Recombinant Porcine 12-Lipoxygenase Catalytic Domain: Effect of Inhibitors, Selectivity of Substrates and Specificity of Oxidation Products of Linoleic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Recombinant Porcine 12-LOXcd
2.3. Determination of Activity of Recombinant Porcine 12-LOXcd
2.4. Kinetics of Recombinant Porcine 12-LOXcd Oxidation
2.5. Oxidation of LA by Porcine 12-LOXcd and Extraction of Oxidation Products
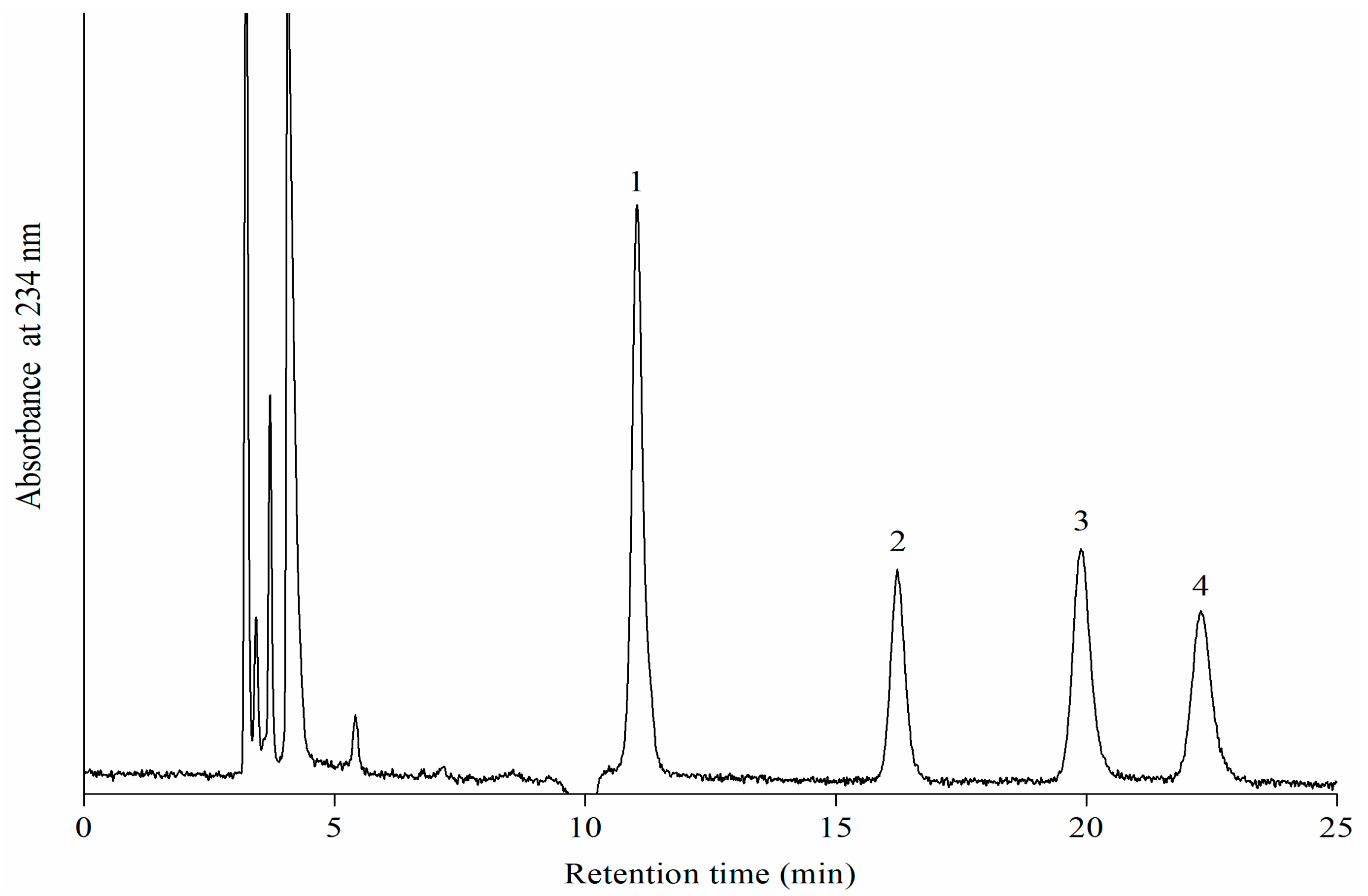
2.6. Normal Phase High Performance Liquid Chromatography (NP-HPLC) Analysis of Oxidation Products of LA by Porcine 12-LOXcd
2.7. Statistic Analysis
3. Results and Discussion
3.1. Effects of Inhibitors on the Activity of Recombinant Porcine 12-LOXcd
3.2. Substrate Selectivity of Recombinant Porcine 12-LOXcd
3.3. Specificity of Oxidation Products of LA by Recombinant Porcine 12-LOXcd
3.4. The Impacts of pH on Yield and Specificity of Oxidation Products of LA by Recombinant Porcine 12-LOXcd
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Kospwska, M.; Majcher, M.A.; Fortuna, T. Volatile compounds in meat and meat products. Food Sci. Tech. 2017, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Brettonnet, A.; Hewavitarana, A.; Delong, S.; Lanari, M.C. Phenolic acids composition and antioxidant activity of canola extracts in cooked beef, chicken and pork. Food Chem. 2010, 121, 927–933. [Google Scholar] [CrossRef]
- Sousa, B.C.; Pitt, A.R.; Spickett, C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 294–308. [Google Scholar] [CrossRef]
- Robinson, D.S.; Wu, Z.; Domoney, C.; Casey, R. Lipoxygenases and the quality of foods. Food Chem. 1995, 54, 33–43. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.T.; Cao, J.X.; Chen, Y.J.; Sun, Y.Y.; Zeng, X.Q.; Pan, D.D.; Ou, R.C.; Gan, N. Study on lipolysis-oxidation and volatile flavor compounds of dry-cured goose with different curing salt content during production. Food Chem. 2016, 190, 33–40. [Google Scholar]
- Jin, G.F.; Zhang, J.H.; Xiang, Y.; Lei, Y.X.; Wang, J.M. Crude lipoxygenase from pig muscle: Partial characterization and interactions of temperature, NaCl and pH on its activity. Meat Sci. 2011, 87, 257–263. [Google Scholar] [CrossRef]
- Grossman, S.; Bergman, M.; Sklan, D. Lipoxygenase in chicken muscle. J. Agric. Food Chem. 1988, 36, 1268–1270. [Google Scholar] [CrossRef]
- German, J.B.; Creveling, R.K. Identification and characterization of a 15-lipoxygenase from fish gills. J. Agric. Food Chem. 1990, 38, 2144–2147. [Google Scholar] [CrossRef]
- Fu, X.J.; Xu, S.Y.; Wang, Z. Kinetics of lipid oxidation and off-odor formation in silver carp mince: The effect of lipoxygenase and hemoglobin. Food Res. Int. 2009, 42, 85–90. [Google Scholar] [CrossRef]
- Song, H.; Wu, H.H.; Geng, Z.M.; Sun, C.; Ren, S.; Wang, D.Y.; Zhang, M.H.; Liu, F.; Xu, W.M. Simultaneous Determination of 13-HODE, 9,10-DHODE and 9,10,13-THODE in cured meat products by LC-MS/MS. Food Anal. Methods 2016, 9, 2832–2841. [Google Scholar] [CrossRef]
- Ma, J.J.; Geng, Z.M.; Sun, C.; Li, P.P.; Zhang, M.H.; Wang, D.Y.; Xu, W.M. Novel sample treatment method for the determination of free (E)-4-hydroxy-2-nonenal in meat products by liquid chromatography-tandem mass spectrometry using d3-HNE as internal standard. Rapid Commun. Mass Spectrom. 2021, 35, e9023. [Google Scholar] [CrossRef]
- Wang, J.J.; Wang, T.; Zhang, X.X.; Bian, H.; Geng, Z.M.; Li, P.P.; Wang, D.Y.; Xu, W.M. Expression, purification and characterization of 12-lipoxygenase catalytic domain from pig muscle. Food Sci. 2019, 40, 41–47. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of proteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kemal, C.; Flamberg, P.L.; Krupinski-Olsen, R.; Shorter, L. Reductive inactivation of soybean lipoxygenase 1 by catechols: A possible mechanism for regulation of lipoxygenase activity. Biochemistry 1987, 26, 7064–7072. [Google Scholar] [CrossRef] [PubMed]
- Sudína, G.F.; Mirzoeva, O.K.; Pushkareva, M.A.; Korshunova, G.A.; Sumbatyan, N.V. Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidants properties. FEBS Lett. 1993, 329, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.; Mugas, M.L.; Aguilar, J.J.; Marioni, J.; Contigiani, M.S.; Montoya, S.C.N.; Konigheim, B.S. First report of antiviral activity of nordihydroguaiaretic acid against Fort Sherman virus (Orthobunyavirus). Antivir. Res. 2021, 187, 104976. [Google Scholar] [CrossRef]
- Lucia, D.D.; Lucio, O.M.; Musio, B.; Bender, A.; Listing, M.; Dennhardt, S. Design, synthesis and evaluation of semi-synthetic triazole-containing caffeic acid analogues as 5-lipoxygenase inhibitors. Eur. J. Med. Chem. 2015, 101, 573–583. [Google Scholar] [CrossRef]
- Macías, P.; Pinto, M.C.; Campillo, J.E. Purification and partial characterization of rat liver lipoxygenase. Z. Naturforsch. 1987, 42B, 1343–1348. [Google Scholar] [CrossRef]
- Moon, C.; Ahn, M.; Wie, M.B.; Kim, H.M.; Koh, C.S.; Hong, S.C.; Kim, M.D.; Tanuma, N.; Matsumoto, Y.; Shin, T. Phenidone, a dual inhibitor of cyclooxygenases and lipoxygenases, ameliorates rat paralysis in experimental autoimmune encephalomyelitis by suppressing its target enzymes. Brain Res. 2005, 1035, 206–210. [Google Scholar] [CrossRef]
- Orafaie, A.; Mousavian, M.; Orafai, H.; Sadeghian, H. An overview of lipoxygenase inhibitors with approach of in vivo studies. Prostag. Other Lipid Med. 2020, 148, 106411. [Google Scholar] [CrossRef] [PubMed]
- Osipova, E.V.; Chechetkin, I.R.; Gogolev, Y.V.; Tarasova, N.B. Recombinant maize 9-lipoxygenase: Expression, purification, and properties. Biochemistry 2010, 75, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Gata, J.L.; Pinto, M.C.; Macías, P. Lipoxygenase activity in pig muscle: Purification and partial characterization. J. Agric. Food Chem. 1996, 44, 2573–2577. [Google Scholar] [CrossRef]
- Huang, L.S.; Kim, M.R.; Jeong, T.-S.; Sok, D.-E. Linoleoyl ysophosphatidic acid and linoleoyl lysophosphatidylcholine are efficient substrates for mammalian lipoxygenases. Biochim. Biophys. Acta 2007, 1770, 1062–1070. [Google Scholar] [CrossRef]
- Spiteller, G. Peroxidation of linoleic acid and its relation to aging and age dependent diseases. Mech. Ageing Dev. 2001, 122, 617–657. [Google Scholar] [CrossRef]
- Kuhn, H.; Banthiya, S.; van Leyen, K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta 2015, 1851, 308–330. [Google Scholar] [CrossRef] [Green Version]
- Hornung, E.; Walther, M.; Kuhn, H.; Feussner, I. Conversion of cucumber linoleate 13-lipoxygenase to a 9-lipoxygenating species by site-directed mutagenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 4192–4197. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, H.; Humeniuk, L.; Kozlov, N.; Roigas, S.; Adel, S.; Heydeck, D. The evolutionary hypothesis of reaction specificity of mammalian ALOX15 orthologs. Prog. Lipid Res. 2018, 72, 55–74. [Google Scholar] [CrossRef]
- Sugio, A.; Østergaard, L.H.; Matsui, K.; Takagi, S. Characterization of two fungal lipoxygenases expressed in Aspergillus oryzae. J. Biosci. Bioeng. 2018, 126, 436–444. [Google Scholar] [CrossRef]
- Walther, M.; Roffeis, J.; Jansen, C.; Anton, M.; Ivanov, I.; Kuhn, H. Structural basis for pH-dependent alterations of reaction specificity of vertebrate lipoxygenase isoforms. Biochim. Biophys. Acta 2009, 1791, 827–835. [Google Scholar] [CrossRef]
- Chechetkin, I.R.; Osipova, E.V.; Tarasova, N.B.; Mukhitova, F.K.; Hamberg, M.; Gogolev, Y.V.; Grechkin, A.N. Specificity of Oxidation of linoleic acid homologs by plant lipoxygenases. Biochemistry 2009, 74, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Lampi, A.-M.; Yang, Z.; Mustonen, O.; Piironen, V. Potential of faba bean lipase and lipoxygenase to promote formation of volatile lipid oxidation products in food models. Food Chem. 2020, 311, 125982. [Google Scholar] [CrossRef] [PubMed]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef] [PubMed]

| Compound | Concentration | Residual Activity (%) |
|---|---|---|
| NDGA | 0.03 µM | 38.0 ± 1.9 c |
| NDGA | 2.5 µM | 3.8 ± 0.4 g |
| caffeic acid | 0.01 mM | 57.0 ± 2.3 a |
| caffeic acid | 0.1 mM | 4.8 ± 0.1 f |
| phenidone | 0.12 µM | 46.0 ± 1.6 b |
| phenidone | 20 µM | 6.1 ± 0.5 e |
| iodoacetamide | 0.12 mM | 64.0 ± 5.3 a |
| iodoacetamide | 4 mM | 6.4 ± 0.5 e |
| β-mercaptoethanol | 0.2 mM | 38.2 ± 1.8 c |
| β-mercaptoethanol | 1.4 mM | 9.2 ± 0.9 d |
| Substrate | Km (µM) | Vmax (U/mg) | Vmax/Km (U/mg/µM) |
|---|---|---|---|
| LA | 149 ± 1.2 d | 0.019 ± 0.001 d | 0.1275 ± 0.0033 a |
| LN | 592 ± 2.7 c | 0.031 ± 0.003 c | 0.0524 ± 0.0011 c |
| AA | 909 ± 2.0 b | 0.076 ± 0.007 b | 0.0836 ± 0.0015 b |
| DHA | 8766 ± 5.5 a | 0.214 ± 0.011 a | 0.0244 ± 0.0012 d |
| pH | 13-Z,E-HODE | 13-E,E-HODE | 9-Z,E-HODE | 9-E,E-HODE | Total HODEs 2 |
|---|---|---|---|---|---|
| 5.5 | 0.35 ± 0.005 b | 0.23 ± 0.005 a | 0.20 ± 0.006 d | 0.22 ± 0.005 a | 4.78 ± 0.14 b |
| 6.0 | 0.36 ± 0.008 b | 0.22 ± 0.007 a | 0.22 ± 0.007 c | 0.20 ± 0.007 b | 5.22 ± 0.09 a |
| 6.5 | 0.36 ± 0.004 b | 0.19 ± 0.005 b | 0.24 ± 0.008 b | 0.21 ± 0.004 ab | 4.62 ± 0.11 b |
| 7.0 | 0.37 ± 0.006 ab | 0.15 ± 0.006 c | 0.28 ± 0.006 a | 0.20 ± 0.008 b | 4.26 ± 0.16 c |
| 7.5 | 0.40 ± 0.007 a | 0.14 ± 0.004 c | 0.25 ± 0.009 b | 0.22 ± 0.009 a | 3.83 ± 0.13 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Liu, Y.; Ma, J.; Li, P.; Geng, Z.; Wang, D.; Zhang, M.; Xu, W. Recombinant Porcine 12-Lipoxygenase Catalytic Domain: Effect of Inhibitors, Selectivity of Substrates and Specificity of Oxidation Products of Linoleic Acid. Foods 2022, 11, 980. https://doi.org/10.3390/foods11070980
Xu J, Liu Y, Ma J, Li P, Geng Z, Wang D, Zhang M, Xu W. Recombinant Porcine 12-Lipoxygenase Catalytic Domain: Effect of Inhibitors, Selectivity of Substrates and Specificity of Oxidation Products of Linoleic Acid. Foods. 2022; 11(7):980. https://doi.org/10.3390/foods11070980
Chicago/Turabian StyleXu, Jiamei, Yu Liu, Jingjing Ma, Pengpeng Li, Zhiming Geng, Daoying Wang, Muhan Zhang, and Weimin Xu. 2022. "Recombinant Porcine 12-Lipoxygenase Catalytic Domain: Effect of Inhibitors, Selectivity of Substrates and Specificity of Oxidation Products of Linoleic Acid" Foods 11, no. 7: 980. https://doi.org/10.3390/foods11070980
APA StyleXu, J., Liu, Y., Ma, J., Li, P., Geng, Z., Wang, D., Zhang, M., & Xu, W. (2022). Recombinant Porcine 12-Lipoxygenase Catalytic Domain: Effect of Inhibitors, Selectivity of Substrates and Specificity of Oxidation Products of Linoleic Acid. Foods, 11(7), 980. https://doi.org/10.3390/foods11070980




