Antibiotic Resistance and Molecular Characterization of Cronobacter sakazakii Strains Isolated from Powdered Infant Formula Milk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of PIFM Samples
2.2. Isolation and Identification of C. sakazakii
2.3. Phenotypic and Genotypic Antimicrobial Susceptibility Profile of C. sakazakii Isolates
2.4. DNA Extraction
2.5. ERIC-PRC Genotyping
2.6. Statistical Analysis
3. Results
3.1. Prevalence of C. sakazakii
3.2. Antibiotic Resistance Phenotypes and Genotypes in C. sakazakii Isolates
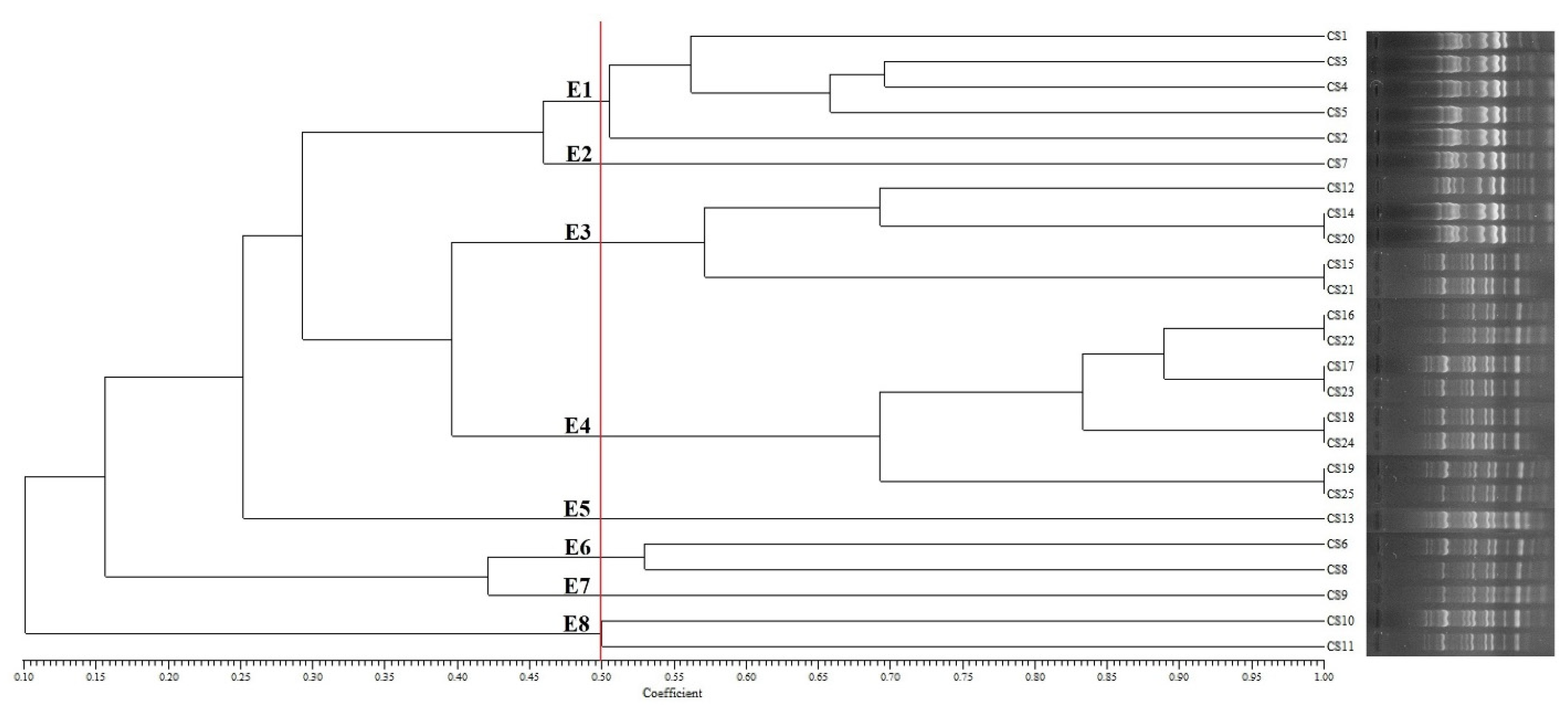
3.3. Genotyping of C. sakazakii Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Healy, B.; Cooney, S.; O’Brien, S.; Iversen, C.; Whyte, P.; Nally, J.; Callanan, J.J.; Fanning, S. Cronobacter (Enterobacter sakazakii): An opportunistic foodborne pathogen. Foodborne Pathog. Dis. 2010, 7, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Guo, B.; Shi, X.; Cheng, T.; Chen, M.; Jiang, X.; Ye, Y.; Wang, J.; Xie, G.; Ding, J. An investigation of an acute gastroenteritis outbreak: Cronobacter sakazakii, a potential cause of food-borne illness. Front. Microbiol. 2018, 9, 2549. [Google Scholar] [CrossRef] [PubMed]
- Holý, O.; Forsythe, S. Cronobacter spp. as emerging causes of healthcare-associated infection. J. Hosp. Infect. 2014, 86, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529. [Google Scholar] [CrossRef]
- McMullan, R.; Menon, V.; Beukers, A.G.; Jensen, S.O.; van Hal, S.J.; Davis, R. Cronobacter sakazakii infection from expressed breast milk, Australia. Emerg. Infect. Dis. 2018, 24, 393. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Fouladkhah, A. Outbreak history, biofilm formation, and preventive measures for control of Cronobacter sakazakii in infant formula and infant care settings. Microorganisms 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Chap, J.; Jackson, P.; Siqueira, R.; Gaspar, N.; Quintas, C.; Park, J.; Osaili, T.; Shaker, R.; Jaradat, Z.; Hartantyo, S. International survey of Cronobacter sakazakii and other Cronobacter spp. in follow up formulas and infant foods. Int. J. Food Microbiol. 2009, 136, 185–188. [Google Scholar] [CrossRef]
- Carvalho, G.G.; Calarga, A.P.; Teodoro, J.R.; Queiroz, M.M.; Astudillo-Trujillo, C.A.; Levy, C.E.; Brocchi, M.; Kabuki, D.Y. Isolation, comparison of identification methods and antibiotic resistance of Cronobacter spp. in infant foods. Food Res. Int. 2020, 137, 109643. [Google Scholar] [CrossRef]
- Franz, C.; den Besten, H.M.; Boehnlein, C.; Gareis, M.; Zwietering, M.H.; Fusco, V. Reprint of: Microbial food safety in the 21st century: Emerging challenges and foodborne pathogenic bacteria. Trends Food Sci. Technol. 2019, 84, 34–37. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Osaili, T.M.; Elabedeen, N.A.Z.; Jaradat, Z.W.; Shaker, R.R.; Kheirallah, K.A.; Tarazi, Y.H.; Holley, R.A. Impact of environmental stress desiccation, acidity, alkalinity, heat or cold on antibiotic susceptibility of Cronobacter sakazakii. Int. J. Food Microbiol. 2011, 146, 137–143. [Google Scholar] [CrossRef]
- Lepuschitz, S.; Ruppitsch, W.; Pekard-Amenitsch, S.; Forsythe, S.J.; Cormican, M.; Mach, R.L.; Piérard, D.; Allerberger, F.; Group, E.S. Multicenter study of Cronobacter sakazakii infections in humans, Europe, 2017. Emerg. Infect. Dis. 2019, 25, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakbin, B.; Mahmoudi, R.; Mousavi, S.; Allahyari, S.; Amani, Z.; Peymani, A.; Qajarbeygi, P.; Hoseinabadi, Z. Genotypic and antimicrobial resistance characterizations of Cronobacter sakazakii isolated from powdered milk infant formula: A comparison between domestic and imported products. Food Sci. Nutr. 2020, 8, 6708–6717. [Google Scholar] [CrossRef] [PubMed]
- Reade, M. Trends in DNA Fingerprinting Research; Nova Publishers: New York, NY, USA, 2005. [Google Scholar]
- Armstrong, G.L.; MacCannell, D.R.; Taylor, J.; Carleton, H.A.; Neuhaus, E.B.; Bradbury, R.S.; Posey, J.E.; Gwinn, M. Pathogen genomics in public health. N. Engl. J. Med. 2019, 381, 2569–2580. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.-H.; Lee, M.-F.; Chuang, Y.-C.; Yu, W.-L. Detection of plasmid-mediated β-lactamase genes and emergence of a novel AmpC (CMH-1) in Enterobacter cloacae at a medical center in Southern Taiwan. J. Clin. Med. 2018, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Gan, X.; Li, M.; Yan, S.; Wang, X.; Wang, W.; Li, F. Genomic Landscape and Phenotypic Assessment of Cronobacter sakazakii Isolated From Raw Material, Environment, and Production Facilities in Powdered Infant Formula Factories in China. Front. Microbiol. 2021, 12, 686189. [Google Scholar] [CrossRef]
- Fei, P.; Jiang, Y.; Feng, J.; Forsythe, S.J.; Li, R.; Zhou, Y.; Man, C. Antibiotic and desiccation resistance of Cronobacter sakazakii and C. malonaticus isolates from powdered infant formula and processing environments. Front. Microbiol. 2017, 8, 316. [Google Scholar] [CrossRef] [Green Version]
- Parra-Flores, J.; Aguirre, J.; Juneja, V.; Jackson, E.E.; Cruz-Córdova, A.; Silva-Sanchez, J.; Forsythe, S. Virulence and antibiotic resistance profiles of Cronobacter sakazakii and Enterobacter spp. involved in the diarrheic hemorrhagic outbreak in Mexico. Front. Microbiol. 2018, 9, 2206. [Google Scholar] [CrossRef] [Green Version]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Wu, Q.; Xu, X.; Yang, X.; Dong, X.; Zhang, J. The phenotypic and genotypic characterization of Enterobacter sakazakii strains from infant formula milk. J. Dairy Sci. 2010, 93, 2315–2320. [Google Scholar] [CrossRef] [Green Version]
- Pavel, A.B.; Vasile, C.I. PyElph-a software tool for gel images analysis and phylogenetics. BMC Bioinform. 2012, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Rolf, J. NTSYS-PC, Numerical Taxonomy and Multivariate Analysis System, Version 2.11; T Exeter Software: Setauket, NY, USA, 2000. [Google Scholar]
- Chauhan, R.; Singh, N.; Pal, G.K.; Goel, G. Trending biocontrol strategies against Cronobacter sakazakii: A recent updated review. Food Res. Int. 2020, 137, 109385. [Google Scholar] [CrossRef] [PubMed]
- Elkhawaga, A.A.; Hetta, H.F.; Osman, N.S.; Hosni, A.; El-Mokhtar, M.A. Emergence of Cronobacter sakazakii in cases of neonatal sepsis in upper Egypt: First report in North Africa. Front. Microbiol. 2020, 11, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, N.; Forsythe, S.; Wu, Q.; Ding, Y.; Zhang, J.; Zeng, H. Insights into Cronobacter sakazakii biofilm formation and control strategies in the food industry. Engineering 2020, 6, 393–405. [Google Scholar] [CrossRef]
- O’Brien, S.; Healy, B.; Negredo, C.; Anderson, W.; Fanning, S.; Iversen, C. Prevalence of Cronobacter species (Enterobacter sakazakii) in follow-on infant formulae and infant drinks. Lett. Appl. Microbiol. 2009, 48, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ge, W.; Li, K.; Gan, J.; Zhang, Y.; Zhang, Q.; Luo, R.; Chen, L.; Liang, Y.; Wang, Q. Prevalence and characterization of Cronobacter sakazakii in retail milk-based infant and baby foods in Shaanxi, China. Foodborne Pathog. Dis. 2016, 13, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo-Nthenge, A.; Rotich, E.; Godwin, S.; Nahashon, S.; Chen, F. Prevalence and antimicrobial resistance of Cronobacter sakazakii isolated from domestic kitchens in middle Tennessee, United States. J. Food Prot. 2012, 75, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Jang, S.S.; Kim, S.K.; Park, J.-H.; Heu, S.; Ryu, S. Prevalence and genetic diversity of Enterobacter sakazakii in ingredients of infant foods. Int. J. Food Microbiol. 2008, 122, 196–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Tang, J.; Wang, S.; Wang, L.; Zhu, G.; Li, X.; Liu, Y. Thermal inactivation of Cronobacter sakazakii ATCC 29544 in powdered infant formula milk using thermostatic radio frequency. Food Control 2020, 114, 107270. [Google Scholar] [CrossRef]
- Dalkilic-Kaya, G.; Heperkan, D.; Juneja, V.K.; Heperkan, H.A. Thermal resistance of Cronobacter sakazakii isolated from baby food ingredients of dairy origin in liquid medium. J. Food Processing Preserv. 2018, 42, e13463. [Google Scholar] [CrossRef]
- Dancer, G.; Mah, J.H.; Rhee, M.S.; Hwang, I.G.; Kang, D.H. Resistance of Enterobacter sakazakii (Cronobacter spp.) to environmental stresses. J. Appl. Microbiol. 2009, 107, 1606–1614. [Google Scholar] [CrossRef]
- Mancilla-Becerra, L.M.; Lías-Macías, T.; Ramírez-Jiménez, C.L.; León, J.B. Multidrug-resistant bacterial foodborne pathogens: Impact on human health and economy. Pathog Bact 2019, 2019, 1–18. [Google Scholar]
- Sun, D.; Jeannot, K.; Xiao, Y.; Knapp, C.W. Horizontal gene transfer mediated bacterial antibiotic resistance. Front. Microbiol. 2019, 10, 1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Han, Z.; Li, P.; Zhang, H.; Du, X.; Wang, S. Outer Membrane Protein F Is Involved in Biofilm Formation, Virulence and Antibiotic Resistance in Cronobacter sakazakii. Microorganisms 2021, 9, 2338. [Google Scholar] [CrossRef] [PubMed]
- Caniça, M.; Manageiro, V.; Abriouel, H.; Moran-Gilad, J.; Franz, C.M. Antibiotic resistance in foodborne bacteria. Trends Food Sci. Technol. 2019, 84, 41–44. [Google Scholar] [CrossRef]
- Zurfluh, K.; Nüesch-Inderbinen, M.; Morach, M.; Zihler Berner, A.; Hächler, H.; Stephan, R. Extended-spectrum-β-lactamase-producing Enterobacteriaceae isolated from vegetables imported from the Dominican Republic, India, Thailand, and Vietnam. Appl. Environ. Microbiol. 2015, 81, 3115–3120. [Google Scholar] [CrossRef] [Green Version]
- Holý, O.; Alsonosi, A.; Hochel, I.; Röderová, M.; Zatloukalova, S.; Mlynárčik, P.; Kolář, M.; Petrželová, J.; Alazraq, A.; Chmelař, D. Antibiotic susceptibility of Cronobacter spp. isolated from clinical samples. Pol. J. Microbiol. 2019, 68, 5. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Wu, Q.; Zhou, Y.; Dong, X.; Zhang, J. Analysis of major band of Enterobacter sakazakii by ERIC-PCR and development of a species-specific PCR for detection of Ent. sakazakii in dry food samples. J. Microbiol. Methods 2008, 75, 392–397. [Google Scholar] [CrossRef]
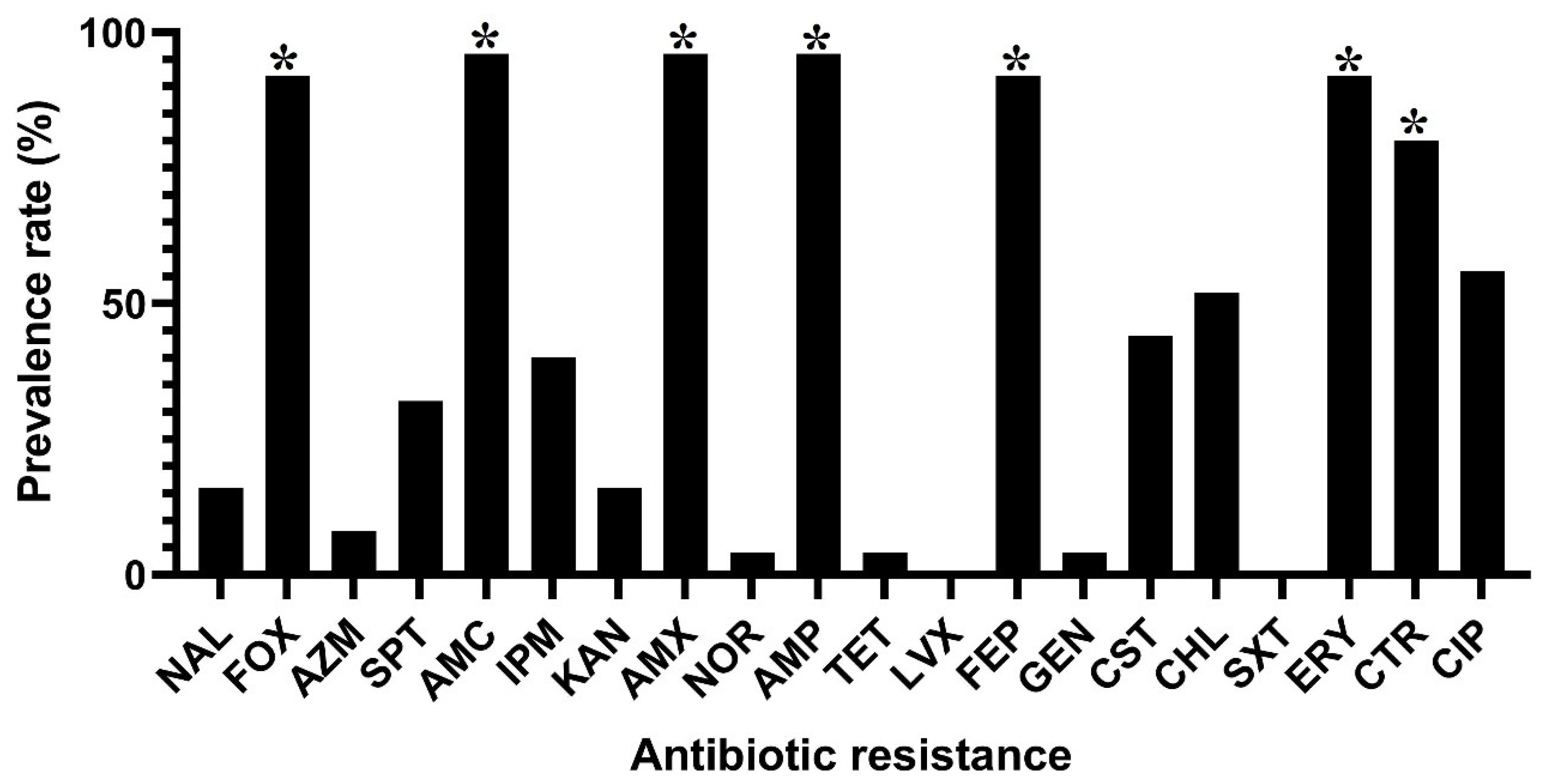
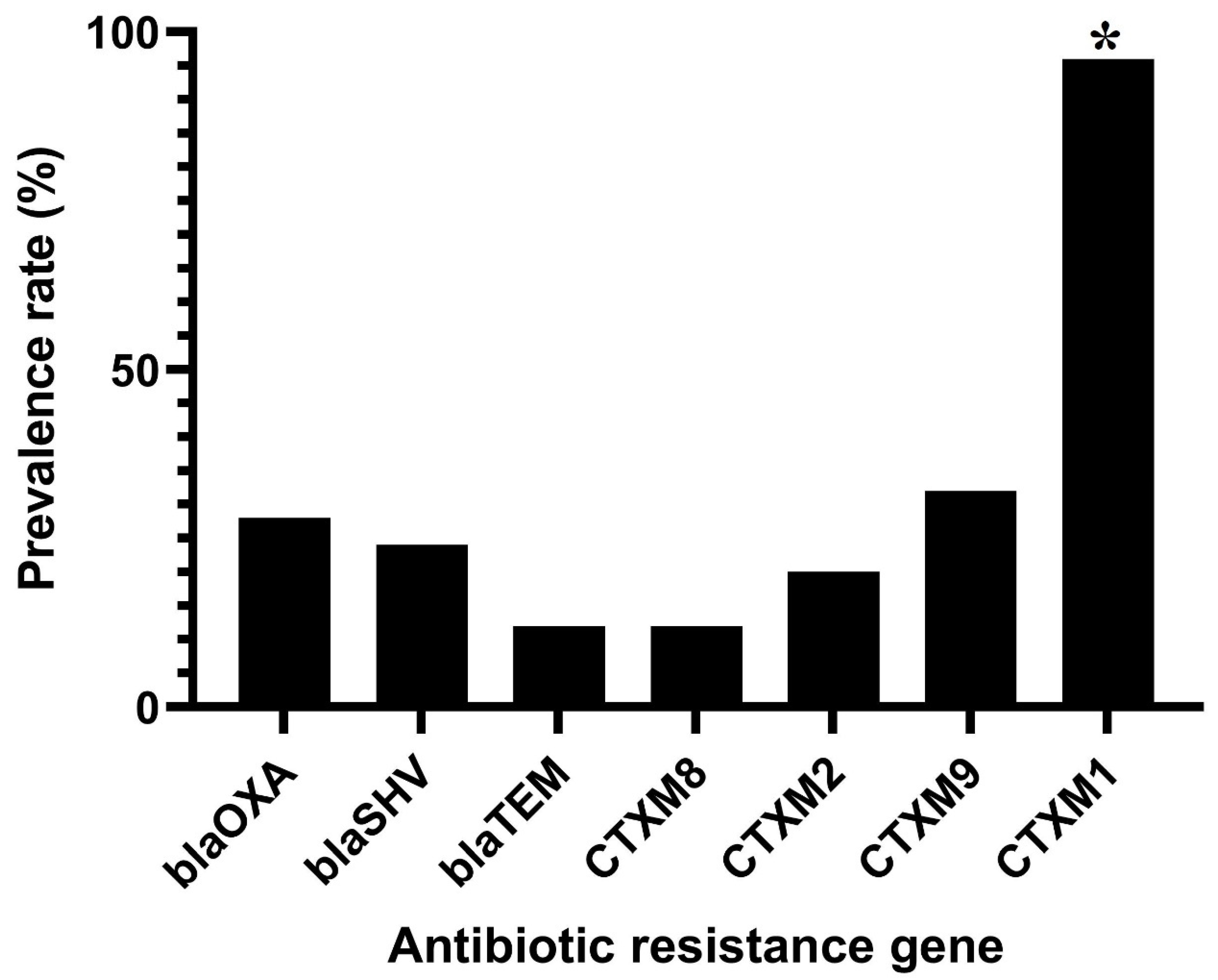
| No. Classes of Antibiotics | Multidrug Resistance Patterns a (No. Isolates in Each Pattern) | No. Total Isolates (%) (n = 25) |
|---|---|---|
| Two | βLs-QNs (n = 1) | 1 (4) |
| Three | βLs-QNs-MLs (n = 7) | 11 (44) |
| βLs-PNs-MLs (n = 4) | ||
| Four | βLs-AGs-LPs-MLs (n = 1) | 3 (12) |
| βLs-PNs-MLs-QNs (n = 1) | ||
| βLs-AGs-LPs-QNs (n = 1) | ||
| Five | βLs-QNs-MLs-AGs-TCs (n = 1) | 2 (8) |
| βLs-QNs-MLs-AGs-LPs (n = 1) | ||
| Six | βLs-QNs-AGs-LPs-MLs-PNs (n = 8) | 8 (32) |
| No. | Isolate | Resistance Phenotype a | Resistance Genotype b | ERIC-PCR Type |
|---|---|---|---|---|
| 1 | CS1 | NAL, FOX, AZM, AMC, IPM, KAN, AMX, AMP, TET, FEP, GEN, ERY | C1, S, O, T | E1 |
| 2 | CS2 | NAL, FOX, AMC, IPM, KAN, AMX, AMP, FEP, ERY, CST | C1, C2, C9 | E1 |
| 3 | CS3 | FOX, AZM, AMC, IPM, KAN, AMX, AMP, FEP, ERY, CST | C1, S, O | E1 |
| 4 | CS4 | FOX, AMC, IPM, KAN, NOR, AMP, FEP, CST | C1 | E1 |
| 5 | CS5 | NAL, FOX, AMC, AMX, AMP, FEP, ERY, CTR | C1 | E1 |
| 6 | CS6 | FOX, AMC, AMX, AMP, FEP, CHL, ERY, CTR | C1 | E6 |
| 7 | CS7 | FOX, SPT, AMC, AMX, AMP, FEP, CST, CHL, ERY, CTR, CIP | C1, S, O, T | E2 |
| 8 | CS8 | FOX, AMC, IPM, AMX, AMP, FEP, ERY, CTR, CIP | C1 | E6 |
| 9 | CS9 | FOX, AMC, AMX, AMP, FEP, CHL, ERY, CTR | C1 | E7 |
| 10 | CS10 | SPT, AMC, AMX, AMP, FEP, CST, CHL, ERY, CTR, CIP | C1, C8, C9 | E8 |
| 11 | CS11 | FOX, AMC, IPM, AMX, AMP, FEP, ERY, CTR, CIP | C1, C2, C9 | E8 |
| 12 | CS12 | FOX, SPT, AMC, AMX, AMP, CST, CHL, ERY, CTR, CIP | C1, S, O | E3 |
| 13 | CS13 | FOX, AMC, IPM, AMX, AMP, FEP, ERY, CIP | C1 | E5 |
| 14 | CS14 | FOX, AMC, AMX, AMP, FEP, CHL, ERY, CIP | C1 | E3 |
| 15 | CS15 | FOX, AMC, IPM, AMX, AMP, FEP, CST, CHL, ERY, CTR, CIP | C1, C8, C9 | E3 |
| 16 | CS16 | NAL, FOX, AMC, AMX, AMP, FEP, ERY, CTR | C1 | E4 |
| 17 | CS17 | FOX, AMC, AMX, FEP, CHL, ERY, CTR, | C1 | E4 |
| 18 | CS18 | FOX, SPT, AMC, AMX, AMP, FEP, CST, CHL, ERY, CTR, CIP | C1, C2, C9 | E4 |
| 19 | CS19 | IPM, AMX, AMP, FEP, CTR, CIP | C1 | E4 |
| 20 | CS20 | FOX, AMC, AMX, AMP, FEP, CHL, ERY, CTR | C1, S, O | E3 |
| 21 | CS21 | FOX, SPT, AMC, AMX, AMP, CST, CHL, ERY, CTR, CIP | C1, S, O, T | E3 |
| 22 | CS22 | FOX, AMC, IPM, AMX, AMP, FEP, ERY, CTR, CIP | C8 | E4 |
| 23 | CS23 | FOX, SPT, AMC, AMX, AMP, FEP, CST, CHL, ERY, CTR, CIP | C1, C2, C9 | E4 |
| 24 | CS24 | FOX, AMC, IPM, AMX, AMP, FEP, ERY, CTR, CIP | C1, C2, C9 | E4 |
| 25 | CS25 | FOX, SPT, AMC, AMX, AMP, FEP, CST, CHL, ERY, CTR, CIP | C1, O, C9 | E4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakbin, B.; Brück, W.M.; Allahyari, S.; Rossen, J.W.A.; Mahmoudi, R. Antibiotic Resistance and Molecular Characterization of Cronobacter sakazakii Strains Isolated from Powdered Infant Formula Milk. Foods 2022, 11, 1093. https://doi.org/10.3390/foods11081093
Pakbin B, Brück WM, Allahyari S, Rossen JWA, Mahmoudi R. Antibiotic Resistance and Molecular Characterization of Cronobacter sakazakii Strains Isolated from Powdered Infant Formula Milk. Foods. 2022; 11(8):1093. https://doi.org/10.3390/foods11081093
Chicago/Turabian StylePakbin, Babak, Wolfram Manuel Brück, Samaneh Allahyari, John W. A. Rossen, and Razzagh Mahmoudi. 2022. "Antibiotic Resistance and Molecular Characterization of Cronobacter sakazakii Strains Isolated from Powdered Infant Formula Milk" Foods 11, no. 8: 1093. https://doi.org/10.3390/foods11081093
APA StylePakbin, B., Brück, W. M., Allahyari, S., Rossen, J. W. A., & Mahmoudi, R. (2022). Antibiotic Resistance and Molecular Characterization of Cronobacter sakazakii Strains Isolated from Powdered Infant Formula Milk. Foods, 11(8), 1093. https://doi.org/10.3390/foods11081093






