Sensory and Volatile Compounds Characteristics of the Sauce in Bean Paste Fish Treated with Ultra-High-Pressure and Representative Thermal Sterilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
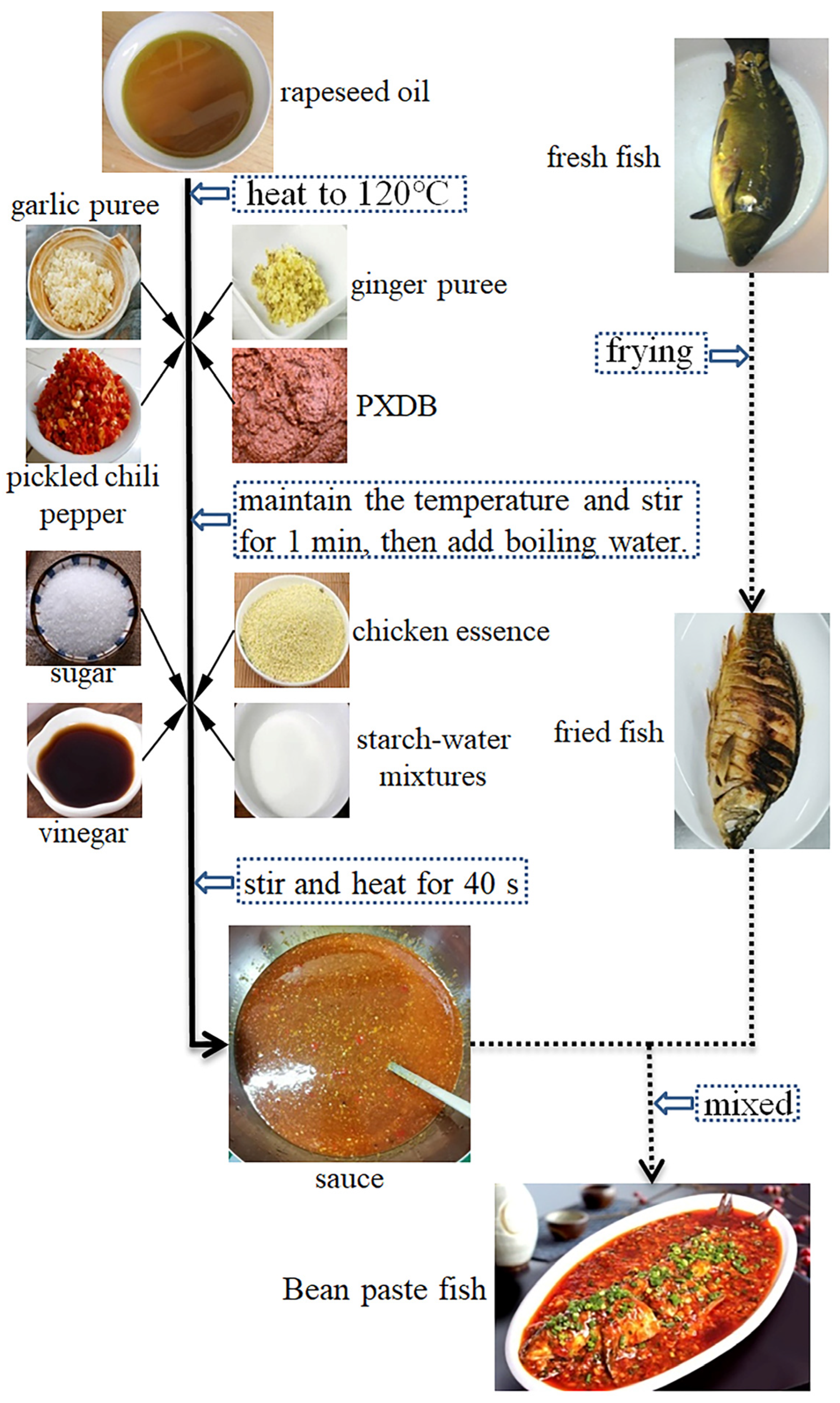
2.2. Sample Preparation
2.3. Quantitative Descriptive Analysis (QDA)
2.4. Characterizing the Volatile Compounds
2.4.1. E-Nose Analysis
2.4.2. Extraction of Volatile Compounds
2.4.3. Identification of Volatile Compounds by Comprehensive Two-Dimensional Gas Chromatography
2.4.4. Determination of Aroma-Active Components by GC-O
2.5. Statistical Analysis
3. Results and Discussion
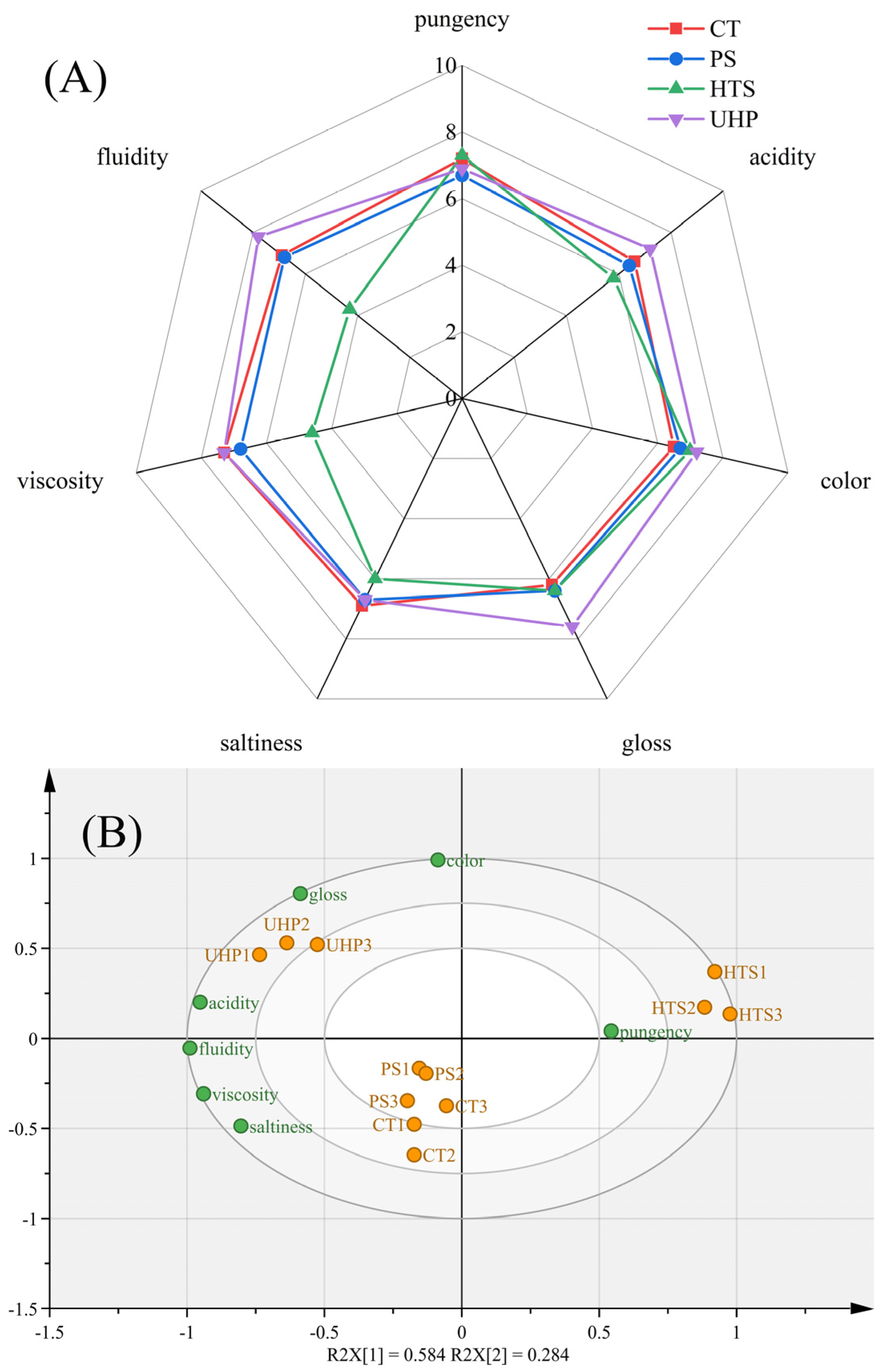
3.1. Quantitative Descriptive Analysis (QDA) of Sauces
3.2. Electronic Nose Analysis
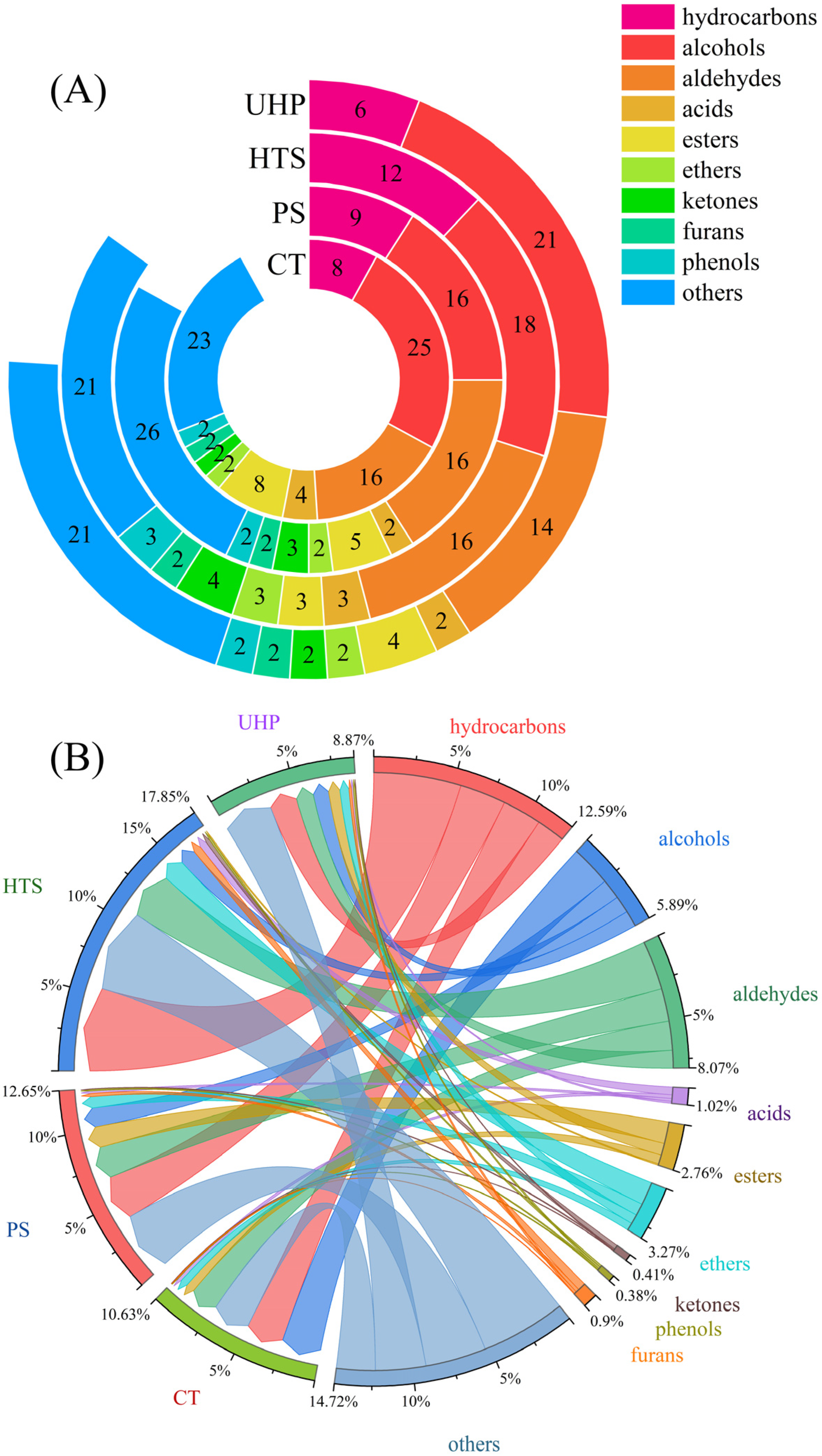
3.3. Characterizing the Volatiles Compounds of the Sauce
3.4. Effect of Sterilization Methods on the Volatile Compounds of Sauces
3.5. Identification of Aroma-active Compounds by GC-O and OAV Analysis
3.6. Cluster Analysis of Volatile Compounds
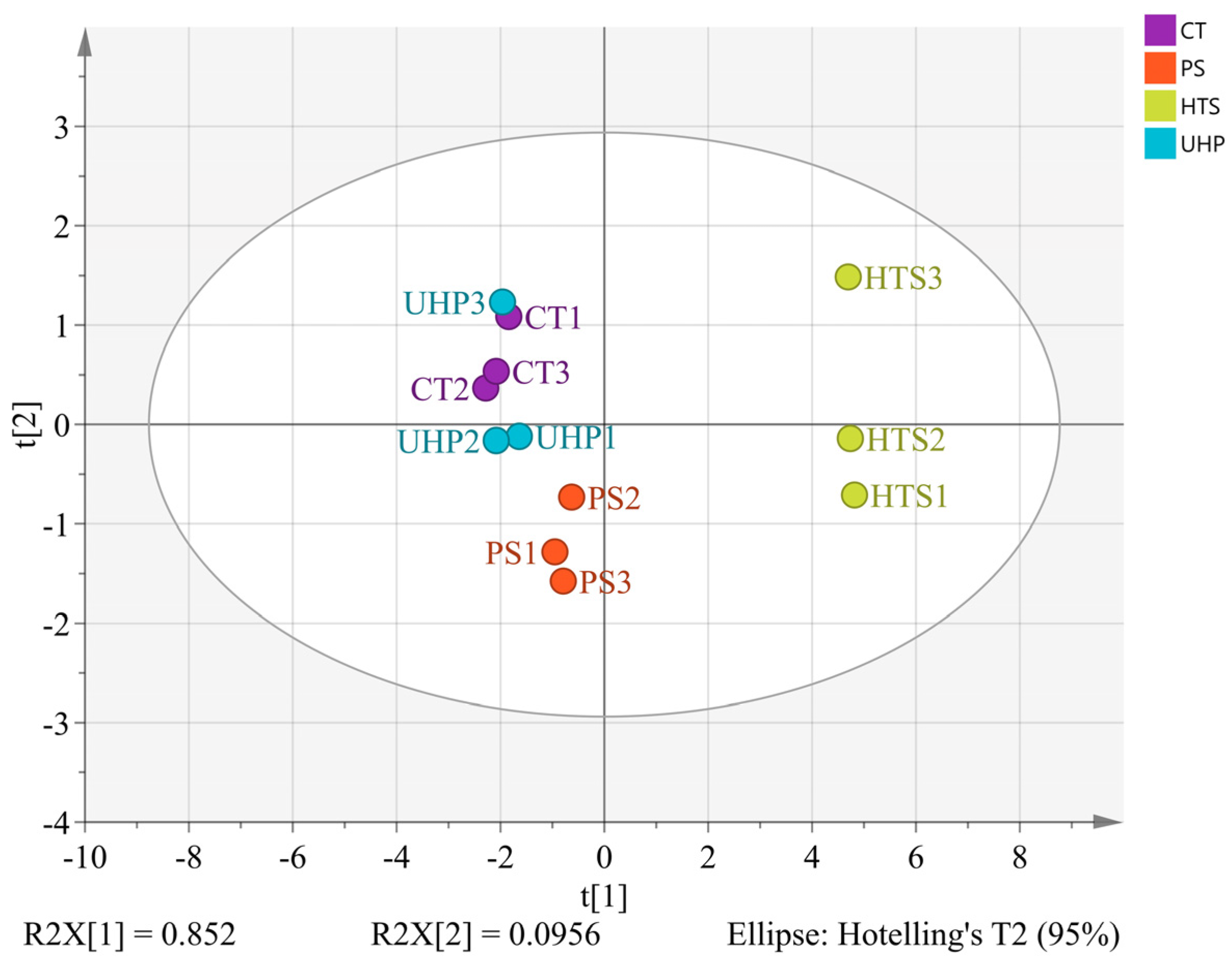
3.7. Discrimination of Flavor Characteristics Based on Various Indicators
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PXDB | Pixian Douban |
| CT | control |
| PS | pasteurized |
| HTS | high-temperature sterilization |
| UHP | ultra-high-pressure treatment |
| HS-SPME | headspace solid-phase microextraction |
| GC×GC-MS | comprehensive two-dimensional gas chromatography-mass spectrometry |
| GC-O | gas chromatograph-olfactometry |
| IS | internal standard |
| NIST | National Institute of Standards and Technology |
| OAV | odor activity value |
| QDA | quantitative descriptive analysis |
| PLS-DA | partial least squares discriminant analysis |
| PCA | principal component analysis |
References
- Sun, Y.; Zhang, L.L.; Zhang, H.; Zhang, Y.; Sun, B.-G. Effects of two sterilization methods on the taste compositions of sweet and sour spare ribs flavor. J. Food Compos. Anal. 2021, 104, 104143. [Google Scholar] [CrossRef]
- Shen, H.; Wei, T.; Zhang, Z.; Zheng, Q.; Guo, R.; Jiang, H.; Zhang, G.; Zheng, J. Discrimination of five brands of instant vermicelli seasonings by HS-SPME/GC-MS and electronic nose. J. Food Sci. Technol.-Mysore 2020, 57, 4160–4170. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ma, M.; Sun, B.; Ren, F.; Chen, H.; Zhang, N.; Zhang, Y. Identification of characteristic aroma components of butter from Chinese butter hotpot seasoning. Food Chem. 2021, 338, 127838. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, M.; Song, H.; Meng, Q.; Guan, X. Characterization of key odor-active compounds in commercial high-salt liquid-state soy sauce by switchable GC/GC × GC-olfactometry-MS and sensory evaluation. Food Chem. 2021, 342, 128224. [Google Scholar] [CrossRef] [PubMed]
- Sushama Babu, P.; Kundukulangara Pulissery, S.; Jaganath, B.; Chitradurga Obaiah, M. Effect of thermal processing on quality of tender jackfruit in tin-free-steel cans. J. Food Sci. Technol.-Mysore 2022, 59, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Truong, B.Q.; Buckow, R.; Nguyen, M.H.; Nguyen, H.T. High pressure thermal sterilization of barramundi (Lates calcarifer) muscles in brine: Effects on selected physicochemical properties. J. Food Process. Preserv. 2021, 45, e15523. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Pataro, G.; Tiwari, B.; Gozzi, M.; Meireles, M.Á.A.; Wang, S.; Guamis, B.; Pan, Z.; Ramaswamy, H.; Sastry, S.; et al. Guidelines on reporting treatment conditions for emerging technologies in food processing. Crit. Rev. Food Sci. Nutr. 2022, 62, 5925–5949. [Google Scholar] [CrossRef]
- Xu, X.; Deng, J.; Luo, D.; Bao, Y.; Liao, X.; Gao, H.; Wu, J. Comparative study of high hydrostatic pressure and high temperature short time processing on quality of clear and cloudy Se-enriched kiwifruit juices. Innov. Food Sci. Emerg. Technol. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Yi, J.; Kebede, B.T.; Grauwet, T.; Loey, A.V.; Hu, X.; Hendrickx, M. A multivariate approach into physicochemical, biochemical and aromatic quality changes of puree based on Hayward kiwifruit during the final phase of ripening. Postharvest Biol. Technol. 2016, 117, 206–216. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Q.; Su, D.; Huang, H. Effects of Ultra-High Pressure Tenderizing Treatment on the Quality Characteristics of Venison. J. Food Process Eng. 2016, 39, 196–203. [Google Scholar] [CrossRef]
- Gao, H.; Zeng, J.; Ma, H.; Wang, Z.; Pan, R. Improving Tenderness of Goose Breast by Ultra-High Pressure. Int. J. Food Prop. 2015, 18, 1693–1701. [Google Scholar] [CrossRef]
- Shao, Y.; Xiong, G.; Ling, J.; Hu, Y.; Shi, L.; Qiao, Y.; Yu, J.; Cui, Y.; Liao, L.; Wu, W.; et al. Effect of ultra-high pressure treatment on shucking and meat properties of red swamp crayfish (Procambarus clarkia). LWT-Food Sci. Technol. 2018, 87, 234–240. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M. Process development of high pressure-based technologies for food: Research advances and future perspectives. Curr. Opin. Food Sci. 2021, 42, 270–277. [Google Scholar] [CrossRef]
- Apichartsrangkoon, A.; Srisajjalertwaja, S.; Chaikham, P.; Hirun, S. Physical and chemical properties of Nam Prig Noom, a Thai green-chili paste, following ultra-high pressure and thermal processes. High Press. Res. 2013, 33, 83–95. [Google Scholar] [CrossRef]
- Park, Y.S.; Ham, K.S.; Park, Y.K.; Leontowicz, H.; Leontowicz, M.; Namieśnik, J.; Katrich, E.; Gorinstein, S. The effects of treatment on quality parameters of smoothie-type ‘Hayward’ kiwi fruit beverages. Food Control 2016, 70, 221–228. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, P.; Zhan, P.; Tian, H.; Lu, C.; Tian, P. Aroma characteristics of cloudy kiwifruit juices treated with high hydrostatic pressure and representative thermal processes. Food Res. Int. 2021, 139, 109841. [Google Scholar] [CrossRef]
- Ding, W.; Liu, Y.; Zhao, X.; Peng, C.; Ye, X.; Che, Z.; Liu, Y.; Liu, P.; Lin, H.; Huang, J.; et al. Characterization of volatile compounds of Pixian Douban fermented in closed system of gradient steady-state temperature field. Food Sci. Nutr. 2021, 9, 2862–2876. [Google Scholar] [CrossRef]
- Xiao, Z.; Ma, S.; Niu, Y.; Chen, F.; Yu, D. Characterization of odour-active compounds of sweet orange essential oils of different regions by gas chromatography-mass spectrometry, gas chromatography-olfactometry and their correlation with sensory attributes. Flavour Fragr. J. 2016, 31, 41–50. [Google Scholar] [CrossRef]
- Ding, W.; Liu, Y.; Peng, C.; Ye, X.; Zhang, M.; Che, Z.; Liu, Y.; Liu, P.; Lin, H.; Xu, M. Characterization of volatile compounds between industrial closed fermentation and traditional open fermentation doubanjiang-meju. Eur. Food Res. Technol. 2021, 247, 2067–2077. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Shi, Y.; Zhang, M.; Liu, Y.; Che, Z.; Lin, H.; Lv, G.; Zhu, Q.; Dong, S.; et al. Flavor quality evaluation of Pixian Douban fermented in the closed system of multi-scale temperature and flow fields. LWT-Food Sci. Technol. 2022, 163, 113598. [Google Scholar] [CrossRef]
- Pu, D.; Zhang, Y.; Zhang, H.; Sun, B.; Ren, F.; Chen, H.; Tang, Y. Characterization of the Key Aroma Compounds in Traditional Hunan Smoke-Cured Pork Leg (Larou, THSL) by Aroma Extract Dilution Analysis (AEDA), Odor Activity Value (OAV), and Sensory Evaluation Experiments. Foods 2020, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Mastello, R.B.; Janzantti, N.S.; Bisconsin-Júnior, A.; Monteiro, M. Impact of HHP processing on volatile profile and sensory acceptance of Pera-Rio orange juice. Innov. Food Sci. Emerg. Technol. 2018, 45, 106–114. [Google Scholar] [CrossRef]
- Gao, L.; Liu, T.; An, X.; Zhang, J.; Ma, X.; Cui, J. Analysis of volatile flavor compounds influencing Chinese-type soy sauces using GC-MS combined with HS-SPME and discrimination with electronic nose. J. Food Sci. Technol.-Mysore 2017, 54, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, Y.; Wang, G.; Tao, L.; Yu, J.; Ai, L. Effects of boiling, ultra-high temperature and high hydrostatic pressure on free amino acids, flavor characteristics and sensory profiles in Chinese rice wine. Food Chem. 2019, 275, 407–416. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, G.; Ma, C.; Li, G.; Wang, R. Effect of ultra-high pressure and ultra-high temperature treatments on the quality of watermelon juice during storage. J. Food Process. Preserv. 2021, 45. [Google Scholar] [CrossRef]
- Lee, S.J.; Ahn, B. Comparison of volatile components in fermented soybean pastes using simultaneous distillation and extraction (SDE) with sensory characterisation. Food Chem. 2009, 114, 600–609. [Google Scholar] [CrossRef]
- Feng, Y.; Cai, Y.; Su, G.; Zhao, H.; Wang, C.; Zhao, M. Evaluation of aroma differences between high-salt liquid-state fermentation and low-salt solid-state fermentation soy sauces from China. Food Chem. 2014, 145, 126–134. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, J.; Regenstein, J.M.; Yin, Y.; Zhou, C. Characterization of taste and aroma compounds in Tianyou, a traditional fermented wheat flour condiment. Food Res. Int. 2018, 106, 156–163. [Google Scholar] [CrossRef]
- Horchani, H.; Aissa, I.; Ouertani, S.; Zarai, Z.; Gargouri, Y.; Sayari, A. Staphylococcal lipases: Biotechnological applications. J. Mol. Catal. B-Enzym. 2012, 76, 125–132. [Google Scholar] [CrossRef]
- Li, Q.; Shi, X.; Zhao, Q.; Cui, Y.; Ouyang, J.; Xu, F. Effect of cooking methods on nutritional quality and volatile compounds of Chinese chestnut (Castanea mollissima Blume). Food Chem. 2016, 201, 80–86. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Ren, H.; Yang, H.; Liu, Y.; Gao, Z.; Long, F. Changes in Physicochemical Properties and Volatiles of Kiwifruit Pulp Beverage Treated with High Hydrostatic Pressure. Foods 2020, 9, 485. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Quek, S.-Y.; Stevenson, R.J.; Winz, R.A. Kiwifruit flavour: A review. Trends Food Sci. Technol. 2012, 24, 82–91. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Qiao, Y.; Liao, L.; Shi, D.; Wang, J.; Shi, L. Effects of ultrasound and ultra-high pressure pretreatments on volatile and taste compounds of vacuum-freeze dried strawberry slice. LWT-Food Sci. Technol. 2022, 160, 113232. [Google Scholar] [CrossRef]
- Liu, P.; Xiang, Q.; Gao, L.; Wang, X.; Li, J.; Cui, X.; Lin, J.; Che, Z. Effects of Different Fermentation Strains on the Flavor Characteristics of Fermented Soybean Curd. J. Food Sci. 2019, 84, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Mall, V.; Sellami, I.; Schieberle, P. New Degradation Pathways of the Key Aroma Compound 1-Penten-3-one during Storage of Not-from-Concentrate Orange Juice. J. Agric. Food Chem. 2018, 66, 11083–11091. [Google Scholar] [CrossRef]
- Liu, C.; Yang, P.; Wang, H.; Song, H. Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS. LWT-Food Sci. Technol. 2022, 154, 112689. [Google Scholar] [CrossRef]
- Wang, M.-Q.; Ma, W.-J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.-P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
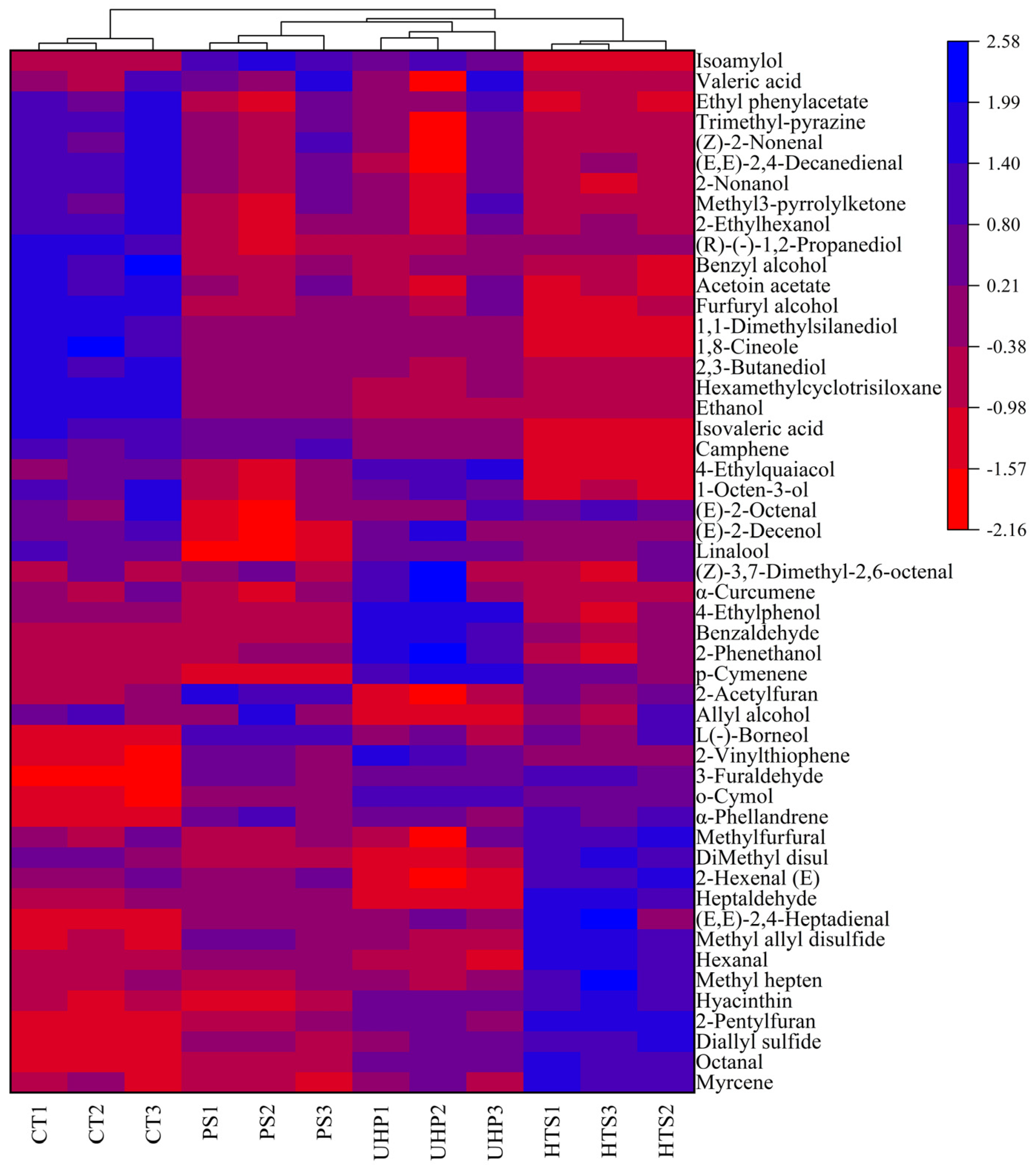
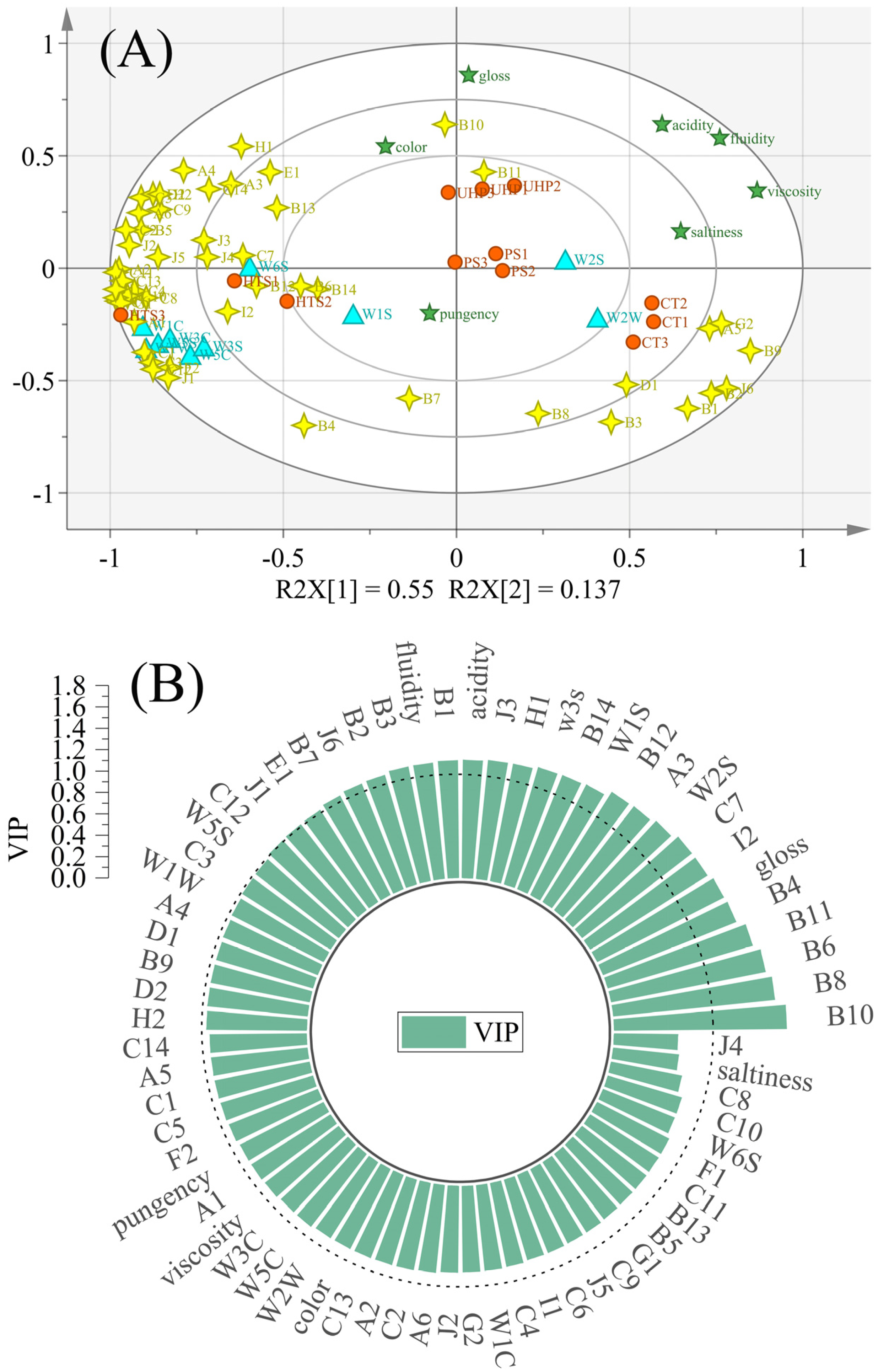
| No. | Compound | CT | PS | HTS | UHP a |
|---|---|---|---|---|---|
| A1 | Myrcene | 19.225 ± 0.253 | 21.754 ± 0.467 | 68.56 ± 0.593 | 25.396 ± 1.089 |
| A2 | α-Phellandrene | 14.429 ± 1.439 | 58.687 ± 1.195 | 94.679 ± 0.892 | 43.084 ± 1.744 |
| A3 | p-Cymenene | 6.332 ± 1.375 | ND # | 41.343 ± 15.921 | 43.454 ± 8.84 |
| A4 | α-Curcumene | 2.699 ± 1.659 | 7.161 ± 1.917 | 11.882 ± 1.581 | 10.774 ± 1.809 |
| A5 | Camphene | 346.032 ± 119.564 | 294.264 ± 88.074 | ND | 129.521 ± 37.397 |
| A6 | o-Cymol | 76.922 ± 11.96 | 235.538 ± 48.891 | 346.819 ± 52.286 | 247.077 ± 62.435 |
| B1 | Ethanol | 140.662 ± 45.906 | 32.233 ± 8.803 | 7.053 ± 1.456 | 8.26 ± 1.265 |
| B2 | 1,8-Cineole | 225.333 ± 41.125 | 87.021 ± 12.099 | 37.214 ± 9.329 | 59.486 ± 13.598 |
| B3 | 2,3-Butanediol | 139.294 ± 44.98 | 72.728 ± 13.562 | 64.817 ± 10.991 | 49.047 ± 14.31 |
| B4 | Allyl alcohol | 18.162 ± 2.35 | 21.366 ± 4.65 | 29.302 ± 3.605 | 4.693 ± 1.127 |
| B5 | 2-Phenethanol | 13.567 ± 2.925 | 19.955 ± 3.428 | 27.974 ± 2.813 | 22.145 ± 2.331 |
| B6 | Linalool | 11.759 ± 2.082 | ND | 18.251 ± 1.457 | 14.294 ± 1.557 |
| B7 | Furfuryl alcohol | 13.564 ± 2.409 | 9.837 ± 2.429 | 12.823 ± 1.384 | 10.098 ± 2.21 |
| B8 | (R)-(-)-1,2-Propanediol | 19.778 ± 2.242 | ND | 10.708 ± 1.515 | 5.726 ± 1.799 |
| B9 | 1,1-Dimethylsilanediol | 30.907 ± 4.773 | 10.188 ± 1.854 | ND | 10.256 ± 1.685 |
| B10 | Isoamylol | ND | 25.355 ± 1.89 | 4.933 ± 1.31 | 16.884 ± 1.128 |
| B11 | 1-Octen-3-ol | 3.031 ± 1.731 | ND | 2.184 ± 1.372 | 6.335 ± 1.395 |
| B12 | 2-Ethylhexanol | 2.78 ± 1.394 | ND | 5.633 ± 1.444 | 4.079 ± 1.438 |
| B13 | 2-Nonanol | 2.031 ± 0.836 | 3.282 ± 1.423 | 4.026 ± 0.7 | 3.788 ± 1.578 |
| B14 | Benzyl alcohol | 1.841 ± 0.92 | ND | 2.88 ± 1.347 | 2.269 ± 0.983 |
| C1 | Hexanal | 39.014 ± 8.473 | 94.861 ± 16.756 | 245.455 ± 62.34 | 30.105 ± 4.762 |
| C2 | 3-Furaldehyde | ND | 83.814 ± 13.866 | 138.168 ± 30.258 | 72.846 ± 13.998 |
| C3 | Heptaldehyde | 7.621 ± 1.441 | 23.502 ± 5.071 | 70.486 ± 14.993 | ND |
| C4 | (E)-2-Octenal | 4.976 ± 1.867 | 6.257 ± 1.908 | 17.556 ± 3.935 | 9.061 ± 1.618 |
| C5 | Benzaldehyde | 7.279 ± 1.454 | 12.626 ± 1.517 | 21.744 ± 2.451 | 17.696 ± 1.917 |
| C6 | Methylfurfural | 4.04 ± 1.354 | 9.911 ± 1.966 | 22.758 ± 2.665 | 8.627 ± 2.277 |
| C7 | (E)-2-Decenol | 5.158 ± 1.669 | ND | 12.687 ± 1.953 | 9.579 ± 1.453 |
| C8 | (E,E)-2,4-Decanedienal | 2.895 ± 0.869 | 4.639 ± 1.383 | 8.017 ± 1.752 | 4.327 ± 1.541 |
| C9 | (Z)-2-Nonenal | ND | 2.628 ± 1.436 | 4.674 ± 1.346 | 3.142 ± 1.421 |
| C10 | (E,E)-2,4-Heptadienal | 9.115 ± 2.102 | 21.593 ± 3.44 | 43.182 ± 13.778 | 18.107 ± 1.582 |
| C11 | Octanal | 8.486 ± 1.453 | 20.545 ± 3.157 | 52.979 ± 10.234 | 24.639 ± 4.104 |
| C12 | 2-Hexenal (E) | 3.583 ± 1.381 | 10.323 ± 1.742 | 30.29 ± 4.059 | ND |
| C13 | Hyacinthin | 4.57 ± 1.333 | 9.762 ± 1.973 | 47.242 ± 9.864 | 19.307 ± 3.058 |
| C14 | (Z)-3,7-Dimethyl-2,6-octenal | 19.674 ± 1.874 | 25.532 ± 1.347 | 34.466 ± 3.189 | 29.824 ± 4.037 |
| D1 | Isovaleric acid | 45.522 ± 12.588 | 35.814 ± 6.488 | 23.574 ± 6.401 | 24.677 ± 3.587 |
| D2 | Valeric acid | ND | 7.18 ± 1.529 | 9.599 ± 1.61 | 6.589 ± 1.764 |
| E1 | Ethyl phenylacetate | 2.287 ± 1.337 | 3.247 ± 1.743 | 5.039 ± 1.873 | 6.18 ± 1.329 |
| F1 | Diallyl sulfide | 50.563 ± 8.873 | 108.325 ± 17.905 | 247.839 ± 47.325 | 102.679 ± 23.25 |
| F2 | Methyl allyl disulfide | 75.27 ± 15.856 | 94.173 ± 13.776 | 149.808 ± 36.008 | 62.935 ± 13.382 |
| G1 | Methyl hepten | 3.849 ± 1.24 | 10.084 ± 1.96 | 32.38 ± 8.886 | 10.926 ± 2.341 |
| G2 | Acetoin acetate | 5.415 ± 1.099 | 2.492 ± 1.362 | ND | 2.366 ± 1.348 |
| H1 | 4-Ethylquaiacol | 5.787 ± 1.254 | 8.117 ± 2.486 | 11.791 ± 2.238 | 13.579 ± 2.268 |
| H2 | 4-Ethylphenol | 10.198 ± 1.646 | 13.397 ± 1.653 | 21.209 ± 1.727 | 18.731 ± 2.662 |
| I1 | 2-Pentylfuran | 7.862 ± 1.87 | 33.258 ± 8.989 | 114.378 ± 21.602 | 39.217 ± 5.01 |
| I2 | 2-Acetylfuran | 1.95 ± 0.829 | 22.821 ± 3.273 | 20.444 ± 3.135 | 2.843 ± 1.871 |
| J1 | DiMethyl disul | 16.87 ± 2.024 | 14.791 ± 2.659 | 39.654 ± 8.906 | 11.475 ± 1.434 |
| J2 | 2-Vinylthiophene | 12.665 ± 1.453 | 26.068 ± 3.248 | 34.628 ± 5.479 | 23.231 ± 2.87 |
| J3 | L(-)-Borneol | ND | 43.117 ± 9.886 | 45.019 ± 4.566 | 19.815 ± 2.541 |
| J4 | Trimethyl-pyrazine | 2.015 ± 0.795 | 3.207 ± 1.476 | 4.677 ± 1.244 | 3.418 ± 1.566 |
| J5 | Methyl3-pyrrolylketone | 2.371 ± 1.095 | 3.06 ± 1.201 | 7.276 ± 1.501 | 4.85 ± 1.594 |
| J6 | Hexamethylcyclotrisiloxane | 22.686 ± 3.634 | 6.266 ± 1.46 | ND | 3.362 ± 1.354 |
| Total | 1470.069 ± 370.786 | 1660.779 ± 327.338 | 2308.1 ± 425.314 | 1320.799 ± 271.657 |
| Compounds | CAS | RI | OAV | Odor Quality | |||
|---|---|---|---|---|---|---|---|
| CT | PS | HTS | HHP | ||||
| esters | |||||||
| Ethyl benzoate | 93-89-0 | 1577 | ND | ND | <1 | <1 | Floral, fruity |
| Ethyl phenylacetate | 101-97-3 | 1690 | <1 | <1 | <1 | <1 | Honey, rose |
| elcohols | |||||||
| 1,8-Cineole | 470-82-6 | 1162 | 204.85 | 79.11 | 33.83 | 54.08 | Eucalyptus, minty, balsamic |
| 1-Octen-3-ol | 3391-86-4 | 1386 | 2.02 | ND | 1.46 | 4.22 | Mushroom-like |
| 2-Nonanol | 628-99-9 | 1455 | <1 | <1 | <1 | <1 | Floral |
| Linalool | 78-70-6 | 1478 | 36.75 | ND | 57.03 | 44.67 | Sweet, flower |
| Furfuryl alcohol | 98-00-0 | 1566 | <1 | <1 | <1 | <1 | Burnt sugar |
| Benzyl alcohol | 100-51-6 | 1766 | <1 | ND | <1 | <1 | Sweet, flower |
| Phenethyl alcohol | 60-12-8 | 1801 | <1 | <1 | <1 | <1 | Rose-like, floral |
| aldehydes | |||||||
| Hexanal | 66-25-1 | 1025 | 7.80 | 18.97 | 49.09 | 6.02 | Cut grass, green |
| (E)-2-Octenal | 2548-87-0 | 1364 | 1.66 | 2.09 | 5.85 | 3.02 | green leaf |
| Benzaldehyde | 100-52-7 | 1437 | <1 | <1 | <1 | <1 | Roasted nuts |
| ketones | |||||||
| 2-Nonanone | 821-55-6 | 1331 | ND | <1 | <1 | ND | Fruits-like, flowers-like |
| furans | |||||||
| 2-Pentylfuran | 3777-69-3 | 1179 | 1.36 | 5.73 | 19.72 | 6.76 | Bean, fruit, green |
| 2-Acetylfuran | 1192-62-7 | 1419 | <1 | <1 | <1 | <1 | Roasted, smoky |
| pyrazines | |||||||
| Trimethyl-pyrazine | 14667-55-1 | 1339 | <1 | <1 | <1 | <1 | Roast |
| thioether | |||||||
| Dimethyl trisulfide | 3658-80-8 | 1306 | 75.71 | 122.23 | 382.09 | 116.18 | Mint smell, fresh onion-like |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Zhang, Y.; Chen, Y.; Zheng, Y.; Peng, C.; Lin, H.; Che, Z.; Ding, W. Sensory and Volatile Compounds Characteristics of the Sauce in Bean Paste Fish Treated with Ultra-High-Pressure and Representative Thermal Sterilization. Foods 2023, 12, 109. https://doi.org/10.3390/foods12010109
Zhao J, Zhang Y, Chen Y, Zheng Y, Peng C, Lin H, Che Z, Ding W. Sensory and Volatile Compounds Characteristics of the Sauce in Bean Paste Fish Treated with Ultra-High-Pressure and Representative Thermal Sterilization. Foods. 2023; 12(1):109. https://doi.org/10.3390/foods12010109
Chicago/Turabian StyleZhao, Jie, Yimao Zhang, Yu Chen, Yuhui Zheng, Changbo Peng, Hongbin Lin, Zhenming Che, and Wenwu Ding. 2023. "Sensory and Volatile Compounds Characteristics of the Sauce in Bean Paste Fish Treated with Ultra-High-Pressure and Representative Thermal Sterilization" Foods 12, no. 1: 109. https://doi.org/10.3390/foods12010109
APA StyleZhao, J., Zhang, Y., Chen, Y., Zheng, Y., Peng, C., Lin, H., Che, Z., & Ding, W. (2023). Sensory and Volatile Compounds Characteristics of the Sauce in Bean Paste Fish Treated with Ultra-High-Pressure and Representative Thermal Sterilization. Foods, 12(1), 109. https://doi.org/10.3390/foods12010109





