W [48]. The survival rate of W. coagulans PL-W at 1% bile salt concentration and pH 2.0 further validated the genomic data.
Adhesion to the intestinal epithelium has been considered an important probiotic property since adhesion can promote intestinal colonization while competitively excluding harmful pathogens [49,50]. We searched the genes of adhesion, colonization, mucin binding, flagellar hook, and fibrinogen/fibronectin binding from annotated data. There were 11 genes found to encode adhesion-related proteins in W. coagulans PL-W, including hook-associated protein FlgK and FlgL, flagellar filament capping protein FliD, flagellar hook–basal body complex protein FliE, flagellar hook–length control protein FliK, flagellar hook assembly protein FlgD, flagellar basal body rod protein FlgF, fibronectin/fibrinogen-binding protein (FbpA), DUF817 domains (fibronectin/fibrinogen binding protein), and segregation and condensation protein (scpA and scpB) (fibronectin-binding protein). Flagella can act directly as adhesins and play a key role in colonization by facilitating bacterial motility [51]. Thus, several proteins related to flagellar formation were detected in W. coagulans PL-W, indicating that W. coagulans PL-W may have good motility. Fibronectin is an important multidomain glycoprotein with various adhesion properties and serves as an essential link between cells and their extracellular matrix [52]. Additionally, some similar types of adhesion proteins have been identified in genome-wide analyses of W. coagulans S-Lac and W. coagulans GBI-30, helping the W. coagulans to colonize in host intestines and act as probiotic [50].
3.7. Antimicrobial Compound Gene Prediction and Validation
The characteristics of synthesizing antimicrobial compounds play an important role in the competition of survival exclusion when probiotics are colonized in the gut [11]. Genome analysis is expected to pave the way to finding new antimicrobial compounds and understanding the mechanisms of antimicrobial compound production in bacteria [53]. Therefore, BAGEL 4.0 and AntiSMASH 5.0 were used to predict antimicrobial compounds in W. coagulans PL-W. Polyketides (PKs)-T3PK3 biosynthesis, betalactone, and two RiPP-like compounds were identified by antiSMASH. The two RiPP-like compounds were both identified as Circularin A and Amylocyclicin by BAGEL 4.0 (Figure 6). Circularin A and Amylocyclicin are typically produced as a propeptide after the leader peptide is cleaved, and then ligation between the N- and C-termini results in cyclic antimicrobial peptides. Circularin A produced by Clostridium beijerinckii ATCC 25,752 has been reported to have broad-spectrum antibacterial activity [54], and Amylocyclicin produced by Bacillus amyloliticus FZB42 has a wide range of antibacterial activity against Gram-positive bacteria [55]. It is worth noting that the putative Circularin A and Amylocyclicin synthetic gene clusters of W. coagulans PL-W were not found to be similar to any known cluster by antiSMASH servicer. The ABC transporter may be responsible for the transport of mature peptides to protect itself from bacteriocin attack [56], which was both found on the putative Circularin A and Amylocyclicin synthesis gene cluster, suggesting that these two bacteriocins may be secreted in the form of ABC transport. A gene annotated with CirC protein that had a modification function related to bacteriocin cyclization was detected in the Circularin A synthesis gene cluster [57]. Meanwhile, a histidine kinase gene was detected on the putative Amylocyclicin synthesis gene cluster, which predicted that Amylocyclicin synthesis might be regulated by quorum sensing [58]. A transcriptional regulatory protein and competence regulatory protein may be involved in the transcription and cyclization of Amylocyclicin.
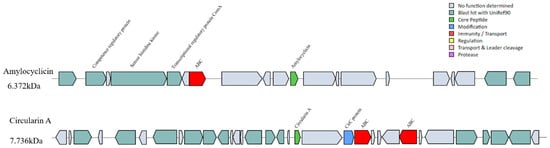
Figure 6.
Bacteriocin synthesis gene cluster predicted by BAGEL4. Two cluster-encoding genes related to Circularin A and Amylocyclicin were identified in the W. coagulans PL-W genome.
Tricine-SDS-PAGE analysis of the bacteriocins indicated that its molecular mass was near 7 kDa (Figure 3C); combining the genomic information, we concluded the bacteriocin extracted from W. coagulans PL-W was Circular A and the entire amino acid sequence was MGLFHVASKFHVSAGIASGVVTAVLHAGTIASIIGAVTVVMSGGVDAILDMGWTAFIAEVKHLAKEYGKKRAIAW. Although the genome was confirmed for the presence of the gene of Amylocyclicin, purification of crude bacteriocin did not find it. Thus, we speculate that Amylocyclicin may be expressed only under certain specific growth conditions, or Amylocyclicin did not show inhibitory activity against the L. monocytogenes CMCC 54,004 that we used in the purification process. In this regard, it may be possible to use heterologous expression methods in the future to detect the antimicrobial ability of Amylocyclicin or attempt to use other bacteria rather than L. monocytogenes as the indicator strain when purifying the bacteriocins. In general, the expression of Circularin A may provide a competitive advantage for W. coagulans PL-W.
4. Conclusions
The current study identifies a bacteriocins-producing W. coagulans PL-W from Mongolian traditional cheese. The crude bacteriocin extracted from W. coagulans PL-W by 80% ammonium sulfate not only exhibited a broad antimicrobial spectrum including a Gram-positive bacterium and a Gram-negative bacterium, some of which are common foodborne pathogens, but has good stability to surfactant, heat, and pH. Genome analysis indicated that W. coagulans PL-W is a safe strain to use. In addition, the strain harbors genes encoding two bacteriocins which might ensure W. coagulans PL-W has excellent antibacterial ability. Research of the crude bacteriocin characteristics and genomic sequencing suggested that W. coagulans PL-W is a safe candidate for controlling foodborne pathogens and is promising for use in the food industry. Moreover, the whole genomic results will contribute to further in vitro and in vivo investigations of W. coagulans PL-W to prospect its application as a probiotic.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/foods12010216/s1, Figure S1: Phylogenetic analysis of 16S rRNA sequence of W. coagulans PL-W; Figure S2: KEGG category distribution of functional annotation results; Figure S3: WEGO category distribution of functional annotation results. Figure S4: Evaluation of hemolytic activity of W. coagulans PL-W in Columbia blood agar plates.
Author Contributions
Conceptualization, P.L. and Y.W.; methodology, Y.W., Z.G. and S.Z.; software, Y.W.; formal analysis, Y.W.; investigation, Y.W.; resources, Y.W.; data curation, Y.W.; writing—original draft preparation, Y.W.; writing—review and editing, P.L., Z.G. and S.Z.; visualization, Y.W.; supervision, P.L.; funding acquisition, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 32172172).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, W.; Pires, S.M.; Liu, Z.; Ma, X.; Guo, Y. Surveillance of foodborne disease outbreaks in China, 2003–2017. Food Control 2020, 118, 107359. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A one health perspective. Microbiol. Spectr. 2018, 6, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M. Antibiotic overuse: Stop the killing of beneficial bacteria. Nature 2011, 476, 393–394. [Google Scholar] [CrossRef]
- Heilbronner, S.; Krismer, B.; Brotz-Oesterhelt, H.; Peschel, A. The microbiome-shaping roles of bacteriocins. Nat. Rev. Microbiol. 2021, 19, 726–739. [Google Scholar] [CrossRef]
- Ahmad, V.; Khan, M.S.; Jamal, Q.M.S.; Alzohairy, M.A.; Al Karaawi, M.A.; Siddiqui, M.U. Antimicrobial potential of bacteriocins: In therapy, agriculture and food preservation. Int. J. Antimicrob. Agents 2017, 49, 1–11. [Google Scholar] [CrossRef]
- Choi, G.H.; Holzapfel, W.H.; Todorov, S.D. Diversity of the bacteriocins, their classification and potential applications in combat of antibiotic resistant and clinically relevant pathogens. Crit. Rev. Microbiol. 2022, 48, 1–20. [Google Scholar] [CrossRef]
- O’Connor, P.M.; Kuniyoshi, T.M.; Oliveira, R.P.; Hill, C.; Ross, R.P.; Cotter, P.D. Antimicrobials for food and feed; a bacteriocin perspective. Curr. Opin. Biotechnol. 2020, 61, 160–167. [Google Scholar] [CrossRef]
- Ko, K.Y.; Park, S.R.; Lim, H.S.; Park, S.J.; Kim, M. Improved pretreatment method for determination of Nisins A and Z and monitoring in cheese using liquid chromatrography-tandem mass spectrometry. Food Anal. Method 2016, 9, 122–130. [Google Scholar] [CrossRef]
- Santiago-Silva, P.; Soares, N.F.F.; Nobrega, J.E.; Junior, M.A.W.; Barbosa, K.B.F.; Volp, A.C.P.; Zerdas, E.R.M.A.; Wurlitzer, N.J. Antimicrobial efficiency of film incorporated with pediocin (ALTA (R) 2351) on preservation of sliced ham. Food Control 2009, 20, 85–89. [Google Scholar] [CrossRef]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.N.; Konings, W.N.; Driessen, A.J. Bacteriocins: Mechanism of membrane insertion and pore formation. Antonie Van Leeuwenhoek 1999, 76, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Babasaki, K.; Takao, T.; Shimonishi, Y.; Kurahashi, K. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: Isolation, structural analysis, and biogenesis. J. Biochem. 1985, 98, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Shelburne, C.E.; An, F.Y.; Dholpe, V.; Ramamoorthy, A.; Lopatin, D.E.; Lantz, M.S. The spectrum of antimicrobial activity of the bacteriocin subtilosin A. J. Antimicrob. Chemother. 2007, 59, 297–300. [Google Scholar] [CrossRef]
- Stein, T.; Dusterhus, S.; Stroh, A.; Entian, K.D. Subtilosin production by two Bacillus subtilis subspecies and variance of the sbo-alb cluster. Appl. Environ. Microbiol. 2004, 70, 2349–2353. [Google Scholar] [CrossRef]
- Abriouel, H.; Franz, C.M.; Ben Omar, N.; Galvez, A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol. Rev. 2011, 35, 201–232. [Google Scholar] [CrossRef]
- Konuray, G.; Erginkaya, Z. Potential use of Bacillus coagulans in the food industry. Foods 2018, 7, 92. [Google Scholar] [CrossRef]
- Shinde, T.; Vemuri, R.; Shastri, M.D.; Perera, A.P.; Tristram, S.; Stanley, R.; Eri, R. Probiotic Bacillus coagulans MTCC 5856 spores exhibit excellent in-vitro functional efficacy in simulated gastric survival, mucosal adhesion and immunomodulation. J. Funct. Foods 2019, 52, 100–108. [Google Scholar] [CrossRef]
- Nath, S.; Chowdhury, S.; Dora, K. Application of Bacillus sp. as a biopreservative for food preservation. Int. J. Eng. Res. Appl. 2015, 5, 85–95. [Google Scholar]
- Riazi, S.; Dover, S.E.; Chikindas, M.L. Mode of action and safety of lactosporin, a novel antimicrobial protein produced by Bacillus coagulans ATCC 7050. J. Appl. Microbiol. 2012, 113, 714–722. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Ruan, X.; Li, G.; Zhao, Y.; Wang, Y. Preservation of large yellow croaker (Pseudosciaena crocea) by Coagulin L1208, a novel bacteriocin produced by Bacillus coagulans L1208. Int J Food Microbiol. 2018, 266, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Vanithamani, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Bacteriocinogenic potential of a probiotic strain Bacillus coagulans [BDU3] from Ngari. Int. J. Biol. Macromol. 2015, 79, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Orru, L.; Salvetti, E.; Cattivelli, L.; Lamontanara, A.; Michelotti, V.; Capozzi, V.; Spano, G.; Keller, D.; Cash, H.; Martina, A.; et al. Draft genome sequence of Bacillus coagulans GBI-30, 6086, a widely used spore-forming probiotic strain. Genome Announc. 2014, 2, e01080-14. [Google Scholar] [CrossRef] [PubMed]
- Saroj, D.B.; Gupta, A.K. Genome based safety assessment for Bacillus coagulans strain LBSC (DSM 17654) for probiotic application. Int. J. Food Microbiol. 2020, 318, 108523. [Google Scholar] [CrossRef]
- Sreenadh, M.; Kumar, K.R.; Nath, S. In vitro evaluation of Weizmannia coagulans strain LMG S-31876 isolated from fermented rice for potential probiotic properties, safety assessment and technological properties. Life 2022, 12, 1388. [Google Scholar] [CrossRef]
- Aulitto, M.; Martinez-Alvarez, L.; Fiorentino, G.; Limauro, D.; Peng, X.; Contursi, P. A comparative analysis of Weizmannia coagulans genomes unravels the genetic potential for biotechnological applications. Int. J. Mol. Sci. 2022, 23, 3135. [Google Scholar] [CrossRef]
- Abdhul, K.; Ganesh, M.; Shanmughapriya, S.; Kanagavel, M.; Anbarasu, K.; Natarajaseenivasan, K. Antioxidant activity of exopolysaccharide from probiotic strain Enterococcus faecium (BDU7) from Ngari. Int. J. Biol. Macromol. 2014, 70, 450–454. [Google Scholar] [CrossRef]
- Voulgari, K.; Hatzikamari, M.; Delepoglou, A.; Georgakopoulos, P.; Litopoulou-Tzanetaki, E.; Tzanetakis, N. Antifungal activity of non-starter lactic acid bacteria isolates from dairy products. Food Control 2010, 21, 136–142. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Y.; Xie, Q.; Zhang, Y.; Hu, J.; Li, P. Purification and characterization of plantaricin LPL-1, a novel class IIa bacteriocin produced by Lactobacillus plantarum LPL-1 isolated from fermented fish. Front. Microbiol. 2018, 9, 2276. [Google Scholar] [CrossRef]
- An, Y.; Wang, Y.; Liang, X.; Yi, H.; Zuo, Z.; Xu, X.; Zhang, D.; Yu, C.; Han, X. Purification and partial characterization of M1-UVs300, a novel bacteriocin produced by Lactobacillus plantarum isolated from fermented sausage. Food Control 2017, 81, 211–217. [Google Scholar] [CrossRef]
- Zhu, H.; Han, L.; Ni, Y.; Yu, Z.; Wang, D.; Zhou, J.; Li, B.; Zhang, W.; He, K. In vitro and in vivo antibacterial effects of Nisin against Streptococcus suis. Probiotics Antimicrob. Proteins 2021, 13, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Teng, K.; Liu, Y.; Cao, Y.; Wang, T.; Ma, C.; Zhang, J.; Zhong, J. Bacteriocins: Potential for human health. Oxid. Med. Cell Longev. 2021, 2021, 5518825. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jung, Y.G.; Jin, Y.Y.; Jayabalan, R.; Yang, S.H.; Suh, J.W. Bacteriocins as food preservatives: Challenges and emerging horizons. Crit. Rev. Food Sci. 2018, 58, 2743–2767. [Google Scholar] [CrossRef] [PubMed]
- Vijay Simha, B.; Sood, S.K.; Kumariya, R.; Garsa, A.K. Simple and rapid purification of pediocin PA-1 from Pediococcus pentosaceous NCDC 273 suitable for industrial application. Microbiol. Res. 2012, 167, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, P.K.; Ekblad, B.; Kristiansen, P.E.; Kaznessis, Y.N. Interactions of a class IIb bacteriocin with a model lipid bilayer, investigated through molecular dynamics simulations. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 824–835. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Maheshwari, D.K.; Bajpai, V.K. Isolation and preliminary characterization of a bacteriocin-producer Bacillus strain inhibiting methicillin resistant Staphylococcus aureus. Acta Biol. Hung. 2017, 68, 208–219. [Google Scholar] [CrossRef][Green Version]
- Mercado, V.; Olmos, J. Bacteriocin production by Bacillus Species: Isolation, characterization, and application. Probiotics Antimicrob. Proteins 2022, 14, 1151–1169. [Google Scholar] [CrossRef]
- Kapse, N.G.; Engineer, A.S.; Gowdaman, V.; Wagh, S.; Dhakephalkar, P.K. Functional annotation of the genome unravels probiotic potential of Bacillus coagulans HS243. Genomics 2019, 111, 921–929. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Goker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Goker, M. TYGS and LPSN: A database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef]
- Richter, M.; Rossello-Mora, R.; Oliver Glockner, F.; Peplies, J. JSpeciesWS: A web server for prokaryotic species circumscription based on pairwise genome comparison. Bioinformatics 2016, 32, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Sanchez, B.; de Los Reyes-Gavilan, C.G.; Margolles, A. Antibiotic resistance in probiotic bacteria. Front Microbiol. 2013, 4, 202. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zheng, D.; Jin, Q.; Chen, L.; Yang, J. VFDB 2019: A comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019, 47, D687–D692. [Google Scholar] [CrossRef]
- Ruiz, L.; Ruas-Madiedo, P.; Gueimonde, M.; de los Reyes-Gavilan, C.G.; Margolles, A.; Sanchez, B. How do bifidobacteria counteract environmental challenges? Mechanisms involved and physiological consequences. Genes Nutr. 2011, 6, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Padan, E.; Venturi, M.; Gerchman, Y.; Dover, N. Na+/H+ antiporters. Biochim. Biophys. Acta 2001, 1505, 144–157. [Google Scholar] [CrossRef]
- D’Souza, S.; Garcia-Cabado, A.; Yu, F.; Teter, K.; Lukacs, G.; Skorecki, K.; Moore, H.P.; Orlowski, J.; Grinstein, S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J. Biol. Chem. 1998, 273, 2035–2043. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sanchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef]
- Granato, D.; Bergonzelli, G.E.; Pridmore, R.D.; Marvin, L.; Rouvet, M.; Corthesy-Theulaz, I.E. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 2004, 72, 2160–2169. [Google Scholar] [CrossRef]
- Khatri, I.; Sharma, S.; Ramya, T.N.; Subramanian, S. Complete genomes of Bacillus coagulans S-lac and Bacillus subtilis TO-A JPC, two phylogenetically distinct probiotics. PLoS ONE 2016, 11, e0156745. [Google Scholar] [CrossRef]
- Haiko, J.; Westerlund-Wikstrom, B. The role of the bacterial flagellum in adhesion and virulence. Biology 2013, 2, 1242–1267. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Nair, S.; Pallas, J.; Williams, M.A. Fibronectin: A multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol. Rev. 2011, 35, 147–200. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligne, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.; Kuipers, A.; Karsens, H.; Nauta, A.; Kuipers, O.; Kok, J. Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Appl. Environ. Microbiol. 2003, 69, 1589–1597. [Google Scholar] [CrossRef]
- Scholz, R.; Vater, J.; Budiharjo, A.; Wang, Z.; He, Y.; Dietel, K.; Schwecke, T.; Herfort, S.; Lasch, P.; Borriss, R. Amylocyclicin, a novel circular bacteriocin produced by Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2014, 196, 1842–1852. [Google Scholar] [CrossRef]
- Havarstein, L.S.; Diep, D.B.; Nes, I.F. A family of bacteriocin Abc transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 1995, 16, 229–240. [Google Scholar] [CrossRef]
- Johny, L.C.; Suresh, P.V. Complete genome sequencing and strain characterization of a novel marine Bacillus velezensis FTL7 with a potential broad inhibitory spectrum against foodborne pathogens. World J. Microbiol. Biotechnol. 2022, 38, 164. [Google Scholar] [CrossRef]
- Teng, K.; Zhang, J.; Zhang, X.; Ge, X.; Gao, Y.; Wang, J.; Lin, Y.; Zhong, J. Identification of ligand specificity determinants in Lantibiotic bovicin HJ50 and the receptor BovK, a multitransmembrane histidine kinase. J. Biol. Chem. 2014, 289, 9823–9832. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).