Effect of Acute Intake of Fermented Orange Juice on Fasting and Postprandial Glucose Metabolism, Plasma Lipids and Antioxidant Status in Healthy Human
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
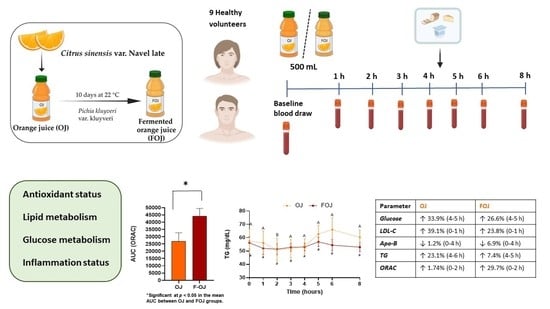
2.2. Production of Fermented Orange Juice
2.3. Subjects
2.4. Experimental Design
2.5. Blood Sample Collection and Handling
2.6. Anthropometric Evaluation
2.7. Glucose Metabolism
2.8. Lipid Metabolism
2.9. Antioxidant and Inflammation Status
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of Acute FOJ Consumption on Postprandial Glucose Metabolism
3.2. Effect of Acute FOJ Consumption on Postprandial Lipid Metabolism
3.3. Effect of Acute FOJ Consumption on Antioxidant and Inflammation Status
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000–2016; WHO: Geneva, Switzerland, 2018.
- World Health Organization (WHO). World Health Statistics 2018: Monitoring Health for the SDGs; WHO: Geneva, Switzerland, 2018.
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, R.; Rocic, P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: The great exploration. Exp. Diabetes Res. 2012, 2012, 271028. [Google Scholar] [CrossRef]
- Perrotta, I.; Aquila, S. The role of oxidative stress and autophagy in atherosclerosis. Oxid. Med. Cell. Longev. 2015, 2015, 130315. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Tango, T. Effects of lifestyle modification on metabolic syndrome: A systematic review and meta-analysis. BMC Med. 2012, 10, 138. [Google Scholar] [CrossRef]
- Kimokoti, R.W.; Millen, B.E. Nutrition for the prevention of chronic diseases. Med. Clin. N. Am. 2016, 100, 1185–1198. [Google Scholar] [CrossRef]
- D’Alessandro, A.; De Pergola, G. The mediterranean diet: Its definition and evaluation of a priori dietary indexes in primary cardiovascular prevention. Int. J. Food Sci. Nutr. 2018, 69, 647–659. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384–392. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; Yang, J.; Micek, A.; Pajak, A.; Scalfi, L.; Galvano, F.; Kales, S.N. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit. Rev. Food Sci. Nutr. 2017, 57, 3218–3232. [Google Scholar] [CrossRef]
- Martini, D. Health benefits of mediterranean diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef]
- Rosato, V.; Temple, N.J.; La Vecchia, C.; Castellan, G.; Tavani, A.; Guercio, V. Mediterranean diet andcardiovascular disease: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2019, 58, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Franke, S.I.; Guecheva, T.N.; Henriques, J.A.; Prá, D. Orange juice and cancer chemoprevention. Nutr. Cancer 2013, 65, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Cirmi, S.; Minciullo, P.L.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Citrus fruits and inflammaging: A systematic review. Phytochem. Rev. 2019, 18, 1025–1049. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, beer, alcohol and polyphenols on cardiovascular disease and cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Escudero-López, B.; Cerrillo, I.; Herrero-Martin, G.; Hornero-Méndez, D.; Gil-Izquierdo, A.; Medina, S.; Ferreres, F.; Berna, G.; Martin, F.; Fernández-Pachón, M.S. Fermented orange juice: Source of higher carotenoid and flavanone contents. J. Agric. Food Chem. 2013, 61, 8773–8782. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Pachón, M.S.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-López, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of red orange juice intake on endothelial function and inflammatory markers in adult subjects with increased cardiovascular risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef]
- Aptekmann, N.P.; Cesar, T.B. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013, 112, 119–128. [Google Scholar] [CrossRef]
- Coelho, R.C.; Hermsdorff, H.H.M.; Bressan, J. Antiinflammatory properties of orange juice: Possible favorable molecular and metabolic effects. Plant Foods Hum. Nutr. 2013, 68, 1–10. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Colletti, A. Effects of carotenoids on health: Are all the same? Results from clinical trials. Curr. Pharm. Des. 2017, 23, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, F.D.; Spencer, J.P.E. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Kay, C.D.; Crozier, A. The bioavailability, transport, and bioactivity of dietary flavonoids: A review from a historical perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Mena, P.; Ascacio-Valdés, J.A.; Gironés-Vilaplana, A.; Del Rio, D.; Moreno, D.A.; García-Viguera, C. Assessment of pomegranate wine lees as a valuable source for the recovery of (poly)phenolic compounds. Food Chem. 2014, 145, 327–334. [Google Scholar] [CrossRef]
- Adamenko, K.; Kawa-Rygielska, J.; Kucharska, A.Z. Characteristics of Cornelian cherry sour non-alcoholic beers brewed with the special yeast Saccharomycodes ludwigii. Food Chem. 2020, 312, 125968. [Google Scholar] [CrossRef]
- Dey, G.; Sireswar, S. Tailoring functional beverages from fruits and vegetables for specific disease conditions-are we there yet? Crit. Rev. Food Sci. Nutr. 2020, 61, 2034–2046. [Google Scholar] [CrossRef]
- Mantzourani, I.; Terpou, A.; Bekatorou, A.; Mallouchos, A.; Alexopoulos, A.; Kimbaris, A.; Bezirtzoglou, E.; Koutinas, A.A.; Plessas, S. Functional pomegranate beverage production by fermentation with a novel synbiotic L. paracasei biocatalyst. Food Chem. 2020, 308, 125658. [Google Scholar] [CrossRef]
- Cerrillo, I.; Escudero-López, B.; Hornero-Méndez, D.; Martín, F.; Fernández-Pachón, M.S. Effect of alcoholic fermentation on the carotenoid composition and provitamin A content of orange juice. J. Agric. Food Chem. 2014, 62, 842–849. [Google Scholar] [CrossRef]
- Oliveras-López, M.J.; Cerezo, A.B.; Escudero-López, B.; Cerrillo, I.; Berná, G.; Martín, F.; García-Parrilla, M.C.; Fernández-Pachón, M.S. Changes in orange juice (poly)phenol composition induced by controlled alcoholic fermentation. Anal. Methods 2016, 8, 8151–8164. [Google Scholar] [CrossRef]
- Escudero-López, B.; Berná, G.; Ortega, A.; Herrero-Martín, G.; Cerrillo, I.; Martín, F.; Fernández-Pachón, M.S. Consumption of orange fermented beverage reduces cardiovascular risk factors in healthy mice. Food Chem. Toxicol. 2015, 78, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Escudero-López, B.; Fernández-Pachón, M.S.; Herrero-Martín, G.; Ortega, A.; Cerrillo, I.; Martín, F.; Berná, G. Orange beverage amelioates high-fat-diet-induced metabolic disorder in mice. J. Funct. Foods 2016, 24, 254–263. [Google Scholar] [CrossRef]
- Escudero-López, B.; Ortega, A.; Cerrillo, I.; Rodríguez-Griñolo, M.R.; Muñoz-Hernández, R.; Macher, H.C.; Martín, F.; Hornero-Méndez, D.; Mena, P.; Del Rio, D.; et al. Consumption of orange fermented beverage improves antioxidant status and reduces peroxidation lipid and inflammatory markers in healthy humans. J. Sci. Food Agric. 2018, 98, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, I.; Escudero-López, B.; Ortega, A.; Martin, F.; Fernández-Pachón, M.S. Effect of daily intake of a low-alcohol orange beverage on cardiovascular risk factors in hypercholesterolemic humans. Food Res. Int. 2019, 116, 168–174. [Google Scholar] [CrossRef]
- Escudero-Lopez, B.; Calani, L.; Fernandez-Pachon, M.S.; Ortega, A.; Brighenti, F.; Crozier, A.; Del Rio, D. Absorption, metabolism, and excretion of fermented orange juice (poly)phenols in rats. Biofactors 2014, 40, 327–335. [Google Scholar] [CrossRef]
- Castello, F.; Fernández-Pachón, M.S.; Cerrillo, I.; Escudero-López, B.; Ortega, A.; Rosi, A.; Bresciani, L.; Del Rio, D.; Mena, P. Absorption, metabolism, and excretion of orange juice (poly)phenols in humans: The effect of a controlled alcoholic fermentation. Arch. Biochem. Biophys. 2020, 30, 695–108627. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Cerrillo, I.; Ortega, A.; Rodríguez-Griñolo, M.R.; Escudero-López, B.; Martín, F.; Fernández-Pachón, M.S. β-Cryptoxanthin is more bioavailable in humans from fermented orange juice than from orange juice. Food Chem. 2018, 262, 215–220. [Google Scholar] [CrossRef]
- Vitale, M.; Giacco, R.; Laiola, M.; Della Pepa, G.; Luongo, D.; Mangione, A.; Salamone, D.; Vitaglione, P.; Ercolini, D.; Rivellese, A.A. Acute and chronic improvement in postprandial glucose metabolism by a diet resembling the traditional Mediterranean dietary pattern: Can SCFAs play a role? Clin. Nutr. 2021, 40, 428–437. [Google Scholar] [CrossRef]
- Berryman, C.E.; Grieger, J.A.; West, S.G.; Chen, C.Y.O.; Blumberg, J.B.; Rothblat, G.H.; Sankaranarayanan, S.; Kris-Etherton, P.M. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J. Nutr. 2013, 143, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzmán, O.J.; Álvarez-Quintero, R.; Osorio, E.; Naranjo-Cano, M.; Muñoz-Durango, K. GC/MS method to quantify bioavailable phenolic compounds and antioxidant capacity determination of plasma after acute coffee consumption in human volunteers. Food Res. Int. 2016, 89, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Copetti, C.; Franco, F.W.; Machado, E.D.R.; Soquetta, M.B.; Quatrin, A.; Ramos, V.M.; Moreira, J.; Emanuelli, T.; Sautter, C.K.; Penna, N.G. Acute consumption of bordo grape juice and wine improves serum antioxidant status in healthy individuals and inhibits reactive oxygen species production in human neuron-like cells. J. Nutr. Metab. 2018, 2018, 4384012. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef]
- Fuchs, D.; Nyakayiru, J.; Draijer, R.; Mulder, T.P.; Hopman, M.T.; Eijsvogels, T.M.; Thijssen, D.H. Impact of flavonoid-rich black tea and beetroot juice on postprandial peripheral vascular resistance and glucose homeostasis in obese, insulin-resistant men: A randomized controlled trial. Nutr. Metab. 2016, 13, 34. [Google Scholar] [CrossRef]
- Stookey, J.D.; Hamer, J.; Espinoza, G.; Higa, A.; Ng, V.; Tinajero-Deck, L.; Havel, P.J.; King, J.C. Orange juice limits postprandial fat oxidation after breakfast in normal-weight adolescents and adults. Adv. Nutr. 2012, 3, 629S–635S. [Google Scholar] [CrossRef]
- Cerletti, C.; Gianfagna, F.; Tamburrelli, C.; De Curtis, A.; D’Imperio, M.; Coletta, W.; Giordano, L.; Lorenzet, R.; Rapisarda, P.; Reforgiato Recupero, G.; et al. Orange juice intake during a fatty meal consumption reduces the postprandial low-grade inflammatory response in healthy subjects. Thromb. Res. 2015, 135, 255–259. [Google Scholar] [CrossRef]
- Geraldi, M.V.; Cazarin, C.; Cristianini, M.; Vasques, A.C.; Geloneze, B.; Maróstica Júnior, M.R. Jabuticaba juice improves postprandial glucagon-like peptide-1 and antioxidant status in healthy adults: A randomized crossover trial. Br. J. Nutr. 2021, 1–29. [Google Scholar] [CrossRef]
- Kasuya, N.; Inoue, N.; Inoue, Y.; Murata, I.; Kanamoto, I. Effect of vegetable juice consumption prior to eating rice on postprandial blood glucose and insulin levels. Exp. Ther. Med. 2019, 18, 3817–3822. [Google Scholar] [CrossRef]
- Barros, C.P.; Grom, L.C.; Guimarães, J.T.; Balthazar, C.F.; Rocha, R.S.; Silva, R.; Almada, C.N.; Pimentel, T.C.; Venâncio, E.L.; Collopy Junior, I.; et al. Paraprobiotic obtained by ohmic heating added in whey-grape juice drink is effective to control postprandial glycemia in healthy adults. Food Res. Int. 2021, 140, 109905. [Google Scholar] [CrossRef]
- de Araujo, R.L.; Tomas-Barberan, F.A.; Ferreira dos Santos, R.; Martinez-Blazquez, J.A.; Genovese, M.I. Postprandial glucose-lowering effect of cagaita (Eugenia dysenterica DC) fruit juice in dysglycemic subjects with metabolic syndrome: An exploratory study. Food Res. Int. 2021, 142, 110209. [Google Scholar] [CrossRef] [PubMed]
- Gouws, C.A.; McKune, A.; Tee, N.; Somerset, S.; Mortazavi, R. Prickly pear juice consumption after fat intake affects postprandial heart rate variability but not traditional risk factors of cardiovascular disease in healthy men. Nutrition 2022, 96, 111555. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Sierra, N.; Marqués-Cardete, R.; Gurrea-Martínez, A.; Grau-del Valle, C.; Morillas, C.; Hernández-Mijares, A.; Bañuls, C. Effect of fibre-enriched orange juice on postprandial glycaemic response and satiety in healthy individuals: An acute, randomised, placebo-controlled, double-blind, crossover study. Nutrients 2019, 11, 3014. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef]
- Redfern, K.M.; Cammack, V.L.; Sweet, N.; Preston, L.A.; SoBHCS Student Team; Jarvis, M.A.; Rees, G.A. Nutrient-extraction blender preparation reduces postprandial glucose responses from fruit juice consumption. Nutr. Diabetes 2017, 7, e288. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Analysis; OIV: Paris, France, 2017.
- Lohman, T.G.; Roche, A.F.; Martorell, R. (Eds.) Anthoropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1998. [Google Scholar]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufián-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Moser, S.E.; Shin, J.E.; Kasturi, P.; Hamaker, B.R.; Ferruzzi, M.G.; Bordenave, N. Formulation of orange juice with dietary fibers enhances bioaccessibility of orange flavonoids in juice but limits their ability to inhibit in vitro glucose transport. J. Agric. Food Chem. 2020, 68, 9387–9397. [Google Scholar] [CrossRef]
- Dong, H.; Rendeiro, C.; Kristek, A.; Sargent, L.J.; Saunders, C.; Harkness, L.; Rowland, I.; Jackson, K.G.; Spencer, J.P.; Lovegrove, J.A. Addition of orange pomace to orange juice attenuates the increases in peak glucose and insulin concentescudero ions after sequential meal ingestion in men with elevated cardiometabolic risk. J. Nutr. 2016, 146, 1. [Google Scholar] [CrossRef] [PubMed]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Samie, A.; Sedaghat, R.; Baluchnejadmojarad, T.; Roghani, M. Hesperetin, a citrus flavonoid, attenuates testicular damage in diabetic rats via inhibition of oxidative stress, inflammation, and apoptosis. Life Sci. 2018, 210, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.; Hermsdorff, H.; Gomide, R.S.; Alves, R.; Bressan, J. Orange juice with a high-fat meal prolongs postprandial lipemia in apparently healthy overweight/obese women. Arch. Endocrinol. Metab. 2017, 61, 263–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barter, P.J.; Ballantyne, C.M.; Carmena, R.; Castro Cabezas, M.; Chapman, M.J.; Couture, P.; de Graaf, J.; Durrington, P.N.; Faergeman, O.; Frohlich, J.; et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: Report of the thirthy-person/ten-country panel. J. Int. Med. 2006, 259, 247–258. [Google Scholar] [CrossRef]
- Lin, T.; Wang, L.; Guo, J.; Liu, P.; Chen, L.; Wei, M.; Li, G. Association between serum LDL-C and ApoB and SYNTAX Score in patients with stable coronary artery. Angiology 2018, 69, 724–729. [Google Scholar] [CrossRef]
- Murkovic, M.; Abuja, P.M.; Bergmann, A.R.; Zirngast, A.; Adam, U.; Winklhofer-Roob, B.M.; Toplak, H. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: A randomized, double-blind, placebo-controlled study. Eur. J. Clin. Nutr. 2004, 58, 244–249. [Google Scholar] [CrossRef]
- Curtis, P.J.; Berends, L.; van der Velpen, V.; Jennings, A.; Haag, L.; Chandra, P.; Kay, C.D.; Rimm, E.B.; Cassidy, A. Blueberry anthocyanin intake attenuates the postprandial cardiometabolic effect of an energy-dense food challenge: Results from a double blind, randomized controlled trial in metabolic syndrome participants. Clin. Nutr. 2022, 41, 165–176. [Google Scholar] [CrossRef]
- Franke, A.A.; Cooney, R.V.; Henning, S.M.; Custer, L.J. Bioavailability and antioxidant effects of orange juice components in humans. J. Agric. Food Chem. 2005, 53, 5170–5178. [Google Scholar] [CrossRef]
- Wang, D.D.; Sievenpiper, J.L.; de Souza, R.J.; Cozma, A.I.; Chiavaroli, L.; Ha, V.; Mirrahimi, A.; Carleton, A.J.; Di Buono, M.; Jenkins, A.L.; et al. Effect of fructose on postprandial triglycerides: A systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014, 232, 125–133. [Google Scholar] [CrossRef]
- Roza, J.M.; Xian-Liu, Z.; Guthrie, N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern. Ther. Health Med. 2007, 13, 44–48. [Google Scholar] [PubMed]
- Kolovou, G.; Ooi, T.C. Postprandial lipaemia and vascular disease. Curr. Opin. Cardiol. 2013, 28, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Sandhu, M.S.; Ricketts, S.L.; Butterworth, A.S.; Di Angelantonio, E.; Boekholdt, S.M.; Ouwehand, W.; Watkins, H.; Samani, N.J.; Saleheen, D.; et al. Triglyceride-mediated pathways and coronary disease: Collaborative analysis of 101 studies. Lancet 2010, 375, 634–639. [Google Scholar]
- Hyson, D.; Rutledge, J.C.; Berglund, L. Postprandial lipemia and cardiovascular disease. Curr. Atheroscler. Rep. 2003, 5, 437–444. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; de Diego, E.H.; Riomoros-Arranz, M.; Reynoso-Camacho, R.; Saura-Calixto, F.; Pérez-Jiménez, J. Effects of acute intake of grape/pomegranate pomace dietary supplement on glucose metabolism and oxidative stress in adults with abdominal obesity. Int. J. Food Sci. Nutr. 2020, 71, 94–105. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoglu, E. Antioxidant activity/capacity measurement. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Vieira, G.K.; Di Pietro, P.F.; da Silva, E.L.; Borges, G.S.C.; Nunes, E.C.; Fett, R. Improvement of serum antioxidant status in humans after the acute intake of apple juices. Nutr. Res. 2012, 32, 229–232. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of Iavonoid-rich foods and increases plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2016, 41, 1727–1746. [Google Scholar] [CrossRef]
- Toaldo, I.M.; Cruz, F.A.; da Silva, E.L.; Bordignon-Luiz, M.T. Acute consumption of organic and conventional tropical grape juices (Vitis labrusca L.) increases antioxidants in plasma and erythrocytes, but not glucose and uric acid levels, in healthy individuals. Nutr. Res. 2016, 36, 808–817. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Poles, J.; Karhu, E.; McGill, M.; McDaniel, H.R.; Lewis, J.E. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: A narrative review. J. Clin. Transl. Res. 2021, 7, 333–376. [Google Scholar] [PubMed]
- Peluso, I.; Villano, D.V.; Roberts, S.A.; Cesqui, E.; Raguzzini, A.; Borges, G.; Crozier, A.; Catasta, G.; Toti, E.; Serafini, M. Consumption of mixed fruit-juice drink and vitamin C reduces postprandial stress induced by a high fat meal in healthy overweight subjects. Curr. Pharm. Des. 2014, 20, 1020–1024. [Google Scholar] [CrossRef] [PubMed]










| Composition | OJ | FOJ |
|---|---|---|
| pH | 3.48 ± 0.20 | 3.48 ± 0.03 |
| TA (g citric acid/L) | 8.48 ± 0.02 | 8.60 ± 0.01 |
| TSS (ºBrix) | 11.00 ± 0.50 | 9.01 ± 0.14 |
| % Pulp | 12.00 ± 2.00 | 5.65 ± 0.22 |
| Alcohol (% v/v) | 0 | 0.90 ± 0.15 |
| Ascorbic acid (mg/L) | 423.00 ± 1.80 | 197.00 ± 6.80 |
| Total sugars (g/L) | 78.20 ± 5.64 | 47.90 ± 4.10 |
| Reducing sugars (g/L) | 48.50 ± 3.63 | 20.30 ± 2.40 |
| Non reducing sugars (g/L) | 29.70 ± 2.01 | 27.10 ± 2.61 |
| Total (poly)phenols (µmol/L) | 659.0 ± 10.5 | 835.7 ± 37.6 |
| Total flavanones (µmol/L) | 502.7 ± 20.7 | 667.2 ± 31.6 |
| Total flavones (µmol/L) | 132.8 ± 6.0 | 142.8 ± 6.1 |
| Total phenolic acids (µmol/L) | 23.5 ± 4.2 | 25.7 ± 0.1 |
| Total individual carotenoids (µg/mL) | 11.04 ± 0.20 | 10.33 ± 0.40 |
| Provitamin A RAEs (µg/L) | 62.93 ± 1.85 | 70.97 ± 3.26 |
| Melatonin (ng/mL) | 0.185 ± 0.033 | 0.192 ± 0.072 |
| ORAC (μmol/L) | 6361 ± 261 | 6353 ± 307 |
| Characteristics | Means ± SEM |
|---|---|
| Age | 22.00 ± 0.44 |
| Body Weight (kg) | 63.39 ± 2.60 |
| Height (m2) | 1.73 ± 0.08 |
| BMI (kg/m2) | 20.87 ± 0.49 |
| WC (cm) | 72.30 ± 1.22 |
| Glucose (mg/dL) | 86.83 ± 1.81 |
| GOT (U/L) | 18.72 ± 1.38 |
| GPT (U/L) | 15.17 ± 1.28 |
| Creatinine (mg/dL) | 0.80 ± 0.03 |
| Hemoglobin (g/dL) | 13.48 ± 0.30 |
| Erythrocytes (×1012/L) | 4.48 ± 0.10 |
| Triglycerides (mg/dL) | 56.83 ± 4.87 |
| Total cholesterol (mg/dL) | 153.72 ± 6.02 |
| HDL cholesterol (mg/dL) | 62.61 ± 3.39 |
| LDL cholesterol (mg/dL) | 79.72 ± 4.84 |
| Parameter | OJ | FOJ | p-Value |
|---|---|---|---|
| ORAC (µmol/L) | 26,951.0 ± 5798.2 | 44,093.3 ± 5361.6 * | 0.04 |
| FRAP (mmol/L) | 16,606.1 ± 1749.5 | 14,986.2 ± 467.1 | 0.38 |
| Albumin (g/dL) | 32.1 ± 1.6 | 33.6 ± 0.6 | 0.98 |
| Bilirubin (mg/dL) | 4.1 ± 1.0 | 5.0 ± 1.8 | 0.81 |
| Uric Acid (mg/dL) | 26.3 ± 1.8 | 27.8 ± 1.7 | 0.53 |
| Glucose (mg/dL) | 550.2 ± 33.0 | 591.4 ± 17.0 | 0.66 |
| TC (mg/dL) | 976.3 ± 67.9 | 1112.4 ± 65.7 | 0.16 |
| LDL-C (mg/dL | 621.9 ± 64.2 | 718.6 ± 58.3 | 0.28 |
| HDL-C (mg/dL) | 410.1 ± 41.9 | 458.6 ± 31.0 | 0.36 |
| TG (mg/dL) | 380.1 ± 56.1 | 375.0 ± 20.8 | 0.93 |
| TC/HDL-C | 16.4 ± 1.4 | 17.3 ± 1.1 | 0.63 |
| LDL-C/HDL-C | 10.7 ± 1.5 | 11.3 ± 1.1 | 0.74 |
| Apo-AI (mg/dL) | 794.3 ± 50.4 | 840.2 ± 26.6 | 0.43 |
| Apo-B (mg/dL) | 366.6 ± 29.3 | 409.7 ± 23.1 | 0.26 |
| C-reactive protein (CRP) (mg/L) | 8.3 ± 4.1 | 11.6 ± 5.8 | 0.43 |
| Fibrinogen (mg/dL) | 1997.9 ± 165.3 | 1998.7 ± 79.2 | 0.99 |
| Parameter | OJ | FOJ | p Value * |
|---|---|---|---|
| GB (mg/dL) | 81.0 ± 2.2 | 84.4 ± 1.3 | 0.10 |
| PGV (mg/dL) | 107.5 ± 7.7 | 106.4 ± 7.4 | 0.92 |
| AIg (mg/dL) | 26.5 ± 8.7 | 22.0 ± 7.9 | 0.70 |
| GIP (%) | 33.9 ± 11.0 | 26.6 ± 9.9 | 0.63 |
| HP (min) | 360 ± 27.8 | 333.3 ± 20.3 | 0.57 |
| GIV (%) | 8.6 ± 3.0 | 6.8 ± 2.3 | 0.64 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-López, B.; Cerrillo, I.; Ortega, Á.; Martín, F.; Fernández-Pachón, M.-S. Effect of Acute Intake of Fermented Orange Juice on Fasting and Postprandial Glucose Metabolism, Plasma Lipids and Antioxidant Status in Healthy Human. Foods 2022, 11, 1256. https://doi.org/10.3390/foods11091256
Escudero-López B, Cerrillo I, Ortega Á, Martín F, Fernández-Pachón M-S. Effect of Acute Intake of Fermented Orange Juice on Fasting and Postprandial Glucose Metabolism, Plasma Lipids and Antioxidant Status in Healthy Human. Foods. 2022; 11(9):1256. https://doi.org/10.3390/foods11091256
Chicago/Turabian StyleEscudero-López, Blanca, Isabel Cerrillo, Ángeles Ortega, Franz Martín, and María-Soledad Fernández-Pachón. 2022. "Effect of Acute Intake of Fermented Orange Juice on Fasting and Postprandial Glucose Metabolism, Plasma Lipids and Antioxidant Status in Healthy Human" Foods 11, no. 9: 1256. https://doi.org/10.3390/foods11091256
APA StyleEscudero-López, B., Cerrillo, I., Ortega, Á., Martín, F., & Fernández-Pachón, M.-S. (2022). Effect of Acute Intake of Fermented Orange Juice on Fasting and Postprandial Glucose Metabolism, Plasma Lipids and Antioxidant Status in Healthy Human. Foods, 11(9), 1256. https://doi.org/10.3390/foods11091256