Protease-Assisted Mild Extraction of Soluble Fibre and Protein from Fruit By-Products: A Biorefinery Perspective
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fruit By-Products Collection and Characterization
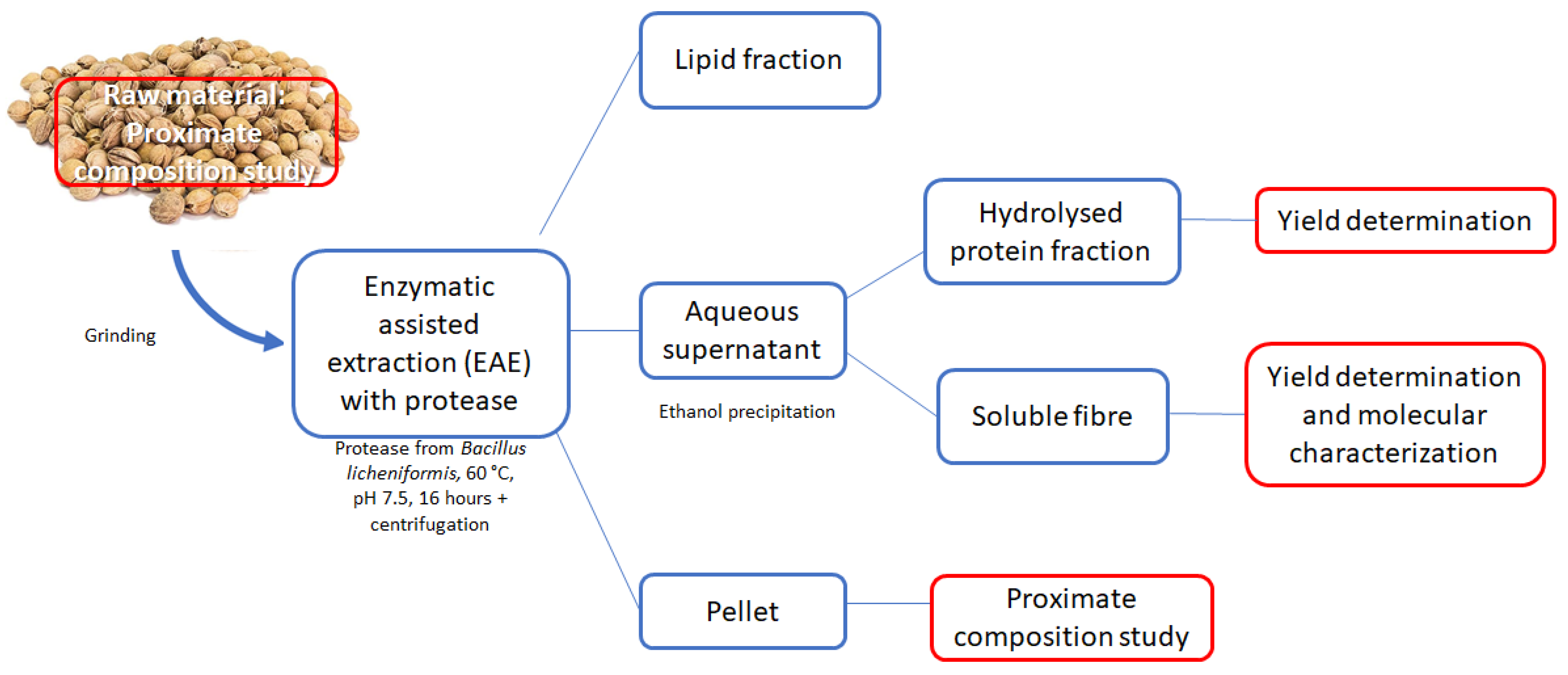
2.3. Protease Assisted Extraction
2.4. Characterization of Soluble Fibre
2.4.1. Residual Ash and Protein
2.4.2. Molecular Weight
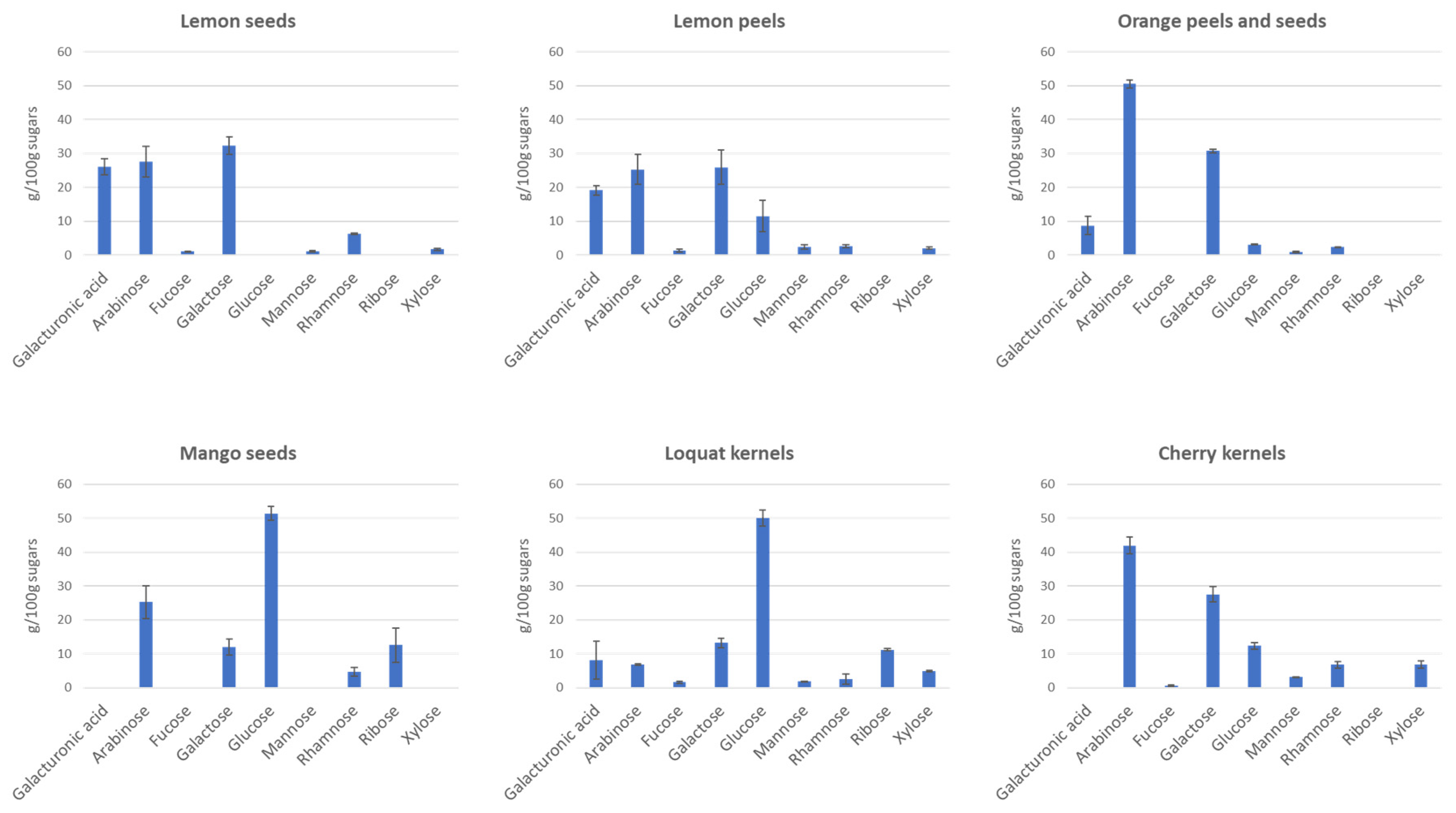
2.4.3. Monosaccharide Composition
2.5. Proximate Composition of Residual Pellet after EAE
2.6. Determination of Extractions Yields
3. Results and Discussion
3.1. Proximate Composition of the Raw Materials (Fruit By-Products)
3.2. Yield Determination for Soluble Fibre and Protein after Protease Assisted Extraction
3.3. Molecular Weight of Soluble Fibre
3.4. Monosaccharide Composition
3.5. Proximate Composition of Residual Pellet
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Food Wastage Footprint: Impacts on Natural Resources—Summary Report; FAO: Rome, Italy, 2013. [Google Scholar]
- Moreno-González, M.; Ottens, M. A Structured Approach to Recover Valuable Compounds from Agri-food Side Streams. Food Bioprocess Technol. 2021, 14, 1387–1406. [Google Scholar] [CrossRef]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Manhongo, T.T.; Chimphango, A.F.A.; Thornley, P.; Röder, M. Current status and opportunities for fruit processing waste biorefineries. Renew. Sustain. Energy Rev. 2022, 155, 111823. [Google Scholar] [CrossRef]
- Kosseva, M.R.; Webb, C. Food Industry Wastes; Academic Press: New York, NY, USA; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 9780123919212. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Henríquez, C.; Speisky, H.; Chiffelle, I.; Valenzuela, T.; Araya, M.; Simpson, R.; Almonacid, S. Development of an ingredient containing apple peel, as a source of polyphenols and dietary fiber. J. Food Sci. 2010, 75, H172–H181. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, Y.; Kawabata, J. Evaluation of antioxidant capacity of non-edible parts of some selected tropical fruits. Food Sci. Technol. Res. 2010, 16, 467–472. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially- available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Alhanif, M.; Wardhani, D.H. A Critical Review on Tropical Fruits Seeds as Prospective Sources of Nutritional and Bioactive Compounds for Functional Foods Development: A Case of Indonesian Exotic Fruits. Int. J. Food Sci. 2020, 2020, 4051475. [Google Scholar] [CrossRef]
- Market Data Forecast. Europe Bioactive Ingredients Market by Type by Application and by Region—Industry Analysis, Size, Share, Growth, Trends, and Forecasts (2018–2023). Available online: https://www.marketdataforecast.com/market-reports/europe-bioactive-ingredients-market (accessed on 27 September 2019).
- Anonymous. Market Research Report; ID: ZCFB1988134EN; Global Industry Analysts, Inc.: San Jose, CA, USA, 2019. [Google Scholar]
- AOAC International. AOAC Official Method of Analysis, 16th ed.; Association of Official Analytical: Washington, DC, USA, 2002. [Google Scholar]
- AOAC. AOAC Official Method 991.43 Total, Soluble, and Insoluble Dietary Fibre in Foods. J. AOAC Int. 2012, 95, 824–844. [Google Scholar] [CrossRef]
- Fuso, A.; Rosso, F.; Rosso, G.; Risso, D.; Manera, I.; Caligiani, A. Production of Xylo-Oligosaccharides (XOS) of Tailored Degree of Polymerization from Acetylated Xylans through Modelling of Enzymatic Hydrolysis. Food Res. Int. 2022, 162, 112019. [Google Scholar] [CrossRef]
- Patiño-Rodríguez, O.; Agama-Acevedo, E.; Ramos-Lopez, G.; Bello-Pérez, L.A. Unripe mango kernel starch: Partial characterization. Food Hydrocoll. 2020, 101, 105512. [Google Scholar] [CrossRef]
- Kasapoğlu, K.N.; Demircan, E.; Eryılmaz, H.S.; Karaça, A.C.; Özçelik, B. Sour Cherry Kernel as an Unexploited Processing Waste: Optimisation of Extraction Conditions for Protein Recovery, Functional Properties and In Vitro Digestibility. Waste Biomass Valor. 2021, 12, 6685–6698. [Google Scholar] [CrossRef]
- Çelik, M.; Güzel, M.; Yildirim, M. Effect of pH on protein extraction from sour cherry kernels and functional properties of resulting protein concentrate. J. Food Sci. Technol. 2019, 56, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de Moreno de LeBlanc, A.; Saad, S.M.I.; LeBlanc, J.G. Tropical fruit by-products water extracts of tropical fruit by-products as sources of soluble fibres and phenolic compounds with potential antioxidant, anti-inflammatory, and functional properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Thakur, A. Extraction and evaluation of structural and physicochemical properties of dietary fiber concentrate from mango peels by using green approach. Biomass Conv. Bioref. 2021, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Cui, Y.; Qu, X. Exopolysaccharides of lactic acid bacteria: Structure, bioactivity and associations: A review. Carbohydr. Polym. 2019, 207, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G. Molecular aspects of cereal β-glucan functionality: Physical properties, technological applications and physiological effects. J. Cereal Sci. 2007, 46, 101–118. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Khodaiyan, F.; Kazemi, M.; Najari, Z. Optimization and characterization of pectin extracted from sour orange peel by ultrasound assisted method. Int. J. Biol. Macromol. 2019, 125, 621–629. [Google Scholar] [CrossRef]
- Mañas, E.; Saura-Calixto, F. Ethanolic precipitation: A source of error in dietary fibre determination. Food Chem. 1993, 47, 351–355. [Google Scholar] [CrossRef]
- Thu Dao, T.A.; Webb, H.K.; Malherbe, F. Optimization of pectin extraction from fruit peels by response surface method: Conventional versus microwave-assisted heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- Hamed, M.; Coelho, E.; Bastos, R.; Evtuguin, D.V.; Ferreira, S.S.; Lima, T.; Vilanova, M.; Sila, A.; Coimbra, M.A.; Bougatef, A. Isolation and identification of an arabinogalactan extracted from pistachio external hull: Assessment of immunostimulatory activity. Food Chem. 2022, 373, 131416. [Google Scholar] [CrossRef] [PubMed]
- Simas-Tosin, F.F.; Abud, A.P.R.; De Oliveira, C.C.; Gorin, P.A.J.; Sassaki, G.L.; Bucchi, D.F.; Iacomini, M. Polysaccharides from peach pulp: Structure and effects on mouse peritoneal macrophages. Food Chem. 2012, 134, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Szczuka, E.; Wydrych, J.; Zdunek, A. Changes in arabinogalactan proteins (AGPs) distribution in apple (Malus x domestica) fruit during senescence. Postharvest Biol. Technol. 2018, 138, 99–106. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Marais, M.F.; Vignon, M.R. An arabinogalactan from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Leivas, C.L.; Iacomini, M.; Cordeiro, L.M.C. Structural characterization of a rhamnogalacturonan I-arabinan-type I arabinogalactan macromolecule from starfruit (Averrhoa carambola L.). Carbohydr. Polym. 2015, 121, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, W.; Qiao, X.; Cui, H.; Yang, X.; Xue, C. Structural characterization of arabinogalactan extracted from Ixeris chinensis (Thunb.) Nakai and its immunomodulatory effect on RAW264.7 macrophages. Int. J. Biol. Macromol 2020, 143, 977–983. [Google Scholar] [CrossRef]
- Islam, A.M.; Phillips, G.O.; Sljivo, A.; Snowden, M.J.; Williams, P.A. A review of recent developments on the regulatory, structural and functional aspects of gum arabic. Food Hydrocoll. 1997, 11, 493–505. [Google Scholar] [CrossRef]
- Han, L.; Hu, B.; Ma, R.; Gao, Z.; Nishinari, K.; Phillips, G.O.; Yang, J.; Fang, Y. Effect of arabinogalactan protein complex content on emulsification performance of gum arabic. Carbohydr. Polym. 2019, 224, 115170. [Google Scholar] [CrossRef]
- Saeidy, S.; Petera, B.; Pierre, G.; Fenoradosoa, T.A.; Djomdi, D.; Michaud, P.; Delattre, C. Plants arabinogalactans: From structures to physico-chemical and biological properties. Biotechnol. Adv. 2021, 53, 107771. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Cepeda, M.R.; Lopes, G.R.; Teixeira-Coelho, M.; Madureira, P.; Nunes, F.M.; Vilanova, M.; Coimbra, M.A. Structural polymeric features that contribute to in vitro immunostimulatory activity of instant coffee. Food Chem. 2018, 242, 548–554. [Google Scholar] [CrossRef]
- Parsons, J.G.; Keeney, P.G.; Patton, S. Identification and Quantitative Analysis of Phospholipids in Cocoa Beans. J. Food Sci. 1969, 34, 497–499. [Google Scholar] [CrossRef]
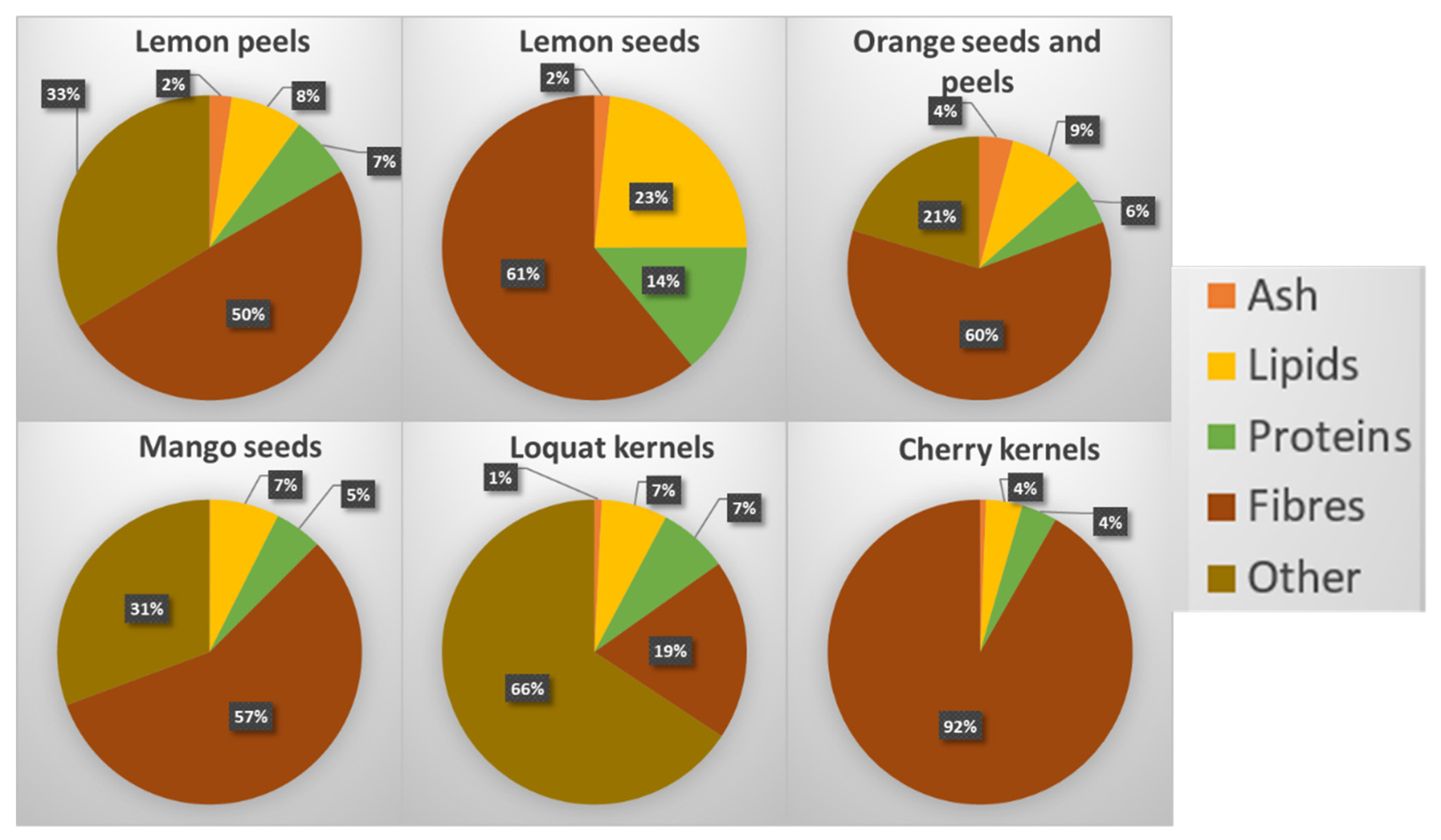
| Ash | Proteins | Lipids | Total Fibre (TDF) | Insoluble Fibre (IDF) | Soluble Fibre (SDF) | Others | |
|---|---|---|---|---|---|---|---|
| Lemon peels | 4.29 ± 0.23 | 7.26 ± 0.47 | 1.62 ± 0.09 | 58.42 ± 2.33 | 37.70 ± 1.23 | 20.72 ± 1.47 | 28.40 ± 1.82 |
| Lemon seeds | 2.45 ± 0.16 | 15.27 ± 1.09 | 6.37 ± 0.19 | 75.91 ± 3.03 | 75.43 ± 4.00 | 3.92 ± 0.23 | - |
| Mango seeds | 1.91 ± 0.06 | 5.11 ± 0.27 | 7.32 ± 0.52 | 37.07 ± 1.10 | 35.97 ± 1.06 | 1.11 ± 0.04 | 48.57 ± 2.57 |
| Loquat kernels | 2.74 ± 0.16 | 6.08 ± 0.36 | 1.12 ± 0.04 | 27.01 ± 1.08 | 14.89 ± 0.48 | 12.11 ± 0.86 | 63.04 ± 3.75 |
| Cherry kernels | 1.48 ± 0.09 | 4.54 ± 0.15 | 7.42 ± 0.22 | 80.90 ± 2.63 | 74.38 ± 2.20 | 6.54 ± 0.35 | 5.64 ± 0.22 |
| Orange peels/seeds | 5.56 ± 0.40 | 4.84 ± 0.34 | 1.91 ± 0.12 | 59.68 ± 1.94 | 26.65 ± 0.87 | 33.03 ± 1.07 | 28.01 ± 1.12 |
| Extraction Yield of Protein (% Respect to the Total Protein in the Raw Sample) | Extraction Yield of Soluble Fibres (% Respect to the Total Soluble Fibre in the Raw Sample) | |
|---|---|---|
| Lemon peels | 93 ± 7 | 6 ± 1 |
| Lemon seeds | 50 ± 2 | 9 ± 1 |
| Mango seeds | 42 ± 6 | 33 ± 2 |
| Loquat kernels | 35 ± 2 | 1.6 ± 0.3 |
| Cherry kernels | 70 ± 2 | 71 ± 5 |
| Orange peels and seeds | 80 ± 2 | 29.8 ± 0.2 |
| Molecular Weight (kDa) | ||||
|---|---|---|---|---|
| <6 | 15–20 | 96–100 | >200 | |
| Peak Area (%) | ||||
| Lemon peels | 2 | 8 | - | 90 |
| Lemon seeds | 5 | - | 19 | 76 |
| Mango seeds | 70 | - | - | 30 |
| Loquat kernels | 21 | - | - | 79 |
| Cherry kernels | 3 | - | - | 97 |
| Orange peels and seeds | 72 | - | - | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuso, A.; Viscusi, P.; Larocca, S.; Sangari, F.S.; Lolli, V.; Caligiani, A. Protease-Assisted Mild Extraction of Soluble Fibre and Protein from Fruit By-Products: A Biorefinery Perspective. Foods 2023, 12, 148. https://doi.org/10.3390/foods12010148
Fuso A, Viscusi P, Larocca S, Sangari FS, Lolli V, Caligiani A. Protease-Assisted Mild Extraction of Soluble Fibre and Protein from Fruit By-Products: A Biorefinery Perspective. Foods. 2023; 12(1):148. https://doi.org/10.3390/foods12010148
Chicago/Turabian StyleFuso, Andrea, Pio Viscusi, Susanna Larocca, Francesco Saverio Sangari, Veronica Lolli, and Augusta Caligiani. 2023. "Protease-Assisted Mild Extraction of Soluble Fibre and Protein from Fruit By-Products: A Biorefinery Perspective" Foods 12, no. 1: 148. https://doi.org/10.3390/foods12010148
APA StyleFuso, A., Viscusi, P., Larocca, S., Sangari, F. S., Lolli, V., & Caligiani, A. (2023). Protease-Assisted Mild Extraction of Soluble Fibre and Protein from Fruit By-Products: A Biorefinery Perspective. Foods, 12(1), 148. https://doi.org/10.3390/foods12010148





