The Effects of Different Processing Methods on the Levels of Biogenic Amines in Zijuan Tea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemicals
2.3. Sample Preparation
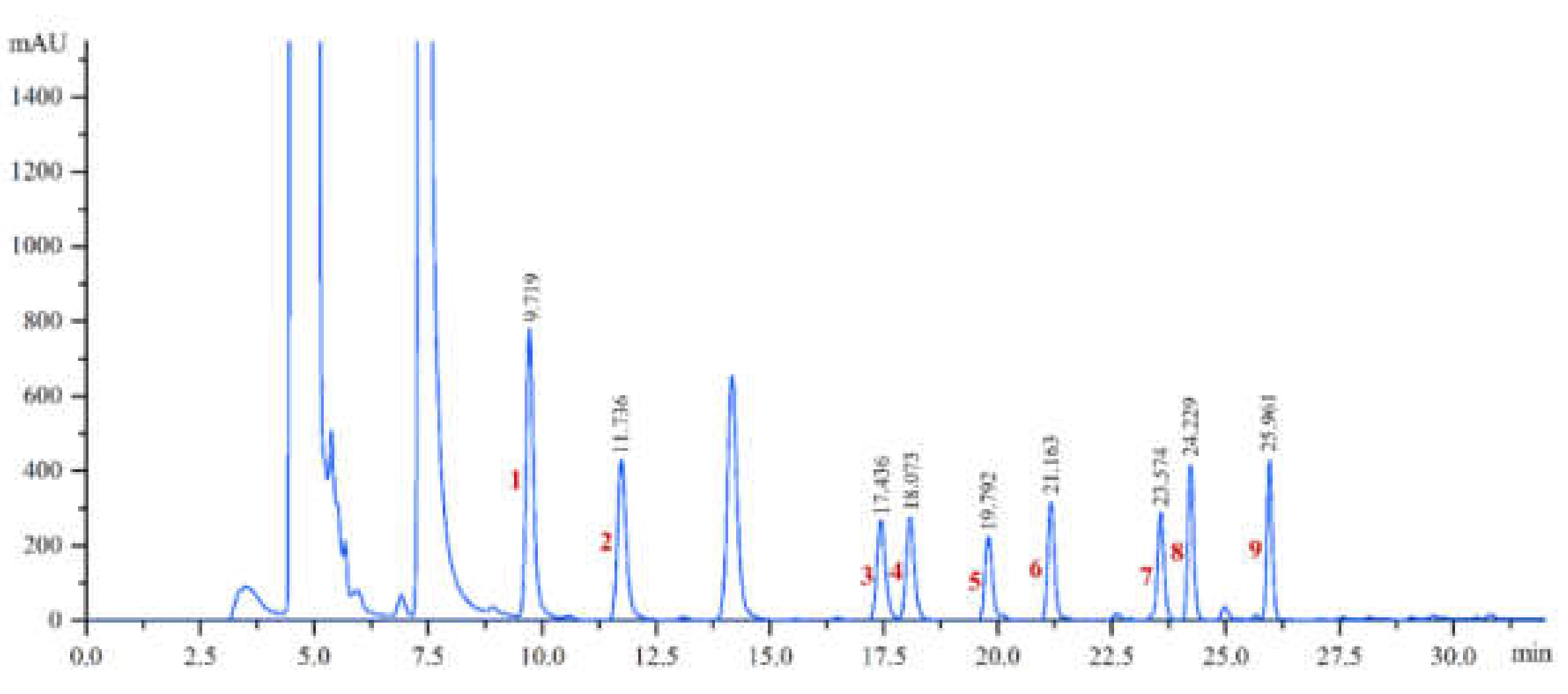
2.4. HPLC Chromatographic Conditions
2.5. Preparation of Amine Standard Solutions
2.6. Validation of the BA Determination Method
2.7. Statistical Analysis
3. Results and Discussion
3.1. Method Performances
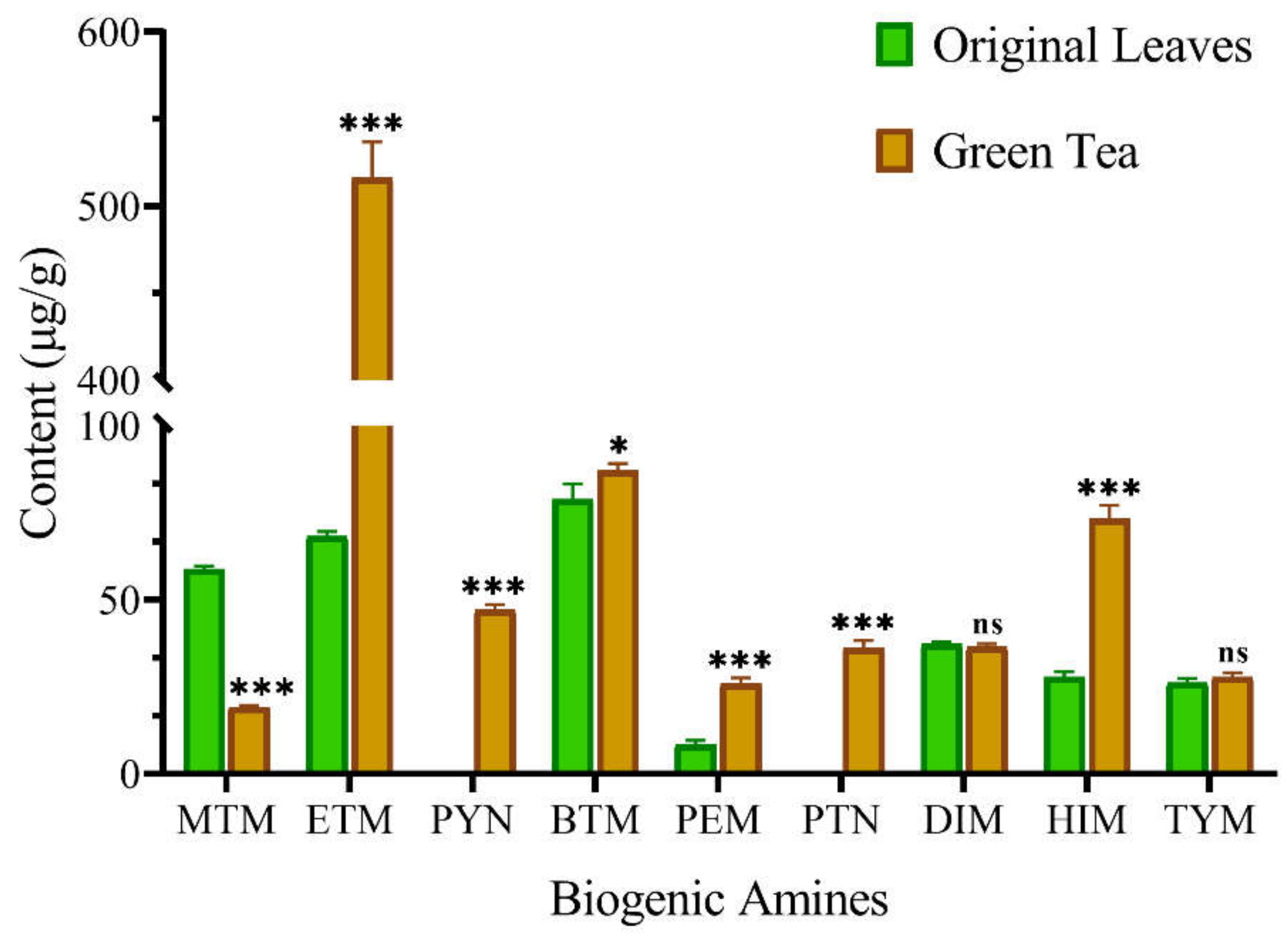
3.2. Levels of BAs in Original Leaves and Green Tea
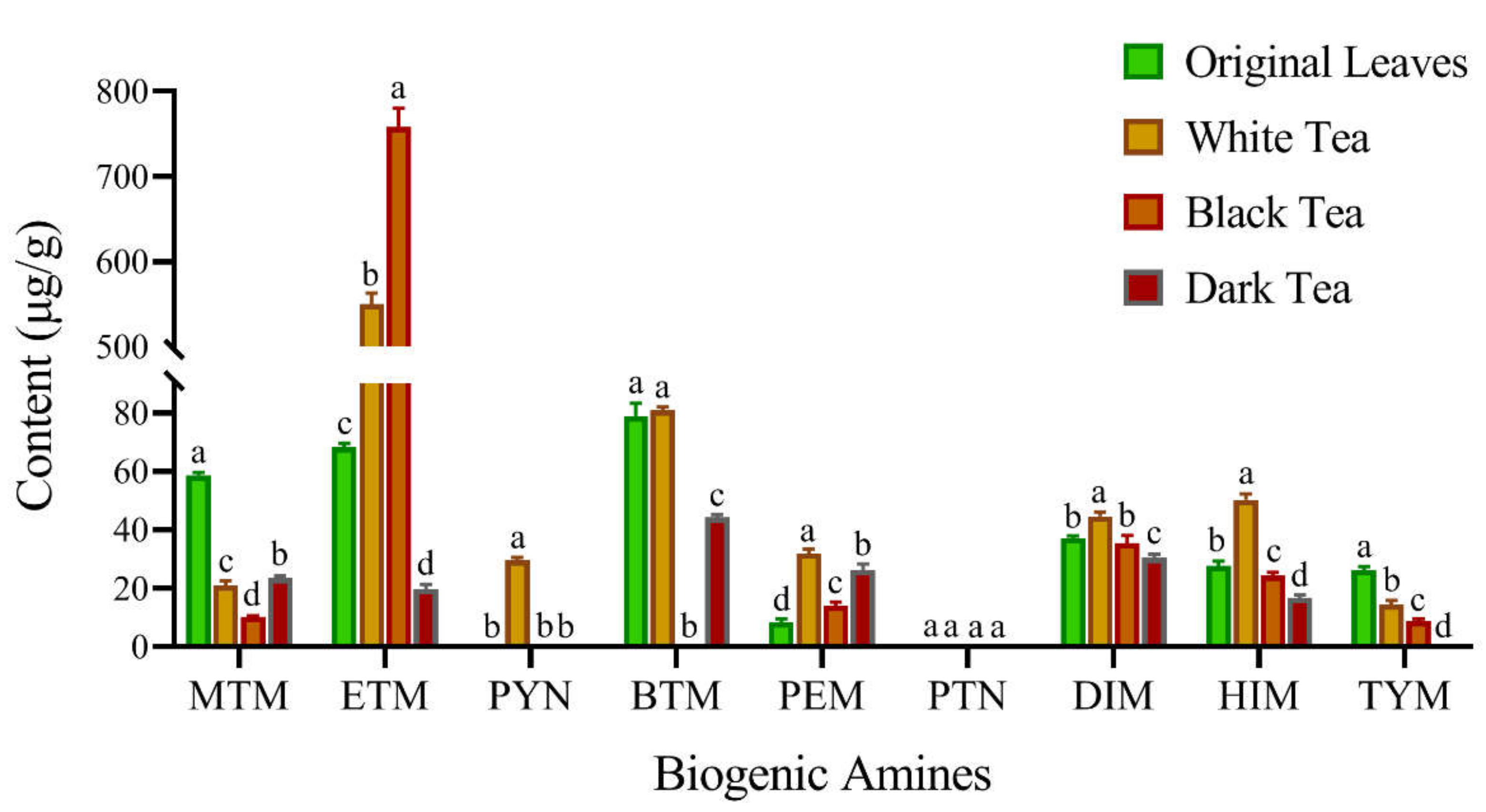
3.3. Levels of BAs in Fermented Teas
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linares, D.M.; Martin, M.C.; Ladero, V.; Alvarez, M.A.; Fernandez, M. Biogenic amines in dairy products. Crit. Rev. Food Sci. 2011, 51, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Gagic, M.; Jamroz, E.; Krizkova, S.; Milosavljevic, V.; Kopel, P.; Adam, V. Current trends in detection of histamine in food and beverages. J. Agric. Food Chem. 2019, 67, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Li, B.B.; Lu, S.L. The importance of amine-degrading enzymes on the biogenic amine degradation in fermented foods: A review. Process Biochem. 2020, 99, 331–339. [Google Scholar] [CrossRef]
- Swider, O.; Roszko, M.L.; Wojcicki, M.; Szymczyk, K. Biogenic amines and free amino acids in traditional fermented vegetables-dietary risk evaluation. J. Agric. Food Chem. 2020, 68, 856–868. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic amines in dairy products: Origin, incidence, and control means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef] [Green Version]
- Erim, F.B. Recent analytical approaches to the analysis of biogenic amines in food samples. TrAC Trends Anal. Chem. 2013, 52, 239–247. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An overview of histamine and other biogenic amines in fish and fish products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- Kannan, S.K.; Ambrose, B.; Sudalaimani, S.; Pandiaraj, M.; Giribabu, K.; Kathiresan, M. A review on chemical and electrochemical methodologies for the sensing of biogenic amines. Anal. Methods 2020, 12, 3438–3453. [Google Scholar] [CrossRef]
- Wojcik, W.; Lukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity-a review. J. Sci. Food Agric. 2020, 101, 2634–2640. [Google Scholar] [CrossRef]
- Gardini, F.; Tofalo, R.; Belletti, N.; Iucci, L.; Suzzi, G.; Torriani, S.; Guerzoni, M.E.; Lanciotti, R. Characterization of yeasts involved in the ripening of Pecorino Crotonese cheese. Food Microbiol. 2014, 39, 146–155. [Google Scholar] [CrossRef]
- Roig-Sagues, A.X.; Molina, A.P.; Hernandez-Herrerok, M.M. Histamine and tyramine-forming microorganisms in Spanish traditional cheeses. Eur. Food Res. Technol. 2002, 215, 96–100. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Moreno-Arribas, M.V. The problem of biogenic amines in fermented foods and the use of potential biogenic amine-degrading microorganisms as a solution. Trends Food Sci. Tech. 2014, 39, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Mohammed, G.I.; Al-Eryani, D.A.; Saigl, Z.M.; Alyoubi, A.O.; Alwael, H.; Bashammakh, A.S.; O’Sullivan, C.K.; El-Shahawi, M.S. Biogenic amines formation mechanism and determination strategies: Future challenges and limitations. Crit. Rev. Anal. Chem. 2020, 50, 485–500. [Google Scholar] [CrossRef]
- Emer, C.D.; Marques, S.; Colla, L.M.; Reinehr, C.O. Biogenic amines and the importance of starter cultures for malolactic fermentation. Aust. J. Grape Wine Res. 2021, 27, 26–33. [Google Scholar] [CrossRef]
- Gardini, F.; Martuscelli, M.; Caruso, M.C.; Galgano, F.; Crudele, M.A.; Favati, F.; Guerzoni, M.E.; Suzzi, G. Effects of pH, temperature and NaCl concentration on the growth kinetics, proteolytic activity and biogenic amine production of Enterococcus faecalis. Int. J. Food. Microbiol. 2001, 64, 105–117. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Huang, H.Z.; Cheng, H.; Ye, X.Q.; Zhi, Z.J. Process improvement to prevent the formation of biogenic amines during soy sauce brewing. Food Chem. 2020, 331, 127347. [Google Scholar] [CrossRef]
- Zhou, X.; Qiu, M.; Zhao, D.; Lu, F.; Ding, Y. Inhibitory effects of spices on biogenic amine accumulation during fish sauce fermentation. J. Food Sci. 2016, 81, 913–920. [Google Scholar] [CrossRef]
- De Borba, B.M.; Rohrer, J.S. Determination of biogenic amines in alcoholic beverages by ion chromatography with suppressed conductivity detection and integrated pulsed amperometric detection. J. Chromatogr. A 2007, 1155, 22–30. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; Simeonov, V.; Namiesnik, J. An in situ derivatization-dispersive liquid-liquid microextraction combined with gas-chromatography-mass spectrometry for determining biogenic amines in home-made fermented alcoholic drinks. J. Chromatogr. A 2016, 1453, 10–18. [Google Scholar] [CrossRef]
- Tameem, A.A.; Saad, B.; Makahleh, A.; Salhin, A.; Saleh, M.I. A 4-hydroxy-N′-[(E)-(2-hydroxyphenyl)methylidene]benzohydrazidebased sorbent material for the extraction-HPLC determination of biogenic amines in food samples. Talanta 2010, 82, 1385–1391. [Google Scholar] [CrossRef]
- An, D.; Chen, Z.; Zheng, J.; Chen, S.; Wang, L.; Huang, Z.; Weng, L. Determination of biogenic amines in oysters by capillary electrophoresis coupled with electrochemiluminescence. Food Chem. 2015, 168, 1–6. [Google Scholar] [CrossRef]
- Chauhan, N.; Jain, U.; Gandotra, R.; Hooda, V. Zeolites-AuNPs assembled interface towards amperometric biosensing of spermidine. Electrochim. Acta 2017, 230, 106–115. [Google Scholar] [CrossRef]
- Ishimaru, M.; Muto, Y.; Nakayama, A.; Hatate, H.; Tanaka, R. Determination of Biogenic Amines in Fish Meat and Fermented Foods Using Column-Switching High-Performance Liquid Chromatography with Fluorescence Detection. Food Anal. Methods 2018, 12, 166–175. [Google Scholar] [CrossRef]
- Plakidi, E.S.; Maragou, N.C.; Dasenaki, M.E.; Megoulas, N.C.; Koupparis, M.A.; Thomaidis, N.S. Liquid Chromatographic Determination of Biogenic Amines in Fish Based on Pyrene Sulfonyl Chloride Pre-Column Derivatization. Foods 2020, 9, 609. [Google Scholar] [CrossRef]
- Jain, A.; Verma, K.K. Strategies in liquid chromatographic methods for the analysis of biogenic amines without and with derivatization. TrAC-Trends Anal. Chem. 2018, 109, 62–82. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Lambropoulou, D.; Morrison, C.; Kłodzińska, E.; Namieśnik, J.; Płotka-Wasylka, J. Literature update of analytical methods for biogenic amines determination in food and beverages. Trends Anal. Chem. 2018, 98, 128–142. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Ho, C.T.; Li, X.F.; Lin, X.R.; Zhang, Y.Y.; Chen, Z.Z.; Li, B. Phytochemicals, anti-inflammatory, antiproliferative, and methylglyoxal trapping properties of Zijuan Tea. J. Food Sci. 2018, 83, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.S.; Zhang, Q.; Peng, C.X.; Fan, J.P.; Dong, W.M. Curie-point pyrolysis-gas chromatography-mass spectroscopic analysis of theabrownins from fermented Zijuan tea. J. Anal. Appl. Pyrolysis 2012, 97, 171–180. [Google Scholar] [CrossRef]
- He, C.J.; Guo, X.M.; Yang, Y.M.; Xie, Y.F.; Ju, F.Y.; Guo, W.B. Characterization of the aromatic profile in “zijuan” and “pu-erh” green teas by headspace solid-phase microextraction coupled with GC-O and GC-MS. Anal. Methods 2016, 8, 4727–4735. [Google Scholar] [CrossRef]
- Lu, H.P.; Dai, W.D.; Tan, J.F.; Guo, L.; Zhu, Y.; Lin, Z. Identification of the anthocyanins from the purple leaf coloured tea cultivar Zijuan (Camellia sinensis var assamica) and characterization of their antioxidant activities. J. Funct. Foods 2015, 17, 449–458. [Google Scholar]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Picci, N. Brewing effect on levels of biogenic amines in different coffee samples as determined by LC-UV. Food Chem. 2015, 175, 143–150. [Google Scholar] [CrossRef]
- Shen, N.Y.; Zheng, S.Y.; Wang, X.Q. Determination of biogenic amines in Pu-erh tea with precolumn derivatization by high-performance liquid chromatography. Food Anal. Method 2017, 10, 1690–1698. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Picci, N.; Restuccia, D. Extraction efficiency of different solvents and LC-UV determination of biogenic amines in tea leaves and infusions. J. Anal. Methods Chem. 2016, 2016, 8715287. [Google Scholar] [CrossRef] [Green Version]
- Bouchereau, A.; Guenot, P.; Lather, F. Analysis of amines in plant materials. J. Chromatogr. B 2000, 747, 49–67. [Google Scholar] [CrossRef]
- Sanchez-Perez, S.; Comas-Baste, O.; Rabell-Gonzalez, J.; Veciana-Nogues, M.T.; Latorre-Moratalla, M.L.; Vidal-Carou, M.C. Biogenic amines in plant-origin foods: Are they frequently underestimated in low-histamine diets? Foods 2018, 7, 205. [Google Scholar] [CrossRef] [Green Version]
- Yadav, K.C.; Parajuli, A.; Khatri, B.B.; Shiwakoti, L.D. Phytochemicals and quality of green and black teas from different clones of tea plant. J. Food Qual. 2020, 2020, 8874271. [Google Scholar]
- Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of fermentation, drying and roasting on biogenic amines and other biocompounds in Colombian Criollo cocoa beans and shells. Foods 2020, 9, 520. [Google Scholar] [CrossRef] [Green Version]
- Gardini, F.; Ozogul, Y.; Suzzi, G.; Tabanelli, G.; Ozogul, F. Technological factors affecting biogenic amine content in foods: A review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [Green Version]
- Guarcello, R.; De Angelis, M.; Settanni, L.; Formiglio, S.; Gaglio, R.; Minervini, F.; Moschetti, G.; Gobbetti, M. Selection of amine-oxidizing dairy lactic acid bacteria and identification of the enzyme and gene involved in the decrease of biogenic amines. Appl. Environ. Microbiol. 2016, 82, 6870–6880. [Google Scholar] [CrossRef] [Green Version]
- Makhamrueang, N.; Sirilun, S.; Sirithunyalug, J.; Chaiyana, W.; Wangcharoen, W.; Peerajan, S.; Chaiyasut, C. Effect of pretreatment processes on biogenic amines content and some bioactive compounds in Hericium erinaceus extract. Foods 2021, 10, 996. [Google Scholar] [CrossRef]
- Zheng, W.J.; Wan, X.C.; Bao, G.H. Brick dark tea: A review of the manufacture, chemical constituents and bioconversion of the major chemical components during fermentation. Phytochem. Rev. 2015, 14, 499–523. [Google Scholar] [CrossRef]
- Wang, Y.F.; Chen, L.; Lai, W.Y.; Zhao, Y.L.; Xu, P. Nonvolatile metabolite alterations during Zijuan black tea processing affect the protective potential on HOECs exposed to nicotine. Food Funct. 2021, 12, 12291–12302. [Google Scholar] [CrossRef]
- Xu, Z.G.; Wu, L.; Liu, S.Q.; Huang, H.M.; Dong, M.; Zhao, Y.L. Review for development of microbial diversity during dark tea fermentation period. J. Biol. 2019, 36, 92–95. [Google Scholar]
| BAs | Calibration Curve Equation | R2 | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|
| Methylamine | y = 228.7x + 82.568 | 0.9995 | 0.15 | 0.50 |
| Ethylamine | y = 113.73x + 115.19 | 0.9999 | 0.31 | 1.04 |
| Pyrrolidine | y = 142.42x − 18.254 | 0.9999 | 0.29 | 0.96 |
| Butylamine | y = 71.14x + 148.51 | 0.9951 | 0.42 | 1.40 |
| 2-Phenethylamine | y = 64.163x + 5.8879 | 0.9998 | 0.44 | 1.51 |
| Putrescine | y = 53.625x + 91.609 | 0.9996 | 0.33 | 0.98 |
| 1,7-Diaminoheptane | y = 35.049x − 18.118 | 0.9983 | 0.96 | 3.22 |
| Histamine | y = 73.276x + 7.4512 | 0.9999 | 0.43 | 1.46 |
| Tyramine | y = 64.951x + 47.487 | 0.9995 | 0.36 | 1.20 |
| BAs | Recovery Rate (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|
| 30 μg/g | 150 μg/g | 300 μg/g | 30 μg/g | 150 μg/g | 300 μg/g | |
| Methylamine | 98.2 | 96.5 | 100.2 | 3.5 | 2.3 | 1.9 |
| Ethylamine | 97.5 | 105.1 | 95.7 | 2.8 | 1.5 | 1.3 |
| Pyrrolidine | 96.8 | 92.2 | 103.4 | 1.6 | 1.1 | 2.4 |
| Butylamine | 99.2 | 94.5 | 95.7 | 4.2 | 3.4 | 4.7 |
| 2-Phenethylamine | 97.1 | 99.7 | 95.9 | 1.3 | 0.9 | 2.8 |
| Putrescine | 93.8 | 96.9 | 98.4 | 1.8 | 2.4 | 4.1 |
| 1,7-Diaminoheptane | 99.2 | 95.8 | 97.5 | 2.2 | 1.2 | 3.8 |
| Histamine | 95.9 | 97.4 | 94.6 | 1.0 | 2.3 | 1.7 |
| Tyramine | 99.1 | 104.4 | 98.7 | 2.5 | 1.4 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Wang, K.; Xue, X.; Wen, Q.; Qin, S.; Suo, Y.; Liang, M. The Effects of Different Processing Methods on the Levels of Biogenic Amines in Zijuan Tea. Foods 2022, 11, 1260. https://doi.org/10.3390/foods11091260
Liu D, Wang K, Xue X, Wen Q, Qin S, Suo Y, Liang M. The Effects of Different Processing Methods on the Levels of Biogenic Amines in Zijuan Tea. Foods. 2022; 11(9):1260. https://doi.org/10.3390/foods11091260
Chicago/Turabian StyleLiu, Dandan, Kang Wang, Xiaoran Xue, Qiang Wen, Shiwen Qin, Yukai Suo, and Mingzhi Liang. 2022. "The Effects of Different Processing Methods on the Levels of Biogenic Amines in Zijuan Tea" Foods 11, no. 9: 1260. https://doi.org/10.3390/foods11091260
APA StyleLiu, D., Wang, K., Xue, X., Wen, Q., Qin, S., Suo, Y., & Liang, M. (2022). The Effects of Different Processing Methods on the Levels of Biogenic Amines in Zijuan Tea. Foods, 11(9), 1260. https://doi.org/10.3390/foods11091260





