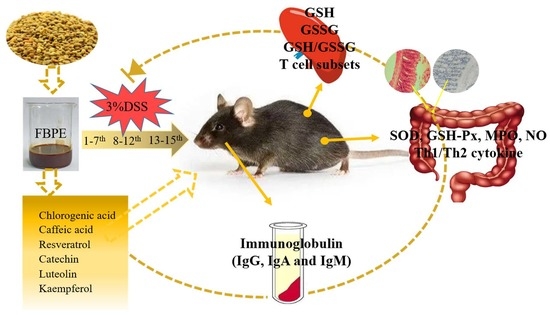
Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparative and Phenolic Composition Analyses of FBPE
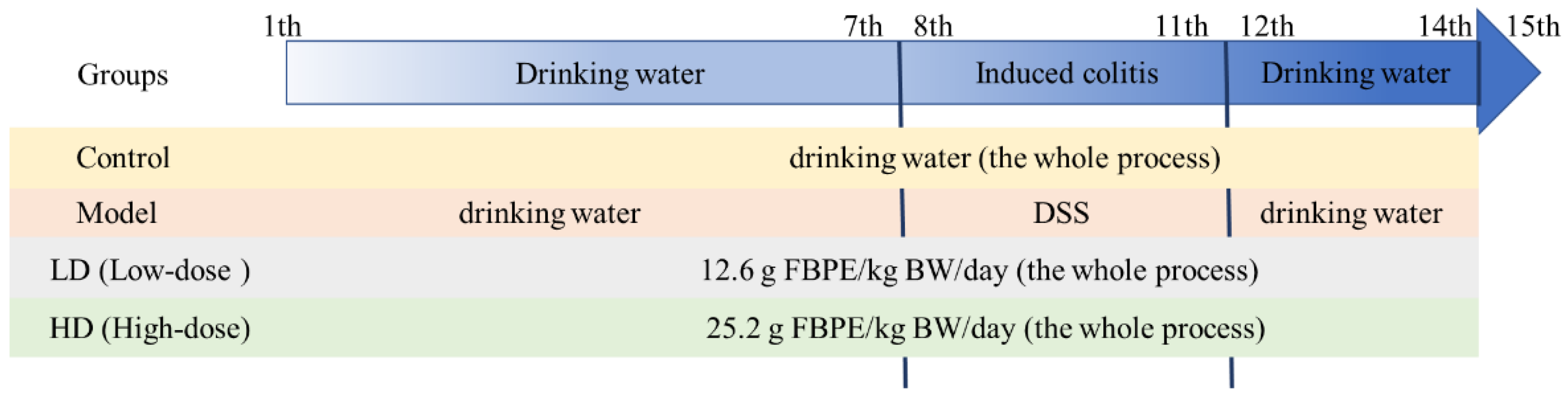
2.2. Animals and Experimental Design
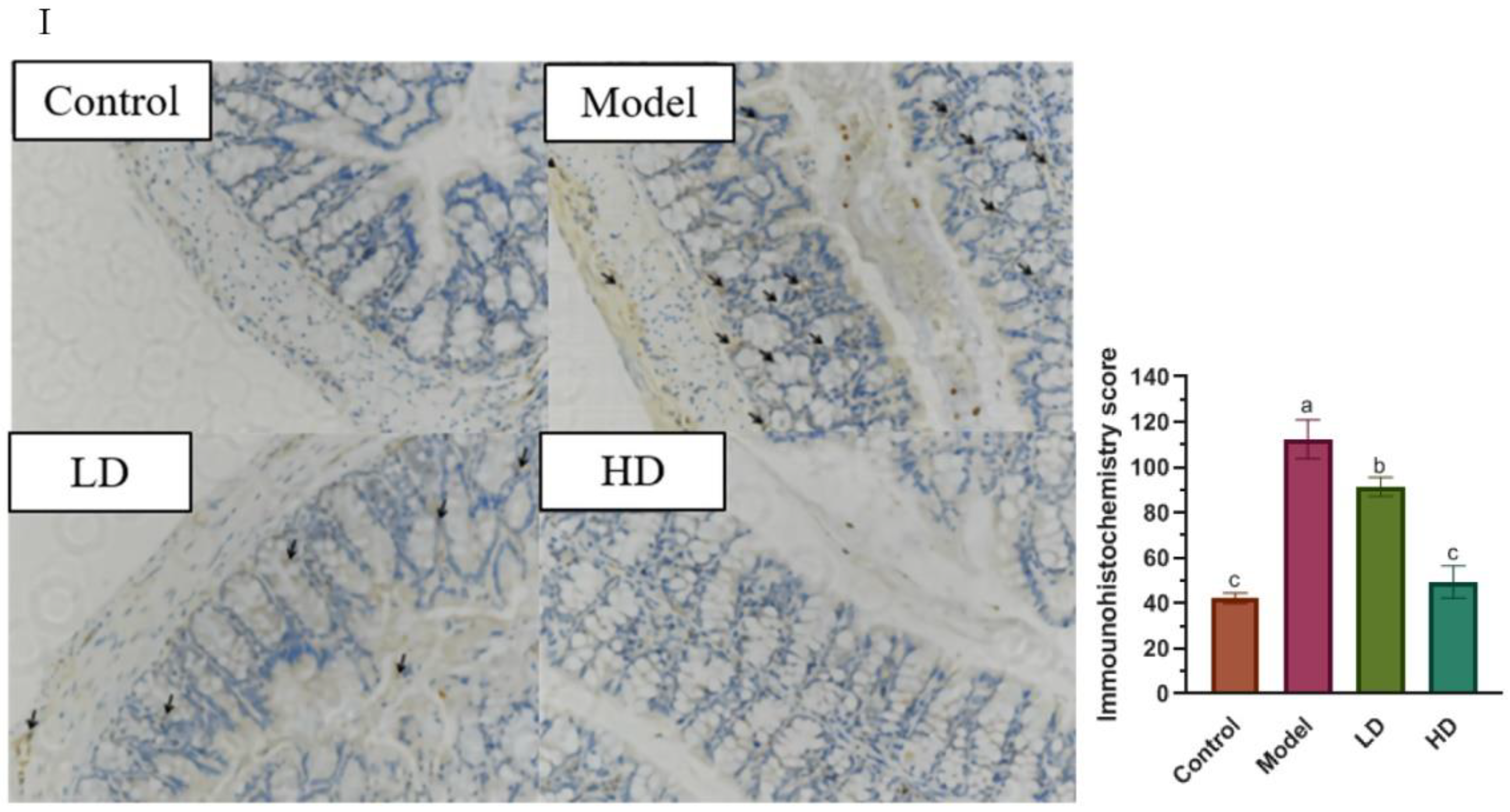
2.3. Histomorphometry and Immunohistochemistry (IHC)
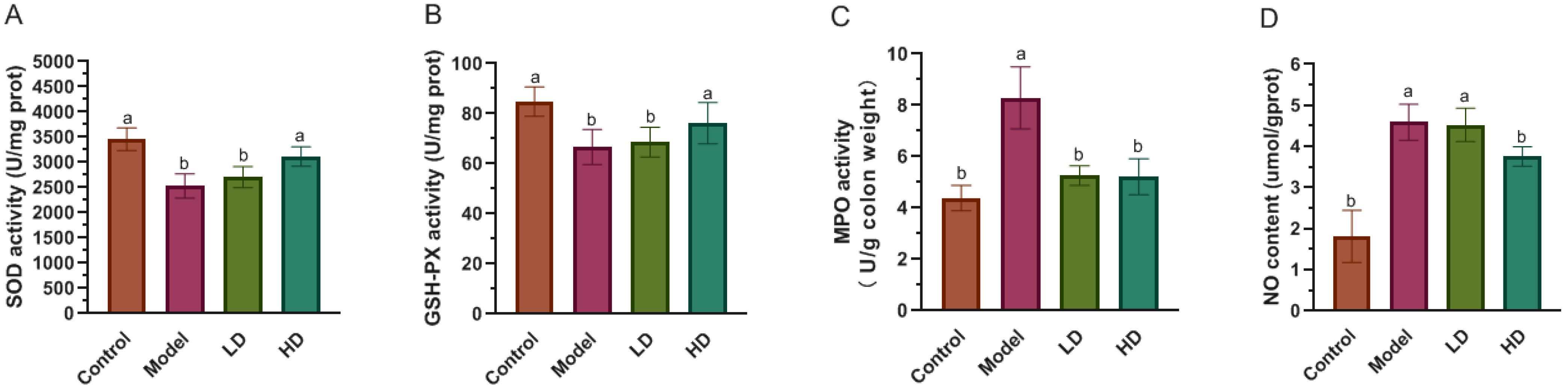
2.4. Measurement of SOD, GSH-Px, MPO Activity and NO Level in Colon
2.5. Determination of GSH, GSSG and the Levels of T Cells in Splenocytes
2.6. Determination of Immunoglobulin Content in Serum
2.7. Quantitative Real-Time PCR (qPCR) Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Phenolics Composition of FBPE
3.2. FBPE Attenuated the Clinical Symptoms of DSS-Induced Colitis
3.3. Effect of FBPE on Inflammatory Mediators in Colon
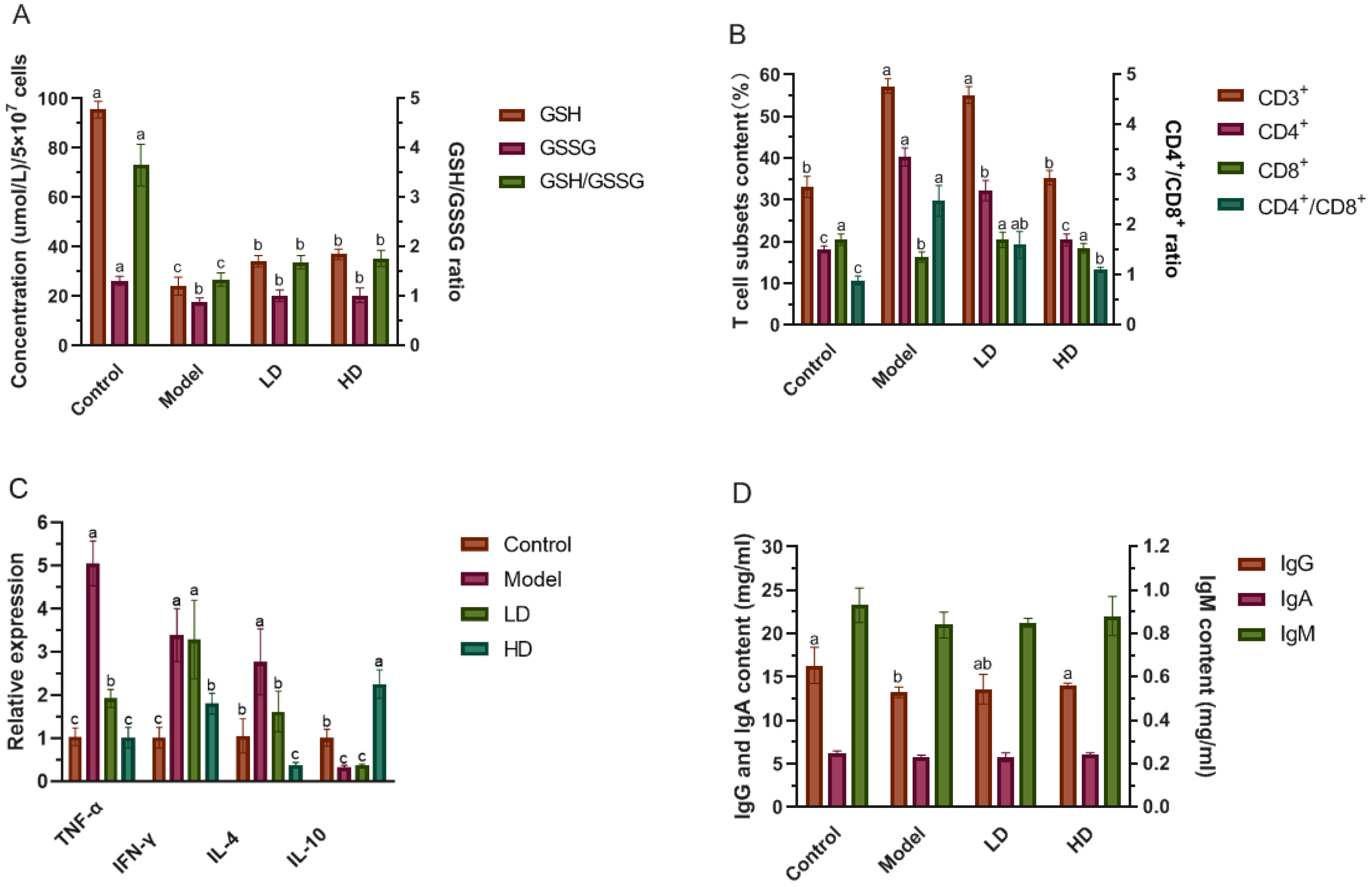
3.4. Effect of FBPE on Immune Response in Splenocytes and Colon
3.5. Effect of FBPE on Immunoglobulin in Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. Inflammatory bowel disease: Past, present, and future. J. Gastroenterol. 2007, 42, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hultqvist, M.; Olsson, L.; Gelderman, K.A.; Holmdahl, R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009, 30, 201–208. [Google Scholar] [CrossRef]
- Suri-Payer, E.; Cantor, H. Differential Cytokine Requirements for Regulation of Autoimmune Gastritis and Colitis by CD4+CD25+T Cells. J. Autoimmun. 2001, 16, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Alex, P.; Zachos, N.C.; Nguyen, T.; Gonzales, L.; Chen, T.E.; Conklin, L.S.; Centola, M.; Li, X. Distinct Cytokine Patterns Identified from Multiplex Profiles of Murine DSS and TNBS-Induced Colitis. Inflamm. Bowel Dis. 2009, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Ogawa, A.; Sakai, F.; Kadooka, Y. A cheese-containing diet modulates immune responses and alleviates dextran sodium sulfate–induced colitis in mice. J. Dairy Sci. 2012, 95, 2810–2818. [Google Scholar] [CrossRef]
- Han, Y.; Song, M.; Gu, M.; Ren, D.; Zhu, X.; Cao, X.; Li, F.; Wang, W.; Cai, X.; Yuan, B.; et al. Dietary intake of whole strawberry inhibited colonic inflammation in dextran-sulfate-sodium-treated mice via restoring immune homeostasis and alleviating gut microbiota dysbiosis. J. Agric. Food Chem. 2019, 67, 9168–9177. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, H.; Cheng, N.; Cao, W. Rape bee pollen alleviates dextran sulfate sodium (DSS)-induced colitis by neutralizing IL-1β and regulating the gut microbiota in mice. Food Res. Int. 2019, 122, 241–251. [Google Scholar] [CrossRef]
- Song, Y.; Xie, F.; Ma, S.; Deng, G.; Li, Y.; Nie, Y.; Wang, F.; Yu, G.; Gao, Z.; Chen, K.; et al. Caveolin-1 protects against DSS-induced colitis through inhibiting intestinal nitrosative stress and mucosal barrier damage in mice. Biochem. Pharmacol. 2020, 180, 114153. [Google Scholar] [CrossRef]
- Lama, A.; Provensi, G.; Amoriello, R.; Pirozzi, C.; Rani, B.; Mollica, M.P.; Raso, G.M.; Ballerini, C.; Meli, R.; Passani, M.B. The anti-inflammatory and immune-modulatory effects of OEA limit DSS-induced colitis in mice. Biomed. Pharmacother. 2020, 129, 110368. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, W.-K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Zheng, J.-J.; Qu, C.; Xiao, Y.; Li, F.-F.; Jin, Q.-X.; Li, H.-H.; Meng, F.-P.; Jin, G.-H.; Jin, D. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance. Artif. Cells Nanomed. Biotechnol. 2019, 47, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Van Bergenhenegouwen, J.; Overbeek, S.; Van De Kant, H.J.G.; Garssen, J.; Folkerts, G.; Vos, P.; Morgan, M.E.; Kraneveld, A.D. Bifidobacterium breve Attenuates Murine Dextran Sodium Sulfate-Induced Colitis and Increases Regulatory T Cell Responses. PLoS ONE 2014, 9, e95441. [Google Scholar] [CrossRef] [PubMed]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2012, 6, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. New Resource Food Management Approach. Available online: http://www.nhc.gov.cn/ (accessed on 15 October 2020).
- Cheng, N.; Ren, N.; Gao, H.; Lei, X.; Zheng, J.; Cao, W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013, 55, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, S.; Zhang, S.; Peng, S.; Cao, W.; Ho, C.-T.; Bai, N. Bioactive Constituents of F. esculentum Bee Pollen and Quantitative Analysis of Samples Collected from Seven Areas by HPLC. Molecules 2019, 24, 2705. [Google Scholar] [CrossRef]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Investig. 1993, 69, 238–249. [Google Scholar]
- Kumar, S.; Patial, V.; Soni, S.; Sharma, S.; Pratap, K.; Kumar, D.; Padwad, Y. Picrorhiza kurroa Enhances β-Cell Mass Proliferation and Insulin Secretion in Streptozotocin Evoked β-Cell Damage in Rats. Front. Pharmacol. 2017, 8, 537–552. [Google Scholar] [CrossRef]
- Hamamoto, N.; Maemura, K.; Hirata, I.; Murano, M.; Sasaki, S.; Katsu, K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin. Exp. Immunol. 1999, 117, 462–468. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, N.; Zhou, W.; Chen, S.; Wang, Q.; Gao, H.; Xue, X.; Wu, L.; Cao, W. Honey Polyphenols Ameliorate DSS-Induced Ulcerative Colitis via Modulating Gut Microbiota in Rats. Mol. Nutr. Food Res. 2019, 63, e1900638. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Chatterjee, I.B. Assay of superoxide dismutase activity in animal tissues. J. Biosci. 1988, 13, 305–315. [Google Scholar] [CrossRef]
- Hintze, K.; Keck, A.-S.; Finley, J.W.; Jeffery, E. Induction of hepatic thioredoxin reductase activity by sulforaphane, both in Hepa1c1c7 cells and in male Fisher 344 rats. J. Nutr. Biochem. 2003, 14, 173–179. [Google Scholar] [CrossRef]
- Krawisz, J.E.; Sharon, P.; Stenson, W.F. Quantit active assay for acute intestinal inflammation based on myeloperocidase activity, assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Ben Ya’acov, A.; Lichtenstein, Y.; Zolotarov, L.; Ilan, Y. The gut microbiome as a target for regulatory T cell-based immunotherapy: Induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol. 2015, 15, 154. [Google Scholar] [CrossRef]
- Sharma, A.; Tirpude, N.V.; Kulurkar, P.M.; Sharma, R.; Padwad, Y. Berberis lycium fruit extract attenuates oxi-inflammatory stress and promotes mucosal healing by mitigating NF-κB/c-Jun/MAPKs signalling and augmenting splenic Treg proliferation in a murine model of dextran sulphate sodium-induced ulcerative colitis. Eur. J. Nutr. 2020, 59, 2663–2681. [Google Scholar] [CrossRef]
- Asmae, E.G.; Nawal, E.M.; Bakour, M.; Lyoussi, B. Moroccan Monofloral Bee Pollen: Botanical Origin, Physicochemical Characterization, and Antioxidant Activities. J. Food Qual. 2021, 2021, 8877266. [Google Scholar] [CrossRef]
- LeBlanc, B.W.; Davis, O.K.; Boue, S.; DeLucca, A.; Deeby, T. Antioxidant activity of Sonoran Desert bee pollen. Food Chem. 2009, 115, 1299–1305. [Google Scholar] [CrossRef]
- Mărghitaş, L.A.; Stanciu, O.G.; Dezmirean, D.S.; Bobis, O.; Popescu, O.; Bogdanov, S.; Campos, M.G. In vitro antioxidant capacity of honeybee-collected pollen of selected floral origin harvested from Romania. Food Chem. 2009, 115, 878–883. [Google Scholar] [CrossRef]
- Zeynep, K.; Hakan, K.; Serhat, D.; Sevgi, K.; Bedia, E.F. Characterization of Turkish honeybee pollens by principal compo-nent analysis based on their individual organic acids, sugars, minerals, and antioxidant activities. LWT 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary Buckwheat Genome Provides Insights into Rutin Biosynthesis and Abiotic Stress Tolerance. Mol. Plant 2017, 10, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an Anti-Inflammatory and Neuroprotective Agent: A Brief Review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Hwang, B.S.; Kim, D.-W.; Kim, J.-Y.; Woo, E.-E.; Lee, Y.-J.; Choi, H.J.; Yun, B.-S. Characterization of Neuraminidase Inhibitors in Korean Papaver rhoeas Bee Pollen Contributing to Anti-Influenza Activities In Vitro. Planta Med. 2016, 82, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.; Milinčić, D.D.; Gašić, U.M.; Nedić, N.; Stanojević, S.P.; Tešić, L.; Pešić, M.B. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. Food Sci. Tech.-Brazil. 2019, 112, 108244. [Google Scholar] [CrossRef]
- Ding, S.; Jiang, H.; Fang, J. Regulation of Immune Function by Polyphenols. J. Immunol. Res. 2018, 2018, 1264074. [Google Scholar] [CrossRef]
- Gaudio, E.; Taddei, G.; Vetuschi, A.; Sferra, R.; Frieri, G.; Ricciardi, G.; Caprilli, R. Dextran sulfate sodium (DSS) colitis in rats: Clinical, structural, and ultrastructural aspects. Am. J. Dig. Dis. 1999, 44, 1458–1475. [Google Scholar] [CrossRef]
- Vincenzo, B.; Pittet, M.J. The spleen in local and systemic regulation of immunity. Immunity 2013, 5, 806–818. [Google Scholar]
- Kitajima, S.; Morimoto, M.; Sagara, E.; Shimizu, C.; Ikeda, Y. Dextran Sodium Sulfate-Induced Colitis in Germ-Free IQI/Jic Mice. Exp. Anim. 2001, 50, 387–395. [Google Scholar] [CrossRef]
- Błaszczyk, K.; Wilczak, J.; Harasym, J.; Gudej, S.; Suchecka, D.; Królikowski, T.; Lange, E.; Gromadzka-Ostrowska, J. Impact of low and high molecular weight oat beta-glucan on oxidative stress and antioxidant defense in spleen of rats with LPS induced enteritis. Food Hydrocoll. 2015, 51, 272–280. [Google Scholar] [CrossRef]
- Xu, G.; Ren, G.; Xu, X.; Yuan, H.; Wang, Z.; Kang, L.; Yu, W.; Tian, K. Combination of curcumin and green tea catechins prevents dimethylhydrazine-induced colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Vainer, B. Intercellular adhesion molecule-1 (ICAM-1) in ulcerative colitis: Presence, visualization, and significance. Agents Actions 2005, 54, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Paradee, N.; Utama-Ang, N.; Uthaipibull, C.; Porter, J.B.; Garbowski, M.W.; Srichairatanakool, S. Extracts of Thai Perilla frutescens nutlets attenuate tumour necrosis factor-α-activated generation of microparticles, ICAM-1 and IL-6 in human endothelial cells. Biosci. Rep. 2020, 40, BSR20192110. [Google Scholar] [CrossRef] [PubMed]
- Boussenna, A.; Cholet, J.; Goncalves-Mendes, N.; Joubert-Zakeyh, J.; Fraisse, D.; Vasson, M.-P.; Texier, O.; Felgines, C. Polyphenol-rich grape pomace extracts protect against dextran sulfate sodium-induced colitis in rats. J. Sci. Food Agric. 2016, 96, 1260–1268. [Google Scholar] [CrossRef]
- Roch, P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture 1999, 172, 125–145. [Google Scholar] [CrossRef]
- Liu, X.-L.; Xi, Q.-Y.; Yang, L.; Li, H.-Y.; Jiang, Q.-Y.; Shu, G.; Wang, S.-B.; Gao, P.; Zhu, X.-T.; Zhang, Y.-L. The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef]
- Sakamoto, T.; Imai, H. Hydrogen peroxide produced by superoxide dismutase SOD-2 activates sperm in Caenorhabditis elegans. J. Biol. Chem. 2017, 292, 14804–14813. [Google Scholar] [CrossRef]
- Stupin, A.; Cosic, A.; Novak, S.; Vesel, M.; Jukic, I.; Popovic, B.; Karalic, K.; Loncaric, Z.; Drenjancevic, I. Reduced Dietary Selenium Impairs Vascular Function by Increasing Oxidative Stress in Sprague-Dawley Rat Aortas. Int. J. Environ. Res. Public Health 2017, 14, 591. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Norhaizan, M.; Mohebali, N.; Yeng, L.C. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Dev. Ther. 2015, ume 9, 3923–3934. [Google Scholar] [CrossRef]
- Kim, H.-R.; Lee, A.; Choi, E.-J.; Kie, J.-H.; Lim, W.; Lee, H.K.; Moon, B.-I.; Seoh, J.-Y. Attenuation of Experimental Colitis in Glutathione Peroxidase 1 and Catalase Double Knockout Mice through Enhancing Regulatory T Cell Function. PLoS ONE 2014, 9, e95332. [Google Scholar] [CrossRef]
- Wang, R.; Feng, X.; Zhu, K.; Zhao, X.; Suo, H. Preventive activity of banana peel polyphenols on CCl4-induced experimental hepatic injury in Kunming mice. Exp. Ther. Med. 2016, 11, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Ripley, B.J.M.; Fujimoto, M.; Serada, S.; Ohkawara, T.; Nishikawa, T.; Terabe, F.; Matsukawa, Y.; Stephanou, A.; Knight, R.A.; Isenberg, D.; et al. Green tea polyphenol epigallocatechin gallate inhibits cell signaling by inducing SOCS1 gene expression. Int. Immunol. 2010, 22, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Keshavarzian, A.; Yong, S.; Wade, M.; Bocckino, S.; Day, B.J.; Banan, A. Novel Antioxidants Zolimid and AEOL11201 Ameliorate Colitis in Rats. Am. J. Dig. Dis. 2001, 46, 2222–2230. [Google Scholar] [CrossRef]
- Guan, L.H.; Gong, Y.F.; Zhang, H.; Shang, Y.Z. Effect of Jiechangkang on MPO, NO and iNOS of colitis mice induced by oxazolone. Chin. Trad. Pat Med. 2013, 35, 669–673. [Google Scholar] [CrossRef]
- Sun, B.; Yuan, J.; Wang, S.; Lin, J.; Zhang, W.; Shao, J.; Wang, R.; Shi, B.; Hu, H. Qingchang Suppository Ameliorates Colonic Vascular Permeability in Dextran-Sulfate-Sodium-Induced Colitis. Front. Pharmacol. 2018, 9, 1235. [Google Scholar] [CrossRef]
- Shen, Y.; Zou, J.; Chen, M.; Zhang, Z.; Liu, C.; Jiang, S.; Qian, D.; Duan, J.-A. Protective effects of Lizhong decoction on ulcerative colitis in mice by suppressing inflammation and ameliorating gut barrier. J. Ethnopharmacol. 2020, 259, 112919. [Google Scholar] [CrossRef]
- Lee, D.H.; Son, D.J.; Park, M.H.; Yoon, D.Y.; Han, S.B.; Hong, J.T. Glutathione peroxidase 1 deficiency attenuates concanavalin A-induced hepatic injury by modulation of T-cell activation. Cell Death Dis. 2016, 7, e2208. [Google Scholar] [CrossRef]
- Chaves, M.A.F.; Leonart, M.S.S.; Nascimento, A.J.D. Oxidative process in erythrocytes of individuals with hemoglobin S. Hematology 2008, 13, 187–192. [Google Scholar] [CrossRef]
- Nur, E.; Verwijs, M.; de Waart, D.R.; Schnog, J.-J.B.; Otten, H.-M.; Brandjes, D.P.; Biemond, B.J.; Elferink, R.P.O. Increased efflux of oxidized glutathione (GSSG) causes glutathione depletion and potentially diminishes antioxidant defense in sickle erythrocytes. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 1412–1417. [Google Scholar] [CrossRef]
- Prchal, J.; Srivastava, S.; Beutler, E. Active transport of GSSG from reconstituted erythrocyte ghosts. Blood 1975, 46, 111–117. [Google Scholar] [CrossRef]
- Holmes, E.; Yong, S.L.; Eiznhamer, D.; Keshavarzian, A. Glutathione content of colonic mucosa: Evidence for oxidative damage in active ulcerative colitis. Am. J. Dig. Dis. 1998, 43, 1088–1095. [Google Scholar] [CrossRef]
- Hardy, K.; Hunt, N.H. Effects of a redox-active agent on lymphocyte activation and early gene expression patterns. Free Radic. Biol. Med. 2004, 37, 1550–1563. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Nakayama, K.; Ishida, H. Low Dose γ-Rays Activate Immune Functions via Induction of Glutathione and Delay Tumor Growth. J. Radiat. Res. 2004, 45, 33–39. [Google Scholar] [CrossRef]
- Olguín, J.E.; Medina-Andrade, I.; Molina, E.; Vázquez, A.; Pacheco-Fernández, T.; Saavedra, R.; Pérez-Plasencia, C.; Chirino, Y.I.; Vaca-Paniagua, F.; Arias-Romero, L.E.; et al. Early and Partial Reduction in CD4+Foxp3+ Regulatory T Cells during Colitis-Associated Colon Cancer Induces CD4+ and CD8+ T Cell Activation Inhibiting Tumorigenesis. J. Cancer 2018, 9, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Annacker, O.; Coombes, J.; Malmstrom, V.; Uhlig, H.H.; Bourne, T.; Johansson-Lindbom, B.; Agace, W.; Parker, C.M.; Powrie, F. Essential role for CD103 in the T cell–mediated regulation of experimental colitis. J. Exp. Med. 2005, 202, 1051–1061. [Google Scholar] [CrossRef]
- McPherson, M.; Wei, B.; Turovskaya, O.; Fujiwara, D.; Brewer, S.; Braun, J. Colitis immunoregulation by CD8+ T cell requires T cell cytotoxicity and B cell peptide antigen presentation. Am. J. Physiol. Liver Physiol. 2008, 295, G485–G492. [Google Scholar] [CrossRef]
- Fujiwara, D.; Chen, L.; Wei, B.; Braun, J. Small intestine CD11c+ CD8+ T cells suppress CD4+ T cell-induced immune colitis. Am. J. Physiol. Liver Physiol. 2011, 300, G939–G947. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef]
- Veltkamp, C.; Anstaett, M.; Wahl, K.; Möller, S.; Gangl, S.; Bachmann, O.; Hardtke-Wolenski, M.; Länger, F.; Stremmel, W.; Manns, M.P.; et al. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNF treatment. Gut 2011, 60, 1345–1353. [Google Scholar] [CrossRef]
- Thompson-Chagoyán, O.C.; Maldonado, J.; Gil, A. Aetiology of inflammatory bowel disease (IBD): Role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin. Nutr. 2005, 24, 339–352. [Google Scholar] [CrossRef]
- Britta, S.; Giamila, F.; Florian, R.; Fabia, G.R.; Hans-Anton, L.; Gunther, H.; Charles, A.D.; Stefan, E.; Andreas, E. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-γ and TNF-α production. Am. J. Physiol.-Cell Physiol. 2001, 281, R1264. [Google Scholar] [CrossRef]
- Warhurst, A.C.; Hopkins, S.J.; Warhurst, G. Interferon γ induces differential upregulation of α and β chemokine secretion in colonic epithelial cell lines. Gut 1998, 42, 208–213. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Z.; Feng, B.S.; Yang, S.B.; Chen, X.; Su, J.; Yang, P.C. Interleukin (IL)-23 suppresses IL-10 in inflammatory bowel dis-ease. J. Biol. Chem. 2012, 287, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.C.; Madsen, K.; Doyle, J.; Meddings, J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009, 58, 41–48. [Google Scholar] [CrossRef]
- Stockinger, S.; Duerr, C.U.; Fulde, M.; Dolowschiak, T.; Pott, J.; Yang, I.; Eibach, D.; Bäckhed, F.; Akira, S.; Suerbaum, S.; et al. TRIF signaling drives homeostaticin testinalepithelial antimicrobial peptide expression. J. Immunol. 2014, 193, 422–423. [Google Scholar] [CrossRef]
- Bjursten, M.; Bland, P.W.; Willén, R.; Hörnquist, E.H. Long-term treatment with anti-α4 integrin antibodies aggravates colitis in Gαi2-deficient mice. Eur. J. Immunol. 2005, 35, 2274–2283. [Google Scholar] [CrossRef]
- Williams, A.R.; Krych, L.; Ahmad, H.F.; Nejsum, P.; Skovgaard, K.; Nielsen, D.S.; Thamsborg, S.M. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS ONE 2017, 12, e0186546. [Google Scholar] [CrossRef]
- Okazaki, Y.; Han, Y.; Kayahara, M.; Watanabe, T.; Arishige, H.; Kato, N. Consumption of Curcumin Elevates Fecal Immunoglobulin A, an Index of Intestinal Immune Function, in Rats Fed a High-Fat Diet. J. Nutr. Sci. Vitaminol. 2010, 56, 68–71. [Google Scholar] [CrossRef]

| Primer | F | R |
|---|---|---|
| TNF-α | 5′-AGCCGATGGGTTGTACCTTG-3′ | 5′-AGTACTTGGGCAGATTGACCTC-3′ |
| IFN-γ | 5′-AGGTCCAGCGCCAAGCATTCAA-3′ | 5′-AGCAGCGACTCCTTTTCCGCTT-3′ |
| IL-4 | 5′-AACGTCCTCACAGCAACGAA-3′ | 5′-AGGCATCGAAAAGCCCGAAA-3′ |
| IL-10 | 5′-CAGTACAGCCGGGAAGACAA-3′ | 5′-CCTGGGGCATCACTTCTACC-3′ |
| β-actin | 5′-CACGATGGAGGGGCCGGACTCATC-3′ | 5′-TAAAGACCTCTATGCCAACACAGT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xu, Y.; Cheng, N.; Li, F.; Zhao, H.; Bai, N.; El-Seedi, H.R.; Cao, W. Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract. Foods 2022, 11, 1293. https://doi.org/10.3390/foods11091293
Chen S, Xu Y, Cheng N, Li F, Zhao H, Bai N, El-Seedi HR, Cao W. Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract. Foods. 2022; 11(9):1293. https://doi.org/10.3390/foods11091293
Chicago/Turabian StyleChen, Sinan, Yifei Xu, Ni Cheng, Feng Li, Haoan Zhao, Naisheng Bai, Hesham R. El-Seedi, and Wei Cao. 2022. "Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract" Foods 11, no. 9: 1293. https://doi.org/10.3390/foods11091293
APA StyleChen, S., Xu, Y., Cheng, N., Li, F., Zhao, H., Bai, N., El-Seedi, H. R., & Cao, W. (2022). Mitigation of DSS-Induced Colitis Potentially via Th1/Th2 Cytokine and Immunological Function Balance Induced by Phenolic-Enriched Buckwheat (Fagopyrum esculentum Moench) Bee Pollen Extract. Foods, 11(9), 1293. https://doi.org/10.3390/foods11091293