1. Introduction
Pig production is critical to Thailand’s food chain as the primary source of protein. Additionally, pig production has increased dramatically during the last decade [
1]. Commercial breeds such as the Large White, Landrace, Duroc, and crosses of these are the main breeds in this production system. Likewise, native pigs or breeds of indigenous and exotic races have been raised, including ‘Rad’, ‘Phuang’, and ‘Kwai’ [
2]. These genotypes are important to smallholder farming and highland agriculture in Thailand. However, the limitation of native pig breeds is that they grow slowly and their reproduction rates are lower than those of commercial breeds. On the other hand, they are well adapted to hot and humid climates and to low-quality feed [
1,
2]. As a result, various studies have been conducted to improve indigenous pigs’ productivity and meat quality through crossbreeding with commercial breeds such as Duroc, Large White, Pietrain, etc. [
3,
4,
5,
6] to respond to consumer demand. Furthermore, Glinoubol et al. [
5] reported that the Thai native pigs are known for their high intramuscular fat content. Therefore, it is interesting to study the potential of Thai crossbred pigs, especially the indicators of meat quality, such as intramuscular fat.
In recent years, a trend in pig production has been toward higher-quality pork. The intramuscular fat (IMF) content of pork is a critical factor in determining consumer acceptance. The critical elements for producing high-quality pork with a high IMF content are achieved through breeding selection and nutritional management [
7]. Numerous studies have been conducted to demonstrate that nutritional control strategies, specifically a reduced lysine level in a low-protein diet, had an effect on the accumulation of IMF in finisher pigs [
7,
8,
9,
10,
11]. Even so, there was concern that lysine- or protein-deficient diets may have an adverse effect on pig productivity. However, previous studies indicated that a lysine shortage in the diet had no effect on pigs’ productive performance [
7,
11]. Additionally, the rise in IMF content should be followed by an increase in high-quality fat in pig muscle. The fatty acid composition of the IMF has a significant impact on the quality aspects of pork processing and the nutritional value of pork for customers. This may be related to the inclusion of feedstuffs containing high-quality fatty acids to the pig diet. Functional substances can be added to feed to improve the n-3 fatty acid composition of pork. In a previous work, Okrouhlá et al. [
12] reported that pigs fed a diet supplemented with linseed exhibited a significantly increased content of polyunsaturated fatty acids (PUFAs), especially n-3 PUFAs. Tonnac and Mourot [
13] also reported that the content of n-3 PUFA content of pigs’
longissimus muscle and subcutaneous backfat increased in response to a dietary intake of linseed and microalgae rich in docosahexaenoic acid (DHA).
Perilla (
Perilla frutescens) is a Lamiaceae/Labiatae family member [
14]. It is extensively cultivated in Asian countries including China, Korea, India, and Thailand. Perilla is frequently utilized in cooking as a source of edible oil. Additionally, it has biological activities, for example antiviral, anti-inflammatory, and antioxidant activities. [
15,
16,
17]. Thailand produces approximately 272 tons of perilla seeds per year for perilla oil production, with approximately 60% of the by-products being perilla meal [
18,
19]. Perilla cake (PC) is a by-product of perilla seeds in the oil refinery industries via screw pressing. [
18]. PC is a rich source of PUFA, particularly alpha-linolenic acid (ALA) (55.97%), with a 10.52% ether extract (EE) content [
18]. Arjin et al. [
20] explained that perilla cake with a high ALA content might have potential as a feed supplement to enhance n-3 PUFA levels in pig tissues. For this reason, PC is interesting to consider as an alternative feedstuff for increasing IMF accumulation and modifying the fatty acid profile in pigs. In several studies, perilla meal was used to improve the fatty acid content in livestock. Hadi and Sudiyano [
21] showed that dietary supplementation of with perilla seed meal in ducks improved omega-3 fatty acid levels (0.94%). In addition, dietary supplementation of perilla oil in broilers results in greater levels of ALA, DHA, PUFA, and n-3 fatty acids [
22]. Moreover, growing pigs that received perilla cake supplementation in diet significantly increased the ALA content, whereas the n-6/n-3 ratio decreased significantly in the
longissimus muscle, backfat, and abdominal fat [
20]. However, no study has been conducted to determine the efficacy of supplementing PC in finishing pig diets with lysine shortage in order to improve IMF and fatty acid profiles. We postulate that lowering lysine consumption promotes IMF formation in late-stage pig muscle and that supplementing with PC improves the fatty acid composition. Therefore, the purpose of this study is to determine the influence of PC supplementation in low-lysine feed on the productive performance, meat quality, and fatty acid profile of finishing crossbred pigs.
4. Discussion
Pork production is critical in the modern world as a protein source. Historically, there was a strong emphasis on fat reduction in the breeding strategy for pigs as consumers demanded leaner pork, and the carcass value was largely determined by its backfat depth [
11]. Consumer preferences have shifted in recent years, with an emphasis on high-quality pork. Intramuscular fat is one of the indicators for determining pork quality. The increase in the IMF content of
longissimus muscle improved the meat tenderness and palatability for consumers purchasing pork loin [
7]. The critical factor in increasing the IMF content of the pigs’
longissimus muscles is nutritional modification, particularly the impact of dietary lysine levels. Consequently, we designed the experiment to reduce the quantity of lysine in the diet of late-phase pigs by roughly 30% relative to the recommended dietary requirement for pigs [
23], with the goal to improve IMF in the
longissimus muscles of finishing crossbred pigs. Additionally, we supplemented PC at 2.5 and 4.5 percent in the pigs’ diet with the assumption that it would increase the fat quality in pigs. We found that the productive performance, including ADFI, ADG, and FCR, did not different significantly from the control group (
p > 0.05). The was in agreement with previous studies showing that the performance of finishing pigs was not affected by lysine deficiency in the diet [
7,
11]. Our result has answered the concern that the decrease in dietary lysine may affect the productive performance of pigs. In terms of carcass features, the dietary lysine shortage and PC supplementation in the diet had no effect on carcass percentage, hot or chilled carcass weight, carcass length, or backfat thickness. However, there is exemption on ham and loin part in primal cutting. This result indicated that the ham and loin in the PC-supplemented low-lysine diet groups were significantly higher than those of the control group. This result contrasts with those of Palma-Granados et al. [
37], who observed that lysine deficiency resulted in reduced carcass components, most notably ham and loin.
The impact of PC supplementation in a low-lysine diet resulted in significantly increased IMF and marbling score in crossbred pigs’
longissimus muscles. Several studies have confirmed that a lower lysine content in the diet is associated with a higher IMF content in pork [
7,
11,
37]. In porcine muscle, a low-lysine diet increases the gene expression of glucose transporter protein 4 and peroxisome proliferator-activated receptor gamma (PPAR-γ), which is associated with increased mitochondrial oxidative enzyme activity [
11]. The PPAR-γ gene plays a central role in the regulation of adipogenesis, a process by which fibroblast-like preadipocytes differentiate into mature adipocytes [
38,
39]. Moreover, the activity of mitochondrial oxidative enzymes is positively correlated with the amount of IMF in bovine muscles [
11,
40]. Additionally, we hypothesized that the rise in IMF in lean pork was also related to PC supplementation. Several studies indicated that dietary fat sources for pigs could increase the fatty acid content and IMF of the pork [
41,
42,
43]. Cold-pressed perilla seed oil yields a by-product known as PC, which is high in protein (31.54%) and crude fat (10.52%). Typically, it is utilized as a source of protein in animal feeds. However, due to its high EE content and high alpha-linolenic acid content, it was added as a functional feedstuff for enhancing the fatty acid composition of animal products [
18,
20]. A recent study demonstrated that supplementation with perilla seed extract significantly increased the IMF content of fattening cattle’s
longissimus muscle. Additionally, it greatly improved the activity of fatty acid accumulation enzymes, such as fatty acid synthetase and acetyl-coenzyme A carboxylase, and upregulated the PPAR-γ gene expression in fattening cattle’s
longissimus muscle [
44]. Moreover, our results demonstrated that the substantial fat deposition in the LTL muscle of pigs receiving PC supplementation in the low-lysine diet groups resulted in a much greater marbling score than those of the control group. This is consistent with earlier research demonstrating the positive correlation of IMF content on the marbling score in
longissimus muscle [
7,
45,
46]. The supplementation of PC in finishing pigs fed low-lysine diet significantly decreased boiling loss in pork when compared to the control group. Drip loss and boiling loss are presented in this research as measures of the water-holding capacity (WHC) of meat. The WHC influences the juiciness of cooked meats, which may affect consumers’ perceptions of tenderness [
47,
48]. In this investigation, there was no difference in drip loss across the groups, which was in agreement with Witte et al. [
49], who showed that the dietary differential levels of lysine did not affect drip loss. One of the cooking loss parameters examined in this research was boiling loss. It was discovered that the control group’s boiling loss was significantly greater than that in the PC supplemented low-lysine diet groups. Aaslyng et al. [
50] reported that meat with a low WHC (high drip loss, high thawing loss, and high internal reflection) and a low pH had a larger cooking loss, but meat with a medium or high WHC or pH had no impact. Additionally, they discovered that meat with a low IMF content had a significantly higher cooking loss at 80 °C center temperature than the meat with a high IMF level [
50]. In this study, it was found that the pigs receiving PC supplementation in the low-lysine diet had a significantly lower shear force value than that in the control group. The shear force technique was utilized to determine the tenderness of meat when the IMF was increased in pig loin muscle. Our findings agreed with Ramsey et al. [
51] and Jankowiak et al. [
46] who reported that the meat with a high IMF content had a lower shear force value and was more tender than meat with a low IMF content.
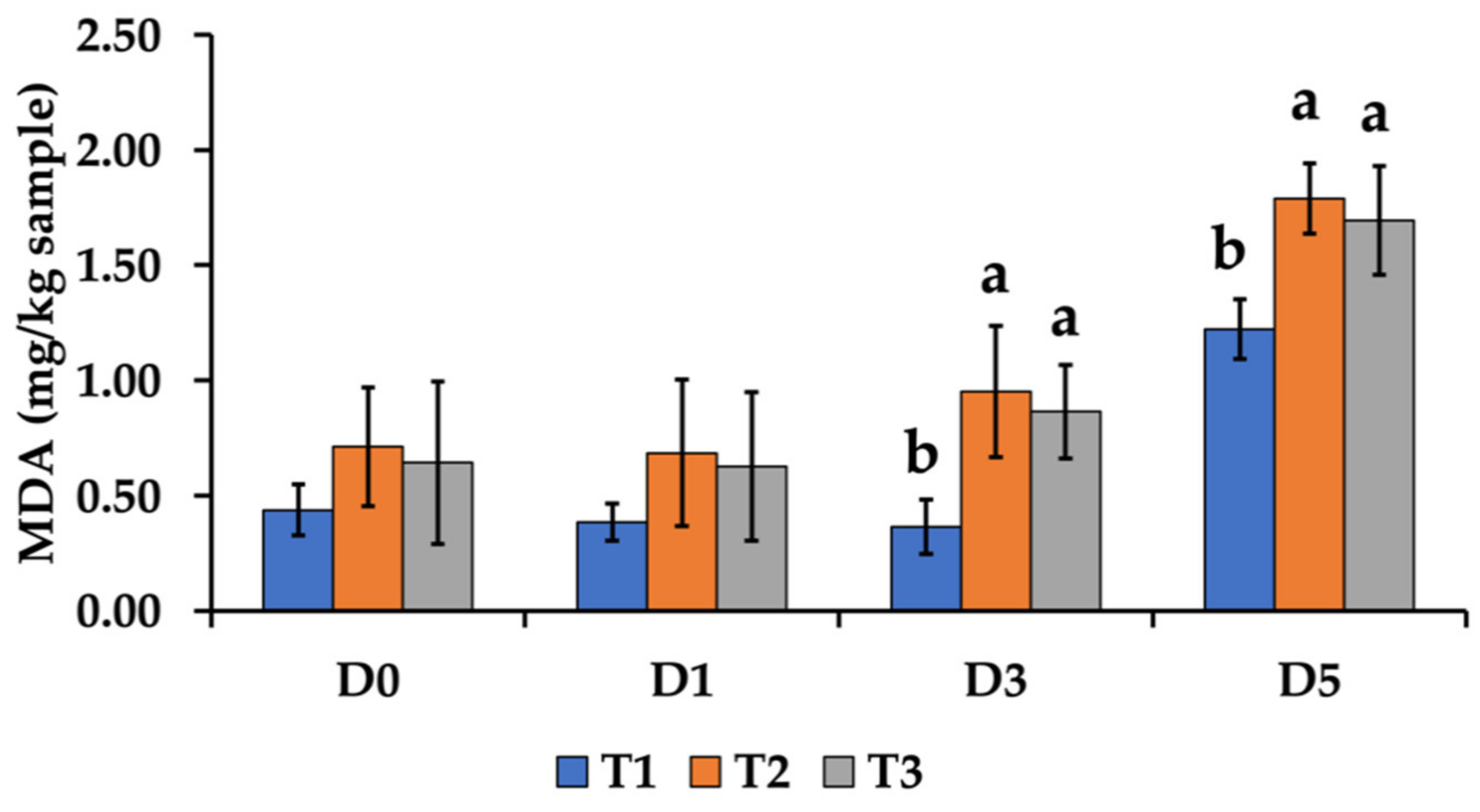
Meat lipid peroxidation is one of the characteristics to consider when developing high-fat meat. Malondialdehyde (MDA) is an ubiquitous aldehyde generated during secondary lipid oxidation and is a widely used oxidation indicator, resulting in an increase in plasma and tissue levels of thiobarbituric acid reactive substances (TBARS) [
52]. Our result show that the supplementation of PC in a low-lysine diet significantly affected MDA content in LDL muscle from day 3. Therefore, we hypothesized that it was related to the high IMF content and fatty acid composition of the meat. However, Chaiwang et al. [
3] explained that the composition of fat was more important than the quantity of fat in meat, as the susceptibility of muscular lipids to peroxidation is dependent on the amount of polyunsaturated fatty acids. This was consistent with our results showing that supplementation of PC to a low-protein diet had a significant effect on the high PUFA composition of LTL muscle. According to Reitznerová et al. [
52], an increase in the MDA value was correlated with an increase in the lipid content; thus, increasing FFA levels in samples resulted in an increase in the MDA content. Furthermore, we added PC to a low-lysine diet in this study to improve the quality of fatty acids in pork. The fatty acid composition of animal products is regulated by both fatty acid production in animal tissues and the lipids included in the feedstuffs ingested by animals [
53]. By integrating a suitable fat source into the diet, it was feasible to modify the fatty acid composition and relationship between n-6 and n-3 fatty acids in pig tissues [
20,
54]. In this study, supplementation of PC to a low-lysine diet contributed to a significant increase in ALA and PUFA levels in the investigated tissues, whereas the ratio of n-6 to n-3 was considerably lower in the groups of PC supplementation. The result agreed with the report of Arjin et al. [
20], who explained that the increase in PUFA and ALA levels while the n-6/n-3 ratio decreased in abdominal fat, backfat, and
longissimus muscle of growing pigs, was affected by PC supplementation in the diet. PC is used as an oily plant that contains high levels of PUFAs, especially ALA (55.97%) [
18]. As a result, we hypothesize that this is a possible explanation for the high ALA content of tissues. However, ALA deposition is dependent on a variety of parameters, including diet and tissue type. Moreover, the PUFA/SFA ratio was significantly higher in the PC-supplemented low-lysine diet groups in backfat and LTL muscle. Genetics had the greatest influence on the PUFA/SFA ratio, followed by nutrition (mostly the animal’s overall fat and intramuscular fat content) [
55]. Nonetheless, only T3 in LTL muscle had a higher PUFA/SFA ratio than that recommended by the UK Department of Health [
56], which is a minimum PUFA/SFA ratio of 0.4 in pork. The increased n-3 fatty acid content in the pigs affected by the supplementation of PC in the low-lysine diet leads to a lower n-6/n-3 ratio. In pig tissues, the fatty acid composition, PUFA/SFA, and n-6/n-3 ratios are greatly impacted by the fat sources in the diet [
20,
57]. In this study, PC supplementation resulted in a high amount of ALA in the food, which may have an effect on the fatty acid composition of pigs, especially the ALA level. ALA is a member of the n-3 (omega-3) fatty acids that play an important role in mitigating the risk of cardiovascular diseases. Therefore, improving the pork quality by reducing lysine level by supplementation with high fat content feedstuffs such as PC is an interesting strategy to create functional meat that is highly palatable and has a high-quality fatty acid profile that meets modern consumer expectations.