Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation
Abstract
1. Introduction
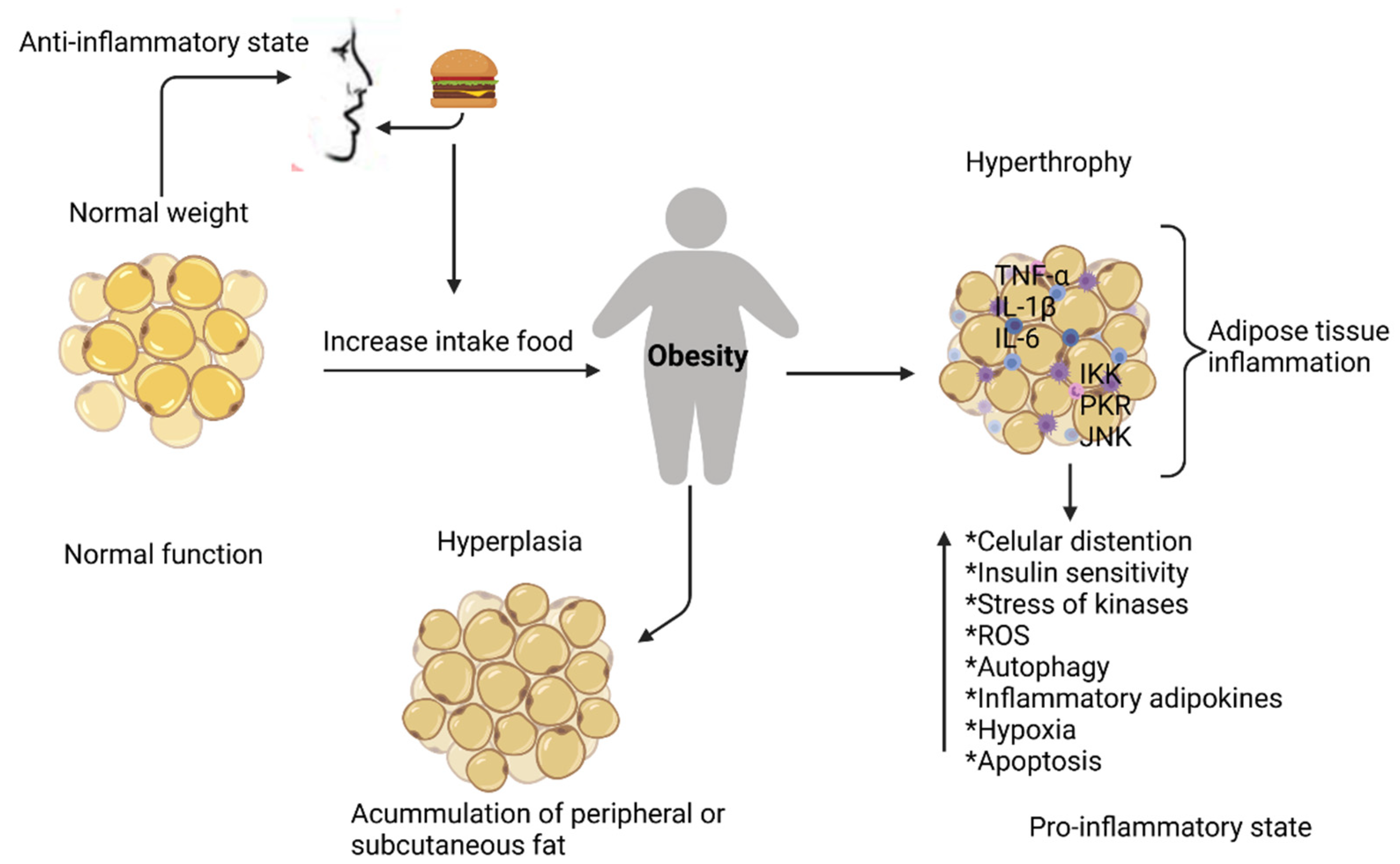
2. Physiopathology of Obesity
2.1. Inflammation in Adipose Tissue
| Adipokines | Segregation Molecules | Effect | Author |
|---|---|---|---|
| CRP | Increases the expression of vascular cell adhesion molecule-1 (VCAM-1), intracellular adhesion (ICAM-1), and E-selectin in vascular endothelial cells Increases the secretion of monocyte chemoattractant protein-1 (MCP-1) | Participates in the coronary and aortic atherosclerosis that leads to cardiac events | [51] |
| TNF-α | Decrease of nitric oxide (NO) Increase of endothelin1 (ET-1), angiotensin II (ATII), oxidized low-density lipoproteins (oxLDL), ICAM-1, VCAM-1, MCP-1, CD40/CD40L, and leukocyte adhesion | Increases foam cell formation Increases smooth muscle cell (SMC) proliferation and migration Expansion of the injury area Increases platelet adhesion Increases leptin concentration | [52] |
| IL-6 | Increases the concentration of free fatty acids (FFAs), C-reactive protein (CRP), and nitric oxide (NO) | Induces insulin resistance Decreases hepatic insulin clearance, insulin-dependent hepatic glycogen synthesis, glucose uptake in adipose cells | [53,54] |
| IL-1β | Inhibition of the insulin-transduction pathway Inhibition of β-cell function Destruction of β-cell mass Induces the transcriptional activation of inflammatory genes | [55,56] | |
| MCP1 | Strongly implicated in adipose tissue macrophage (ATM) recruitment, adipose expansion and remodeling, and angiogenesis | [30] | |
| IFN-γ | Cytokine secretion | Induces attraction of monocytes towards the activation of M1-type macrophages originating from proinflammatory cytokine secretion | [57] |
| PAI-1 | Increases the proliferation and migration of smooth muscle cells (SMCs) | Increases foam cell formation Increases platelet adhesion (thrombosis) Inhibition of the residual plasminogen activator | [58] |
| Resistin | Increases endoteline-1 (ET-1), angiotensine (ATII), oxLDL, intracellular adhesion (ICAM-1), VCAM-1, MCP-1, CD40/CD40L, leukocyte adhesion, and VSMC Stimulates the synthesis and secretion of cytokines in adipocytes and endothelial cells | Decreases NO release Increases in foam cell formation Increases in proliferation and migration of SMC and the expansion of injury area Increases in platelet adhesion and, as a consequence, thrombosis | [59,60] |
| Visfatin | Induces ICAM-1, VCAM-1, E-selectin, IL-8, IL-6, MCP-1, fibroblast growth factor-2(FGF-2), and metalloproteinase MMP-2/-9 production Increases the release of ROS (reactive oxygen species) | [60,61] | |
| Vaspin | Overexpressed in the obesity state Induces phosphatidylinositol 3-kinase/ Protein kinase (PI3K/AKT) activation, increases both glucose transporter type-4 (GLUT4) expression and translocation, and promotes insulin-stimulated glucose | [62] | |
| Angiotensinogen | Stimulates ICAM, VCAM-1, MCP-1, and factors stimulant of colonies of macrophages M-CSF production. | Decreases NO bioavailability Decreases vasorelaxation mechanisms and increases platelet adhesion to the vascular wall | [44,63] |
| Leptin | Increases VCAM-1 | In hyperleptinemia, the inflammatory process increases Increases oxidative stress Improves vasorelaxation Increases vascular permeability | [52,64] |
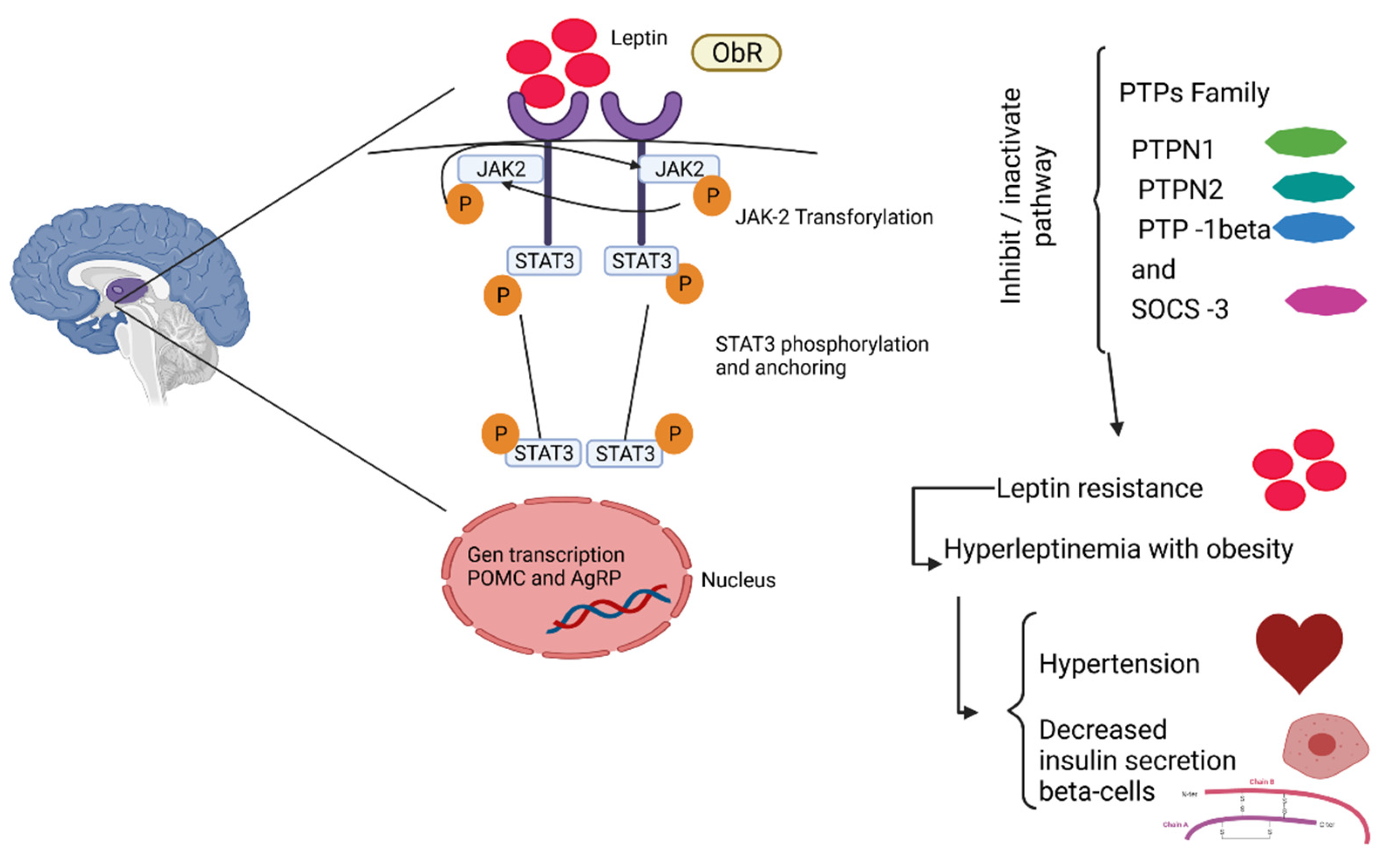
2.2. Obesity and Its Comorbidities
2.2.1. Type-2 Diabetes
2.2.2. Cardiovascular Disease
2.2.3. Cancer
2.2.4. Non-Alcoholic Fatty Liver Disease
| Diseases | Description | Author |
|---|---|---|
| Dyslipidemia | This pathology is due to the consequence of lipolysis produced in the adipocyte, increasing the levels of free fatty acids and increasing the synthesis of hepatic triglycerides, which, in turn, leads to an increase in VLDL. On the other hand, the decline in HDL-c is due to the decrease of Apo A-I, CETP, and LCAT, which inhibits the expression of ABCA1, ABCG1, and SR-B1. The cytokines and adipokines are responsible for these alterations in the adipose tissue | [99] |
| Gallbladder disease | Gallstones originate from the accumulation of cholesterol monohydrate crystals precipitating in gallbladder bile. Therefore, an increase in weight stimulates the risk of gallstones. | [100] |
| Hyperuricemia | An alteration with increased serum uric acid level development to gout due to monosodium urate crystals depositing mainly in the joints. These conditions increase with obesity due to the production of urates. | [101] |
| Osteoarthritis | Although the damage is not clear, it has been found that the dysregulation of adipokines (adiponectin, apelin, leptin, lipocalin-2, visfatin, chemerin, and resistin) and cartilage extracellular matrix degradation in the muscle–skeletal system exerts deleterious effects on the joint. | [102] |
| Hypothyroidism | Lower free irosin 4 and higher tirosin-stimulating Hormone levels are associated with fat accumulation. Modified thyroid function with normal feedback regulation may be the cause of alterations in energy expenditure with subsequent increases in BMI and weight | [103] |
3. A Brief Overview of the Properties and Dietary Effects of Some Bioactive Compounds in Obesity
3.1. Polyphenol Compounds
3.1.1. Phenolic Acids
3.1.2. Flavonoids
3.1.3. Betalains
3.1.4. Carotenoids
3.2. Clinical Evidence
3.3. Antioxidant Fiber Dietary
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, M. Obesity and Diabetes: The Slow-Motion Disaster. Milbank Q. 2017, 95, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenberg, S.J.; Sinaiko, A.R. Obesity and inflammation in children. Paediatr. Respir. Rev. 2006, 7, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Perez Mayorga, M. El Adipocito Como Órgano Endocrino: Implicaciones Fisiopatológicas y Terapéuticas. Revista Med. 2007, 152, 225–242. [Google Scholar]
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef] [PubMed]
- Krüger, K.; Mooren, F.C.; Eder, K.; Ringseis, R. Immune and Inflammatory Signaling Pathways in Exercise and Obesity. Am. J. Lifestyle Med. 2016, 10, 268–279. [Google Scholar] [CrossRef]
- Castro, A.M.; Macedo-de la Concha, L.E.; Pantoja-Meléndez, C.A. Low-grade inflammation and its relation to obesity and chronic degenerative diseases. Rev. Médica del Hosp. Gen. México 2017, 80, 101–105. [Google Scholar] [CrossRef]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef]
- Nyambuya, T.M.; Dludla, P.V.; Mxinwa, V.; Nkambule, B.B. Obesity-induced inflammation and insulin resistance: A mini-review on T-cells. Metab. Open 2019, 3, 100015. [Google Scholar] [CrossRef]
- Kim, S.; Jin, Y.; Choi, Y.; Park, T. Resveratrol exerts anti-obesity effects via mechanisms involving down-regulation of adipogenic and inflammatory processes in mice. Biochem. Pharmacol. 2011, 81, 1343–1351. [Google Scholar] [CrossRef]
- Aguirre, L.; Milton-Laskibar, I.; Hijona, E.; Bujanda, L.; Rimando, A.M.; Portillo, M.P. Effects of pterostilbene in brown adipose tissue from obese rats. J. Physiol. Biochem. 2016, 73, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Paraíso, A.F.; Sousa, J.N.; Oliveira Andrade, J.M.; Mangabeira, E.S.; de Farias Lelis, D.; Batista de Paula, A.M.; Eleutério Barros-Lima, A.M.; Nogueira Lima, W.J.; Sena Guimarães, A.L.; Melo, G.A.; et al. Oral gallic acid improves metabolic profile by modulating SIRT1 expression in obese mice brown adipose tissue: A molecular and bioinformatic approach. Life Sci. 2019, 237, 116914. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wei, Y.; Ning, C.; Zhang, M.; Fan, P.; Lei, D.; Du, J.; Gale, M.; Ma, Y.; Yang, Y. Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocr. J. 2019, 66, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Guo, J.; You, Y.; Zhan, J.; Huang, W. p-Coumaric acid prevents obesity via activating thermogenesis in brown adipose tissue mediated by mTORC1-RPS6. FASEB J. 2020, 34, 7810–7824. [Google Scholar] [CrossRef] [PubMed]
- Calçada, D.; Vianello, D.; Giampieri, E.; Sala, C.; Castellani, G.; de Graaf, A.; Kremer, B.; van Ommen, B.; Feskens, E.; Santoro, A.; et al. The role of low-grade inflammation and metabolic flexibility in aging and nutritional modulation thereof: A systems biology approach. Mech. Ageing Dev. 2014, 136–137, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, S.; Kunz, C.; Domann, E.; Würdemann, N.; Unger, F.; Römpp, A.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins after microbial fermentation in vitro. Nutrients 2016, 8, 411. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smart, J.L.; Rubinstein, M.; Cerdán, M.G.; Diano, S.; Horvath, T.L.; Cone, R.D.; Low, M.J. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001, 411, 480–484. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.-G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Ibrahim Abdalla, M.M. Ghrelin—Physiological Functions and Regulation. Eur. Endocrinol. 2015, 11, 90. [Google Scholar] [CrossRef]
- Svane, M.S.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Dirksen, C.; Nielsen, S.; Kristiansen, V.B.; Toräng, S.; Wewer Albrechtsen, N.J.; Rehfeld, J.F.; Hartmann, B.; et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. 2016, 40, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Kumar Ganesan; Baojun Xu A Critical Review on Polyphenols and Health Benefits of Black Soybeans. Nutrients 2017, 9, 455. [CrossRef] [PubMed]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Stulnig, T.M. Adipose tissue macrophages. Immunol. Lett. 2007, 112, 61–67. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Li, S.; Eguchi, N.; Lau, H.; Ichii, H. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 6973. [Google Scholar] [CrossRef]
- Blancas-Flores, G.; César Almanza-Pérez, J.; Ivette López-Roa, R.; Javier Alarcón-Aguilar, F.; García-Macedo, R.; Cruz, M. Obesity as an inflammatory process. Bol. Med. Hosp. Infant. Mex. 2010, 67, 88–97. [Google Scholar]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef]
- Shah, A.; Mehta, N.; Reilly, M.P. Adipose Inflammation, Insulin Resistance, and Cardiovascular Disease. J. Parenter. Enter. Nutr. 2008, 32, 638–644. [Google Scholar] [CrossRef]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, oxidative stress, and obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- García-Barrado, M.; Iglesias-Osma, M.; Pérez-García, E.; Carrero, S.; Blanco, E.; Carretero-Hernández, M.; Carretero, J. Role of Flavonoids in the Interactions among Obesity, Inflammation, and Autophagy. Pharmaceuticals 2020, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Izaola, O.; de Luis, D.; Sajoux, I.; Domingo, J.C.; Vidal, M. Inflamación y obesidad (Lipoinflamación). Nutr. Hosp. 2015, 31, 2352–2358. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & inflammation: The linking mechanism & the complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 1–25. [Google Scholar] [CrossRef]
- Libby, P. Inflammatory Mechanisms: The Molecular Basis of Inflammation and Disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef]
- Prasad, S.; Sung, B.; Aggarwal, B.B. Age-associated chronic diseases require age-old medicine: Role of chronic inflammation. Prev. Med. 2012, 54, S29–S37. [Google Scholar] [CrossRef]
- Lukens, J.R.; Gross, J.M.; Kanneganti, T.D. IL-1 family cytokines trigger sterile inflammatory disease. Front. Immunol. 2012, 3, 315. [Google Scholar] [CrossRef]
- Rubartelli, A.; Lotze, M.T.; Latz, E.; Manfredi, A. Mechanisms of sterile inflammation. Front. Immunol. 2013, 4, 398. [Google Scholar] [CrossRef]
- Nasef, N.A.; Mehta, S.; Ferguson, L.R. Susceptibility to chronic inflammation: An update. Arch. Toxicol. 2017, 91, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.S.; Alvarez-Leite, J.I. Low-Grade Inflammation, Obesity, and Diabetes. Curr. Obes. Rep. 2014, 3, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Manzur, F.; Alvear, C.; Alayón, A.N. Adipocitos, obesidad visceral, inflamación y enfermedad cardiovascular. Rev. Colomb. Cardiol. 2010, 17, 207–213. [Google Scholar] [CrossRef]
- Tanti, J.F.; Ceppo, F.; Jager, J.; Berthou, F. Implication of inflammatory signaling pathways in obesity-induced insulin resistance. Front. Endocrinol. 2013, 3, 181. [Google Scholar] [CrossRef]
- Engin, A.B.; Engin, A.; Gonul, I.I. The effect of adipocyte-macrophage crosstalk in obesity-related breast cancer. J. Mol. Endocrinol. 2019, 62, R201–R222. [Google Scholar] [CrossRef]
- Lau, D.C.W.; Dhillon, B.; Yan, H.; Szmitko, P.E.; Verma, S. Adipokines: Molecular links between obesity and atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, 2031–2041. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Rajala, M.W.; Scherer, P.E. Minireview: The Adipocyte—At the Crossroads of Energy Homeostasis, Inflammation, and Atherosclerosis. Endocrinology 2003, 144, 3765–3773. [Google Scholar] [CrossRef]
- Azizian, M.; Mahdipour, E.; Mirhafez, S.R.; Shoeibi, S.; Nematy, M.; Esmaily, H.; Ferns, G.A.A.; Ghayour-Mobarhan, M. Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender. Ann. Clin. Biochem. 2016, 53, 663–668. [Google Scholar] [CrossRef]
- Domínguez-Amorocho, O.; Patiño-Cuervo, D. Proteína C reactiva ultrasensible (PCR-us) como marcador de riesgo de enfermedad cardiovascular. Med. Lab. 2008, 14, 457–478. [Google Scholar]
- Sáinz, N.; Barrenetxe, J.; Moreno-Aliaga, M.J.; Martínez, J.A. Leptin resistance and diet-induced obesity: Central and peripheral actions of leptin. Metabolism 2015, 64, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Eder, K.; Baffy, N.; Falus, A.; Fulop, A.K. The major inflammatory mediator interleukin-6 and obesity. Inflamm. Res. 2009, 58, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Meza, M.N.; Carrillo, J.A.B. Biomarkers, Obesity, and Cardiovascular Diseases. In Role of Biomarkers in Medicine; InTech: London, UK, 2016. [Google Scholar]
- Ballak, D.B.; Stienstra, R.; Tack, C.J.; Dinarello, C.A.; van Diepen, J.A. IL-1 family members in the pathogenesis and treatment of metabolic disease: Focus on adipose tissue inflammation and insulin resistance. Cytokine 2015, 75, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Bing, C. Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte 2015, 4, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between Adipocytes and Immune Cells in Adipose Tissue Inflammation and Metabolic Dysregulation in Obesity. Mol. Cells 2014, 37, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Khoukaz, H.B.; Ji, Y.; Braet, D.J.; Vadali, M.; Abdelhamid, A.A.; Emal, C.D.; Lawrence, D.A.; Fay, W.P. Drug Targeting of Plasminogen Activator Inhibitor-1 Inhibits Metabolic Dysfunction and Atherosclerosis in a Murine Model of Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1479–1490. [Google Scholar] [CrossRef]
- Dalamaga, M. Resistin as a biomarker linking obesity and inflammation to cancer: Potential clinical perspectives. Biomark. Med. 2014, 8, 107–118. [Google Scholar] [CrossRef]
- Stofkova, A. Resistin and visfatin: Regulators of insulin sensitivity, inflammation and immunity. Endocr. Regul. 2010, 44, 25–36. [Google Scholar] [CrossRef]
- Singh, M.; Benencia, F. Inflammatory processes in obesity: Focus on endothelial dysfunction and the role of adipokines as inflammatory mediators. Int. Rev. Immunol. 2019, 38, 157–171. [Google Scholar] [CrossRef]
- Nicholson, T.; Church, C.; Tsintzas, K.; Jones, R.; Breen, L.; Davis, E.T.; Baker, D.J.; Jones, S.W. Vaspin promotes insulin sensitivity in elderly muscle and is upregulated in obesity. J. Endocrinol. 2019, 241, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Iantorno, M.; Campia, U.; Di Daniele, N.; Nistico, S.; Forleo, G.; Cardillo, C.; Tesauro, M. Obesity, inflammation and endothelial dysfunction. J. Biol. Regul. Homeost. Agents 2014, 282, 169–176. [Google Scholar]
- Raskin Erusan, R.; Nalini, D.; Manohar, G.; Malathi, R. Correlation between Obesity and Inflammation in Cardiovascular Diseases—Evaluation of Leptin and Inflammatory Cytokines. Open J. Endocr. Metab. Dis. 2012, 2, 7–15. [Google Scholar] [CrossRef][Green Version]
- Pantalone, K.M.; Hobbs, T.M.; Chagin, K.M.; Kong, S.X.; Wells, B.J.; Kattan, M.W.; Bouchard, J.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Prevalence and recognition of obesity and its associated comorbidities: Cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017, 7, e017583. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Calcaterra, V.; Regalbuto, C.; Porri, D.; Pelizzo, G.; Mazzon, E.; Vinci, F.; Zuccotti, G.; Fabiano, V.; Cena, H. Inflammation in Obesity-Related Complications in Children: The Protective Effect of Diet and Its Potential Role as a Therapeutic Agent. Biomolecules 2020, 10, 1324. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Stehouwer, C.D.A.; Emeis, J.J.; Coppack, S.W. C-Reactive Protein in Healthy Subjects: Associations With Obesity, Insulin Resistance, and Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 972–978. [Google Scholar] [CrossRef]
- Stephens, J.M.; Lee, J.; Pilch, P.F. Tumor Necrosis Factor-α-induced Insulin Resistance in 3T3-L1 Adipocytes Is Accompanied by a Loss of Insulin Receptor Substrate-1 and GLUT4 Expression without a Loss of Insulin Receptor-mediated Signal Transduction. J. Biol. Chem. 1997, 272, 971–976. [Google Scholar] [CrossRef]
- Marfella, R.; Di Filippo, C.; Portoghese, M.; Siniscalchi, M.; Martis, S.; Ferraraccio, F.; Guastafierro, S.; Nicoletti, G.; Barbieri, M.; Coppola, A.; et al. The ubiquitin–proteasome system contributes to the inflammatory injury in ischemic diabetic myocardium: The role of glycemic control. Cardiovasc. Pathol. 2009, 18, 332–345. [Google Scholar] [CrossRef]
- Nandipati, K.C.; Subramanian, S.; Agrawal, D.K. Protein kinases: Mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol. Cell. Biochem. 2017, 426, 27–45. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Bandyopadhyay, A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004, 25, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Umpierrez, G.E.; Cuervo, R.; Kitabchi, A.E. Proinflammatory Cytokines, Markers of Cardiovascular Risks, Oxidative Stress, and Lipid Peroxidation in Patients With Hyperglycemic Crises. Diabetes 2004, 53, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Kahn, B.B. Adipose Tissue, Inter-Organ Communication, and the Path to Type 2 Diabetes: The 2016 Banting Medal for Scientific Achievement Lecture. Diabetes 2019, 68, 3–14. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schön, M.R.; Abumrad, N.A.; Blüher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Viardot, A.; Clément, K.; Tordjman, J.; Tonks, K.; Greenfield, J.R.; Campbell, L.V.; Samocha-Bonet, D.; Heilbronn, L.K. Short-Term Overfeeding May Induce Peripheral Insulin Resistance Without Altering Subcutaneous Adipose Tissue Macrophages in Humans. Diabetes 2010, 59, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Schuster, D.P. Obesity and the development of type 2 diabetes: The effects of fatty tissue inflammation. Diabetes Metab. Syndr. Obes. Targets Therapy. Dovepress 2010, 3, 253–262. [Google Scholar] [CrossRef]
- Esser, N.; Legrand-Poels, S.; Piette, J.; Scheen, A.J.; Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014, 105, 141–150. [Google Scholar] [CrossRef]
- Rodrigues, K.F.; Pietrani, N.T.; Bosco, A.A.; Campos, F.M.F.; Sandrim, V.C.; Gomes, K.B. IL-6, TNF-α, and IL-10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch. Endocrinol. Metab. 2017, 61, 438–446. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, H.; Simental-Mendía, L.E.; Rodríguez-Ramírez, G.; Reyes-Romero, M.A. Obesity and inflammation: Epidemiology, risk factors, and markers of inflammation. Int. J. Endocrinol. 2013, 2013, 678159. [Google Scholar] [CrossRef]
- Tangvarasittichai, S.; Pongthaisong, S.; Tangvarasittichai, O. Tumor Necrosis Factor-A, Interleukin-6, C-Reactive Protein Levels and Insulin Resistance Associated with Type 2 Diabetes in Abdominal Obesity Women. Indian J. Clin. Biochem. 2016, 31, 68–74. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; Naya, I.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; Asúnsolo, Á.; de la Torre, B. Type 2 Diabetes Mellitus Associated with Obesity (Diabesity). The Central Role of Gut Microbiota and Its Translational Applications. Nutrients 2020, 12, 2749. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Ikeoka, D.; Mader, J.K.; Pieber, T.R. Adipose tissue, inflammation and cardiovascular disease. Revista da Associação Médica Brasileira 2010, 56, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Wang, Z. Inflammation, a link between obesity and cardiovascular disease. Mediators Inflamm. 2010, 2010, 535918. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Ermetici, F.; Coman, C.; Corsi, M.M.; Morricone, L.; Ambrosi, B. Influence of epicardial adipose tissue and adipocytokine levels on cardiac abnormalities in visceral obesity. Int. J. Cardiol. 2007, 121, 132–134. [Google Scholar] [CrossRef]
- Correa-Burrows, P.; Rogan, J.; Blanco, E.; East, P.; Lozoff, B.; Gahagan, S.; Burrows, R. Resolving early obesity leads to a cardiometabolic profile within normal ranges at 23 years old in a two-decade prospective follow-up study. Sci. Rep. 2021, 11, 18927. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Hopkins, B.D.; Goncalves, M.D.; Cantley, L.C. Obesity and cancer mechanisms: Cancer metabolism. J. Clin. Oncol. 2016, 34, 4277–4283. [Google Scholar] [CrossRef]
- Lohmann, A.E.; Goodwin, P.J.; Chlebowski, R.T.; Pan, K.; Stambolic, V.; Dowling, R.J.O. Association of obesity-related metabolic disruptions with cancer risk and outcome. J. Clin. Oncol. 2016, 34, 4249–4255. [Google Scholar] [CrossRef]
- Nimptsch, K.; Pischon, T. Obesity biomarkers, metabolism and risk of cancer: An epidemiological perspective. In Recent Results in Cancer Research; Springer: New York, NY, USA, 2016; Volume 208, pp. 199–217. [Google Scholar]
- Kolb, R.; Sutterwala, F.S.; Zhang, W. Obesity and cancer: Inflammation bridges the two. Curr. Opin. Pharmacol. 2016, 29, 77–89. [Google Scholar] [CrossRef]
- Hursting, S.D.; Dunlap, S.M. Obesity, metabolic dysregulation, and cancer: A growing concern and an inflammatory (and microenvironmental) issue. Ann. N. Y. Acad. Sci. 2012, 1271, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Scorletti, E.; Mosca, A.; Alisi, A.; Byrne, C.D.; Targher, G. Complications, morbidity and mortality of nonalcoholic fatty liver disease. Metabolism 2020, 111, 154170. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.; Dumitraşcu, D.L. Cardiovascular Risk in Fatty Liver Disease: The Liver-Heart Axis—Literature Review. Front. Med. 2019, 6, 202. [Google Scholar] [CrossRef]
- Francque, S.M.; van der Graaff, D.; Kwanten, W.J. Non-alcoholic fatty liver disease and cardiovascular risk: Pathophysiological mechanisms and implications. J. Hepatol. 2016, 65, 425–443. [Google Scholar] [CrossRef]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.-J.; Nati, M.; Chavakis, T.; Chatzigeorgiou, A. Innate immune cells in the adipose tissue. Rev. Endocr. Metab. Disord. 2018, 19, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Obesity and Dyslipidemia. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: Dartmouth, MA, USA, 2020; pp. 1–20. [Google Scholar]
- Bonfrate, L.; Wang, D.Q.-H.; Garruti, G.; Portincasa, P. Obesity and the risk and prognosis of gallstone disease and pancreatitis. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 623–635. [Google Scholar] [CrossRef]
- Shirasawa, T.; Ochiai, H.; Yoshimoto, T.; Nagahama, S.; Watanabe, A.; Yoshida, R.; Kokaze, A. Cross-sectional study of associations between normal body weight with central obesity and hyperuricemia in Japan. BMC Endocr. Disord. 2020, 20, 2. [Google Scholar] [CrossRef]
- Francisco, V.; Pérez, T.; Pino, J.; López, V.; Franco, E.; Alonso, A.; Gonzalez-Gay, M.A.; Mera, A.; Lago, F.; Gómez, R.; et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J. Orthop. Res. 2017, 36, 594–604. [Google Scholar] [CrossRef]
- Sanyal, D.; Raychaudhuri, M. Hypothyroidism and obesity: An intriguing link. Indian J. Endocrinol. Metab. 2016, 20, 554. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. Available online: https://www.researchgate.net/publication/263544261 (accessed on 15 March 2020).
- Gamboa-Gómez, C.I.; Rocha-Guzmán, N.E.; Moreno-Jiménez, M.R.; Vázquez-Cabral, B.D.; González-Laredo, R.F. Plants with potential use on obesity and its complications. EXCLI J. 2015, 14, 809–831. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Jahnovi Brahma; Dhananjoy Narzary Bioactive and Nutraceutical Compound Manipulation from the Leaves of Some Wild Edible Medicinal Plants in Chirang District of Assam, India. Am. J. Ethnomed. 2015, 2, 356–364.
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Pramila, G.; Farooqui, M. Biological activity of aqueous extract of some medicinal plants. Der Chem. Sin. 2014, 5, 65–70. [Google Scholar]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef]
- Mrduljaš, N.; Krešić, G.; Bilušić, T. Polyphenols: Food Sources and Health Benefits. In Functional Food—Improve Health through Adequate Food; IntechOpen: London, UK, 2017. [Google Scholar]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Bilal Hussain, M.; Hassan, S.; Waheed, M.; Javed, A.; Adil Farooq, M.; Tahir, A. Bioavailability and Metabolic Pathway of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds—Biological Activity; IntechOpen: London, UK, 2017. [Google Scholar]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.S.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Rychlik, J.; Grajek, W. Cranberries (Oxycoccus quadripetalus) inhibit adipogenesis and lipogenesis in 3T3-L1 cells. Food Chem. 2014, 148, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.-T.; Hung, P.-F.; Chen, H.-C.; Huang, R.-N.; Chang, H.-H.; Kao, Y.-H. The Apoptotic Effect of Green Tea (−)-Epigallocatechin Gallate on 3T3-L1 Preadipocytes Depends on the Cdk2 Pathway. J. Agric. Food Chem. 2005, 53, 5695–5701. [Google Scholar] [CrossRef] [PubMed]
- Boqué, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Bañuelos, Ó.; Soria, A.C.; Rodríguez-Sánchez, S.; Martínez, J.A.; Campión, J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic Acid Protects Hyperglycemia-Induced Kidney Damage by Regulating Oxidative Insult, Inflammation and Autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Bian, X.; Yao, Z.; Wang, Y.; Gao, W.; Guo, C. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020, 11, 8003–8013. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, H.J.; Lee, D.Y.; Kim, J.S.; Kim, D.H.; Lee, H.J.; Kim, H.D.; Jeon, R.; Ryu, J.-H. In vitro anti-inflammatory activity of lignans isolated from Magnolia fargesii. Bioorg. Med. Chem. Lett. 2009, 19, 937–940. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.A. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A critical review of phenolic compounds extracted from the bark of woody vascular plants and their potential biological activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Aranaz, P.; Navarro-Herrera, D.; Zabala, M.; Miguéliz, I.; Romo-Hualde, A.; López-Yoldi, M.; Alfredo Martínez, J.; Vizmanos, J.L.; Milagro, F.I.; González-Navarro, C.J. Phenolic compounds inhibit 3T3-L1 adipogenesis depending on the stage of differentiation and their binding affinity to PPARγ. Molecules 2019, 24, 1045. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.L.; Yen, G.C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, O.B.; Ajiboye, T.O. Dietary phenolic acids reverse insulin resistance, hyperglycaemia, dyslipidaemia, inflammation and oxidative stress in high-fructose diet-induced metabolic syndrome rats. Arch. Physiol. Biochem. 2018, 124, 410–417. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.Y.; Son, Y.-J.; Yun, J.-M. Gallic Acid Decreases Inflammatory Cytokine Secretion Through Histone Acetyltransferase/Histone Deacetylase Regulation in High Glucose-Induced Human Monocytes. J. Med. Food 2015, 18, 793–801. [Google Scholar] [CrossRef]
- Marranzano, M.; Ray, S.; Godos, J.; Galvano, F. Association between dietary flavonoids intake and obesity in a cohort of adults living in the Mediterranean area. Int. J. Food Sci. Nutr. 2018, 69, 1020–1029. [Google Scholar] [CrossRef]
- Swick, J.C. Effect of the Flavonoid Quercetin on Adipocytes. Master’s Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2011. [Google Scholar]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S.; Rupasinghe, H.P. Effect of Different Cooking Methods on Polyphenols, Carotenoids and Antioxidant Activities of Selected Edible Leaves. Antioxidants 2018, 7, 117. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Park, J.; Ha, T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 2008, 373, 545–549. [Google Scholar] [CrossRef]
- Gosslau, A.; Zachariah, E.; Li, S.; Ho, C.-T. Effects of a flavonoid-enriched orange peel extract against type 2 diabetes in the obese ZDF rat model. Food Sci. Hum. Wellness 2018, 7, 244–251. [Google Scholar] [CrossRef]
- Nakajima, V.M.; Macedo, G.A.; Macedo, J.A. Citrus bioactive phenolics: Role in the obesity treatment. LWT—Food Sci. Technol. 2014, 59, 1205–1212. [Google Scholar] [CrossRef]
- Esquivel, P. Handbook on Natural Pigments in Food and Beverages; Carle, R., Schweiggert, R.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081003718. [Google Scholar]
- Tan, D.; Wang, Y.; Bai, B.; Yang, X.; Han, J. Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat. Food Chem. Toxicol. 2015, 78, 141–146. [Google Scholar] [CrossRef]
- González-Ponce, H.; Martínez-Saldaña, M.; Rincón-Sánchez, A.; Sumaya-Martínez, M.; Buist-Homan, M.; Faber, K.; Moshage, H.; Jaramillo-Juárez, F. Hepatoprotective Effect of Opuntia robusta and Opuntia streptacantha Fruits against Acetaminophen-Induced Acute Liver Damage. Nutrients 2016, 8, 607. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Salzano, G.; Sarisozen, C.; Torchilin, V. Anti-cancer activity of doxorubicin-loaded liposomes co-modified with transferrin and folic acid. Eur. J. Pharm. Biopharm. 2016, 105, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Abedimanesh, N.; Asghari, S.; Mohammadnejad, K.; Daneshvar, Z.; Rahmani, S.; Shokoohi, S.; Farzaneh, A.H.; Hosseini, S.H.; Jafari Anarkooli, I.; Noubarani, M.; et al. The anti-diabetic effects of betanin in streptozotocin-induced diabetic rats through modulating AMPK/SIRT1/NF-κB signaling pathway. Nutr. Metab. 2021, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Luisa Bonet, M.; Canas, J.A.; Ribot, J.; Palou, A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch. Biochem. Biophys. 2015, 572, 112–125. [Google Scholar] [CrossRef]
- Mounien, L.; Tourniaire, F.; Landrier, J.F. Anti-obesity effect of carotenoids: Direct impact on adipose tissue and adipose tissue-driven indirect effects. Nutrients 2019, 11, 1562. [Google Scholar] [CrossRef]
- Liao, C.-C.; Ou, T.-T.; Wu, C.-H.; Wang, C.-J. Prevention of Diet-Induced Hyperlipidemia and Obesity by Caffeic Acid in C57BL/6 Mice through Regulation of Hepatic Lipogenesis Gene Expression. J. Agric. Food Chem. 2013, 61, 11082–11088. [Google Scholar] [CrossRef]
- Tasaki, M.; Umemura, T.; Maeda, M.; Ishii, Y.; Okamura, T.; Inoue, T.; Kuroiwa, Y.; Hirose, M.; Nishikawa, A. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem. Toxicol. 2008, 46, 1119–1124. [Google Scholar] [CrossRef]
- Techer, D.; Milla, S.; Fontaine, P.; Viot, S.; Thomas, M. Acute toxicity and sublethal effects of gallic and pelargonic acids on the zebrafish Danio rerio. Environ. Sci. Pollut. Res. 2015, 22, 5020–5029. [Google Scholar] [CrossRef]
- Aldaba-Muruato, L.; Ventura-Juárez, J.; Perez-Hernandez, A.; Hernández-Morales, A.; Muñoz-Ortega, M.; Martínez-Hernández, S.; Alvarado-Sánchez, B.; Macías-Pérez, J. Therapeutic perspectives of p-coumaric acid: Anti-necrotic, anti-cholestatic and anti-amoebic activities. World Acad. Sci. J. 2021, 3, 47. [Google Scholar] [CrossRef]
- MIRZA, A.C.; PANCHAL, S.S. Safety Assessment of Vanillic Acid: Subacute Oral Toxicity Studies in Wistar Rats. Turkish J. Pharm. Sci. 2020, 17, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Park, J.; Kim, H.; Sim, J.; Youn, D.; Kang, J.; Lim, S.; Jeong, M.; Yang, W.M.; Lee, S.; et al. Vanillic acid attenuates obesity via activation of the AMPK pathway and thermogenic factors in vivo and in vitro. FASEB J. 2018, 32, 1388–1402. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- SAITO, A.; YAMAMOTO, M. Acute oral toxicity of capsaicin in mice and rats. J. Toxicol. Sci. 1996, 21, 195–200. [Google Scholar] [CrossRef]
- Benn, T.; Kim, B.; Park, Y.K.; Wegner, C.J.; Harness, E.; Nam, T.G.; Kim, D.O.; Lee, J.S.; Lee, J.Y. Polyphenol-rich blackcurrant extract prevents inflammation in diet-induced obese mice. J. Nutr. Biochem. 2014, 25, 1019–1025. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Anthocyanins. Adv. Nutr. 2015, 6, 620–622. [Google Scholar] [CrossRef]
- Medrano-Padial, C.; Prieto, A.I.; Puerto, M.; Pichardo, S. Toxicological Evaluation of Piceatannol, Pterostilbene, and ε-Viniferin for Their Potential Use in the Food Industry: A Review. Foods 2021, 10, 592. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Ejaz, A.; Wu, D.; Kwan, P.; Meydani, M. Curcumin Inhibits Adipogenesis in 3T3-L1 Adipocytes and Angiogenesis and Obesity in C57/BL Mice. J. Nutr. 2009, 139, 919–925. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef]
- Seo, S.; Lee, M.-S.; Chang, E.; Shin, Y.; Oh, S.; Kim, I.-H.; Kim, Y. Rutin Increases Muscle Mitochondrial Biogenesis with AMPK Activation in High-Fat Diet-Induced Obese Rats. Nutrients 2015, 7, 5385. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Vicente, L.; Prieto, M.; Morales, A.I. Eficacia y seguridad de la quercetina como complemento alimenticio. Rev. Toxicol. 2013, 30, 171–181. [Google Scholar]
- Su, T.; Huang, C.; Yang, C.; Jiang, T.; Su, J.; Chen, M.; Fatima, S.; Gong, R.; Hu, X.; Bian, Z.; et al. Apigenin inhibits STAT3/CD36 signaling axis and reduces visceral obesity. Pharmacol. Res. 2020, 152, 104586. [Google Scholar] [CrossRef]
- DeRango-Adem, E.F.; Blay, J. Does Oral Apigenin Have Real Potential for a Therapeutic Effect in the Context of Human Gastrointestinal and Other Cancers? Front. Pharmacol. 2021, 12, 681477. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, N.; Li, S.; Chen, M.; Pu, P. Novel anti-obesity effect of scutellarein and potential underlying mechanism of actions. Biomed. Pharmacother. 2019, 117, 109042. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Li, Y.; Bai, L.; Xue, M. Acute and subacute toxicological evaluation of scutellarin in rodents. Regul. Toxicol. Pharmacol. 2011, 60, 106–111. [Google Scholar] [CrossRef]
- Kwon, E.Y.; Choi, M.S. Luteolin targets the toll-like receptor signaling pathway in prevention of hepatic and adipocyte fibrosis and insulin resistance in diet-induced obese mice. Nutrients 2018, 10, 1415. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.X.; Wang, X.; Zhang, L.; Qu, W.; Bao, B.; Liu, C.A.; Liu, J. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1α pathway-mediated mechanism. Int. J. Obes. 2016, 40, 1841–1849. [Google Scholar] [CrossRef]
- KANAI, K.; NAGATA, S.; HATTA, T.; SUGIURA, Y.; SATO, K.; YAMASHITA, Y.; KIMURA, Y.; ITOH, N. Therapeutic anti-inflammatory effects of luteolin on endotoxin-induced uveitis in Lewis rats. J. Vet. Med. Sci. 2016, 78, 1381–1384. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Zhu, Y.; Liao, M.; Chu, L.; Li, X.; Lin, L.; Zheng, G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKα-LXRα/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019, 10, 7489–7497. [Google Scholar] [CrossRef]
- Li, Y.O.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Lim, S.-M.; Lee, H.S.; Jung, J.I.; Kim, S.M.; Kim, N.Y.; Seo, T.S.; Bae, J.-S.; Kim, E.J. Cyanidin-3-O-Galactoside-Enriched Aronia melanocarpa Extract Attenuates Weight Gain and Adipogenic Pathways in High-Fat Diet-Induced Obese C57BL/6 Mice. Nutrients 2019, 11, 1190. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Chu, Q.; Yan, F.; Yang, Y.; Han, W.; Zheng, X. Red pitaya betacyanins protects from diet-induced obesity, liver steatosis and insulin resistance in association with modulation of gut microbiota in mice. J. Gastroenterol. Hepatol. 2016, 31, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Liu, Z.; Martin, C.K.; Kai-yuan, W.; Nofziger, J.; Rood, J.C.; Yu, Y.; Amen, R.J. Safety and efficacy of NT, an herbal supplement, in treating human obesity. Int. J. Obes. 2006, 30, 1737–1741. [Google Scholar] [CrossRef]
- Basu, A.; Wilkinson, M.; Penugonda, K.; Simmons, B.; Betts, N.M.; Lyons, T.J. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr. J. 2009, 8, 43. [Google Scholar] [CrossRef]
- Abubakar, S.M.; Ukeyima, M.T.; Spencer, J.P.E.; Lovegrove, J.A. Acute Effects of Hibiscus Sabdariffa Calyces on Postprandial Blood Pressure, Vascular Function, Blood Lipids, Biomarkers of Insulin Resistance and Inflammation in Humans. Nutrients 2019, 11, 341. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Perna, S.; Faliva, M.; Solerte, S.B.; Fioravanti, M.; Klersy, C.; Cava, E.; Edda, C.; Paolini, M.; et al. Improvement in insulin resistance and favourable changes in plasma inflammatory adipokines after weight loss associated with two months’ consumption of a combination of bioactive food ingredients in overweight subjects. Endocrine 2013, 44, 391–401. [Google Scholar] [CrossRef]
- Ghadimi, M.; Foroughi, F.; Hashemipour, S.; Nooshabadi, M.R.; Ahmadi, M.H.; Yari, M.G.; Kavianpour, M.; Haghighian, H.K. Decreased insulin resistance in diabetic patients by influencing Sirtuin1 and Fetuin-A following supplementation with ellagic acid: A randomized controlled trial. Diabetol. Metab. Syndr. 2021, 13, 16. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; Platero, J.L.; Yang, I.H.; Ceron, J.J.; Tvarijonaviciute, A.; Sabater, P.S.; Benlloch, M.; Sancho-Cantus, D.; Sancho, S. Possible Role of Butyrylcholinesterase in Fat Loss and Decreases in Inflammatory Levels in Patients with Multiple Sclerosis after Treatment with Epigallocatechin Gallate and Coconut Oil: A Pilot Study. Nutrients 2021, 13, 3230. [Google Scholar] [CrossRef]
- Most, J.; Warnke, I.; Boekschoten, M.V.; Jocken, J.W.E.; de Groot, P.; Friedel, A.; Bendik, I.; Goossens, G.H.; Blaak, E.E. The effects of polyphenol supplementation on adipose tissue morphology and gene expression in overweight and obese humans. Adipocyte 2018, 7, 190–196. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; van de Weijer, T.; Goossens, G.H.; Hoeks, J.; van der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie Restriction-like Effects of 30 Days of Resveratrol Supplementation on Energy Metabolism and Metabolic Profile in Obese Humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Kirch, N.; Berk, L.; Liegl, Y.; Adelsbach, M.; Zimmermann, B.F.; Stehle, P.; Stoffel-Wagner, B.; Ludwig, N.; Schieber, A.; Helfrich, H.-P.; et al. A nutritive dose of pure (−)-epicatechin does not beneficially affect increased cardiometabolic risk factors in overweight-to-obese adults-a randomized, placebo-controlled, double-blind crossover study. Am. J. Clin. Nutr. 2018, 107, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salmeán, G.; Meaney, E.; Lanaspa, M.A.; Cicerchi, C.; Johnson, R.J.; Dugar, S.; Taub, P.; Ramírez-Sánchez, I.; Villarreal, F.; Schreiner, G.; et al. A randomized, placebo-controlled, double-blind study on the effects of (−)-epicatechin on the triglyceride/HDLc ratio and cardiometabolic profile of subjects with hypertriglyceridemia: Unique in vitro effects. Int. J. Cardiol. 2016, 223, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kürbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Egert, S.; Boesch-Saadatmandi, C.; Wolffram, S.; Rimbach, G.; Muüller, M.J. Serum Lipid and Blood Pressure Responses to Quercetin Vary in Overweight Patients by Apolipoprotein E Genotype. J. Nutr. 2010, 140, 278–284. [Google Scholar] [CrossRef]
- Brüll, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Müller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: A randomized double-blinded, placebo-controlled crossover trial. Eur. J. Nutr. 2017, 56, 2265–2275. [Google Scholar] [CrossRef]
- Chuengsamarn, S.; Rattanamongkolgul, S.; Phonrat, B.; Tungtrongchitr, R.; Jirawatnotai, S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: A randomized controlled trial. J. Nutr. Biochem. 2014, 25, 144–150. [Google Scholar] [CrossRef]
- Ganjali, S.; Sahebkar, A.; Mahdipour, E.; Jamialahmadi, K.; Torabi, S.; Akhlaghi, S.; Ferns, G.; Parizadeh, S.M.R.; Ghayour-Mobarhan, M. Investigation of the Effects of Curcumin on Serum Cytokines in Obese Individuals: A Randomized Controlled Trial. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin supplementation on markers of inflammation and oxidative stress among healthy overweight and obese girl adolescents: A randomized placebo-controlled clinical trial. Phyther. Res. 2019, 33, 2015–2022. [Google Scholar] [CrossRef]
- Shirmohammadi, L.; Ghayour-Mobarhan, M.; Saberi-Karimian, M.; Iranshahi, M.; Tavallaie, S.; Emamian, M.; Sahebkar, A. Effect of Curcumin on Serum Cathepsin D in Patients with Metabolic Syndrome. Cardiovasc. Hematol. Disord. Targets 2020, 20, 116–121. [Google Scholar] [CrossRef]
- Vors, C.; Couillard, C.; Paradis, M.-E.; Gigleux, I.; Marin, J.; Vohl, M.-C.; Couture, P.; Lamarche, B. Supplementation with Resveratrol and Curcumin Does Not Affect the Inflammatory Response to a High-Fat Meal in Older Adults with Abdominal Obesity: A Randomized, Placebo-Controlled Crossover Trial. J. Nutr. 2018, 148, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kuszewski, J.C.; Wong, R.H.X.; Wood, L.G.; Howe, P.R.C. Effects of fish oil and curcumin supplementation on cerebrovascular function in older adults: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Hwang, W.; Kim, J.Y.; Lee, C.H.; Kim, Y.-J.; Lee, D.; Kwon, O. Synergistic mechanisms of Sanghuang–Danshen phytochemicals on postprandial vascular dysfunction in healthy subjects: A network biology approach based on a clinical trial. Sci. Rep. 2019, 9, 9746. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Antioxidant Dietary Fiber Product: A New Concept and a Potential Food Ingredient. J. Agric. Food Chem. 1998, 46, 4303–4306. [Google Scholar] [CrossRef]
- Eskicioglu, V.; Kamiloglu, S.; Nilufer-Erdil, D. Antioxidant dietary fibres: Potential functional food ingredients from plant processing by-products. Czech J. Food Sci. 2016, 33, 487–499. [Google Scholar] [CrossRef]
- Ozyurt, V. ye H.; Ötles, S. Effect of food processing on the physicochemical properties of dietary fibre. Acta Sci. Pol. Technol. Aliment. 2016, 15, 233–245. [Google Scholar] [CrossRef]
- Requena, M.C.; González, C.N.A.; Barragán, L.A.P.; Correia, T.; Esquivel, J.C.C.; Herrera, R.R. Functional and physico-chemical properties of six desert-sources of dietary fiber. Food Biosci. 2016, 16, 26–31. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, J.; Tang, J.; Wang, J.-J.; Lu, C.-H.; Wang, P.-X. Beneficial Effect of Higher Dietary Fiber Intake on Plasma HDL-C and TC/HDL-C Ratio among Chinese Rural-to-Urban Migrant Workers. Int. J. Environ. Res. Public Health 2015, 12, 4726. [Google Scholar] [CrossRef]
- Adam, C.L.; Thomson, L.M.; Williams, P.A.; Ross, A.W. Soluble Fermentable Dietary Fibre (Pectin) Decreases Caloric Intake, Adiposity and Lipidaemia in High-Fat Diet-Induced Obese Rats. PLoS ONE 2015, 10, e0140392. [Google Scholar] [CrossRef]
- Aoe, S.; Ichinose, Y.; Kohyama, N.; Komae, K.; Takahashi, A.; Abe, D.; Yoshioka, T.; Yanagisawa, T. Effects of high β-glucan barley on visceral fat obesity in Japanese individuals: A randomized, double-blind study. Nutrition 2017, 42, 1–6. [Google Scholar] [CrossRef]
- Im, H.J.; Yoon, K.Y. Production and characterisation of alcohol-insoluble dietary fibre as a potential sourcefor functional carbohydrates produced by enzymatic depolymerisation of buckwheat hulls. Czech J. Food Sci. 2016, 33, 449–457. [Google Scholar] [CrossRef]
- Mosikanon, K.; Arthan, D.; Kettawan, A.; Tungtrongchitr, R.; Prangthip, P. Yeast β–Glucan Modulates Inflammation and Waist Circumference in Overweight and Obese Subjects. J. Diet. Suppl. 2017, 14, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Samout, N.; Ettaya, A.; Bouzenna, H.; Ncib, S.; Elfeki, A.; Hfaiedh, N. Beneficial effects of Plantago albicans on high-fat diet-induced obesity in rats. Biomed. Pharmacother. 2016, 84, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Almaraz, R.; Martín Fuentes, M.; Palma Milla, S.; López Plaza, B.; Bermejo López, L.M.; Gómez Candela, C. Indicaciones de diferentes tipos de fibra en distintas patologías. Nutr. Hosp. 2015, 31, 2372–2383. [Google Scholar]
- Tester, R.; Al-Ghazzewi, F. Glucomannans and nutrition. Food Hydrocoll. 2017, 68, 246–254. [Google Scholar] [CrossRef]
- Zalewski, B.M.; Chmielewska, A.; Szajewska, H. The effect of glucomannan on body weight in overweight or obese children and adults: A systematic review of randomized controlled trials. Nutrition 2015, 31, 437–442.e2. [Google Scholar] [CrossRef]
- Moyano, G.; Sáyago-Ayerdi, S.G.; Largo, C.; Caz, V.; Santamaria, M.; Tabernero, M. Potential use of dietary fibre from Hibiscus sabdariffa and Agave tequilana in obesity management. J. Funct. Foods 2016, 21, 1–9. [Google Scholar] [CrossRef]
- Zou, J.; Chassaing, B.; Singh, V.; Pellizzon, M.; Ricci, M.; Fythe, M.D.; Kumar, M.V.; Gewirtz, A.T. Fiber-Mediated Nourishment of Gut Microbiota Protects against Diet-Induced Obesity by Restoring IL-22-Mediated Colonic Health. Cell Host Microbe 2018, 23, 41–53.e4. [Google Scholar] [CrossRef]
- Sánchez, D.; Quiñones, M.; Moulay, L.; Muguerza, B.; Miguel, M.; Aleixandre, A. Soluble fiber-enriched diets improve inflammation and oxidative stress biomarkers in Zucker fatty rats. Pharmacol. Res. 2011, 64, 31–35. [Google Scholar] [CrossRef]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J.; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef]
- Dahiya, D.K.; Renuka; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut microbiota modulation and its relationship with obesity using prebiotic fibers and probiotics: A review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
| Bioactive Compounds | In Vivo | Mechanisms of Action | Toxicity | Author |
|---|---|---|---|---|
| Phenol acids | ||||
| Caffeic acid | C57BL/6 mice with diet HFD | The mechanism focuses on an increase of the phosphorylation of AMP-activated protein kinase and decreasing acetyl carboxylase, a downstream target of AMP-activated-protein kinase (AMPK). | No maternal toxicity | [145] |
| Ellagic acid | High-fat diet-induced obesity SD rats. | Decreases the mRNA expression of Zfp423 and Aldh1a1 (responsibilities of WAT plasticity) and increases the mRNA expression of the brown adipocyte, as well as markers UCP1, PRDM16, Cidea, PGC1α, and Ppar-α; and beige markers, including CD137and TMEM26. It also elevates the expression of UPC1 in iWAT (specific protein of brown adipocyte). | No-observed-effect level 3011 mg/kg bw/day (males) No-observed-effect level 3254 mg/kg bw/day and 778 mg/kg (females) (rats) | [13,146] |
| Gallic acid | Mice (Swiss) model fed with high-fat diet | Induces an increase in SIRT1 and PGC1-α, might be responsible for thermogenesis activation under a high-fat diet. | Non-toxic >100 mg /L | [12,147] |
| p-Coumaric acid | Mouse model of high-fat diet-induced obesity | The mechanism of the action on obesity is mediated by the mTORC1-RPS6 pathway, regulating the Ucp1, HSL, and GUT-4 proteins | Low toxicity 2850 mg/kg bodyweight (mice) | [14,148] |
| Vanillic acid | High-fat diet (HFD)-induced obese mice and genetically obese db/db mice | The mechanism of action is due to the increase in the cellular NAD levels, and AMPK activates the NAD-dependent deacetylase SIRT1, which results in the deacetylation or activation of PGC1 and, therefore, a thermogenic effect. | 1000 mg/kg b.w (rats) | [149,150] |
| Flavonoids | ||||
| Capsaicin | Mouse (Adult male WT and TRPV1−/− (B6.129X1Trpv1 tm1Jul/J) model of HFD-induced obesity. | Intracellular Ca2+ rises via TRPV1 channels stimulated by CAP, activating CaMKII/AMPK, which phosphorylates and activates SIRT-1. This causes the deacetylation of PPAR-γ and PRDM-16 and facilitates their interaction to promote the browning of WAT (white adipose tissue). | Oral LD50 118.8 mg/kg for males and 97.4 mg/kg for females (mice) Male rats—161.2 mg/kg, and female rats—148.1 mg/kg | [151,152] |
| Anthocyanins | Male C57BL/6J mice fed a modified AIN-93M control diet containing high fat/high cholesterol | Inhibition of IKKε expression in adipose tissue occurs. Prevents the action of macrophage infiltration by attenuating the action of IKKε in energy preservation. | No toxic effects of anthocyanins identified 20 mg/kg/d mice; >3 g/d guinea pigs and rats; >2.4% body weight in beagle dogs and 9 g/kg/d in rats, mice, and rabbits | [153,154] |
| Pterostilbene | Zucker rats (fa/fa) model of genetic obesity | Present effect thermogenic and oxidative capacity of brown adipose tissue, due to increase of gene expression of Ucp1, peroxisome proliferator-activated receptor γ co-activator 1 α (Pgc-1α), carnitine palmitoyl transferase 1b (Cpt1b), nuclear respiratory factor 1 (Nfr1), and cyclooxygenase-2 (Cox2); PPARα, PGC-1α, p38 mitogen-activated protein kinase (p38 MAPK), UCP1 and glucose transporter (GLUT4); and enzyme activity of CPT 1b and citrate synthase (CS) were assessed in interscapular brown adipose tissue. | No significant toxic effects | [11,155] |
| Resveratrol | High-fat diet (HFD)-induced adipogenesis and inflammation in the epididymal fat tissues of mice C57BL/6J. | There are changes in the GalR1, GalR2, PKCd, and p-ERK protein expressions, with subsequent changes in the Cyc-D and E2F1 expressions, on galanin-mediated adipogenesis cascades in the epididymal adipose tissue. Decrease adipogenic transcription factors (PPARg2, C/EBPa, SREBP-1c, and LXR) and their target genes (FAS, LPL, aP2, and leptin) were suppressed. TLR4 uses MyD88-dependent and MyD88-independent pathways, whereas TLR2 signals only in the MyD88-dependent manner. The MyD88-dependent pathway uses TRAF6 and IRF5, leading to its nuclear translocation and cooperation with NF-kB. The MyD88-independent pathway uses TRIF in activating NF-kB in either a TRAF6-dependent or TRAF6-independent mechanism. TRIF associates with TBK1 and IKKi, which in turn leads p-IRF3. Resveratrol limits changes in the expression of TLR2, TLR4, and downstream molecules (MyD88, Tirap, TRIF, TRAF6, IRF5, p-IRF3, and NF-kB), along with the subsequent changes in the cytokines (TNFα, IFNα, IFNβ, and IL-6) implicated in the TLR2/4-mediated pro-inflammatory signaling cascades on adipose tissue | No toxic effect in humans | [10,156] |
| Curcumin | Mice C57BL/6 fed a high fat diet | There is a suppression of acetyl CoA conversion to malonyl CoA. Lower levels of malonyl CoA increase CPT-1 expression, promoting fatty acid oxidation. The phosphorylated AMPK also suppresses the expression of GPAT-1, which results in reduced fatty acid esterification. The phosphorylated AMPK inhibits PPAR-γ and C/EBP-α transcription factors. | No toxicity from curcumin | [157,158] |
| Quercetin | Diet-induced obese (DIO) ICR mouse | Blocked protein levels of the key adipogenic factors C/EBPβ, C/EBPα, PPARγ, and FABP4, and the TG-synthesis enzymes lipin1, DGAT1, and LPAAT. Inhibited MAPK, ERK1/2, JNK, and p38MAPK, and MCP-1 and TNF-α in adipocytes and macrophages | 285–3000 mg/kg toxicity present | [159,160] |
| Apigenin | High-fat diet (HFD)-induced obese C57BL/6 (C57) mice | Apigenin binds to non-phosphorylated STAT3, reduces STAT3 phosphorylation and transcriptional activity in visceral adipose tissue, and consequently reduces the expression of the STAT3 target gene cluster of differentiation 36 (CD36). The reduced CD36 expression in adipocytes reduces the expression of peroxisome proliferator-activated receptor-gamma (PPAR-γ) which is the critical nuclear factor in adipogenesis. | 300 mg/kg (mice) No toxicity | [161,162] |
| Scutellarein | Mouse model of obesity induced by high-fat diet (HFD) feeding. | There is suppression of the expression of cytokine genes TNF-α, IL-6, IL-1β, ICAM-1, VCAM-1, and NF-κB. | Minimally toxic or non-toxic in rodents | [163,164] |
| Luteolin | C57BL/6J mice model of DIO (diet-induced obesity: high-fat diet) | It is modulated the TLR signaling pathway on pro-inflammatory response. There is a decrease in EMR1 and CCL7, which impacts adipose tissue, increases lipolysis and the TCA cycle, reduces the pro-inflammatory response, adipokine dysregulation, adipocyte macrophage infiltration and accumulation, fibrosis, pancreatic β cell dysfunction, hepatic lipotoxicity, insulin resistance, and chronic inflammation. Another mechanism of action is the interaction in the AMPK/PCG1α. Elevates the expressions of thermogenic genes and the activities of AMPK/PCG1a signaling molecules. | No adverse effect or toxicity | [165,166,167] |
| Chlorogenic acid Caffeine | ICR mice with high-fat diet | Increases AMPK phosphorylation and p-AMPK up-regulates the expression of ATGL and HSL, promoting the hydrolysis of triglycerides and the release of FA. Elevates ACO expression by the activation of AMPK (accelerated β-oxidation). Down-regulation of LXR-α and increase in p-AMPK restrain the expression of SPEBP1c, thereby down-regulating the expression of SCD1 and FAS to inhibit lipid synthesis and regulate lipid metabolism. | [168] | |
| Catechin, Picatechin, Procyanidins | High-fat diet-fed C57BL/6 mice | Activated AMPK-α also induces the expression of UCPs and PGC-1a, which are involved in energy expenditure and thermogenesis | [169] | |
| Cyanidin-3 O galactoside | Mice (C57BL/6) model with high-fat diet-induced obesity | Related to adipogenesis-related transcription factors (C/EBPs, PPAR-γ, and SREBP-1c) and coactivators (PGC-1α), and the down-regulation of specific adipogenesis-related genes affected by these transcription factors. | [170,171] | |
| Other compounds | ||||
| Betacyanins | High-fat diet (HFD)-induced obese mice | Reduces HFD-induced body weight gain, and ameliorates adipose tissue hypertrophy, hepatosteatosis, glucose intolerance, and insulin resistance. Increases the expression levels of lipid metabolism-related genes (AdipoR2, Cpt1a, Cpt1b, Acox1, PPAR-γ, Insig1, and Insig2) and FGF21-related genes (β-Klotho and FGFR1/2), and decreases the expression level of Fads2, Fas, and FGF21 | [171] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Moreno, E.; Arias-Rico, J.; Jiménez-Sánchez, R.C.; Estrada-Luna, D.; Jiménez-Osorio, A.S.; Zafra-Rojas, Q.Y.; Ariza-Ortega, J.A.; Flores-Chávez, O.R.; Morales-Castillejos, L.; Sandoval-Gallegos, E.M. Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods 2022, 11, 1232. https://doi.org/10.3390/foods11091232
Ramírez-Moreno E, Arias-Rico J, Jiménez-Sánchez RC, Estrada-Luna D, Jiménez-Osorio AS, Zafra-Rojas QY, Ariza-Ortega JA, Flores-Chávez OR, Morales-Castillejos L, Sandoval-Gallegos EM. Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods. 2022; 11(9):1232. https://doi.org/10.3390/foods11091232
Chicago/Turabian StyleRamírez-Moreno, Esther, José Arias-Rico, Reyna Cristina Jiménez-Sánchez, Diego Estrada-Luna, Angélica Saraí Jiménez-Osorio, Quinatzin Yadira Zafra-Rojas, José Alberto Ariza-Ortega, Olga Rocío Flores-Chávez, Lizbeth Morales-Castillejos, and Eli Mireya Sandoval-Gallegos. 2022. "Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation" Foods 11, no. 9: 1232. https://doi.org/10.3390/foods11091232
APA StyleRamírez-Moreno, E., Arias-Rico, J., Jiménez-Sánchez, R. C., Estrada-Luna, D., Jiménez-Osorio, A. S., Zafra-Rojas, Q. Y., Ariza-Ortega, J. A., Flores-Chávez, O. R., Morales-Castillejos, L., & Sandoval-Gallegos, E. M. (2022). Role of Bioactive Compounds in Obesity: Metabolic Mechanism Focused on Inflammation. Foods, 11(9), 1232. https://doi.org/10.3390/foods11091232









